APD Compressible Aerogel-Like Monoliths with Potential Use in Environmental Remediation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
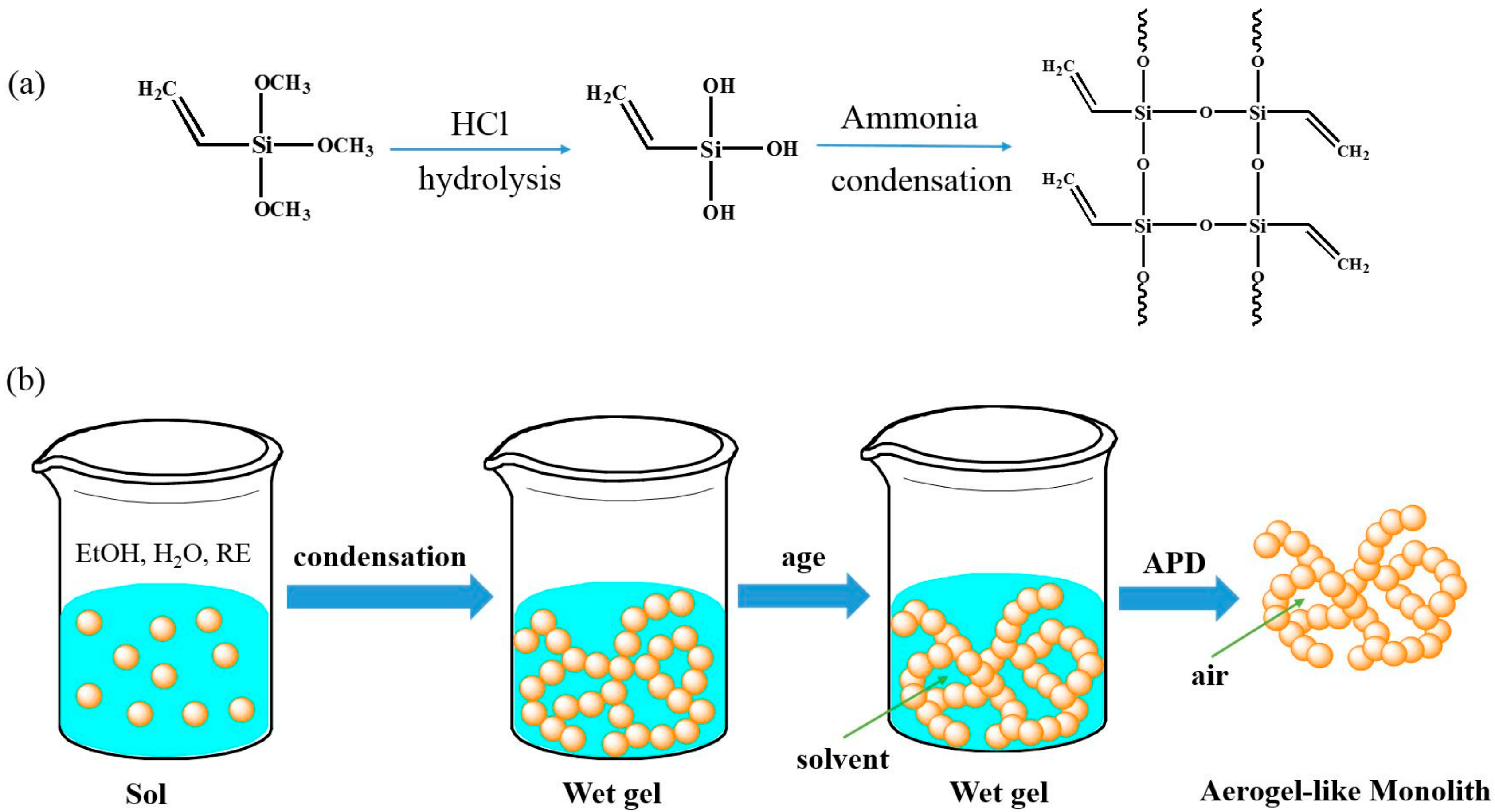
2.2. Sample Preparation and Characterization
3. Results and Discussion
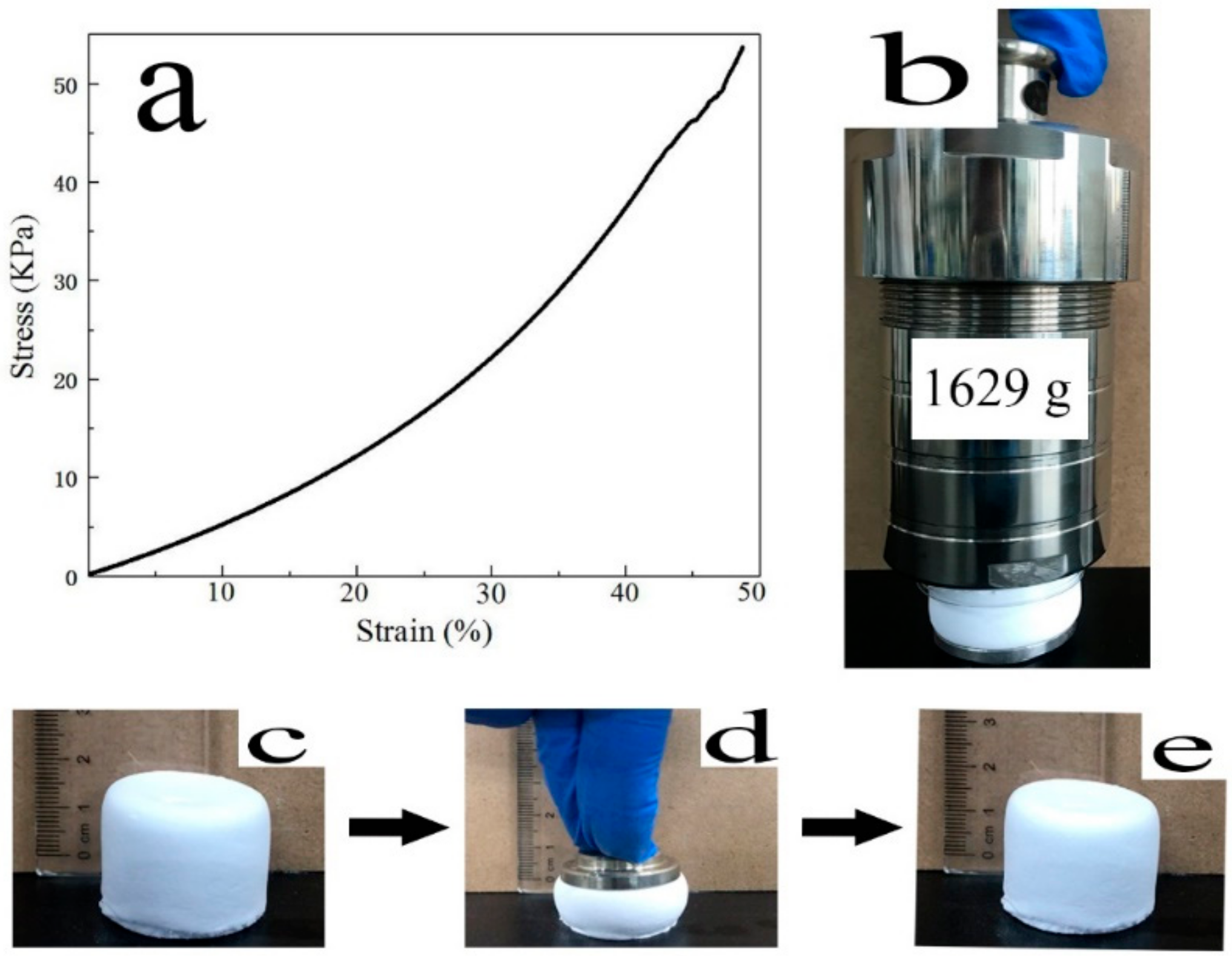
3.1. Formability Studies
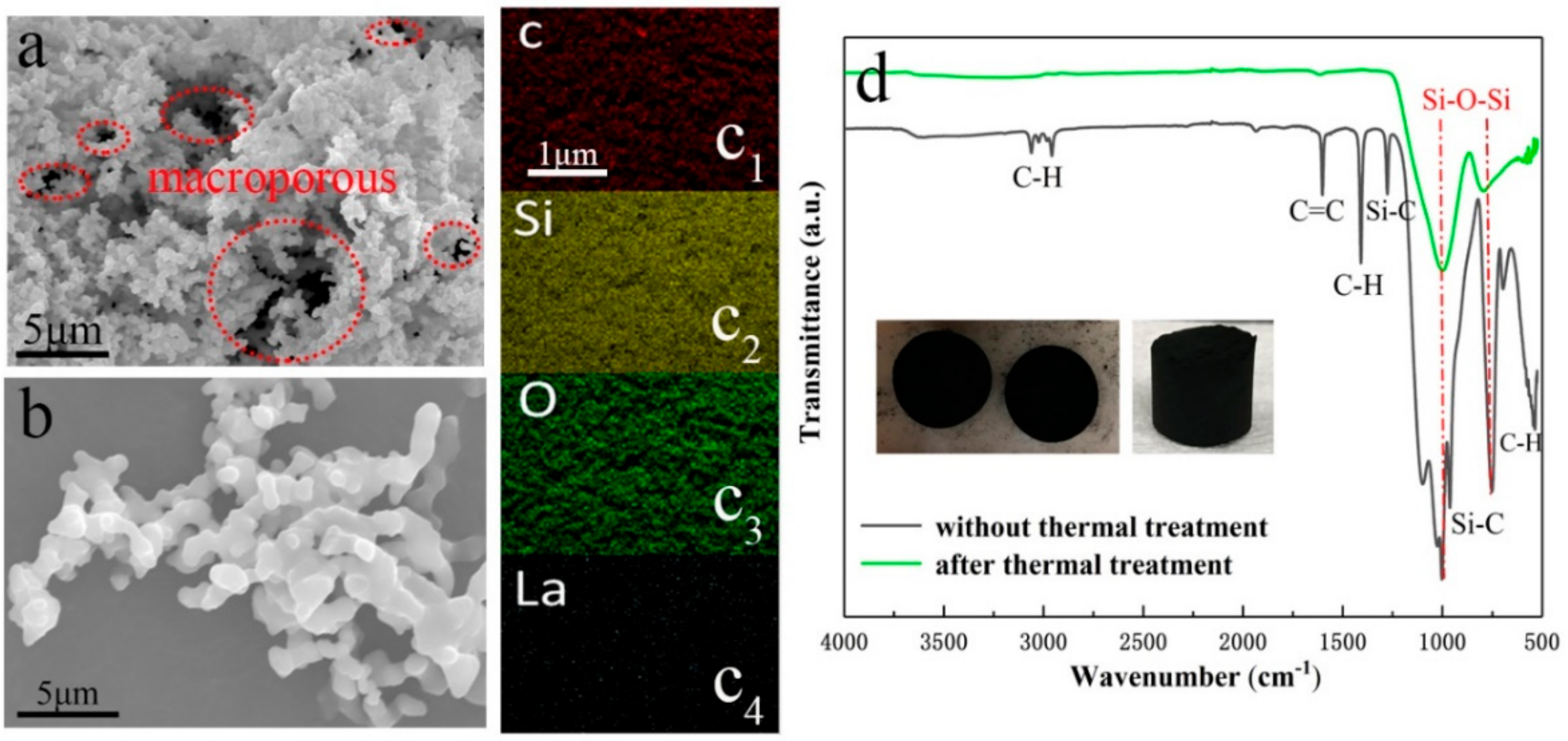
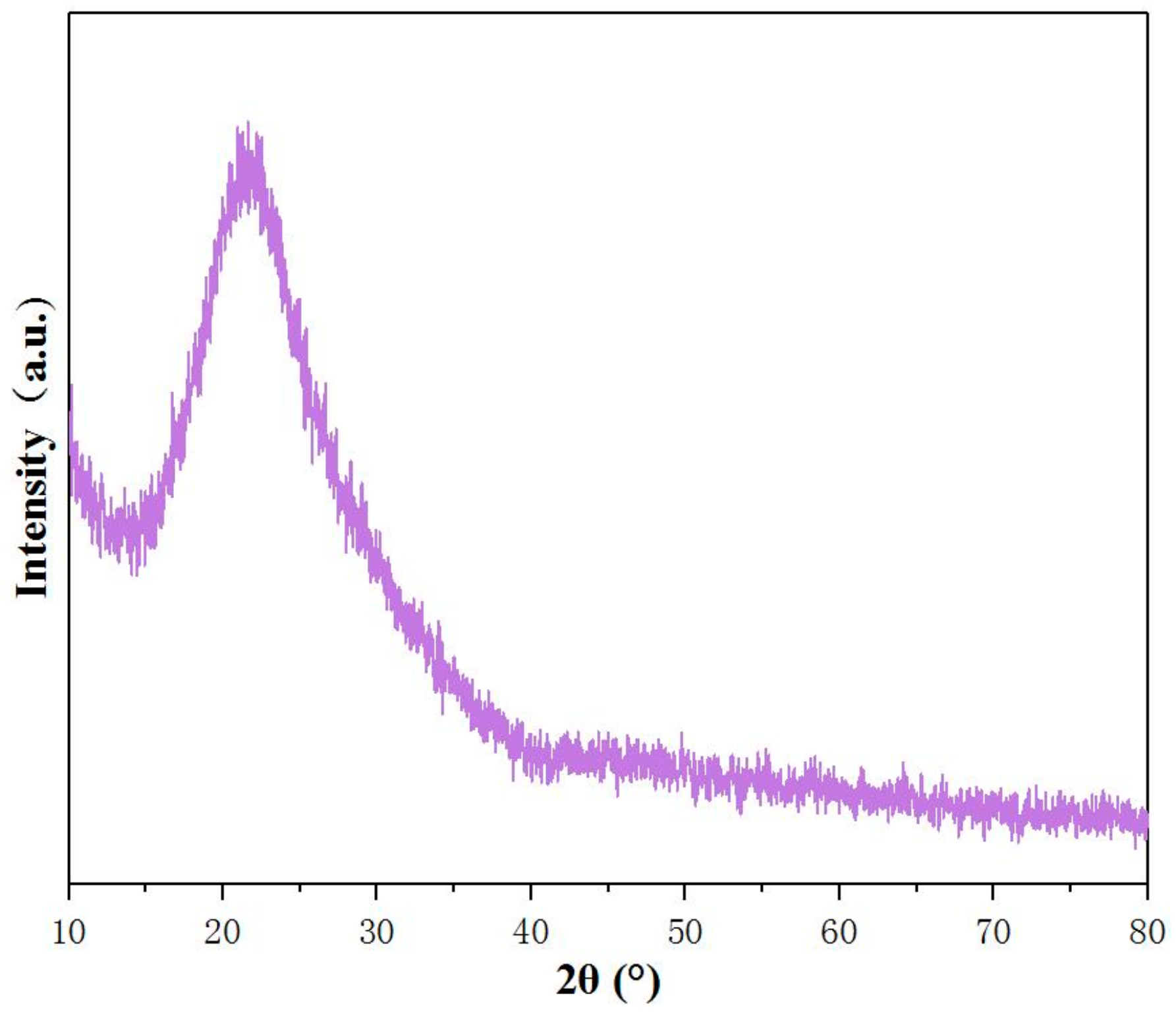
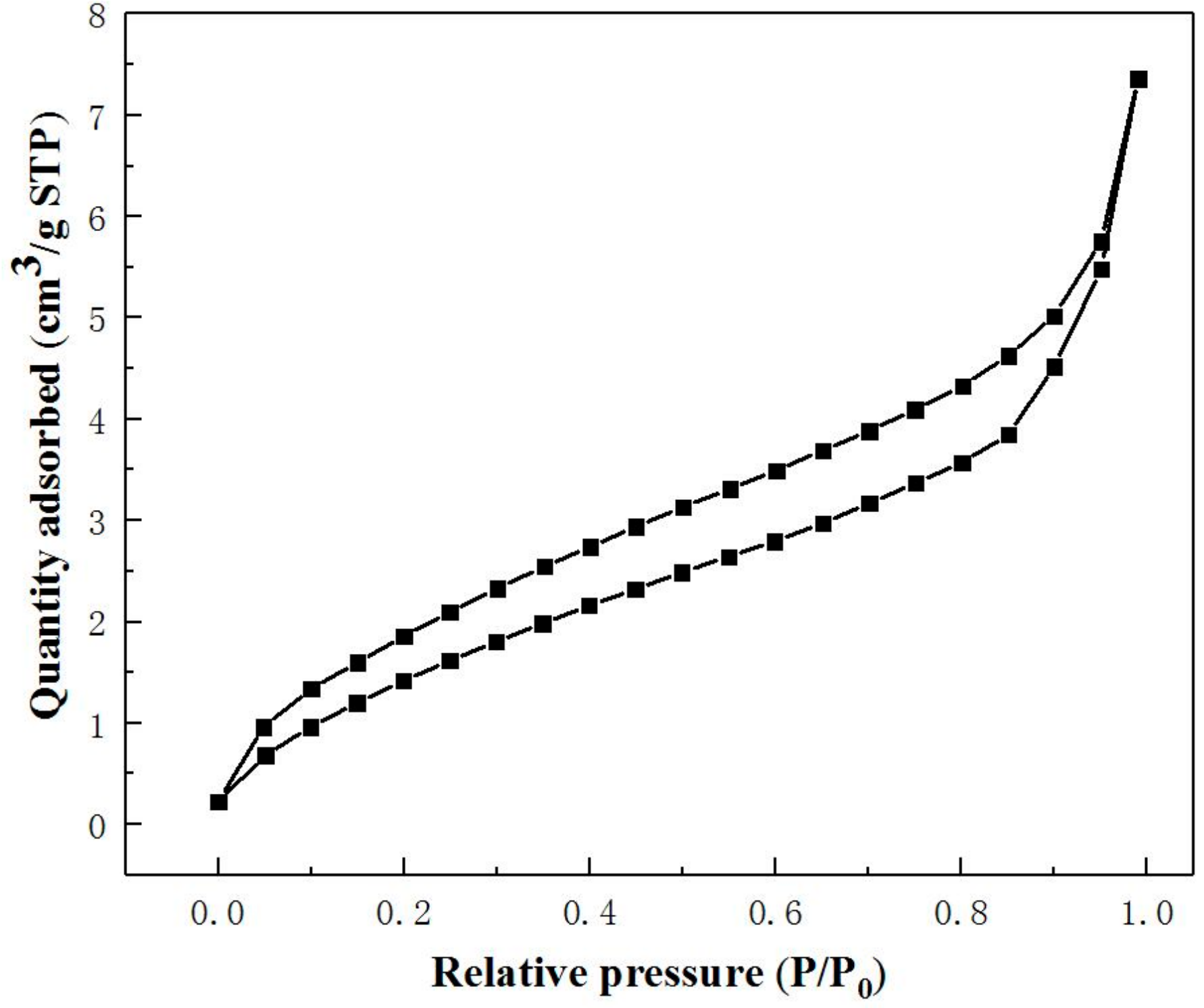
3.2. Physical Properties Studies
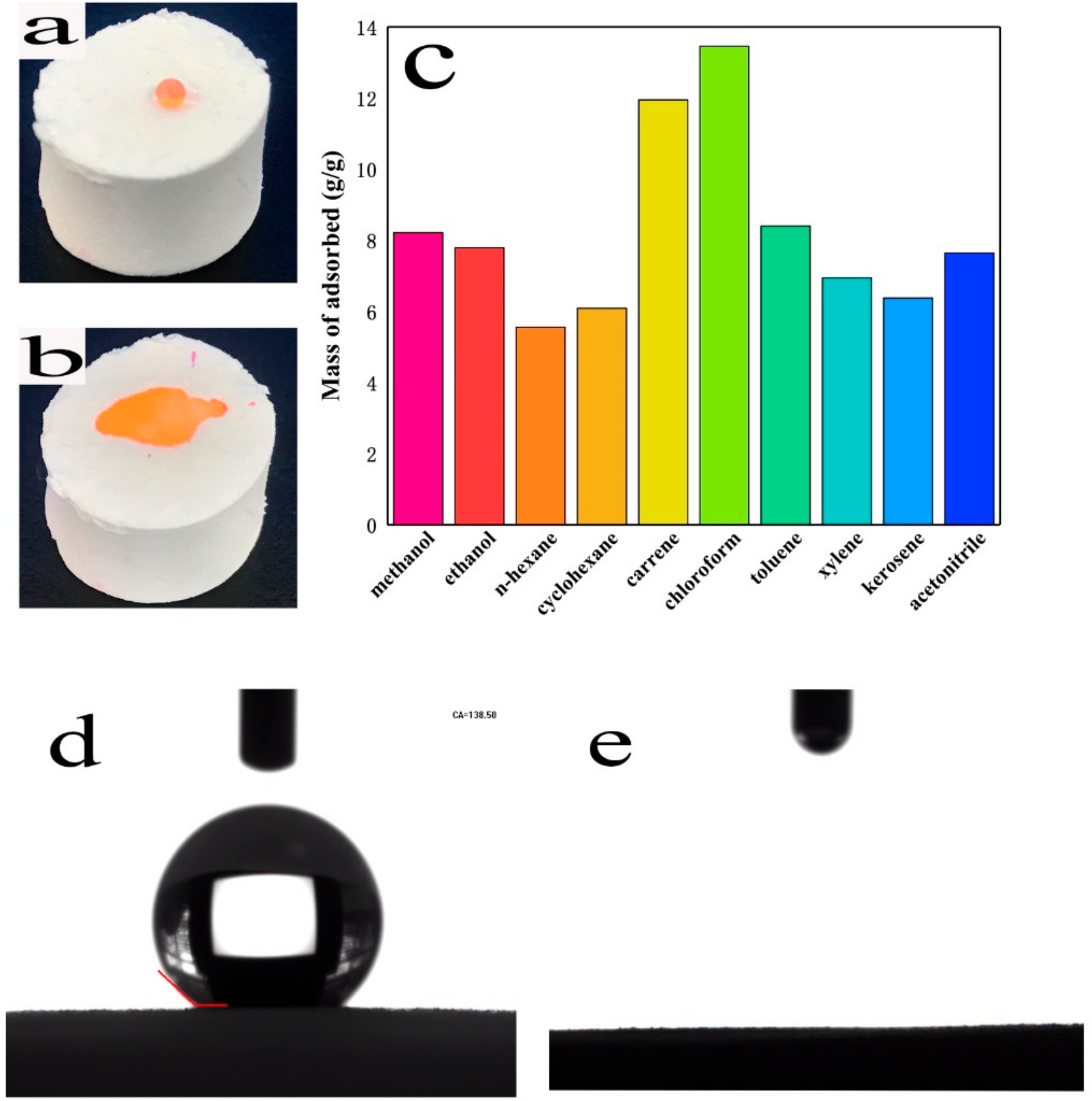
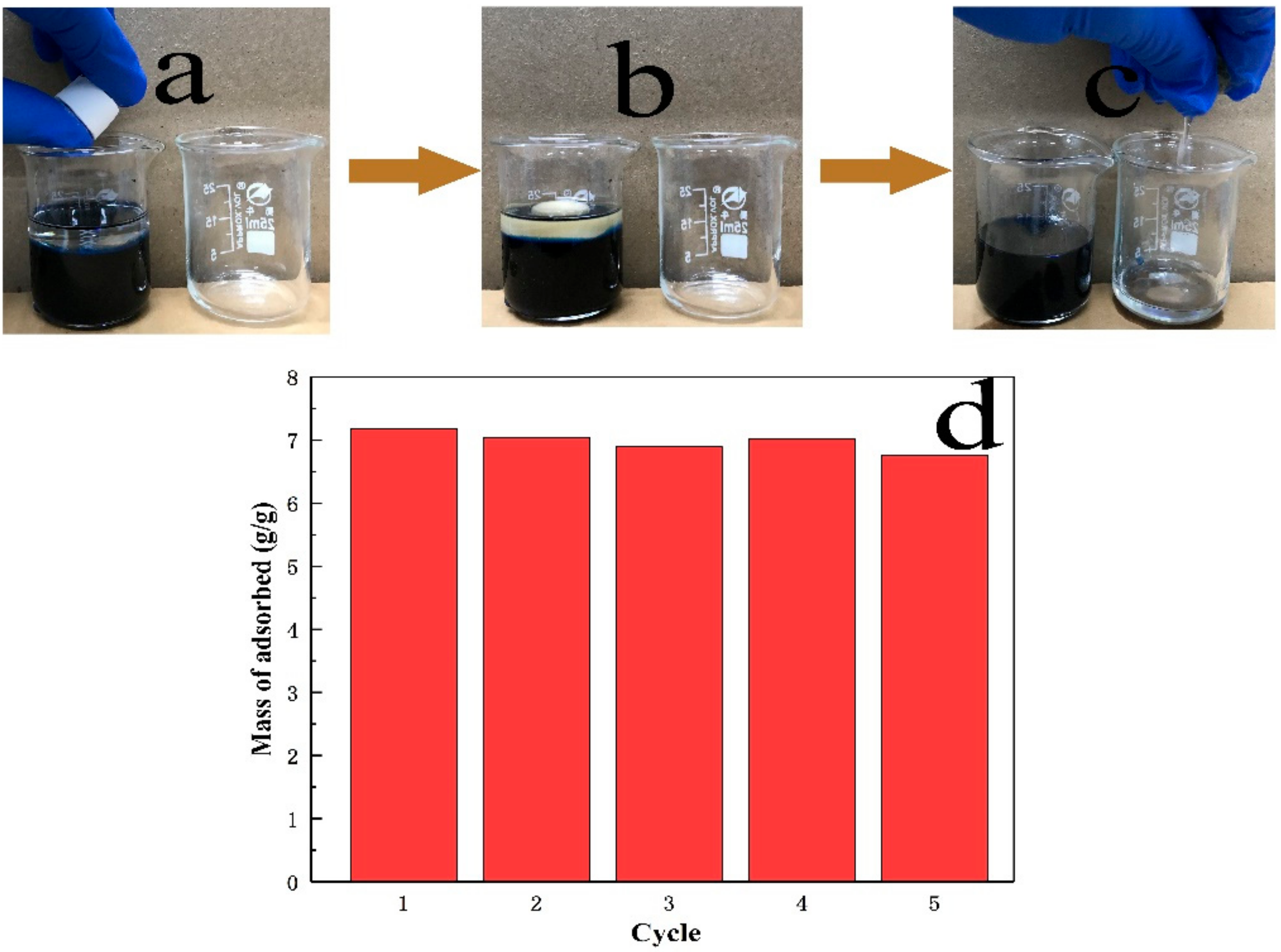
3.3. Adsorption Capacity Studies
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Xu, X.; Zhang, Q.; Hao, M.; Hu, Y.; Lin, Z.; Peng, L.; Wang, T.; Ren, X.; Wang, C.; Zhao, Z.; et al. Double-negative-index ceramic aerogels for thermal superinsulation. Science 2019, 363, 723–727. [Google Scholar] [CrossRef] [PubMed]
- Zu, G.; Shen, J.; Zou, L.; Wang, W.; Lian, Y.; Zhang, Z.; Du, A. Nanoengineering Super Heat-Resistant, Strong Alumina Aerogels. Chem. Mater. 2013, 25, 4757–4764. [Google Scholar] [CrossRef]
- Cai, B.; Eychmuller, A. Promoting Electrocatalysis upon Aerogels. Adv. Mater. 2018, 31, e1804881. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Pan, Z.; Qin, D.; Bai, S.; Peng, Q. Preparation of Ce-TiO2/carbon aerogel electrode and its performance in degradation of 4-chlorophenol. J. Rare Earths 2018, 36, 374–378. [Google Scholar] [CrossRef]
- Gasser-Ramirez, J.L.; Dunn, B.C.; Ramirez, D.W.; Fillerup, E.P.; Turpin, G.C.; Shi, Y.; Ernst, R.D.; Pugmire, R.J.; Eyring, E.M.; Pettigrew, K.A.; et al. A simple synthesis of catalytically active, high surface area ceria aerogels. J. Non-Cryst. Solids. 2008, 354, 5509–5514. [Google Scholar] [CrossRef]
- Zhang, Y.G.; Zhu, Y.J.; Xiong, Z.C.; Wu, J.; Chen, F. Bioinspired Ultralight Inorganic Aerogel for Highly Efficient Air Filtration and Oil-Water Separation. ACS Appl. Mater. Interfaces 2018, 10, 13019–13027. [Google Scholar] [CrossRef] [PubMed]
- Gurav, J.L.; Rao, A.V.; Nadargi, D.Y.; Park, H.-H. Ambient pressure dried TEOS-based silica aerogels: Good absorbents of organic liquids. J. Mater. Sci. 2009, 45, 503–510. [Google Scholar] [CrossRef]
- Tian, X.; Liu, J.; Wang, Y.; Shi, F.; Shan, Z.; Zhou, J.; Liu, J. Adsorption of antibiotics from aqueous solution by different aerogels. J. Non-Cryst. Solids 2019, 505, 72–78. [Google Scholar] [CrossRef]
- Nguyen, D.D.; Vu, C.M.; Vu, H.T.; Choi, H.J. Micron-Size White Bamboo Fibril-Based Silane Cellulose Aerogel: Fabrication and Oil Absorbent Characteristics. Materials 2019, 12, 9. [Google Scholar] [CrossRef] [PubMed]
- Plyushch, A.; Zhai, T.; Xia, H.; Santillo, C.; Verdolotti, L.; Lavorgna, M.; Kuzhir, P. Ultra-Light Reduced Graphene Oxide Based Aerogel/Foam Absorber of Microwave Radiation. Materials 2019, 12, 213. [Google Scholar] [CrossRef] [PubMed]
- Soleimani Dorcheh, A.; Abbasi, M.H. Silica aerogel; synthesis, properties and characterization. J. Mater. Process Technol. 2008, 199, 10–26. [Google Scholar] [CrossRef]
- Li, Y.; Grishkewich, N.; Liu, L.; Wang, C.; Tam, K.C.; Liu, S.; Mao, Z.; Sui, X. Construction of functional cellulose aerogels via atmospheric drying chemically cross-linked and solvent exchanged cellulose nanofibrils. Chem. Eng. J. 2019, 366, 531–538. [Google Scholar] [CrossRef]
- Hegde, N.D.; Hirashima, H.; Venkateswara Rao, A. Two step sol-gel processing of TEOS based hydrophobic silica aerogels using trimethylethoxysilane as a co-precursor. J. Porous. Mater. 2007, 14, 165–171. [Google Scholar] [CrossRef]
- Li, B.; Gao, X.; Li, X.; Liu, Z.; He, N. Monolithic organosilica aerogel consisting of hollow microspheres by a simple ambient pressure drying process. Mater. Lett. 2017, 199, 21–23. [Google Scholar] [CrossRef]
- Li, M.; Jiang, H.; Xu, D.; Hai, O.; Zheng, W. Low density and hydrophobic silica aerogels dried under ambient pressure using a new co-precursor method. J. Non-Cryst. Solids 2016, 452, 187–193. [Google Scholar] [CrossRef]
- Sadri, F.; Nazari, A.M.; Ghahreman, A. A review on the cracking, baking and leaching processes of rare earth element concentrates. J. Rare Earths 2017, 35, 739–752. [Google Scholar] [CrossRef]
- Akah, A. Application of rare earths in fluid catalytic cracking: A review. J. Rare Earths 2017, 35, 941–956. [Google Scholar] [CrossRef]
- Vareda, J.P.; Matias, T.; Durães, L. Facile preparation of ambient pressure dried aerogel-like monoliths with reduced shrinkage based on vinyl-modified silica networks. Ceram. Int. 2018, 44, 17453–17458. [Google Scholar] [CrossRef]
- Nguyen, B.N.; Meador, M.A.B.; Tousley, M.E.; Shonkwiler, B.; McCorkle, L.; Scheiman, D.A.; Palczer, A. Tailoring Elastic Properties of Silica Aerogels Cross-Linked with Polystyrene. ACS Appl. Mater. Interfaces 2009, 1, 621–630. [Google Scholar] [CrossRef] [PubMed]
- Yun, S.; Luo, H.; Gao, Y. Low-density, hydrophobic, highly flexible ambient-pressure-dried monolithic bridged silsesquioxane aerogels. J. Mater. Chem. A 2015, 3, 3390–3398. [Google Scholar] [CrossRef]

| Sample | n(La/Si) (mol/mol, %) | VTMS (mL) | Water (mL) | Ethanol (mL) | Shrinkage (%) | Bulk Density (g/cm3) | Mechanical Properties |
|---|---|---|---|---|---|---|---|
| S1 | 4 | 5 | 0 | 25 | - | - | - |
| S2 | 4 | 5 | 5 | 20 | 69.9 | 0.299 | hard |
| S3 | 4 | 5 | 10 | 15 | 26.4 | 0.121 | soft |
| S4 | 4 | 5 | 15 | 10 | 61.5 | 0.239 | hard |
| S5 | 4 | 5 | 20 | 5 | 56.5 | 0.197 | hard |
| S6 | 4 | 5 | 25 | 0 | 23.1 | 0.113 | fragile |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Yang, F.; Bai, R.; Zhao, Z.; Li, J.; Zeng, X.; Zhang, X. APD Compressible Aerogel-Like Monoliths with Potential Use in Environmental Remediation. Materials 2019, 12, 3459. https://doi.org/10.3390/ma12203459
Zhang H, Yang F, Bai R, Zhao Z, Li J, Zeng X, Zhang X. APD Compressible Aerogel-Like Monoliths with Potential Use in Environmental Remediation. Materials. 2019; 12(20):3459. https://doi.org/10.3390/ma12203459
Chicago/Turabian StyleZhang, Hao, Fan Yang, Ruixi Bai, Zhigang Zhao, Jianguo Li, Xian Zeng, and Xuesong Zhang. 2019. "APD Compressible Aerogel-Like Monoliths with Potential Use in Environmental Remediation" Materials 12, no. 20: 3459. https://doi.org/10.3390/ma12203459
APA StyleZhang, H., Yang, F., Bai, R., Zhao, Z., Li, J., Zeng, X., & Zhang, X. (2019). APD Compressible Aerogel-Like Monoliths with Potential Use in Environmental Remediation. Materials, 12(20), 3459. https://doi.org/10.3390/ma12203459





