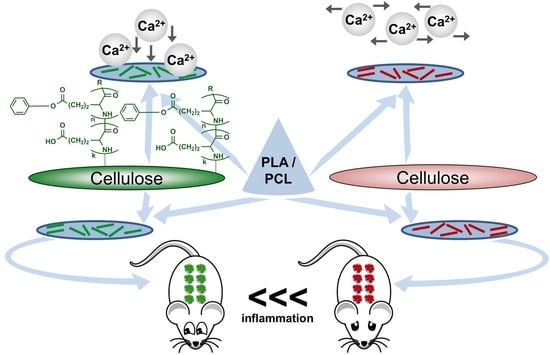
PGlu-Modified Nanocrystalline Cellulose Improves Mechanical Properties, Biocompatibility, and Mineralization of Polyester-Based Composites
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Supplements
2.2. Methods
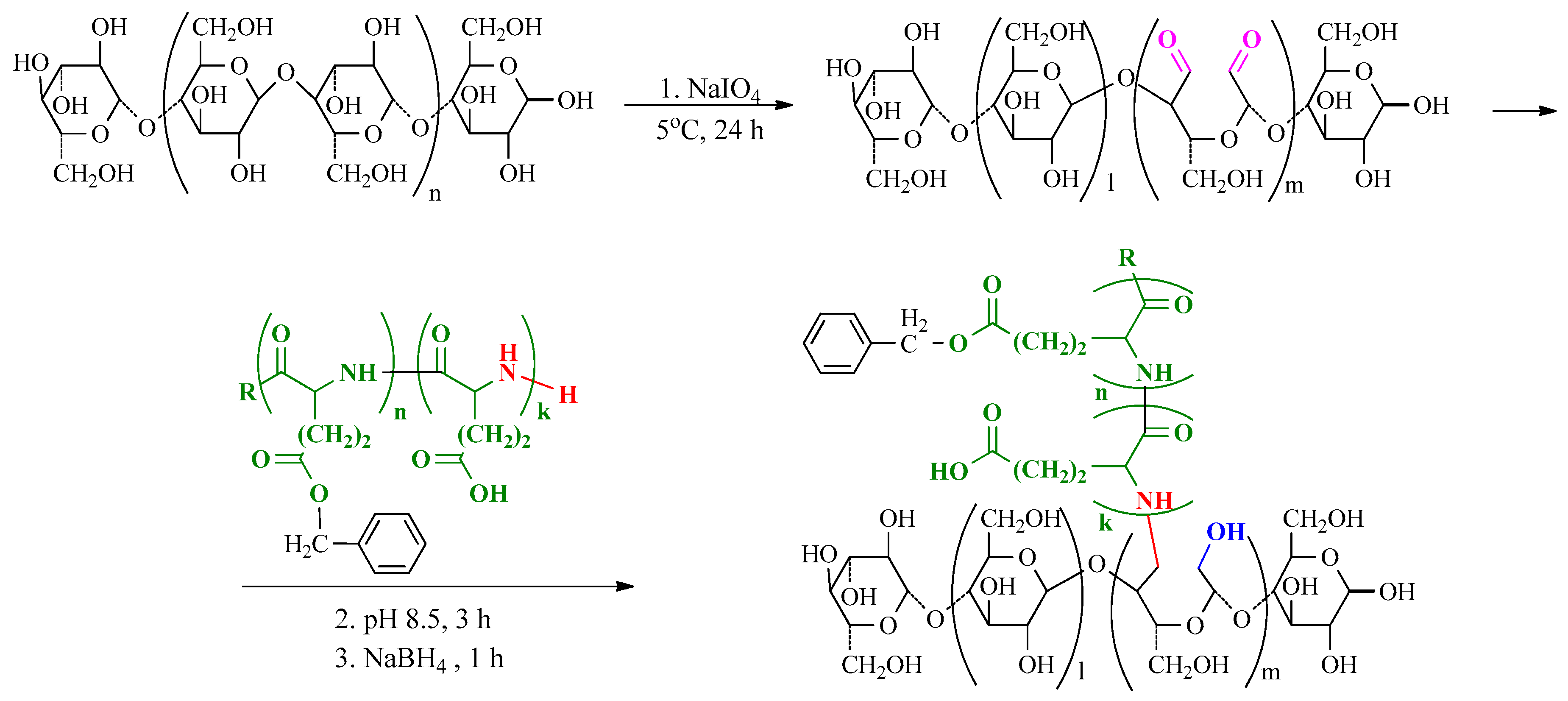
2.2.1. Synthesis and Characterization of Polymers
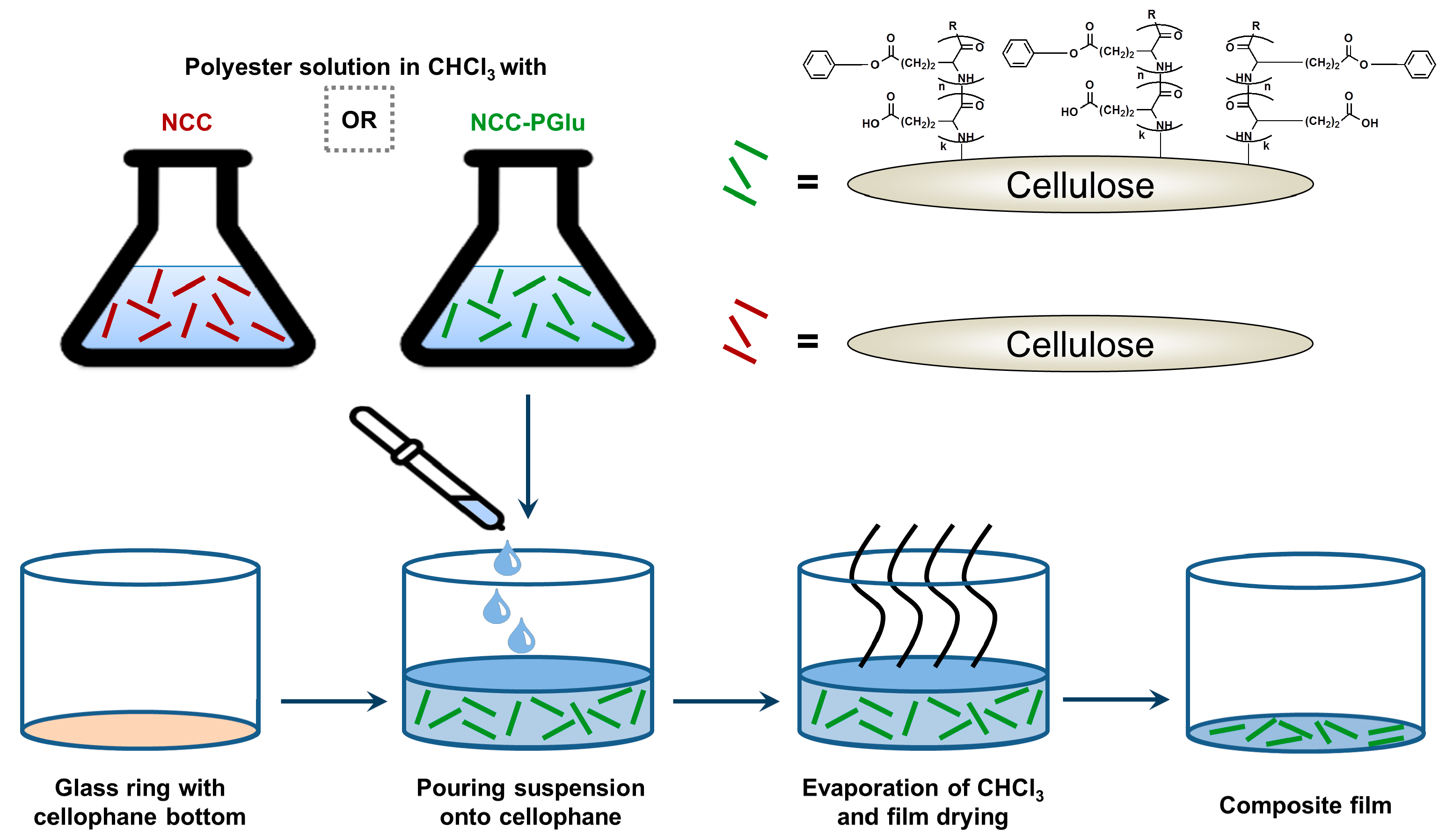
2.2.2. NCC Modification with PGlu
2.2.3. Manufacturing of Films
2.2.4. Study of Mechanical Properties
2.2.5. Microscopy and X-Ray Phase Analysis
2.2.6. Cell Culture Experiments
2.2.7. Mineralization Study
2.2.8. In Vivo Experiments
2.2.9. Histomorphometric Analysis
3. Results and Discussion
3.1. Synthesis of Matrix Polymers and Manufacturing of Composite Fillms
3.2. Physicochemical Characterization of Composite Materials
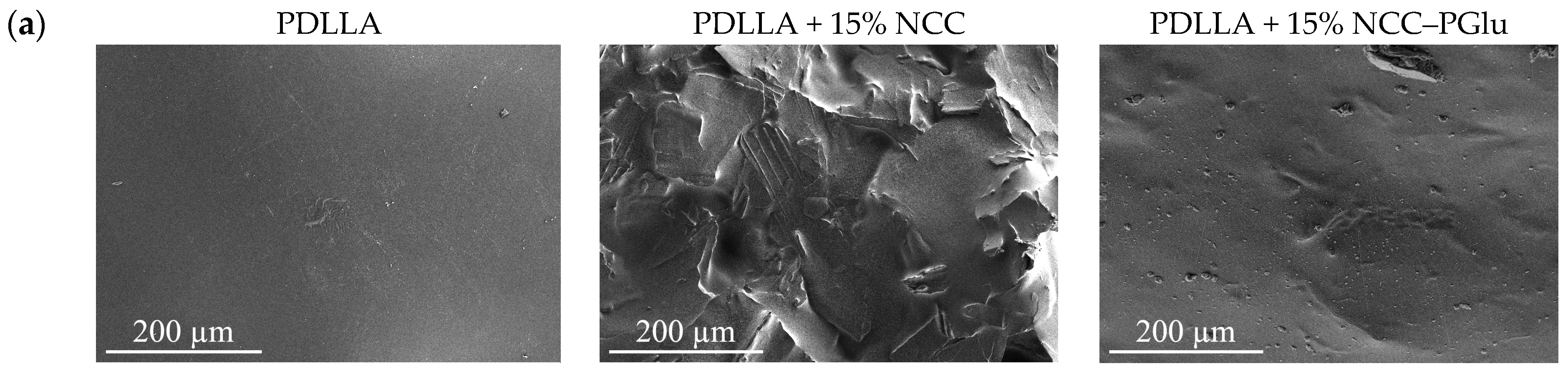
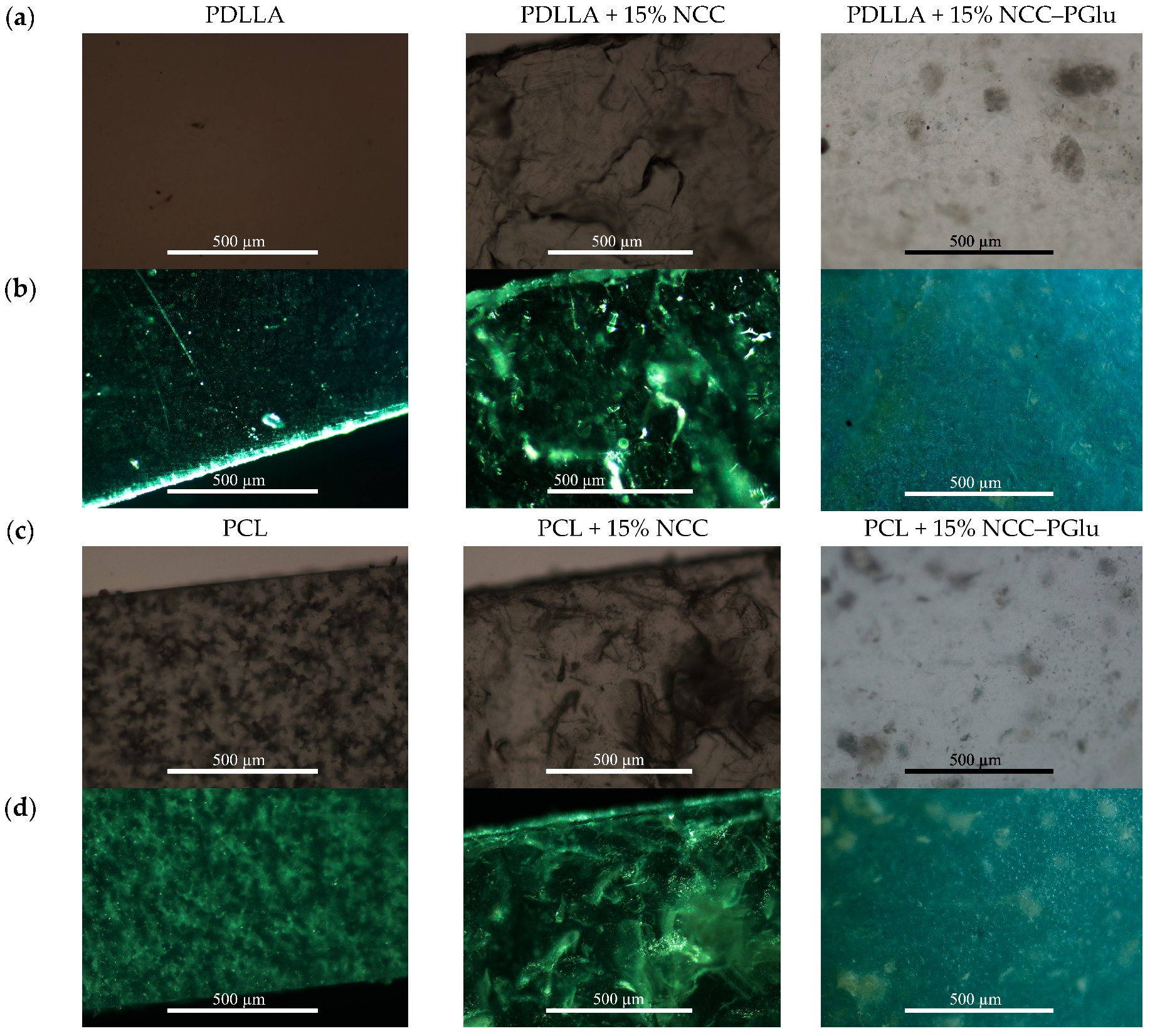
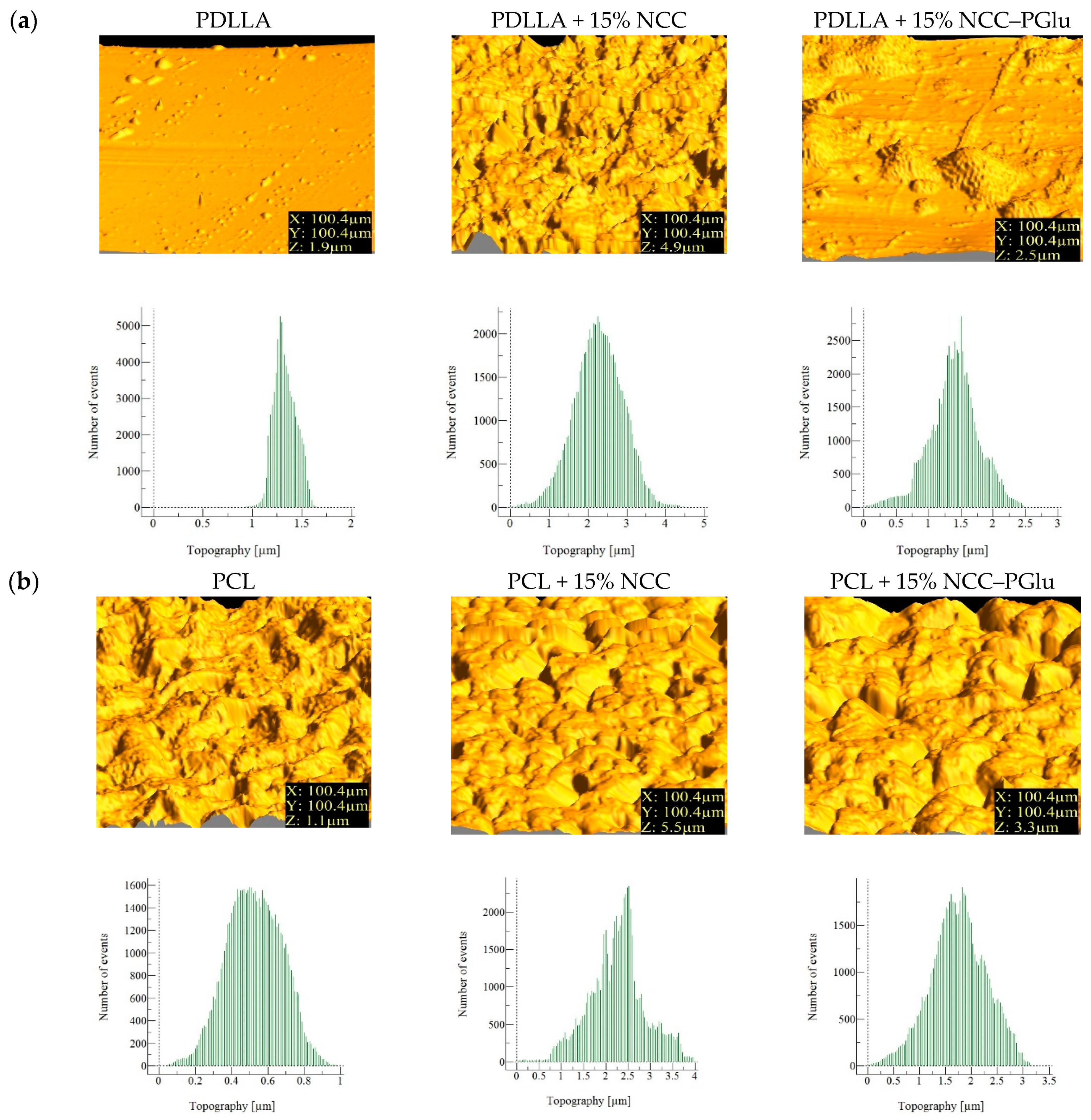
3.2.1. Surface Morphology and Topography
3.2.2. Mechanical Properties
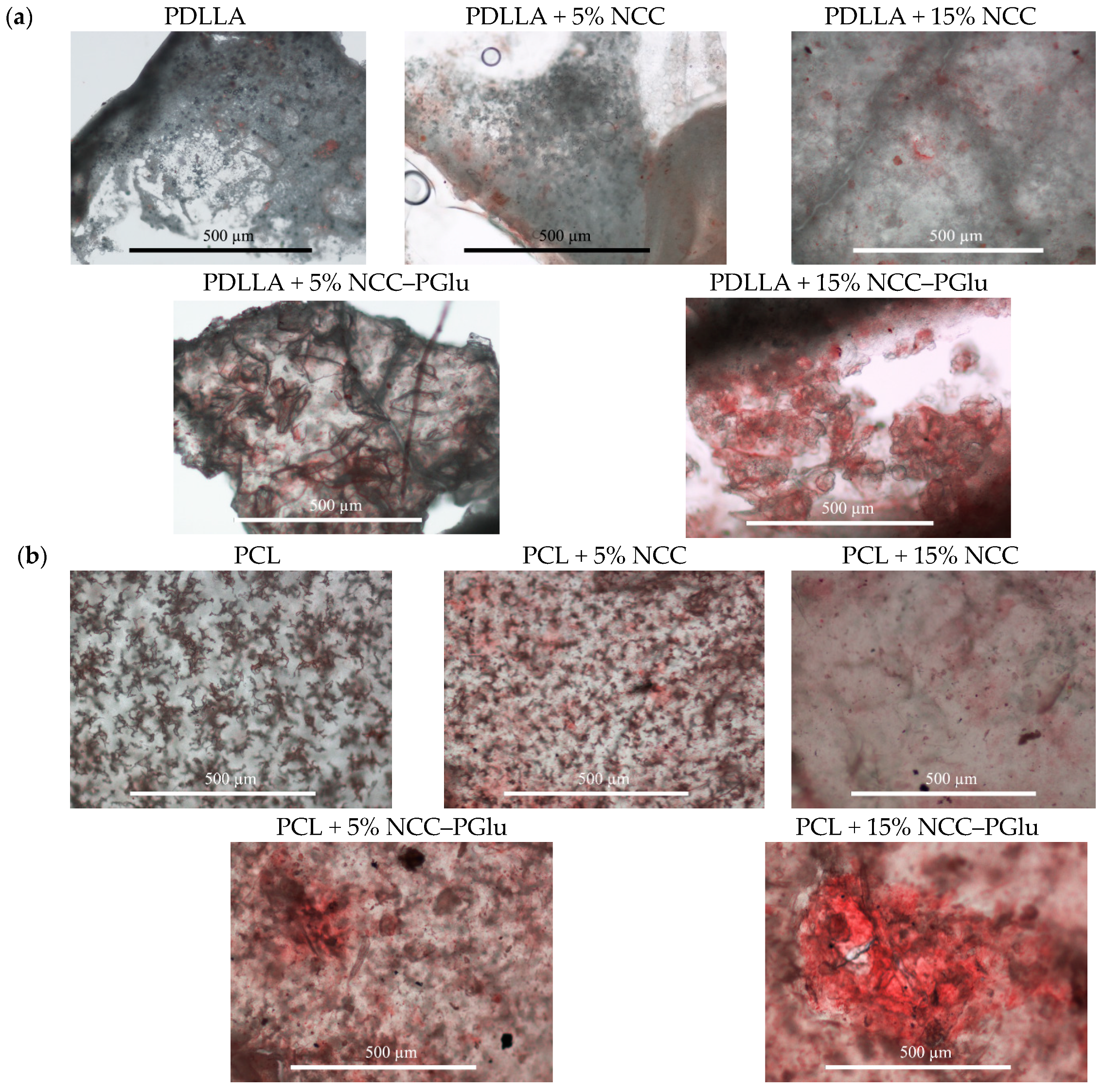
3.3. In Vitro Mineralization
3.4. Biological Evaluation
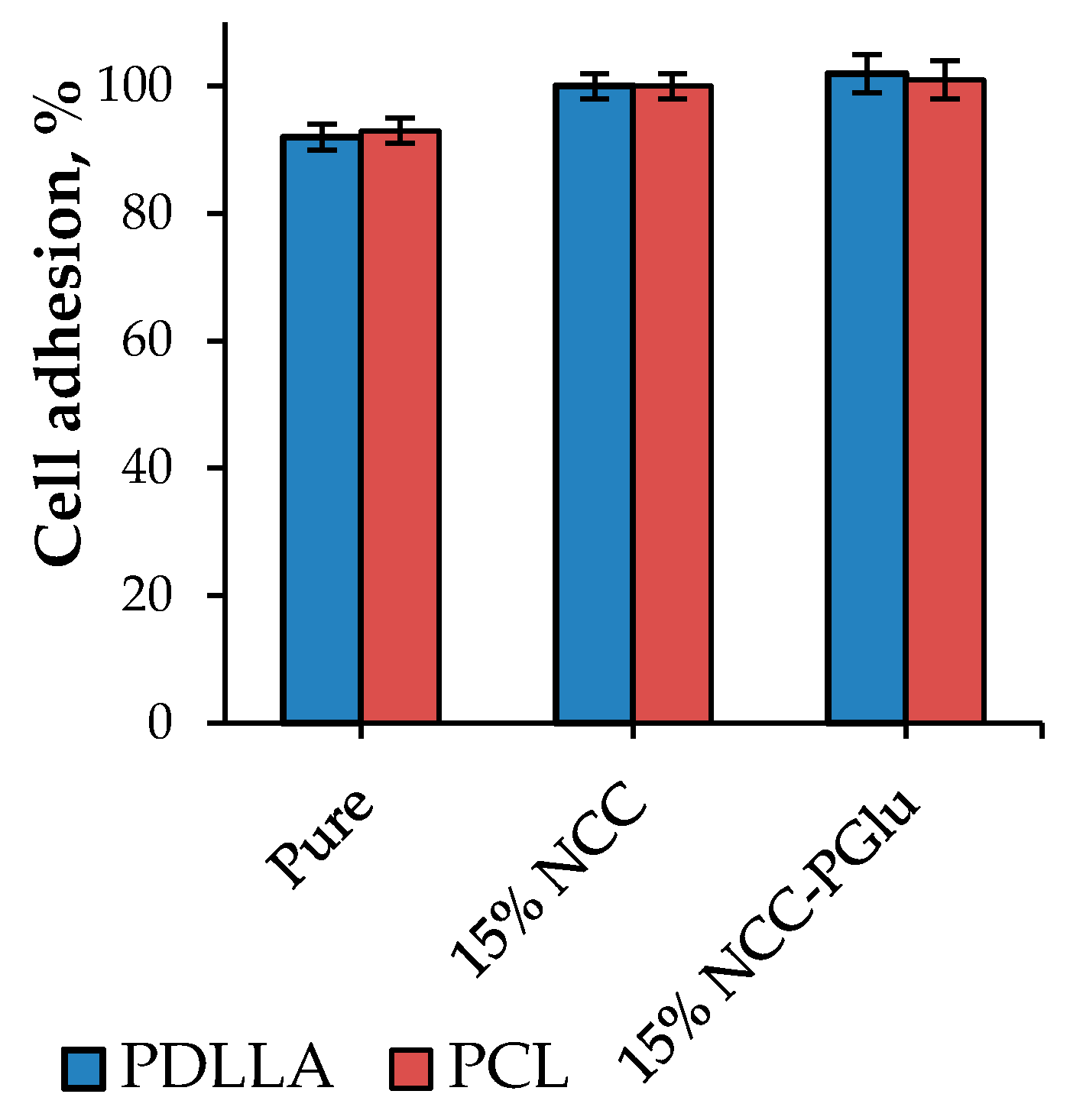
3.4.1. In Vitro Study
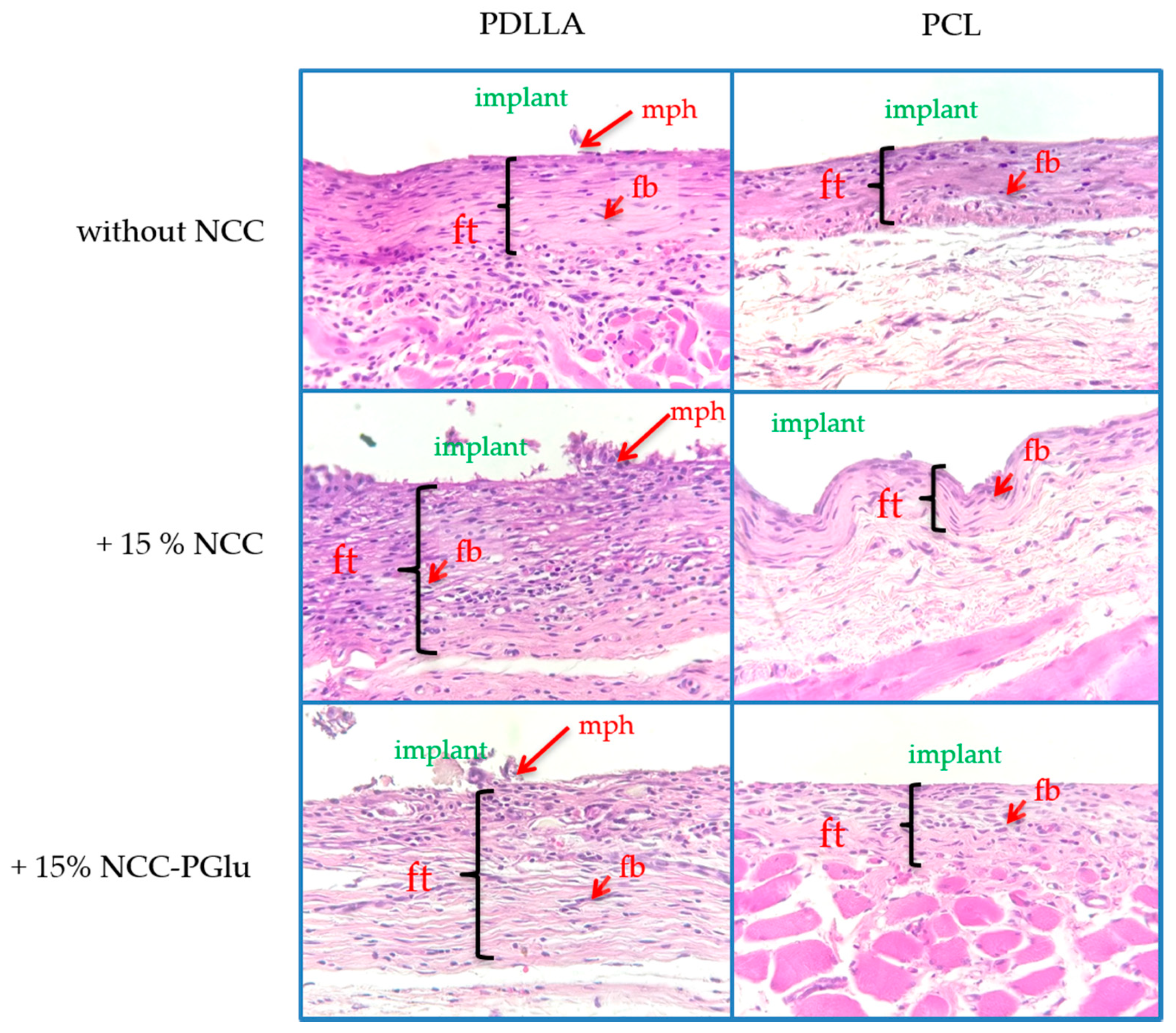
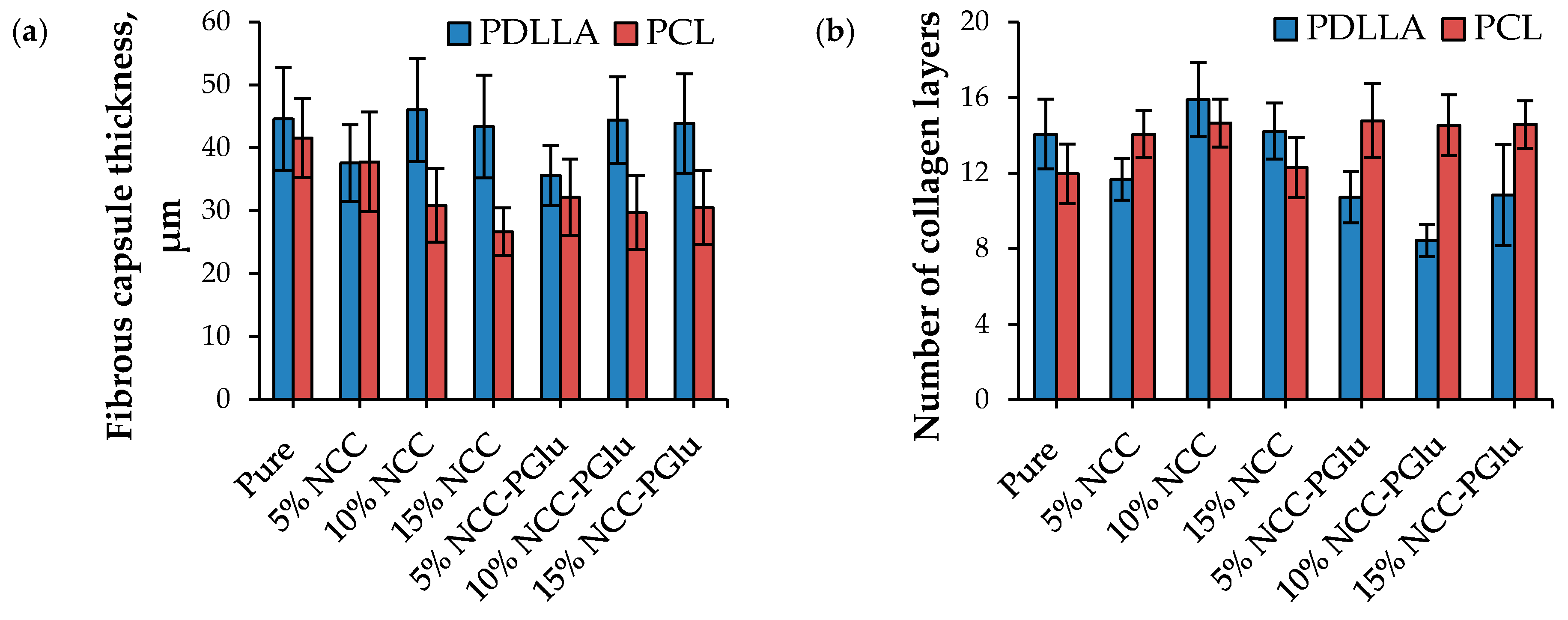
3.4.2. Biocompatibility in Vivo
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Manavitehrani, I.; Fathi, A.; Badr, H.; Daly, S.; Shirazi, A.N.; Dehghani, F. Biomedical applications of biodegradable polyesters. Polymers. 2016, 8, 20. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wen, Q.; Choi, H.S. FDA’s Regulatory science program for generic PLA/PLGA-based drug products. Am. Pharm. Rev. 2016, 19, 5–9. [Google Scholar]
- Pisani, S.; Dorati, R.; Conti, B.; Modena, T.; Bruni, G.; Genta, I. Design of copolymer PLA-PCL electrospun matrix for biomedical applications. React. Funct. Polym. 2018, 124, 77–89. [Google Scholar] [CrossRef]
- Vert, M. After soft tissues, bone, drug delivery and packaging, PLA aims at blood. Eur. Polym. J. 2015, 68, 516–525. [Google Scholar] [CrossRef]
- Lee, B.K.; Yun, Y.; Park, K. PLA micro- and nano-particles. Adv. Drug Deliv. Rev. 2016, 107, 176–191. [Google Scholar] [CrossRef] [PubMed]
- Moroishi, H.; Sonotaki, S.; Murakami, Y.; Moroishi, H.; Sonotaki, S.; Murakami, Y. PLA- and PLA/PLGA-Emulsion Composite Biomaterial Sheets for the Controllable Sustained Release of Hydrophilic Compounds. Materials. 2018, 11, 2588. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Lawrence, J.G.; Bhaduri, S.B. Fabrication aspects of PLA-CaP/PLGA-CaP composites for orthopedic applications: A review. Acta Biomater. 2012, 8, 1999–2016. [Google Scholar] [CrossRef] [PubMed]
- Palma, E.; Pasqua, A.; Gagliardi, A.; Britti, D.; Fresta, M.; Cosco, D. Antileishmanial Activity of Amphotericin B-loaded-PLGA Nanoparticles: An Overview. Mater. (Basel). 2018, 11, 1167. [Google Scholar] [CrossRef]
- Seyednejad, H.; Gawlitta, D.; Dhert, W.J.A.; van Nostrum, C.F.; Vermonden, T.; Hennink, W.E. Preparation and characterization of a three-dimensional printed scaffold based on a functionalized polyester for bone tissue engineering applications. Acta Biomater. 2011, 7, 1999–2006. [Google Scholar] [CrossRef]
- Tallawi, M.; Rosellini, E.; Barbani, N.; Cascone, M.G.; Rai, R.; Saint-Pierre, G.; Boccaccini, A.R. Strategies for the chemical and biological functionalization of scaffolds for cardiac tissue engineering: A review. J. R. Soc. Interface 2015, 12, 20150254. [Google Scholar] [CrossRef]
- Bu, Y.; Ma, J.; Bei, J.; Wang, S. Surface Modification of Aliphatic Polyester to Enhance Biocompatibility. Front. Bioeng. Biotechnol. 2019, 7, 98. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Lin, Z.; Guo, G. Biodegradation Assessment of Poly (Lactic Acid) Filled with Functionalized Titania Nanoparticles (PLA/TiO2) under Compost Conditions. Nanoscale Res. Lett. 2019, 14, 56. [Google Scholar] [CrossRef] [PubMed]
- Mahdavi, M.; Yousefzade, O.; Garmabi, H. A simple method for preparation of microcellular PLA/calcium carbonate nanocomposite using super critical nitrogen as a blowing agent: Control of microstructure. Adv. Polym. Technol. 2018, 37, 3017–3026. [Google Scholar] [CrossRef]
- Korzhikov-Vlakh, V.; Averianov, I.; Sinitsyna, E.; Nashchekina, Y.; Polyakov, D.; Guryanov, I.; Lavrentieva, A.; Raddatz, L.; Korzhikova-Vlakh, E.; Scheper, T.; et al. Novel Pathway for Efficient Covalent Modification of Polyester Materials of Different Design to Prepare Biomimetic Surfaces. Polymers. 2018, 10, 1299. [Google Scholar] [CrossRef]
- Zhao, L.; Yang, C.; Dou, J.; Xi, Y.; Lou, H.; Zhai, G. Development of RGD-Functionalized PEG-PLA Micelles for Delivery of Curcumin. J. Biomed. Nanotechnol. 2015, 11, 436–446. [Google Scholar] [CrossRef]
- Murariu, M.; Dubois, P. PLA composites: From production to properties. Adv. Drug Deliv. Rev. 2016, 107, 17–46. [Google Scholar] [CrossRef]
- Tábi, T.; Suplicz, A.; Czigány, T.; Kovács, J.G. Thermal and mechanical analysis of injection moulded poly(lactic acid) filled with poly(ethylene glycol) and talc. J. Therm. Anal. Calorim. 2014, 118, 1419–1430. [Google Scholar] [CrossRef][Green Version]
- Xu, C.; Luo, X.; Lin, X.; Zhuo, X.; Liang, L. Preparation and characterization of polylactide/thermoplastic konjac glucomannan blends. Polym. (Guildf) 2009, 50, 3698–3705. [Google Scholar] [CrossRef]
- Byun, Y.; Rodriguez, K.; Han, J.H.; Kim, Y.T. Improved thermal stability of polylactic acid (PLA) composite film via PLA–β-cyclodextrin-inclusion complex systems. Int. J. Biol. Macromol. 2015, 81, 591–598. [Google Scholar] [CrossRef]
- Rogovina, S.Z.; Aleksanyan, K.V.; Grachev, A.V.; Berlin, A.A.; Prut, E.V. Mechanical and thermophysical properties of biodegradable polylactide compositions with ethyl cellulose and chitosan containing poly(ethylene glycol). Mendeleev Commun. 2015, 25, 361–363. [Google Scholar] [CrossRef]
- Lizundia, E.; Vilas, J.L.; León, L.M. Crystallization, structural relaxation and thermal degradation in Poly(l-lactide)/cellulose nanocrystal renewable nanocomposites. Carbohydr. Polym. 2015, 123, 256–265. [Google Scholar] [CrossRef] [PubMed]
- Dhar, P.; Bhasney, S.M.; Kumar, A.; Katiyar, V. Acid functionalized cellulose nanocrystals and its effect on mechanical, thermal, crystallization and surfaces properties of poly (lactic acid) bionanocomposites films: A comprehensive study. Polym. (Guildf). 2016, 101, 75–92. [Google Scholar] [CrossRef]
- Lin, N.; Dufresne, A. Nanocellulose in biomedicine: Current status and future prospect. Eur. Polym. J. 2014, 59, 302–325. [Google Scholar] [CrossRef]
- Abdul Khalil, H.P.S.; Bhat, A.H.; Ireana Yusra, A.F. Green composites from sustainable cellulose nanofibrils: A review. Carbohydr. Polym. 2012, 87, 963–979. [Google Scholar] [CrossRef]
- Xiao, L.; Mai, Y.; He, F.; Yu, L.; Zhang, L.; Tang, H.; Yang, G. Bio-based green composites with high performance from poly(lactic acid) and surface-modified microcrystalline cellulose. J. Mater. Chem. 2012, 22, 15732–15739. [Google Scholar] [CrossRef]
- Cheng, D.; Wen, Y.; Wang, L.; An, X.; Zhu, X.; Ni, Y. Adsorption of polyethylene glycol (PEG) onto cellulose nano-crystals to improve its dispersity. Carbohydr. Polym. 2015, 123, 157–163. [Google Scholar] [CrossRef]
- Goffin, A.; Raquez, J.; Duquesne, E.; Siqueira, G.; Habibi, Y.; Dufresne, A.; Dubois, P. From Interfacial Ring-Opening Polymerization to Melt Processing of Cellulose Nanowhisker-Filled Polylactide-Based Nanocomposites. Biomacromolecules 2011, 12, 2456–2465. [Google Scholar] [CrossRef]
- Braun, B.; Dorgan, J.R.; Hollingsworth, L.O. Supra-Molecular EcoBioNanocomposites Based on Polylactide and Cellulosic Nanowhiskers: Synthesis and Properties. Biomacromolecules 2012, 13, 2013–2019. [Google Scholar] [CrossRef]
- Habibi, Y.; Goffin, A.; Schiltz, N.; Duquesne, E.; Dufresne, A. Bionanocomposites based on poly (3-caprolactone) -grafted cellulose nanocrystals by ring-opening polymerization. J. Mater. Chem. 2008, 18, 5002–5010. [Google Scholar] [CrossRef]
- Stepanova, M.; Averianov, I.; Gofman, I.; Solomakha, O.; Nashchekina, Y.; Korzhikov-Vlakh, V.; Korzhikova-Vlakh, E. Poly(ϵ-caprolactone)-based biocomposites reinforced with nanocrystalline cellulose grafted with poly(L-lactic acid). IOP Conf. Ser. Mater. Sci. Eng. 2019, 500, 12021. [Google Scholar] [CrossRef]
- Karaman, O.; Kumar, A.; Moeinzadeh, S.; He, X.; Cui, T.; Jabbari, E. Effect of surface modification of nanofibres with glutamic acid peptide on calcium phosphate nucleation and osteogenic differentiation of marrow stromal cells. J. Tissue Eng. Regen. Med. 2016, 10, E132–E146. [Google Scholar] [CrossRef] [PubMed]
- Vdovchenko, A.A.; Hubina, A.V.; Vlakh, E.G.; Tennikova, T.B. Self-assembled polymer particles based on thermoresponsive biodegradable copolymers of amino acids. Mendeleev Commun. 2017, 27, 153–154. [Google Scholar] [CrossRef]
- Vlakh, E.; Ananyan, A.; Zashikhina, N.; Hubina, A.; Pogodaev, A.; Volokitina, M.; Sharoyko, V.; Tennikova, T. Preparation, characterization, and biological evaluation of poly(glutamic acid)-b-polyphenylalanine polymersomes. Polymers. (Basel). 2016, 8, 212. [Google Scholar] [CrossRef] [PubMed]
- Averianov, I.V.; Stepanova, M.A.; Gofman, I.V.; Nikolaeva, A. l.; Korzhikov-Vlakh, V.A.; Karttunen, M.; Korzhikova-Vlakh, E.G. Chemical modification of nanocrystalline cellulose for enchanced interfacial compatibility with poly(lactic acid). Mendeleev Commun. 2019, 29, 220–222. [Google Scholar] [CrossRef]
- Horcas, I.; Fernández, R.; Gómez-Rodríguez, J.M.; Colchero, J.; Gómez-Herrero, J.; Baro, A.M. WSXM: A software for scanning probe microscopy and a tool for nanotechnology. Rev. Sci. Instrum. 2007, 78, 13705. [Google Scholar] [CrossRef]
- Rao, R.R.; Jiao, A.; Kohn, D.H.; Stegemann, J.P. Exogenous mineralization of cell-seeded and unseeded collagen-chitosan hydrogels using modified culture medium. Acta Biomater. 2012, 8, 1560–1565. [Google Scholar] [CrossRef]
- Korzhikov, V.; Averianov, I.; Litvinchuk, E.; Tennikova, T.B. Polyester-based microparticles of different hydrophobicity: the patterns of lipophilic drug entrapment and release. J. Microencapsul. 2016, 33, 199–208. [Google Scholar] [CrossRef]
- Biggs, D.L.; Lengsfeld, C.S.; Hybertson, B.M.; Ng, K.; Manning, M.C.; Randolph, T.W. In vitro and in vivo evaluation of the effects of PLA microparticle crystallinity on cellular response. J. Control. Release 2003, 92, 147–161. [Google Scholar] [CrossRef]
- Park, J.U.; Ham, J.; Kim, S.; Seo, J.-H.; Kim, S.-H.; Lee, S.; Min, H.J.; Choi, S.; Choi, R.M.; Kim, H.; et al. Alleviation of capsular formations on silicone implants in rats using biomembrane-mimicking coatings. Acta Biomater. 2014, 10, 4217–4225. [Google Scholar] [CrossRef]
- Gredes, T.; Schönitz, S.; Gedrange, T.; Stepien, L.; Kozak, K.; Kunert-Keil, C. In vivo analysis of covering materials composed of biodegradable polymers enriched with flax fibers. Biomater. Res. 2017, 21, 1–12. [Google Scholar] [CrossRef]



| Polymer | Polymer Yield, % | Polymer Characteristics | ||
|---|---|---|---|---|
| Mw | Ɖ | η (CHCl3), dL/g | ||
| PDLLA | 67 | 162,300 | 1.37 | 1.1 |
| PCL | 81 | 155,000 | 1.53 | 1.3 |
| Film | E, GPa | σb, MPa | εb, % |
|---|---|---|---|
| PDLLA | 1.62 ± 0.06 | 35 ± 2 | 67.0 ± 7.0 |
| PDLLA + 5 wt% NCC | 1.12 ± 0.08 | 32 ± 2 | 4.9 ± 0.3 |
| PDLLA + 10 wt% NCC | 1.25 ± 0.06 | 30 ± 1 | 2.9 ± 0.1 |
| PDLLA + 15 wt% NCC | 1.16 ± 0.03 | 21 ± 2 | 2.2 ± 0.2 |
| PDLLA + 5 wt% NCC–PGlu | 2.82 ± 0.30 | 31 ± 1 | 4.8 ± 0.6 |
| PDLLA + 10 wt% NCC–PGlu | 2.66 ± 0.29 | 32 ± 1 | 3.4 ± 0.2 |
| PDLLA + 15 wt% NCC–PGlu | 2.44 ± 0.21 | 31 ± 1 | 3.7 ± 0.2 |
| PCL | 0.33 ± 0.01 | 25 ± 3 | 834.0 ± 65.0 |
| PCL + 5 wt% NCC | 0.35 ± 0.03 | 8 ± 1 | 19.2 ± 5.1 |
| PCL + 10 wt% NCC | 0.34 ± 0.02 | 7 ± 1 | 8.8 ± 1.3 |
| PCL + 15 wt% NCC | 0.32 ± 0.01 | 5 ± 1 | 9.1 ± 1.0 |
| PCL + 5 wt% NCC–PGlu | 0.51 ± 0.01 | 13 ± 1 | 25.0 ± 5.0 |
| PCL + 10 wt% NCC–PGlu | 0.57 ± 0.03 | 14 ± 1 | 17.3 ± 3.2 |
| PCL + 15 wt% NCC–PGlu | 0.58 ± 0.01 | 11 ± 1 | 8.6 ± 0.5 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stepanova, M.; Averianov, I.; Serdobintsev, M.; Gofman, I.; Blum, N.; Semenova, N.; Nashchekina, Y.; Vinogradova, T.; Korzhikov-Vlakh, V.; Karttunen, M.; et al. PGlu-Modified Nanocrystalline Cellulose Improves Mechanical Properties, Biocompatibility, and Mineralization of Polyester-Based Composites. Materials 2019, 12, 3435. https://doi.org/10.3390/ma12203435
Stepanova M, Averianov I, Serdobintsev M, Gofman I, Blum N, Semenova N, Nashchekina Y, Vinogradova T, Korzhikov-Vlakh V, Karttunen M, et al. PGlu-Modified Nanocrystalline Cellulose Improves Mechanical Properties, Biocompatibility, and Mineralization of Polyester-Based Composites. Materials. 2019; 12(20):3435. https://doi.org/10.3390/ma12203435
Chicago/Turabian StyleStepanova, Mariia, Ilia Averianov, Mikhail Serdobintsev, Iosif Gofman, Natalya Blum, Natalya Semenova, Yuliya Nashchekina, Tatiana Vinogradova, Viktor Korzhikov-Vlakh, Mikko Karttunen, and et al. 2019. "PGlu-Modified Nanocrystalline Cellulose Improves Mechanical Properties, Biocompatibility, and Mineralization of Polyester-Based Composites" Materials 12, no. 20: 3435. https://doi.org/10.3390/ma12203435
APA StyleStepanova, M., Averianov, I., Serdobintsev, M., Gofman, I., Blum, N., Semenova, N., Nashchekina, Y., Vinogradova, T., Korzhikov-Vlakh, V., Karttunen, M., & Korzhikova-Vlakh, E. (2019). PGlu-Modified Nanocrystalline Cellulose Improves Mechanical Properties, Biocompatibility, and Mineralization of Polyester-Based Composites. Materials, 12(20), 3435. https://doi.org/10.3390/ma12203435