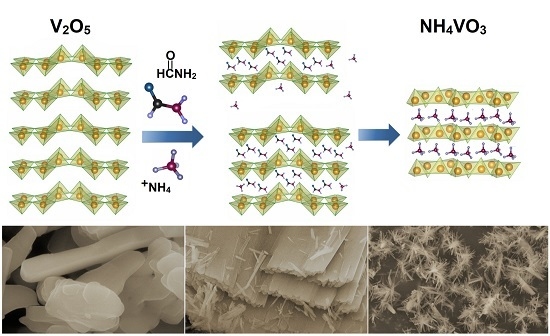
Tailoring the Size and Shape—New Path for Ammonium Metavanadate Synthesis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
Hydrothermal Synthesis of Ammonium Vanadates
2.2. Methods
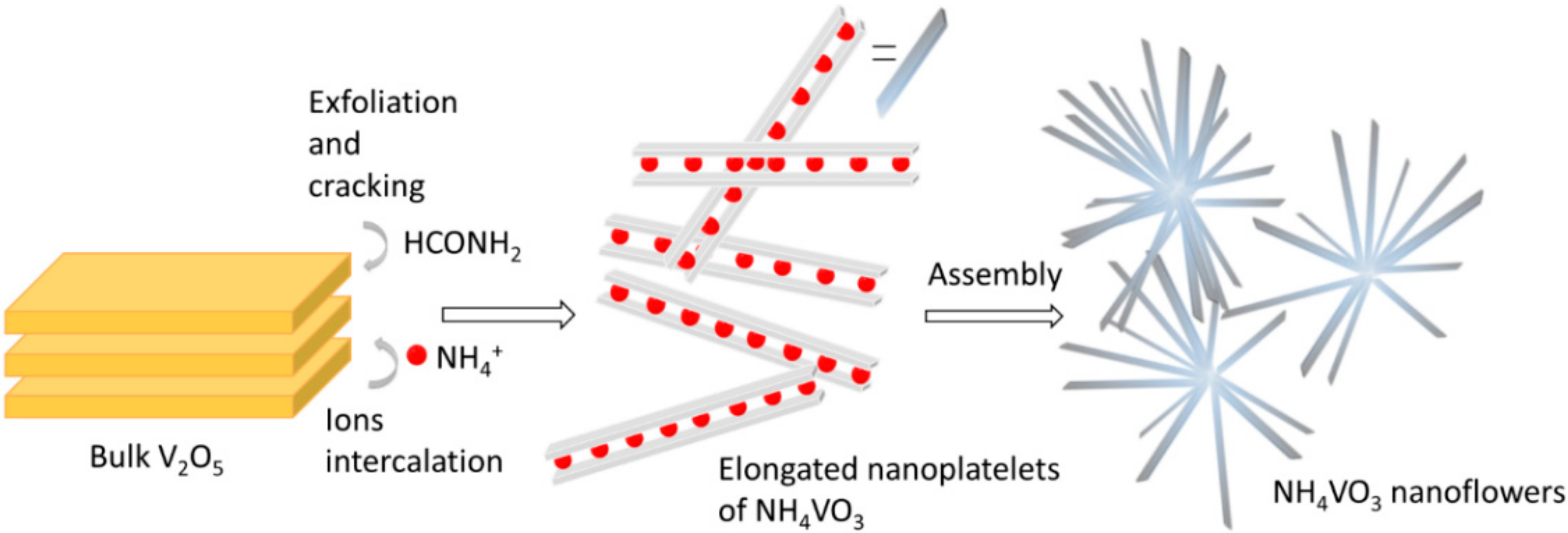
3. Results and Discussion
3.1. Structural Analysis
3.2. Hydrothermal Synthesis of Higher Ammonium Vanadates from NH4VO3
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Liu, Y.; Xu, M.; Shen, B.; Xia, Z.; Li, Y.; Wu, Y.; Li, Q. Facile synthesis of mesoporous NH4V4O10 nanoflowers with high performance as cathode material for lithium battery. J. Mater. Sci. 2018, 53, 2045–2053. [Google Scholar] [CrossRef]
- Mai, L.Q.; Lao, C.S.; Hu, B.; Zhou, J.; Qi, Y.Y.; Chen, W.; Gu, E.D.; Wang, Z.L. Synthesis and electrical transport of single-crystal NH4V3O8 nanobelts. J. Phys. Chem. B 2006, 110, 18138–18141. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Xia, Q.; Xu, Y.; Wang, P.; Tan, Q. NH4V4O10 micro-flowers as cathode material for high performance hybrid magnesium-lithium-ion batteries. Mater. Lett. 2019, 247, 178–181. [Google Scholar] [CrossRef]
- Wang, H.; Ren, Y.; Wang, W.; Huang, X.; Huang, K.; Wang, Y.; Liu, S. NH4V3O8 nanorod as a high performance cathode material for rechargeable Li-ion batteries. J. Power Sources 2012, 199, 315–321. [Google Scholar] [CrossRef]
- Ma, Y.; Ji, S.; Zhou, H.; Zhang, S.; Li, R.; Zhu, J.; Li, W.; Guo, H.; Jin, P. Synthesis of novel ammonium vanadium bronze (NH4) 0.6V2O5 and its application in Li-ion battery. RSC Adv. 2015, 5, 90888–90894. [Google Scholar] [CrossRef]
- Cheng, Y.; Huang, J.; Li, J.; Cao, L.; Xu, Z.; Wu, J.; Cao, S.; Hu, H. Structure-controlled synthesis and electrochemical properties of NH4V3O8 as cathode material for Lithium ion batteries. Electrochim. Acta 2016, 212, 217–224. [Google Scholar] [CrossRef]
- Tian, X.; Xu, X.; He, L.; Wei, Q.; Yan, M.; Xu, L.; Zhao, Y.; Yang, C.; Mai, L. Ultrathin pre-lithiated V6O13 nanosheet cathodes with enhanced electrical transport and cyclability. J. Power Sources 2014, 255, 235–241. [Google Scholar] [CrossRef]
- Vo, TN.; Kim, H.; Hur, J.; Choi, W.; Kim, T. Surfactant-assisted ammonium vanadium oxide as a superior cathode for calcium-ion batteries. J. Mater. Chem. A 2018, 6, 22645–22654. [Google Scholar] [CrossRef]
- Esparcia, E.; Chae, M.; Ocon, J.; Hong, S. Ammonium Vanadium Bronze (NH4V4O10) as a High-Capacity Cathode Material for Nonaqueous Magnesium-Ion Batteries. Chem. Mater. 2018, 30, 3690–3696. [Google Scholar] [CrossRef]
- Wei, T.; Li, Q.; Yang, G.; Wang, C. Highly reversible and long-life cycling aqueous zinc-ion battery based on ultrathin (NH4)2V10O25·8H2O nanobelt. J. Mater. Chem. A 2018, 6, 20402–20410. [Google Scholar] [CrossRef]
- Yang, G.; Wei, T.; Wang, C. Self-Healing Lamellar Structure Boosts Highly Stable Zinc-Storage Property of Bilayered Vanadium Oxides. ACS Appl. Mater. Interfaces 2018, 1041, 35079–35089. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.; Zhu, H.; Zhu, X.; Koritala, H.; Wang, Y. Interlayer-Expanded V6O13 n H2O Architecture Constructed for an Advanced Rechargeable Aqueous Zinc-Ion Battery. ACS Appl. Energy Mater. 2019, 2, 1988–1996. [Google Scholar] [CrossRef]
- Sonar, S.S.; Kategaonkar, A.H.; Ware, M.N.; Gill, C.H.; Shingate, B.B.; Shingare, M.S. Ammonium metavanadate: An effective catalyst for synthesis of α hydroxyphosphonates. Arkivoc 2009, 2, 138–148. [Google Scholar]
- Si, Y.; Xiong, Z.; Zheng, X.; Li, M.; Yang, Q. Improving the Anti-Corrosion Ability of Anodization Film of AZ31B Magnesium Alloy by Addition of NH4VO3 in the Electrolyte. Int. J. Electrochem. Sci. 2016, 11, 3261–3268. [Google Scholar] [CrossRef]
- Brauer, G. Handbook of Preparative Inorganic Chemistry, 2nd ed.; Brauer, G., Ed.; Academic Press Inc.: New York, NY, USA, 1965. [Google Scholar]
- Du, G.; Sun, Z.; Xian, Y.; Jing, H.; Chen, H.; Yin, D. The nucleation kinetics of ammonium metavanadate precipitated by ammonium chloride. J. Cryst. Growth 2016, 441, 117–123. [Google Scholar] [CrossRef]
- Mandhane, P.G.; Joshi, R.S.; Ghawalkar, A.R.; Jadhav, G.R.; Gill, C.H. Ammonium metavanadate: A mild and efficient catalyst for the synthesis of coumarins. Bull. Korean Chem. Soc. 2009, 30, 2969–2972. [Google Scholar] [CrossRef]
- Jadhav, G.R.; Shaikh, M.U.; Kale, R.P.; Gill, C.H. Ammonium metavanadate: A novel catalyst for synthesis of 2-substituted benzimidazole derivatives. Chin. Chem. Lett. 2009, 20, 292–295. [Google Scholar] [CrossRef]
- Niralwad, K.S.; Shingate, B.B.; Shingare, M.S. Microwave-assisted one-pot synthesis of octahydroquinazolinone derivatives using ammonium metavanadate under solvent-free condition. Tetrahedron Lett. 2010, 51, 3616–3618. [Google Scholar] [CrossRef]
- Wu, D.; Wang, C.; Chao, Y.; He, P.; Ma, J. Porous bowl-shaped VS2 nanosheets/graphene composite for high-rate lithium-ion storage. J. Eng. Chem. 2020, 43, 24–32. [Google Scholar] [CrossRef]
- Xie, X.; Mao, M.; Qi, S.; Ma, J. ReS2-Based electrode materials for alkali-metal ion batteries. Cryst. Eng. Commun. 2019, 21, 3755–3769. [Google Scholar] [CrossRef]
- Rui, X.; Lu, Z.; Yu, H.; Yang, D.; Hng, H.H.; Lim, T.M.; Yan, Q. Ultrathin V2O5 nanosheet cathodes: Realizing ultrafast reversible lithium storage. Nanoscale 2013, 5, 556–560. [Google Scholar] [CrossRef] [PubMed]
- Heyns, A.M.; Venter, M.W.; Range, K.J. The vibrational spectra of NH4VO3 at elevated temperatures and pressures. Z. Naturforsch. B 1987, 42, 843–852. [Google Scholar] [CrossRef]
- Onodera, S.; Ikegami, Y. Infrared and Raman spectra of ammonium, potassium, rubidium, and cesium metavanadates. Inorg. Chem. 1980, 19, 615–618. [Google Scholar] [CrossRef]
- Bruyère, V.I.; Morando, P.J.; Blesa, M.A. The dissolution of vanadium pentoxide in aqueous solutions of oxalic and mineral acids. J. Colloid Interface Sci. 1999, 209, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.F.; Zhang, G.Q.; Liu, X.; Su, Z.; Li, H.L. Large scale hydrothermal synthesis and electrochemistry of ammonium vanadium bronze nanobelts. J. Power Sources 2006, 157, 528–532. [Google Scholar] [CrossRef]
- Wang, N.; Chen, W.; Mai, L.; Dai, Y. Selected-control hydrothermal synthesis and formation mechanism of 1D ammonium vanadate. J. Solid State Chem. 2008, 181, 652–657. [Google Scholar] [CrossRef]
- Vernardou, D.; Apostolopoulou, M.; Louloudakis, D.; Katsarakis, N.; Koudoumas, E. Hydrothermal growth and characterization of shape-controlled NH4V3O8. New J. Chem. 2014, 38, 2098–2104. [Google Scholar] [CrossRef]
- Kou, L.; Cao, L.; Huang, J.; Yang, J.; Wang, Y. Facile synthesis of NH4V3O8 nanoflowers as advanced cathodes for high performance of lithium ion battery. J. Mater. Sci. Mater. Electron. 2018, 29, 4830–4834. [Google Scholar] [CrossRef]
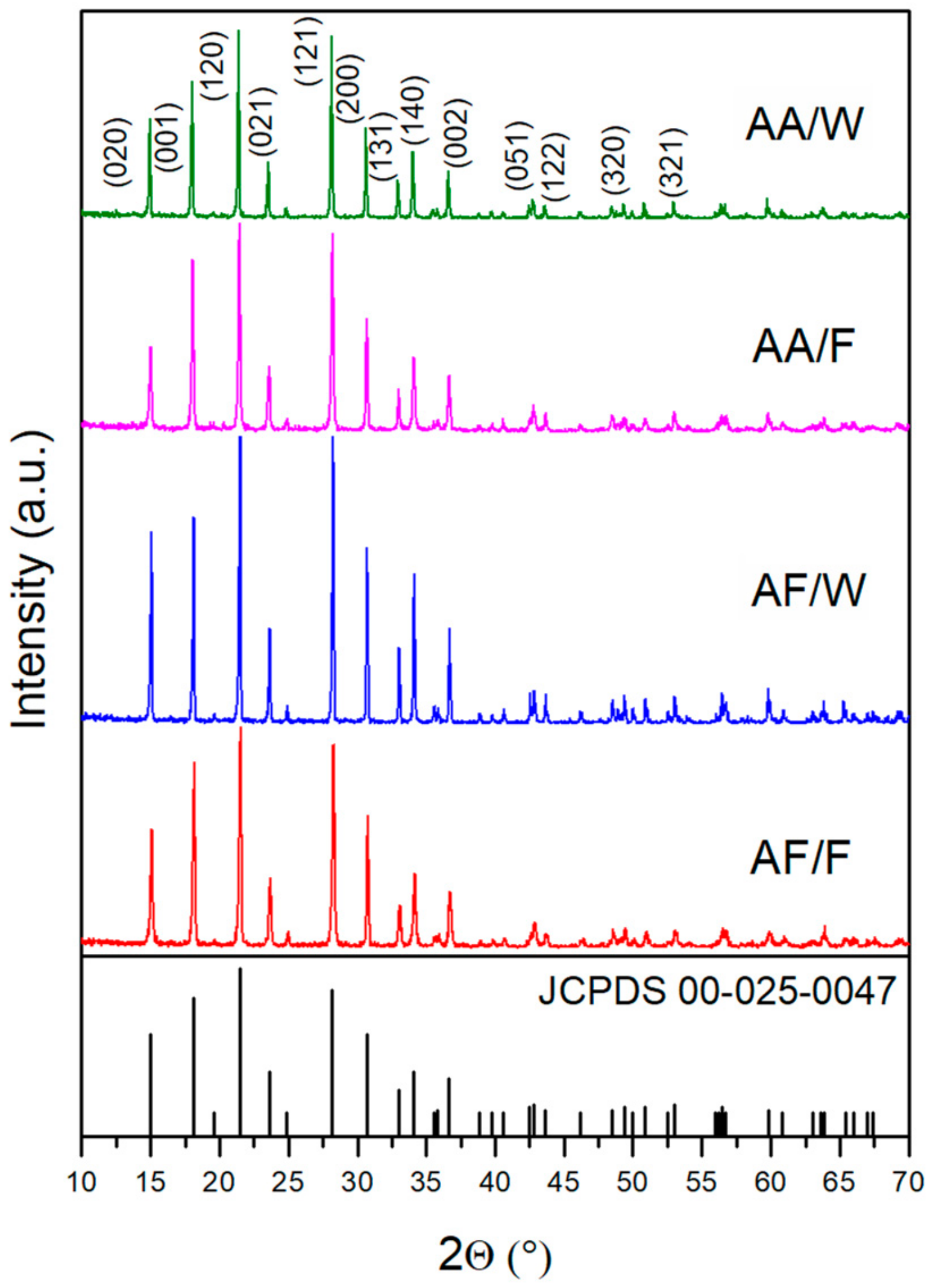
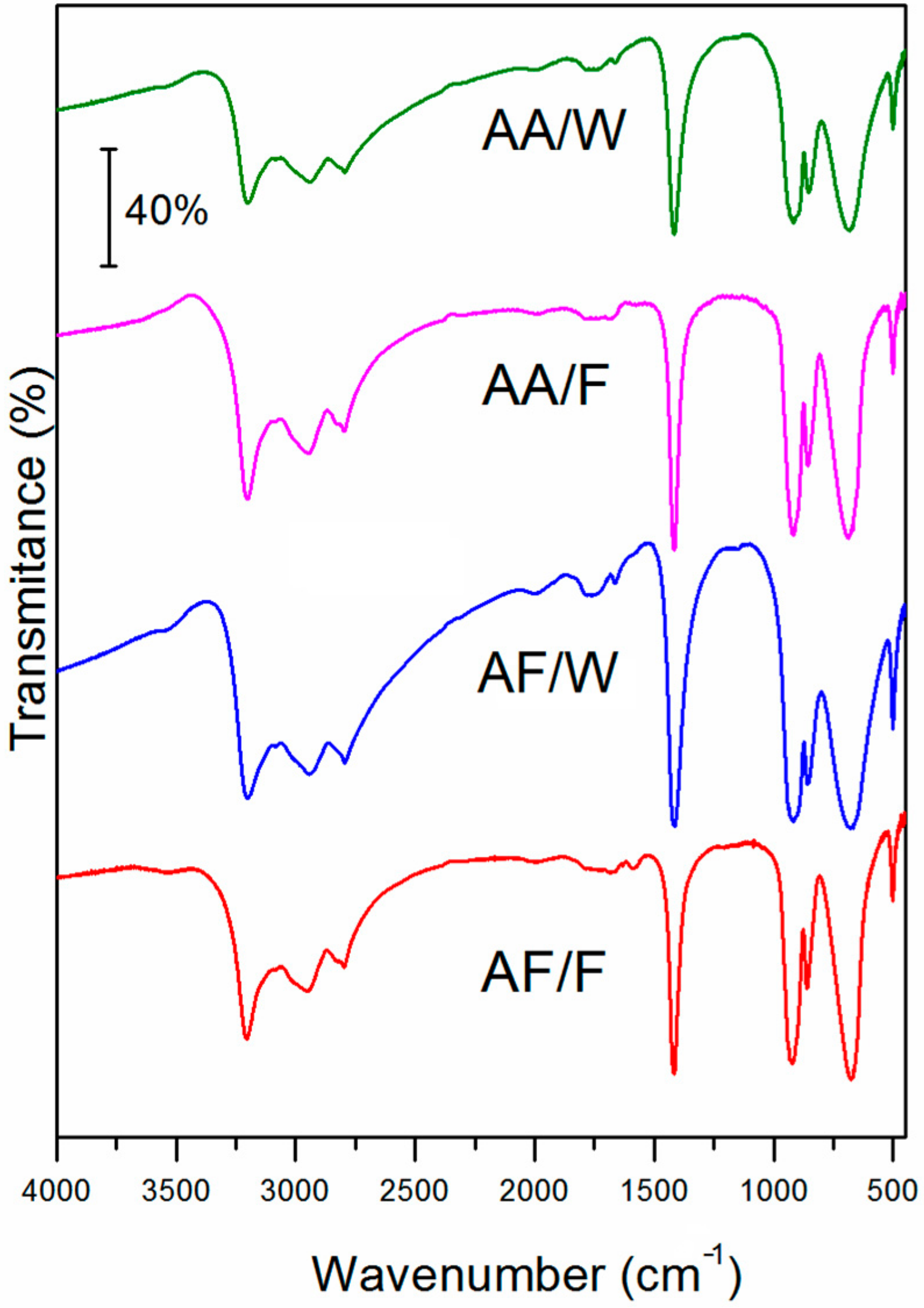

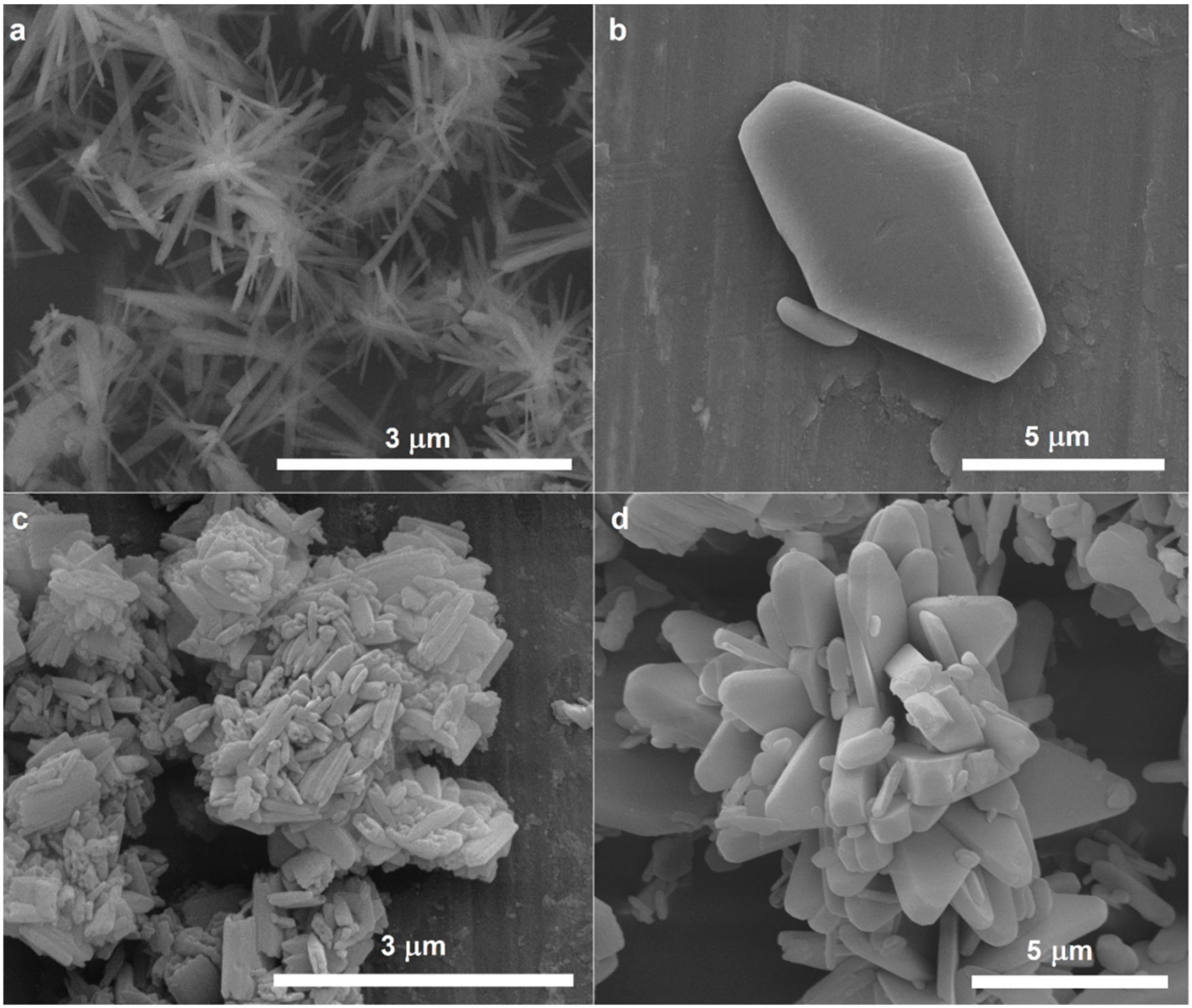


| Solvent | Salt | |
|---|---|---|
| Ammonium Formate | Ammonium Acetate | |
| Water | AF/W | AA/W |
| Formamide | AF/F | AA/F |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prześniak-Welenc, M.; Nadolska, M.; Kościelska, B.; Sadowska, K. Tailoring the Size and Shape—New Path for Ammonium Metavanadate Synthesis. Materials 2019, 12, 3446. https://doi.org/10.3390/ma12203446
Prześniak-Welenc M, Nadolska M, Kościelska B, Sadowska K. Tailoring the Size and Shape—New Path for Ammonium Metavanadate Synthesis. Materials. 2019; 12(20):3446. https://doi.org/10.3390/ma12203446
Chicago/Turabian StylePrześniak-Welenc, Marta, Małgorzata Nadolska, Barbara Kościelska, and Kamila Sadowska. 2019. "Tailoring the Size and Shape—New Path for Ammonium Metavanadate Synthesis" Materials 12, no. 20: 3446. https://doi.org/10.3390/ma12203446
APA StylePrześniak-Welenc, M., Nadolska, M., Kościelska, B., & Sadowska, K. (2019). Tailoring the Size and Shape—New Path for Ammonium Metavanadate Synthesis. Materials, 12(20), 3446. https://doi.org/10.3390/ma12203446