Effect of Thermal Cycle on Microstructure Evolution and Mechanical Properties of Selective Laser Melted Low-Alloy Steel
Abstract
:1. Introduction
2. Experimental Materials and Methods
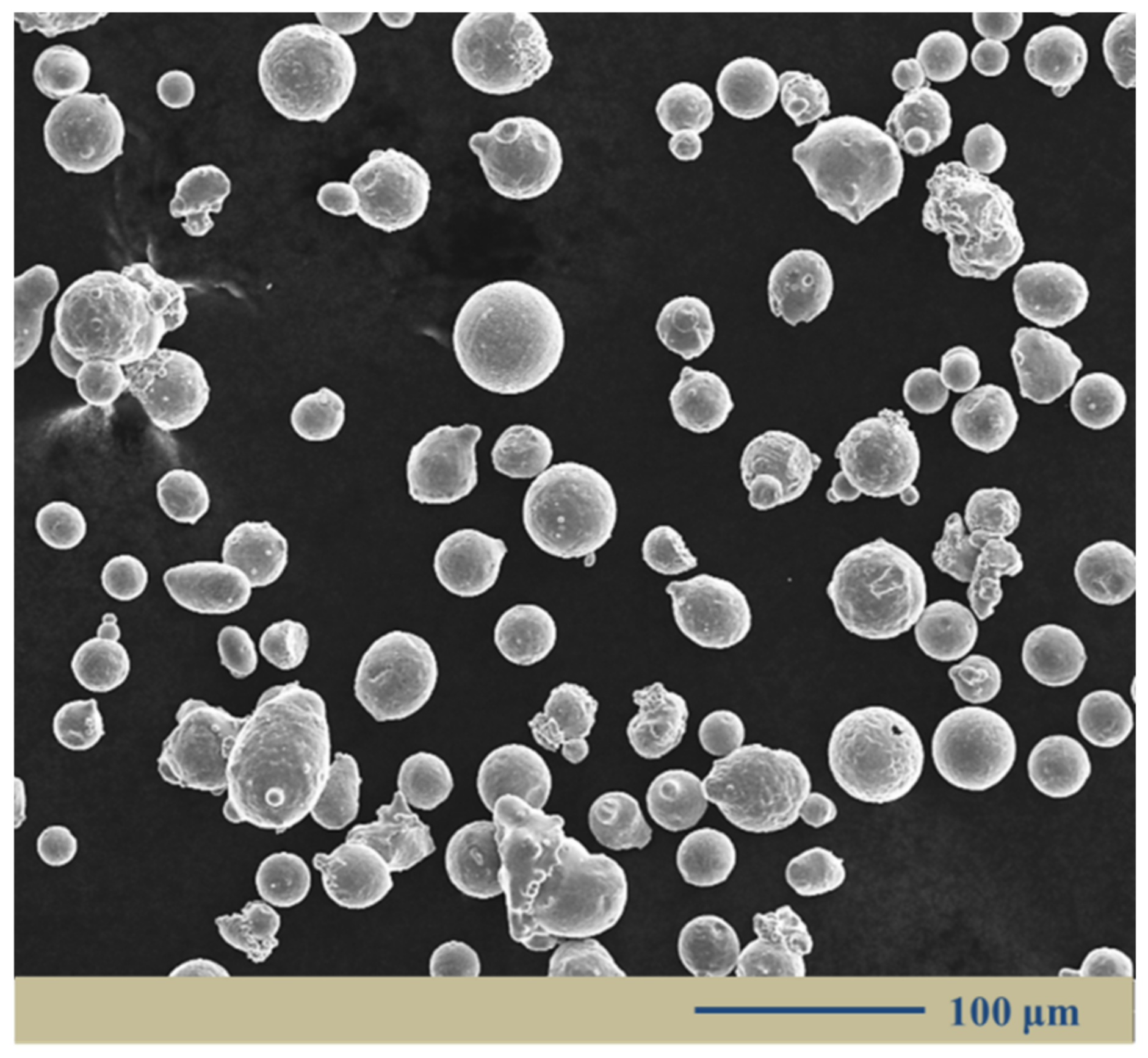
2.1. Powder Material
2.2. Microstructural Characterization and Mechanical Test
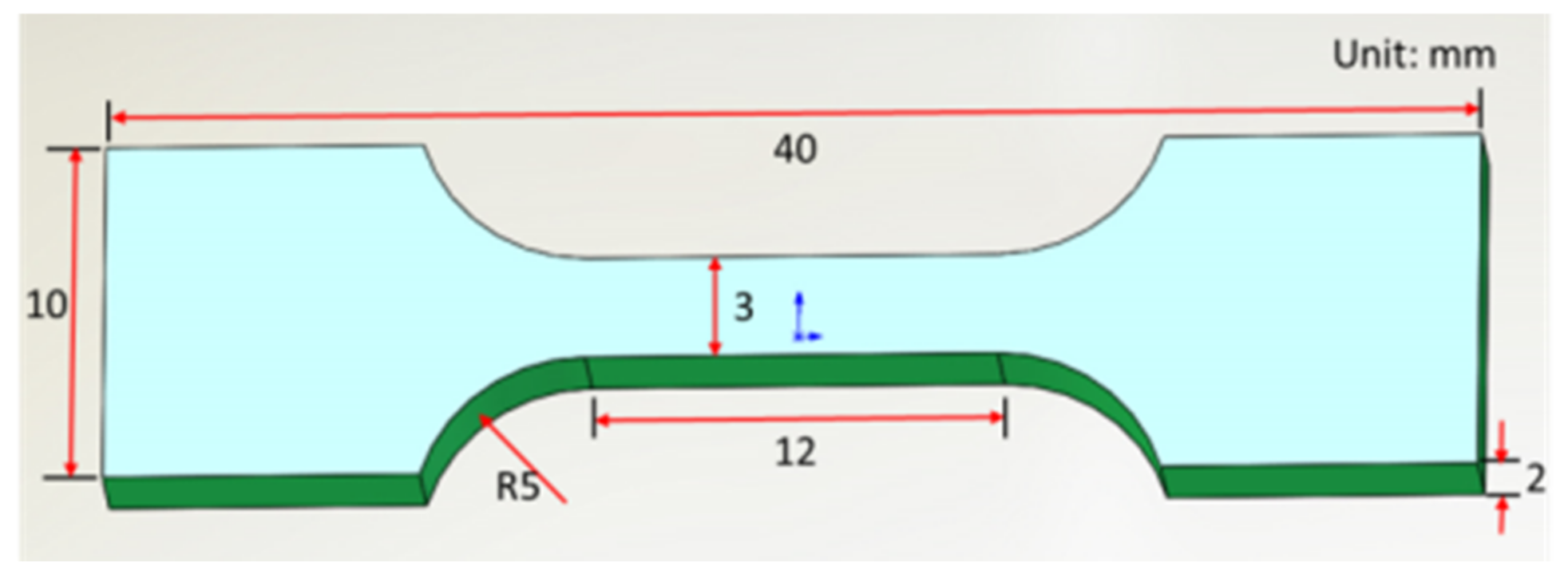
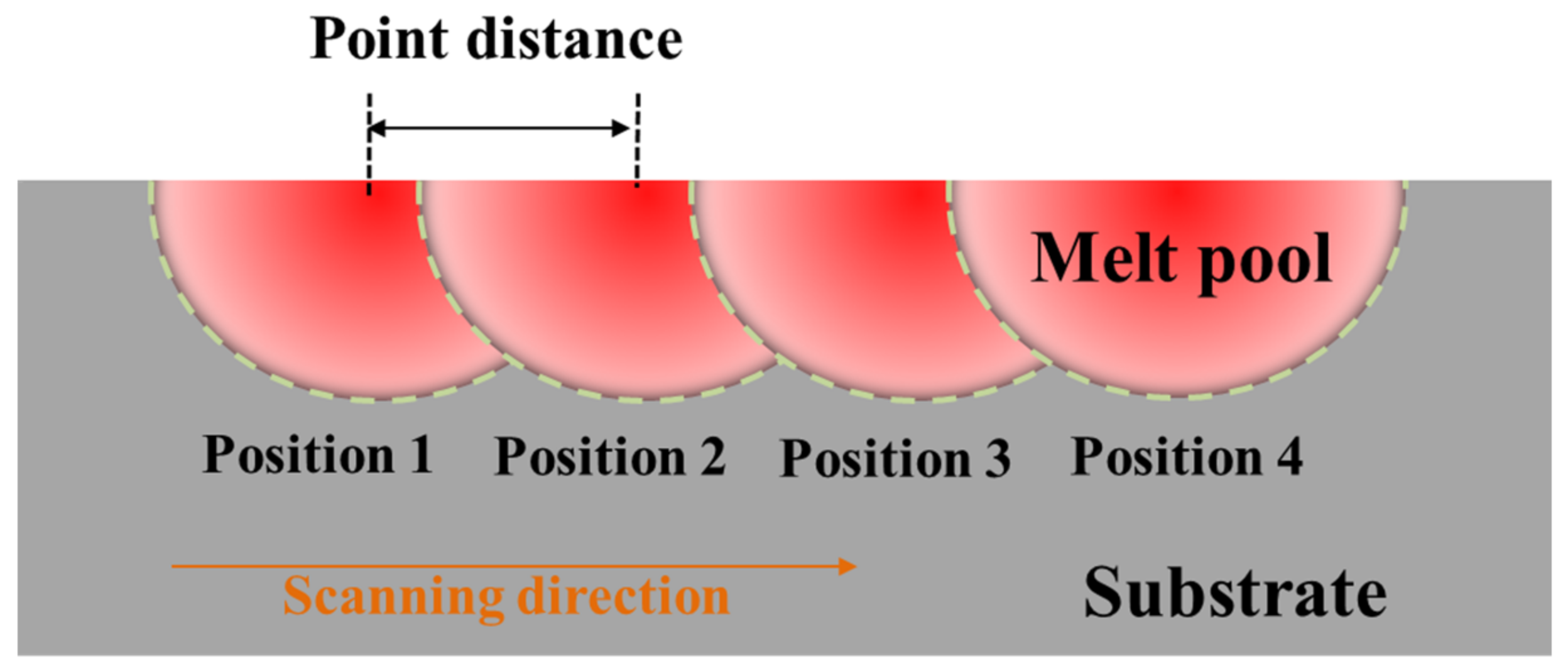
2.3. SLM Process
3. Results and Discussion
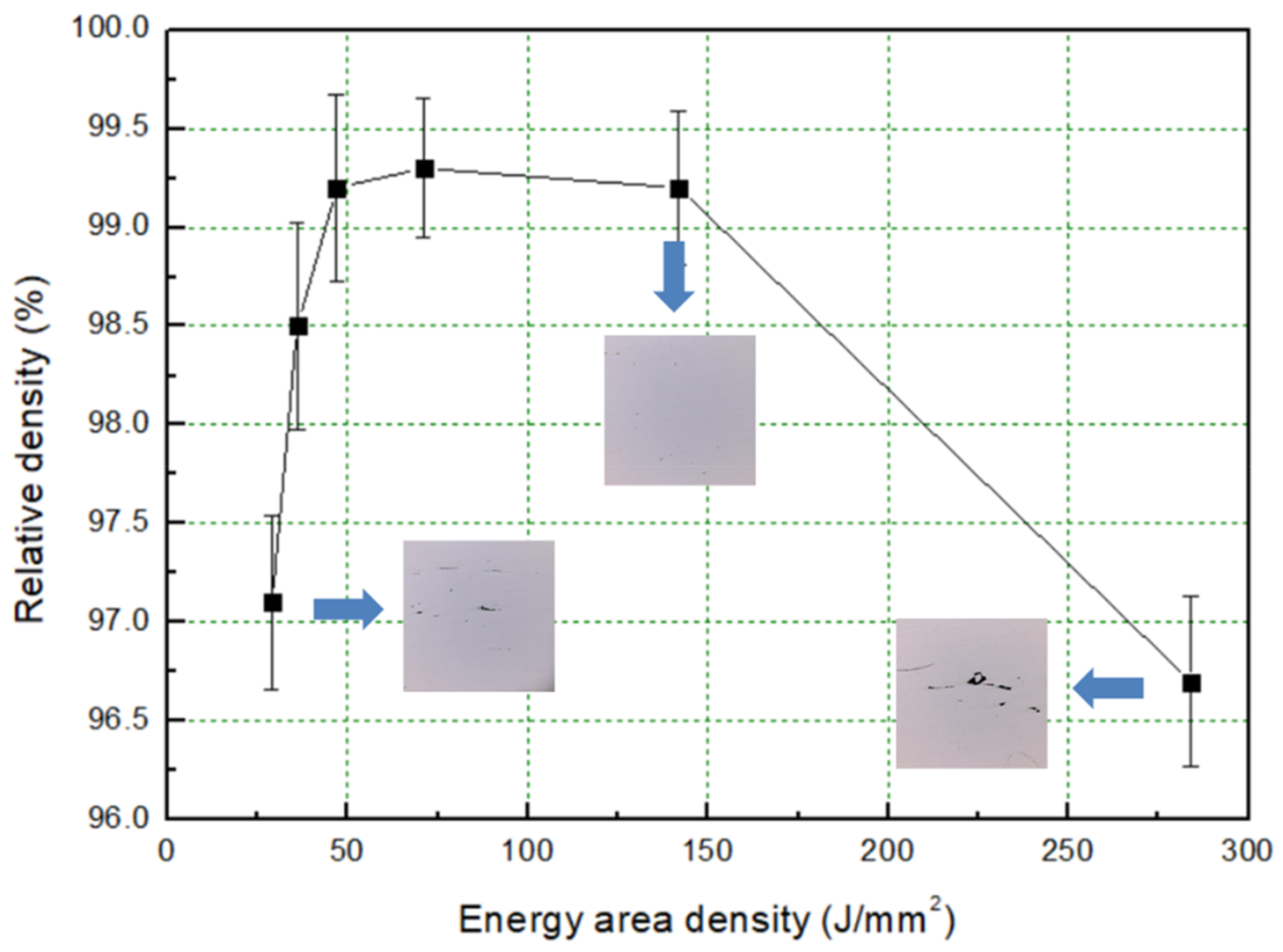
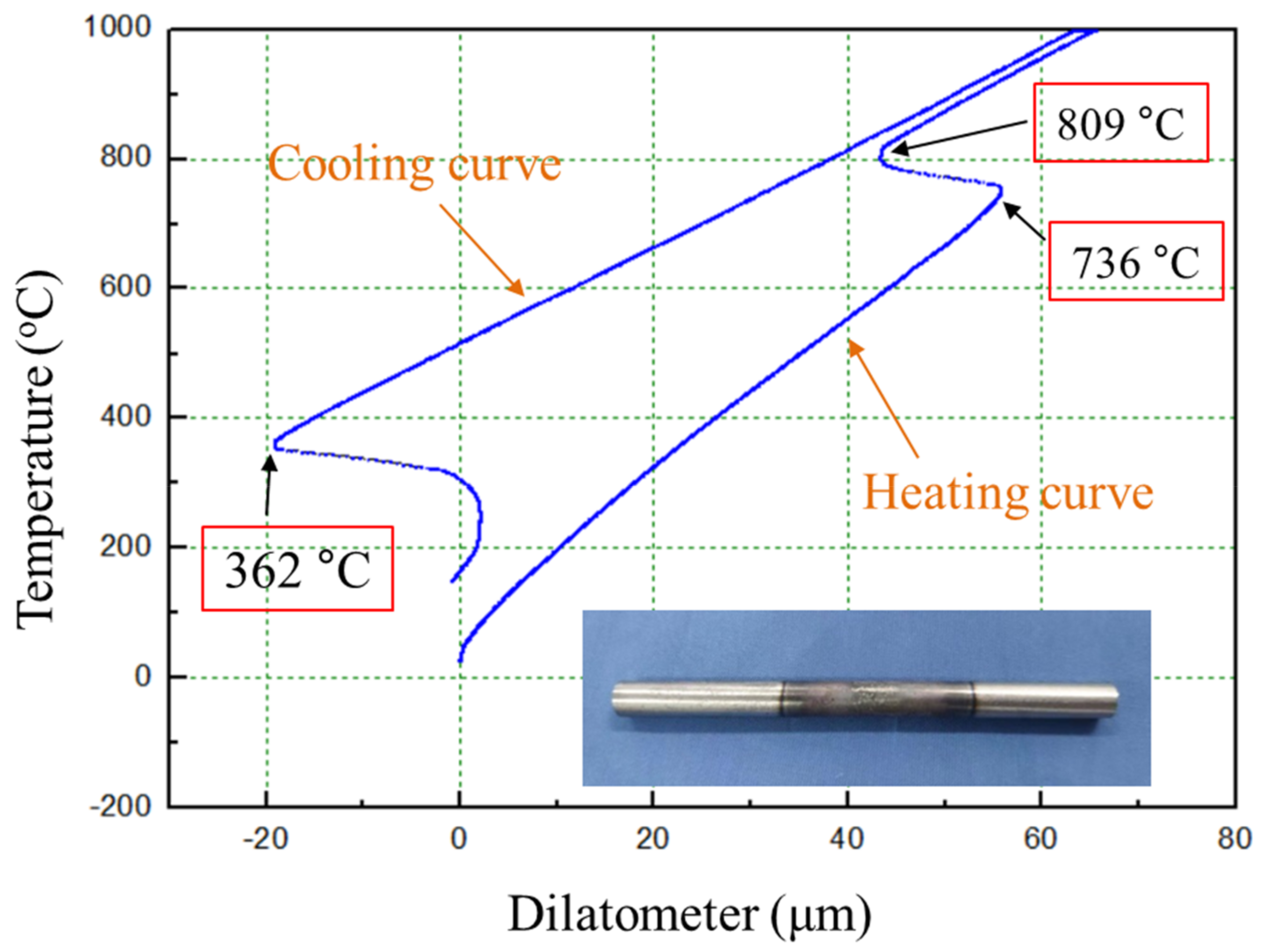
3.1. Formability Characterization
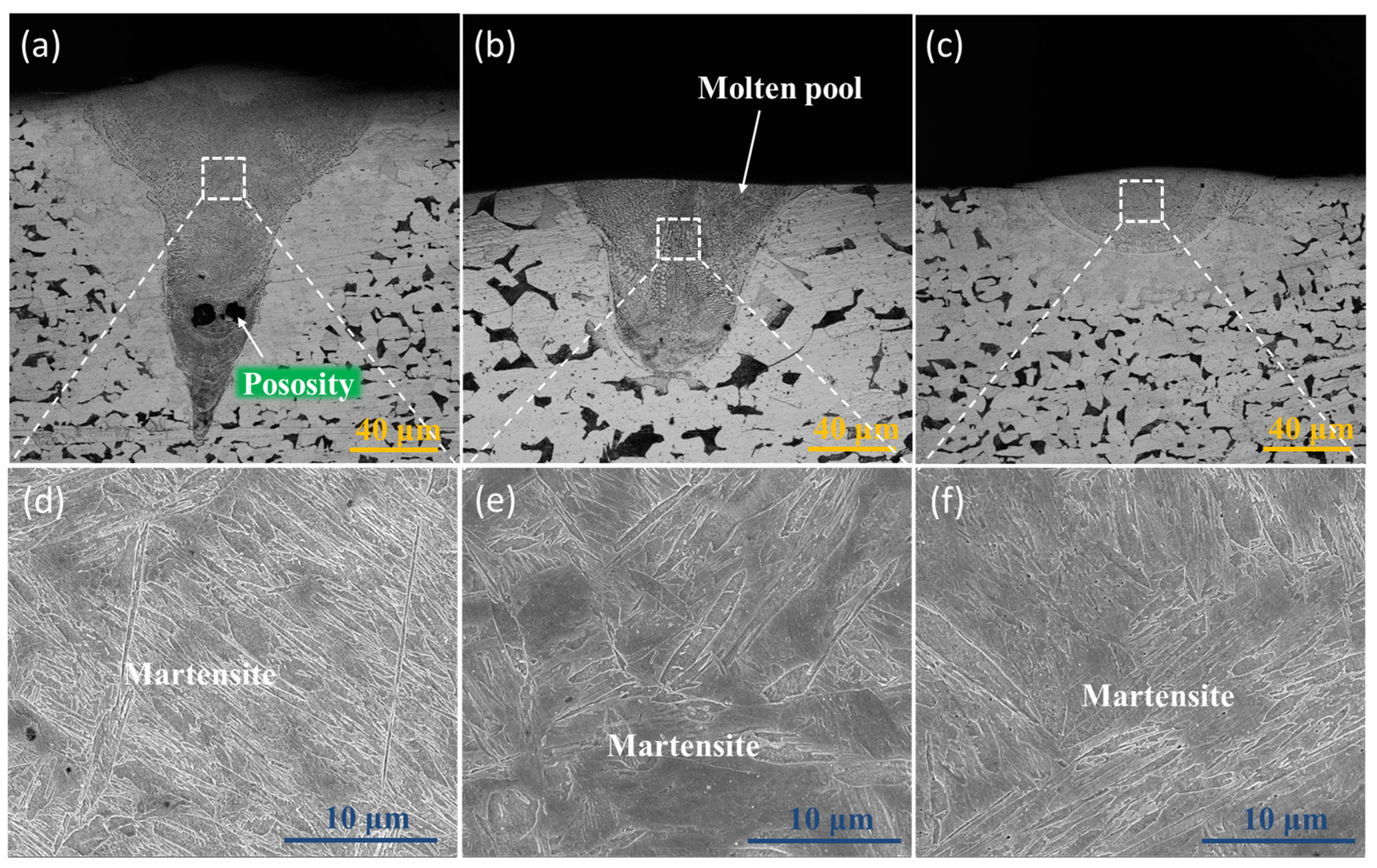
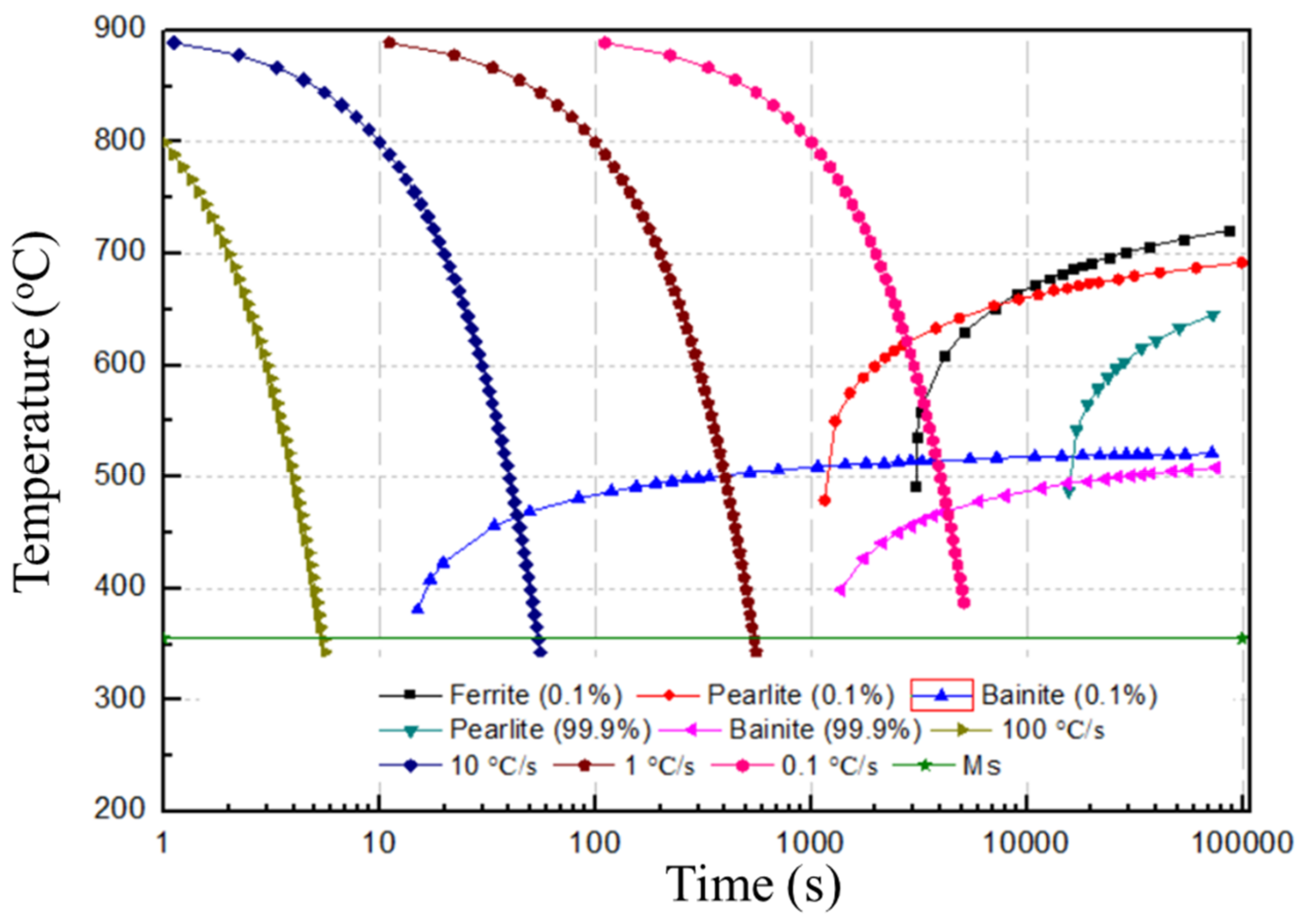
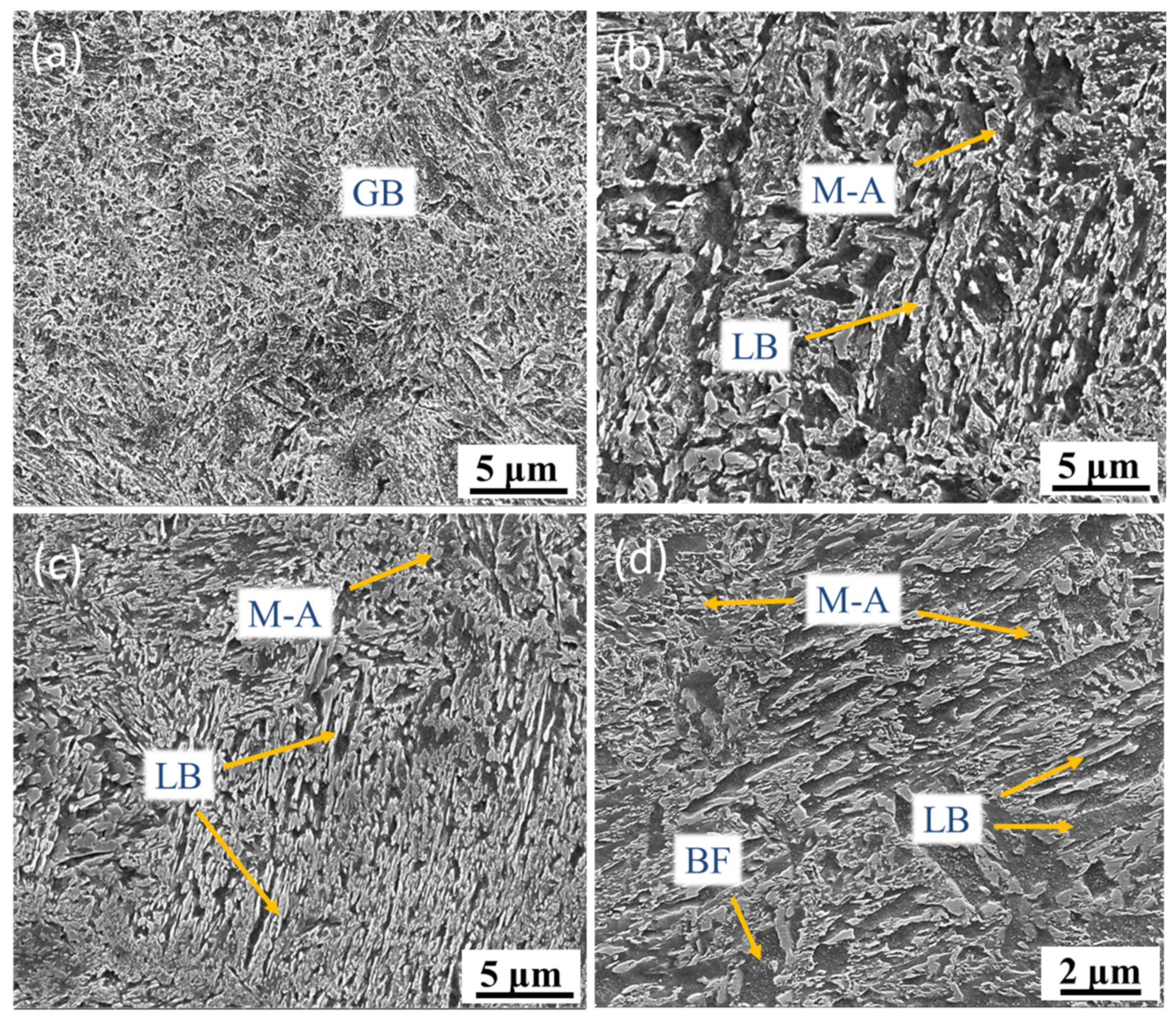
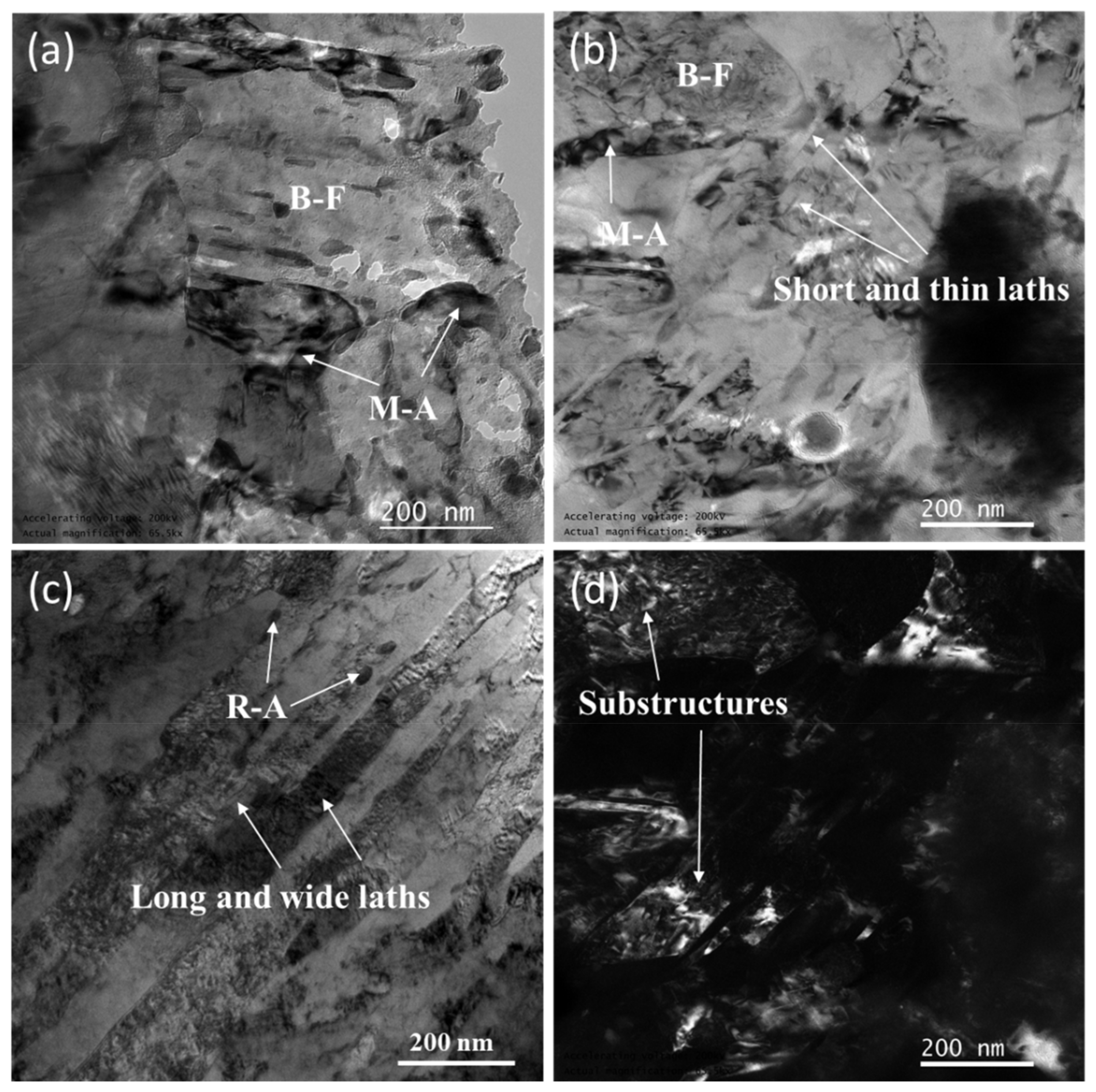
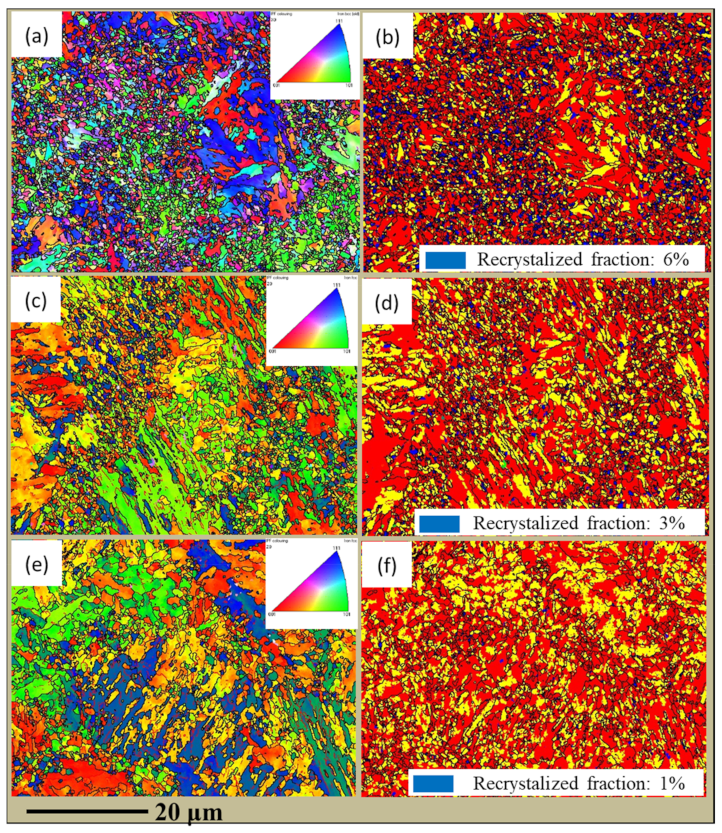
3.2. Microstructural Characterization
3.3. Tensile Tests Analysis
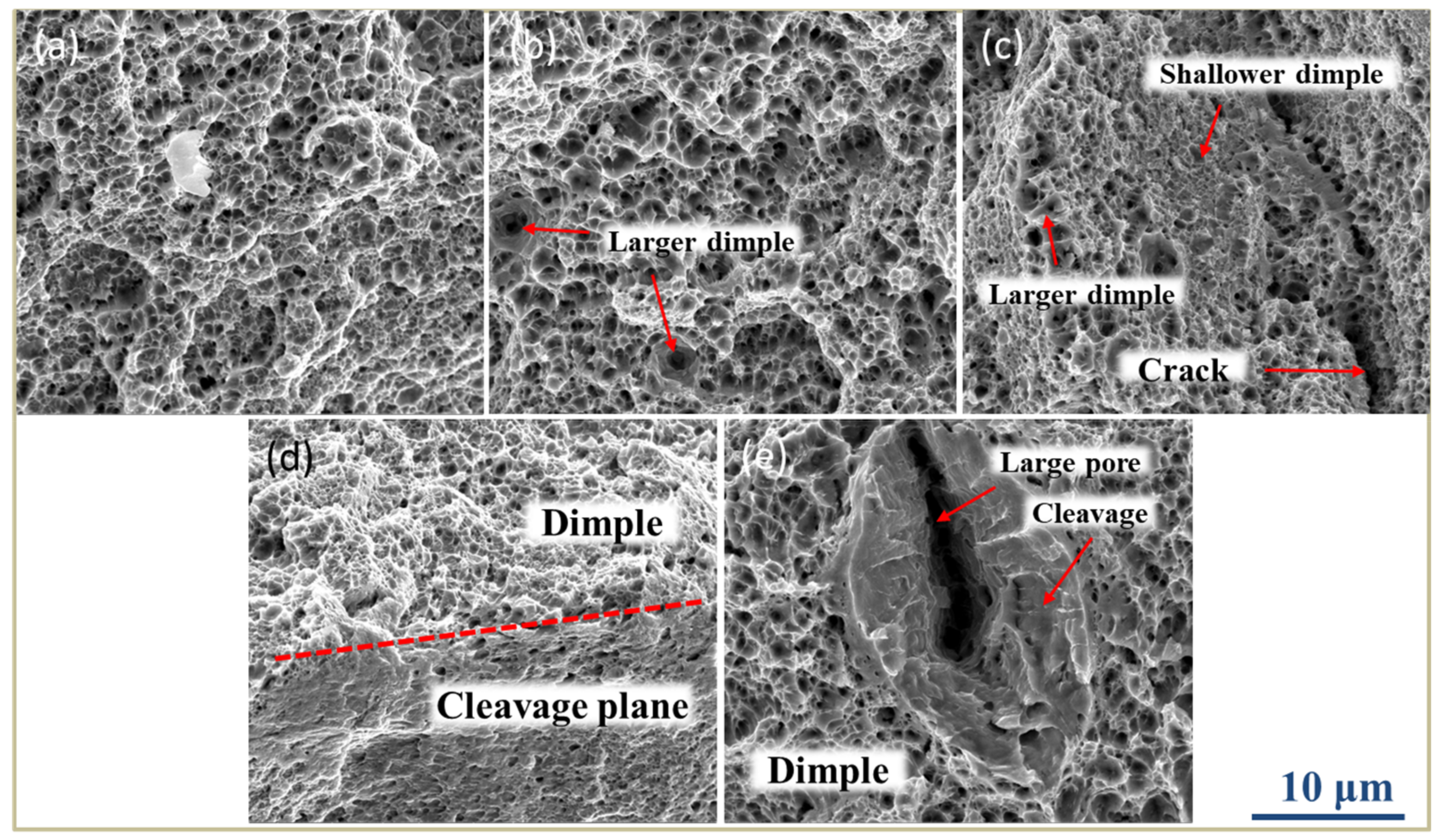
3.4. Fracture Analysis
4. Conclusions
- (1)
- Thermal cycling has a decisive effect on the microstructure evolution of SLM low-alloy steel samples. The initial solidification microstructure of the molten pool is martensite. With the increase of EAD, martensite gradually transforms into a mixed microstructure of bainite and martensite–austenite, and into granular bainite in turn. Bainite ferrite gradually changes from lath to a multilateral structure under the action of subsequent thermal cycles.
- (2)
- Thermal cycling also has a crucial effect on the grain size of the SLM low-alloy steel samples. The average grain size is 1.56 µm, 3.98 µm, and 6.31 µm at EADs of 142 J/mm2, 71 J/mm2, and 47 J/mm2, respectively. Thus, with the increase of EAD, the grain size is remarkably reduced due to the increase of austenitizing cycles and temperature.
- (3)
- With the increase of EAD, yield strength and tensile strength of the low-alloy steel increase and their values far exceed those of the alloys prepared by traditional methods and those of alloy steels reported in literature. The change of elongation with EAD is not obvious.
- (4)
- The grain size and microstructure are uneven in SLM low-alloy steel samples. The inhomogeneity becomes more significant with the decrease of EAD. When EAD is below 47 J/mm2, the fracture mechanism of the SLM low-alloy steel sample changes from ductile to a mixture of ductile and brittle fracture.
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, L.C.; Attar, H.; Calin, M.; Eckert, J. Review on manufacture by selective laser melting and properties of titanium based materials for biomedical applications. Mater. Process. Rep. 2016, 31, 66–76. [Google Scholar] [CrossRef]
- Yan, L.; Yuan, Y.; Ouyang, L.; Li, H.; Mirzasadeghi, A.; Li, L. Improved mechanical properties of the new Ti-15Ta- x Zr alloys fabricated by selective laser melting for biomedical application. J. Alloy. Compd. 2016, 688, 156–162. [Google Scholar] [CrossRef]
- Di, W.; Yimeng, W.; Shibiao, W.; Hui, L.; Yongqiang, Y.; Shicai, F. Customized a Ti6Al4V Bone Plate for Complex Pelvic Fracture by Selective Laser Melting. Materials 2017, 10, 35. [Google Scholar]
- Grigoriev, A.; Polozov, I.; Sufiiarov, V.; Popovich, A. In-situ synthesis of Ti 2 AlNb-based intermetallic alloy by selective laser melting. J. Alloy. Compd. 2017, 704, 434–442. [Google Scholar] [CrossRef]
- Wei, K.; Wang, Z.; Zeng, X. Preliminary investigation on selective laser melting of Ti-5Al-2.5Sn α-Ti alloy: From single tracks to bulk 3D components. J. Mater. Process. Technol. 2017, 244, 73–85. [Google Scholar] [CrossRef]
- Gu, D.; Hagedorn, Y.C.; Meiners, W.; Meng, G.; Poprawe, R. Densification behavior, microstructure evolution, and wear performance of selective laser melting processed commercially pure titanium. Acta Mater. 2012, 60, 3849–3860. [Google Scholar] [CrossRef]
- Mao, Z.; Zhang, D.Z.; Wei, P.; Zhang, K. Manufacturing Feasibility and Forming Properties of Cu-4Sn in Selective Laser Melting. Materials 2017, 10, 333. [Google Scholar] [CrossRef]
- Zhang, G.; Chen, C.; Wang, X.; Wang, P.; Zhang, X.; Gan, X. Additive manufacturing of fine-structured copper alloy by selective laser melting of pre-alloyed Cu-15Ni-8Sn powder. Int. J. Adv. Manuf. Technol. 2018, 96, 1–8. [Google Scholar] [CrossRef]
- Kimura, T.; Nakamoto, T. Microstructures and mechanical properties of A356 (AlSi7Mg0.3) aluminum alloy fabricated by selective laser melting. Mater. Des. 2015, 89, 1294–1301. [Google Scholar] [CrossRef]
- Saedi, S.; Turabi, A.S.; Andani, M.T.; Haberland, C.; Karaca, H.; Elahinia, M. The influence of heat treatment on the thermomechanical response of Ni-rich NiTi alloys manufactured by selective laser melting. J. Alloy. Compd. 2016, 677, 204–210. [Google Scholar] [CrossRef]
- Yan, C.; Hao, L.; Hussein, A.; Young, P.; Raymont, D. Advanced lightweight 316l stainless steel cellular lattice structures fabricated via selective laser melting. Mater. Des. 2014, 55, 533–541. [Google Scholar] [CrossRef]
- Almangour, B.; Grzesiak, D.; Yang, J.M. Rapid fabrication of bulk-form TiB2/316L stainless steel nanocomposites with novel reinforcement architecture and improved performance by selective laser melting. J. Alloy. Compd. 2016, 680, 480–493. [Google Scholar] [CrossRef]
- Shang, Y.; Yuan, Y.; Li, D.; Li, Y.; Chen, J. Effects of scanning speed on in vitro biocompatibility of 316L stainless steel parts elaborated by selective laser melting. Int. J. Adv. Manuf. Technol. 2017, 92, 1–7. [Google Scholar] [CrossRef]
- Kurzynowski, T.; Gruber, K.; Stopyra, W.; Kuźnicka, B.; Chlebus, E. Correlation between process parameters, microstructure and properties of 316L stainless steel processed by selective laser melting. Mater. Sci. Eng. A 2018, 718, 64–73. [Google Scholar] [CrossRef]
- Almangour, B.; Grzesiak, D.; Cheng, J.; Ertas, Y. Thermal behavior of the molten pool, microstructural evolution, and tribological performance during selective laser melting of TiC/316L stainless steel nanocomposites: Experimental and simulation methods. J. Mater. Process. Technol. 2018, 257, 288–301. [Google Scholar] [CrossRef]
- Di, W. Investigation of crystal growth mechanism during selective laser melting and mechanical property characterization of 316L stainless steel parts. Mater. Des. 2016, 100, 291–299. [Google Scholar]
- Zhou, Y.; Chen, S.; Chen, X.; Cui, T.; Liang, J.; Liu, C. The evolution of bainite and mechanical properties of direct laser deposition 12CrNi2 alloy steel at different laser power. Mater. Sci. Eng. A 2019, 742, 150–161. [Google Scholar] [CrossRef]
- Mingwei, W.; Suiyuan, C.; Lianyun, X.; Jing, L.; Changsheng, L. Selective laser melting of 24CrNiMo steel for brake disc: Fabrication efficiency, microstructure evolution, and properties. Opt. Laser Technol. 2018, 107, 99–109. [Google Scholar]
- Zuo, P.; Chen, S.; Wei, M.; Zhou, L.; Liang, J.; Liu, C.; Wang, M. Microstructure evolution of 24CrNiMoY alloy steel parts by high power selective laser melting. J. Manuf. Process. 2019, 44, 28–37. [Google Scholar] [CrossRef]
- Lianfeng, W.; Xiaohui, J.; Yihong, Z.; Xiaogang, Z.; Jing, S.; Biao, Y. An approach to predict the residual stress and distortion during the selective laser melting of AlSi10Mg parts. Int. J. Adv. Manuf. Technol. 2018, 97, 3535–3546. [Google Scholar]
- Yan, J.J.; Zheng, D.L.; Li, H.X.; Jia, X.; Yan, M. Selective laser melting of H13: Microstructure and residual stress. J. Mater. Sci. 2017, 52, 12476–12485. [Google Scholar] [CrossRef]
- Li, Z.; Li, B.-Q.; Bai, P.; Liu, B.; Wang, Y. Research on the Thermal Behaviour of a Selectively Laser Melted Aluminium Alloy: Simulation and Experiment. Materials 2018, 11, 1172. [Google Scholar] [CrossRef] [PubMed]
- Karimi, P.; Raza, T.; Andersson, J.; Svensson, L.E. Influence of laser exposure time and point distance on 75-μm-thick layer of selective laser melted Alloy 718. Int. J. Adv. Manuf. Technol. 2017, 94, 2199–2207. [Google Scholar] [CrossRef]
- Ye, D.; Hong, G.S.; Zhang, Y.; Zhu, K.; Fuh, J.Y.H. Defect detection in selective laser melting technology by acoustic signals with deep belief networks. Int. J. Adv. Manuf. Technol. 2018, 96, 2791–2801. [Google Scholar] [CrossRef]
- Akita, M.; Uematsu, Y.; Kakiuchi, T.; Nakajima, M.; Kawaguchi, R. Defect-dominated fatigue behavior in type 630 stainless steel fabricated by selective laser melting. Mater. Sci. Eng. A 2016, 666, 19–26. [Google Scholar] [CrossRef]
- Das, M.; Balla, V.K.; Basu, D.; Bose, S.; Bandyopadhyay, A. Laser processing of SiC-particle-reinforced coating on titanium. Scr. Mater. 2010, 63, 438–441. [Google Scholar] [CrossRef]
- Xuezhi, S.; Shuyuan, M.; Changmeng, L.; Cheng, C.; Qianru, W.; Xianping, C. Performance of High Layer Thickness in Selective Laser Melting of Ti6Al4V. Materials 2016, 9, 975. [Google Scholar] [Green Version]
- Ran, X.; Liu, D.; Li, A.; Wang, H.; Tang, H.; Cheng, X. Microstructure characterization and mechanical behavior of laser additive manufactured ultrahigh-strength AerMet100 steel. Mater. Sci. Eng. A 2016, 663, 69–77. [Google Scholar] [CrossRef]
- Almangour, B.; Grzesiak, D.; Borkar, T.; Yang, J.M. Densification behavior, microstructural evolution, and mechanical properties of TiC/316L nanocomposites fabricated by selective laser melting. Mater. Des. 2017, 138, 119–128. [Google Scholar] [CrossRef]
- Leslie, W.C. The Physical Metallurgy of Steels; Hempisphere Pub. Corp: New York, NY, USA, 1981. [Google Scholar]
- Cooper, K.P.; Jones, H.N. Phase transformation-induced grain refinement in rapidly solidified ultra-high-carbon steels. Metall. Mater. Trans. A 2002, 33, 2789–2799. [Google Scholar] [CrossRef]
- Grange, R.A. The rapid heat treatment of steel. Metall. Trans. 1971, 2, 65–78. [Google Scholar] [CrossRef]
- Guan, T.; Chen, S.; Chen, X.; Liang, J.; Liu, C.; Wang, M. Effect of laser incident energy on microstructures and mechanical properties of 12CrNi2Y alloy steel by direct laser deposition. J. Mater. Sci. Technol. 2018. [Google Scholar] [CrossRef]



| Element | C | Mn | Ni | Mo | Y | Fe |
|---|---|---|---|---|---|---|
| Content | 0.15–0.25 | 0.6 | 1.0 | 0.5 | 0.01–0.05 | Bal. |
| Layer Thickness (µm) | Laser Power (W) | Point Distance (µm) | Exposure Time (µs) | Scan Speed (mm/s) | EAD (J/mm2) |
|---|---|---|---|---|---|
| 50 | 200 | 10 | 1000 | 10 | 284 |
| 50 | 200 | 10 | 500 | 20 | 142 |
| 50 | 200 | 10 | 250 | 40 | 71 |
| 50 | 200 | 10 | 166 | 60 | 47 |
| 50 | 200 | 10 | 125 | 80 | 36 |
| 50 | 200 | 10 | 100 | 100 | 29 |
| Temperature (°C) | Density (Kg/m3) | Thermal Conductivity (W/m K) | Specific Heat (J/kg K) |
|---|---|---|---|
| 25 | 7841 | 34.9 | 447 |
| 100 | 7820 | 33.4 | 450 |
| 300 | 7818 | 33.6 | 521 |
| 500 | 7827 | 30.4 | 615 |
| 700 | 7717 | 27.3 | 880 |
| 900 | 7609 | 27.0 | 609 |
| 1100 | 7504 | 29.5 | 639 |
| 1300 | 7399 | 31.9 | 673 |
| 1450 | 7347 | 33.2 | 696 |
| 1470 | 7193 | 33.3 | 1071 |
| 1510 | 7071 | 33.5 | 11,507 |
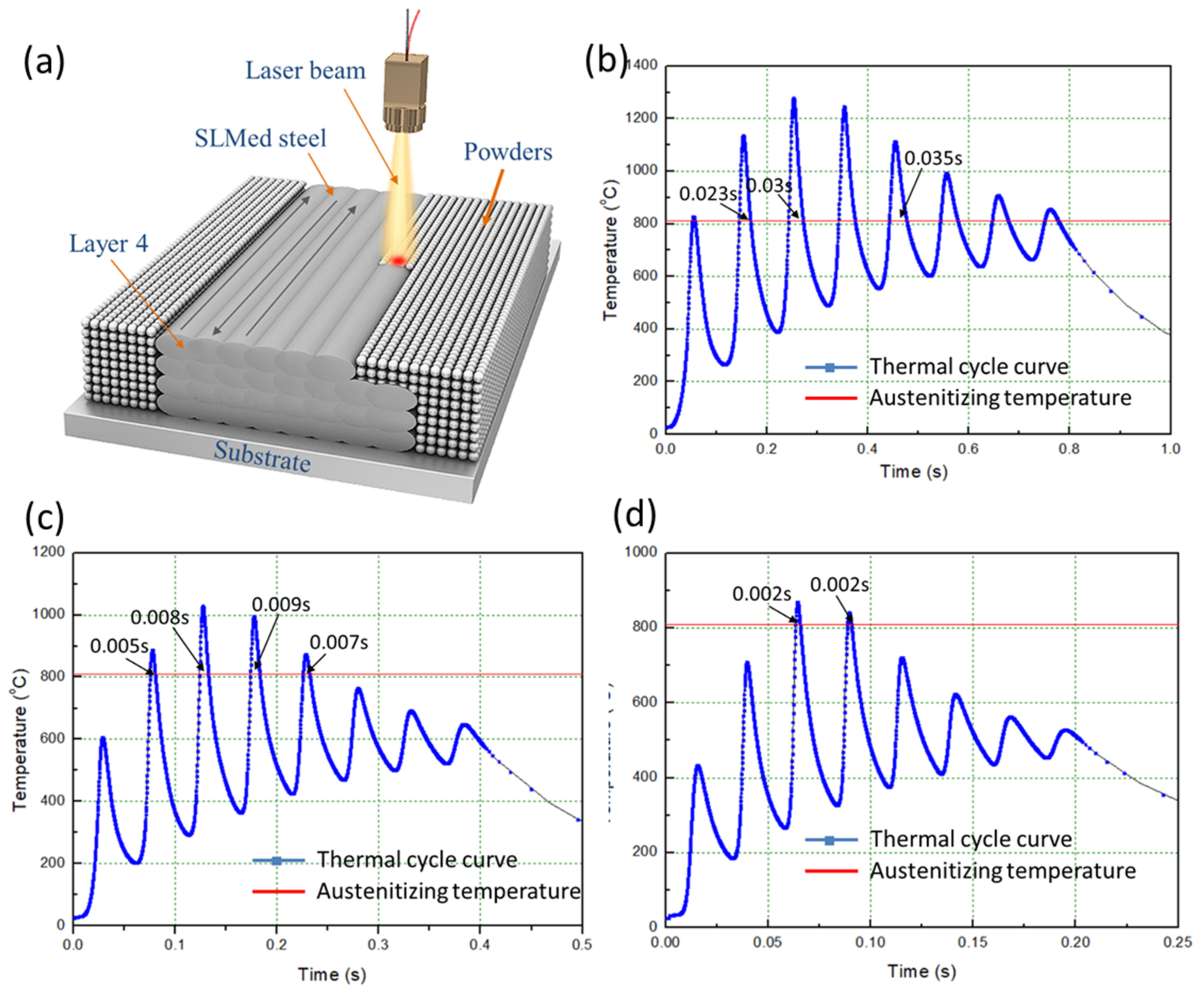
| EAD (J/mm2) | Austenitizing Times | Maximum Heating Temperature (°C) | Heating Rate (°C/s) | Austenitizing Time (s) |
|---|---|---|---|---|
| 142 | 7 | 1279 | 2.7 × 104 | 0.03 |
| 71 | 4 | 1029 | 5.2 × 104 | 0.008 |
| 47 | 2 | 868 | 10 × 104 | 0.002 |
| Sample Description | YS (MPa) | UTS (MPa) | EL (%) |
|---|---|---|---|
| EAD = 142 J/mm2 | 1256 | 1428 | 15.9 |
| EAD = 71 J/mm2 | 1233 | 1385 | 16.2 |
| EAD = 47 J/mm2 | 1205 | 1357 | 16.3 |
| EAD = 36 J/mm2 | 982 | 765 | 5.8 |
| Sample A | 1080 | 1199 | 16.5 |
| Wei [18] | 956 | 1146 | 14.9 |
| Zhouyue [17] | 704.2 | 774.6 | 7.1 |
| Tingting Guan [33] | 702 | 901 | 15.2 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, X.; Dong, S.; Wang, H.; Yan, S.; Liu, X.; Ren, H. Effect of Thermal Cycle on Microstructure Evolution and Mechanical Properties of Selective Laser Melted Low-Alloy Steel. Materials 2019, 12, 3625. https://doi.org/10.3390/ma12213625
Kang X, Dong S, Wang H, Yan S, Liu X, Ren H. Effect of Thermal Cycle on Microstructure Evolution and Mechanical Properties of Selective Laser Melted Low-Alloy Steel. Materials. 2019; 12(21):3625. https://doi.org/10.3390/ma12213625
Chicago/Turabian StyleKang, Xueliang, Shiyun Dong, Hongbin Wang, Shixing Yan, Xiaoting Liu, and Huiping Ren. 2019. "Effect of Thermal Cycle on Microstructure Evolution and Mechanical Properties of Selective Laser Melted Low-Alloy Steel" Materials 12, no. 21: 3625. https://doi.org/10.3390/ma12213625
APA StyleKang, X., Dong, S., Wang, H., Yan, S., Liu, X., & Ren, H. (2019). Effect of Thermal Cycle on Microstructure Evolution and Mechanical Properties of Selective Laser Melted Low-Alloy Steel. Materials, 12(21), 3625. https://doi.org/10.3390/ma12213625





