Preparation of Small-Particle and High-Density Cobalt Carbonate Using a Continuous Carbonate Precipitation Method and Evaluation of Its Growth Mechanism
Abstract
:1. Introduction
2. Experimental
2.1. Material Preparation
2.2. Characterization of CoCO3
3. Results and Discussion
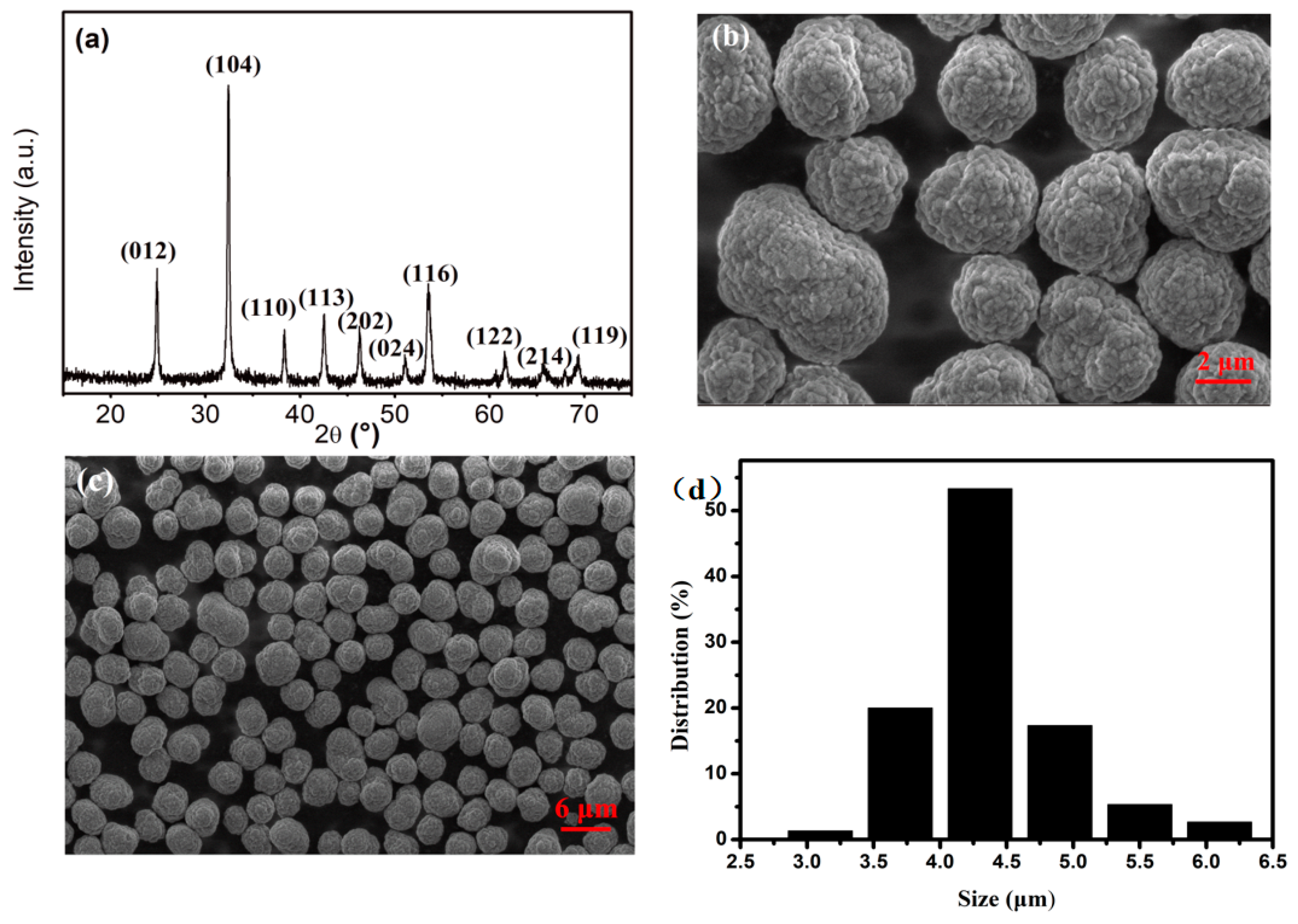
3.1. Density of CoCO3
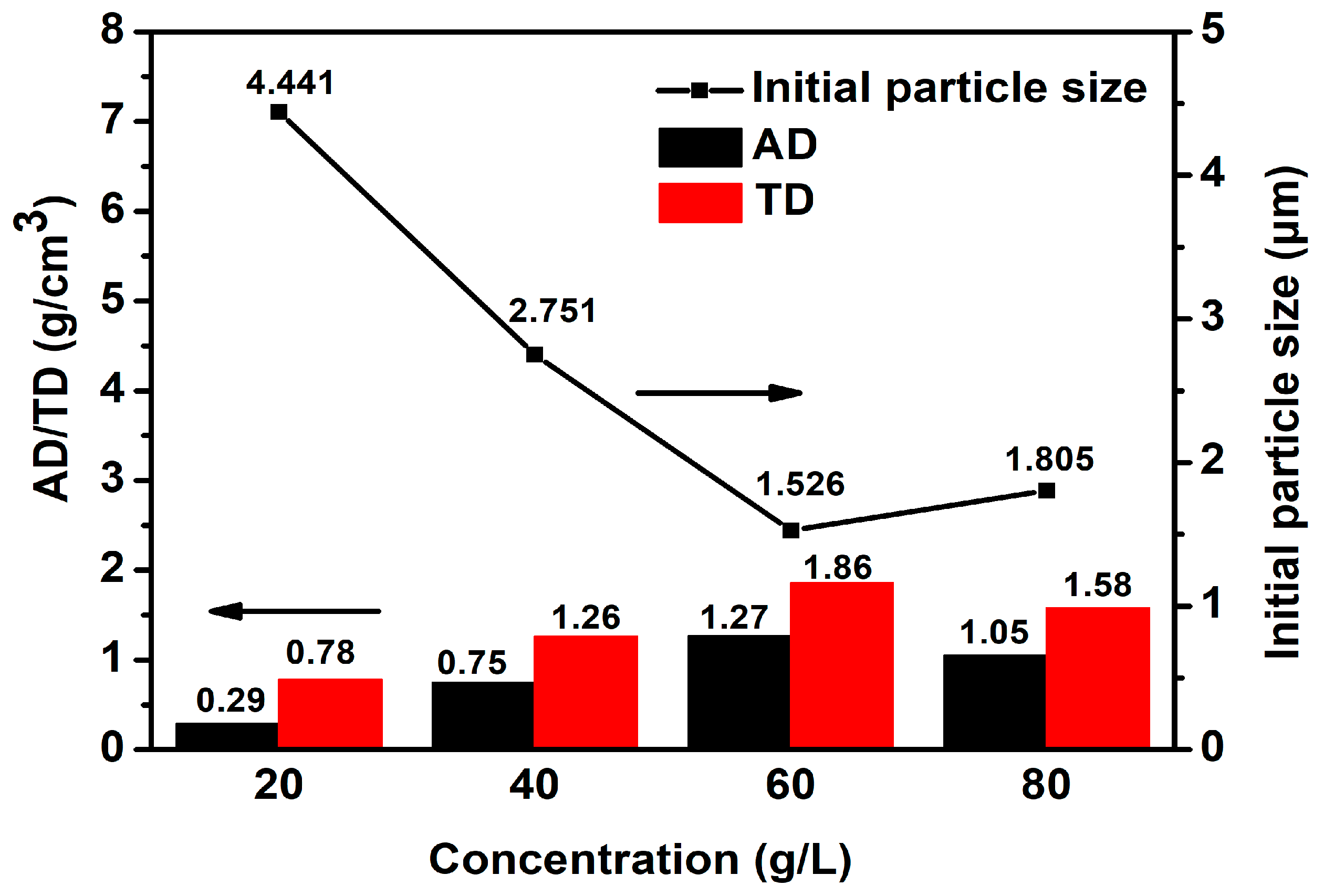
3.1.1. Effect of Concentration of NH4HCO3 on the Density of CoCO3
3.1.2. Effect of pH on the Density of CoCO3
3.1.3. Effect of Feeding Speed on the Density of CoCO3
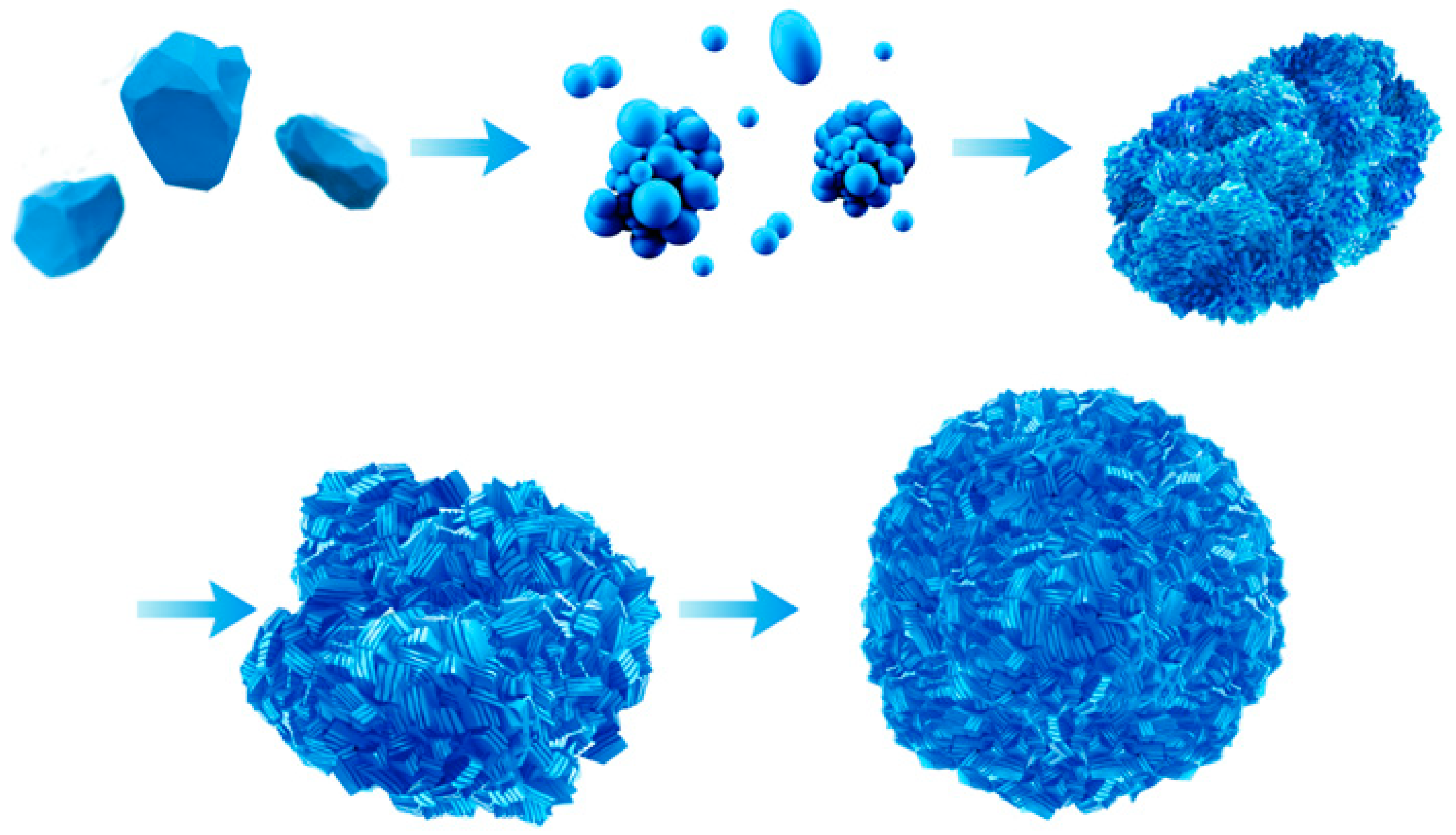
3.2. Growth Mechanism of the Continuous Reaction of CoCO3
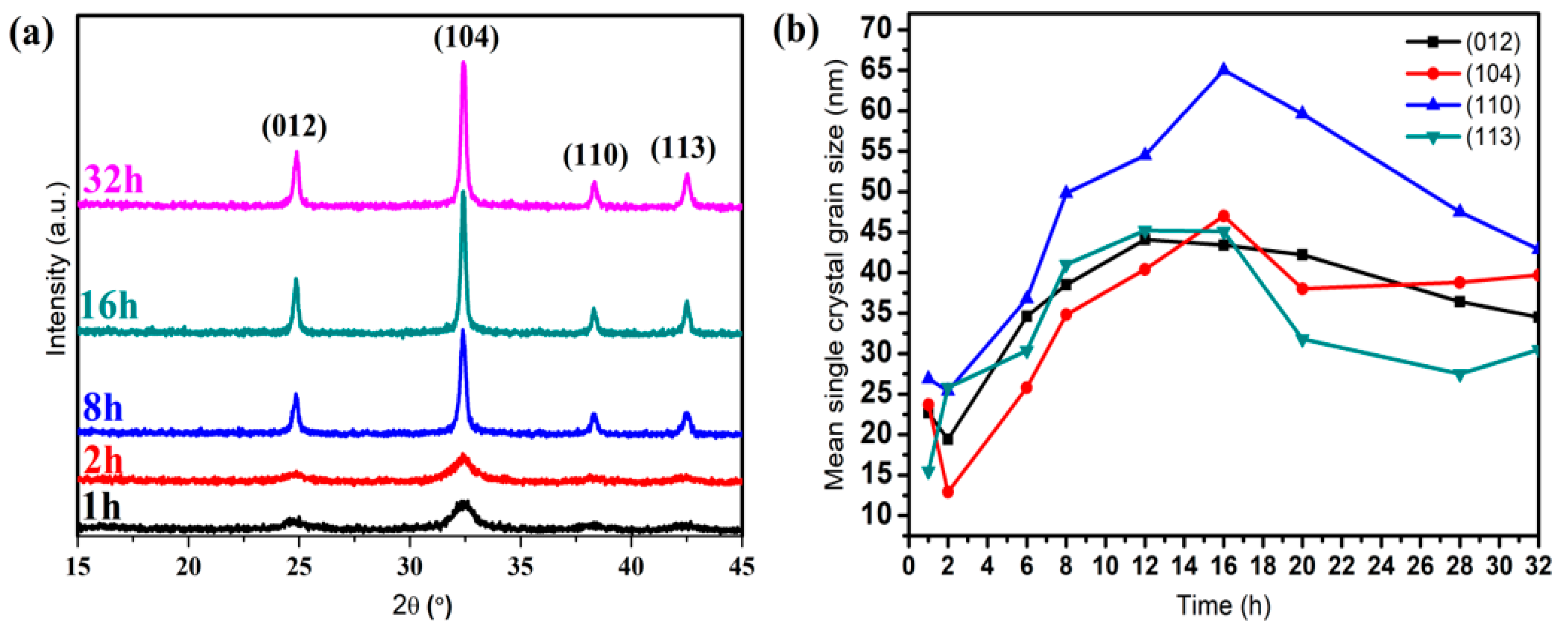
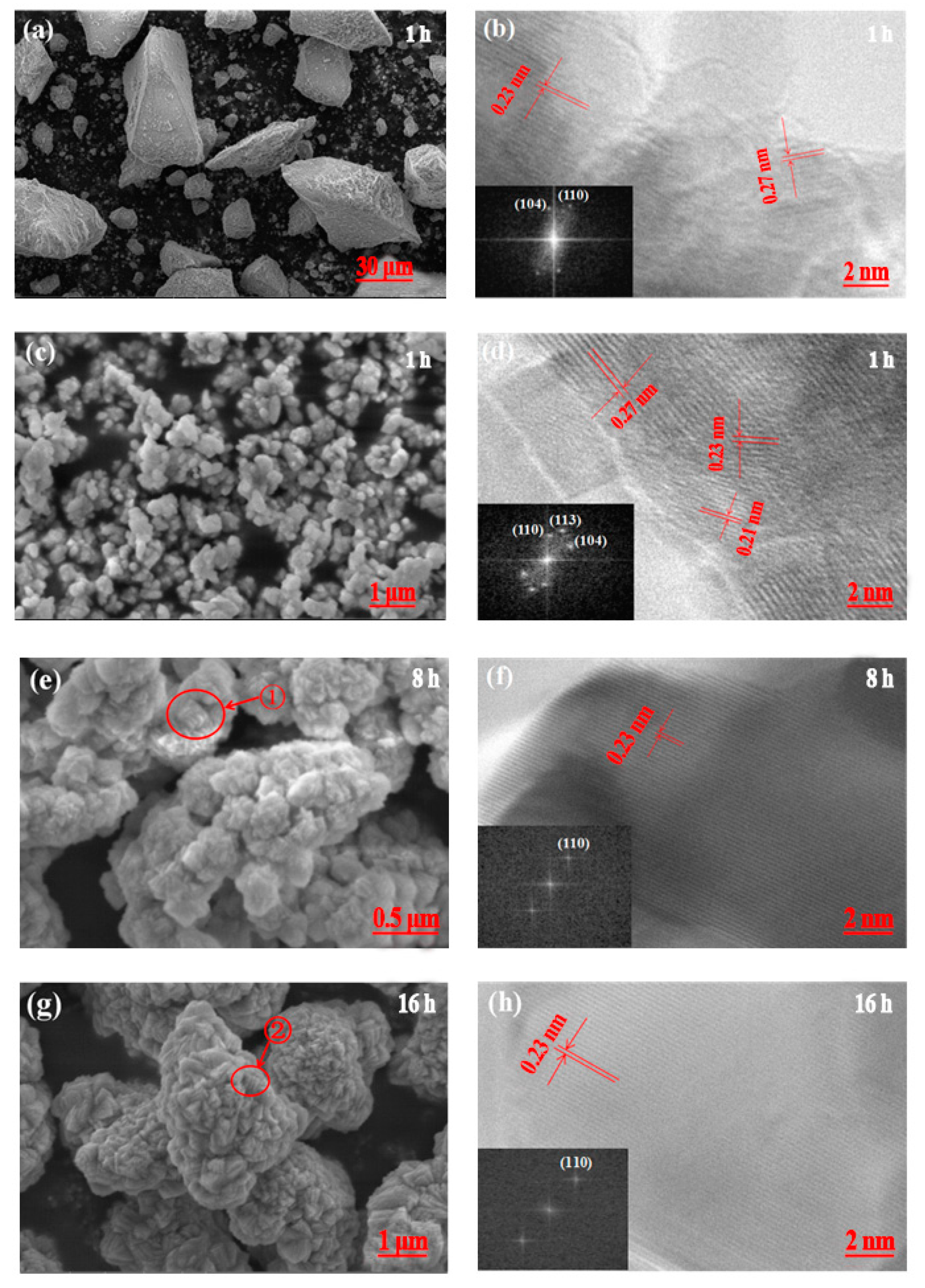
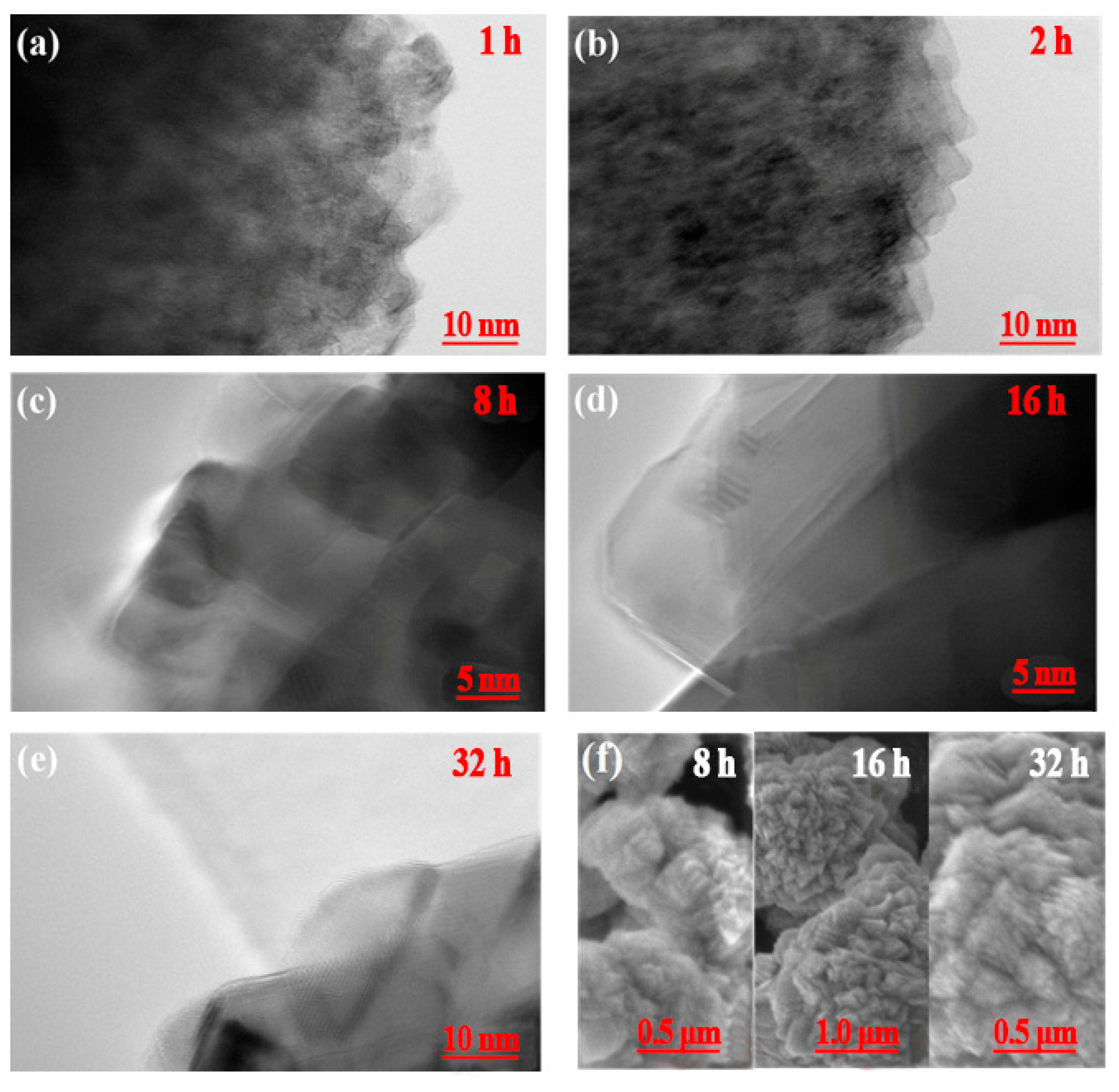
3.2.1. Crystal Anisotropy Growth
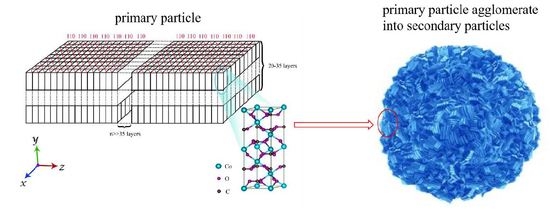
3.2.2. Three-Dimensional Growth Model of Primary Particles
3.2.3. Agglomeration of Secondary Particles
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chariton, S.; Cerantola, V.; Ismailova, L.; Bykova, E.; Bykov, M.; Kupenko, L.; Cammon, C M.; Dubrovinsky, L. The high-pressure behavior of spherocobaltite (CoCO3): A single crystal Raman spectroscopy and XRD study. Phys. Chem. Miner. 2017, 45, 59–68. [Google Scholar] [CrossRef]
- Dohnalová, Ž.; Šulcová, P.; Gorodylova, N. Study of ceramic pigments based on cobalt doped Y2O3–Al2O3 system. J. Therm. Anal. Calorim. 2014, 116, 647–654. [Google Scholar] [CrossRef]
- Singh, M.V. Waste and virgin high-density poly(ethylene) into renewable hydrocarbons fuel by pyrolysis-catalytic cracking with a CoCO3 catalyst. J. Anal. Appl. Pyrolysis 2018, 134, 150–161. [Google Scholar] [CrossRef]
- Huang, G.; Xu, S.; Yang, Y.; Sun, H.; Li, Z.; Chen, Q.; Lu, S. Micro-spherical CoCO3 anode for lithium-ion batteries. Mater. Lett. 2014, 131, 236–239. [Google Scholar] [CrossRef]
- Su, L.; Zhou, Z.; Qin, X.; Tang, Q.; Wu, D.; Shen, P. CoCO3 submicrocube/graphene composites with high lithium storage capability. Nano Energy 2013, 2, 276–282. [Google Scholar] [CrossRef]
- Yin, J.; Ding, Z.; Lei, D.; Tang, L.; Deng, J.; Li, B.; He, Y.-B. Zn-substituted CoCO3 embedded in carbon nanotubes network as high performance anode for lithium-ion batteries. J. Alloys Compd. 2017, 712, 605–612. [Google Scholar] [CrossRef]
- Lu, Z.; Wang, H.; Zhou, T.; Ma, C.; Yin, F.; Jiang, X.; Yang, G. CoCO3 micrometer particles stabilized by carbon nanofibers networks as composite electrode for enhanced rate and cyclic performance of lithium-ion batteries. Electrochim. Acta 2018, 270, 22–29. [Google Scholar] [CrossRef]
- Reddy, M.V.; Prithvi, G.; Loh, K.P.; Chowdari, B.V.R. Li storage and impedance spectroscopy studies on Co3O4, CoO, and CoN for Li-ion batteries. ACS Appl. Mater. Interfaces 2013, 6, 680–690. [Google Scholar] [CrossRef]
- Du, H.; Jiao, L.; Wang, Q.; Wang, Q.; Huan, Q.; Guo, L.; Si, Y.; Wang, Y.; Yuan, H. Morphology control of CoCO3 crystals and their conversion to mesoporous Co3O4 for alkaline rechargeable batteries application. CrystEngComm 2013, 15, 6101. [Google Scholar] [CrossRef]
- Liu, J.; Xia, H.; Lu, L.; Xue, D. Anisotropic Co3O4 porous nanocapsules toward high-capacity Li-ion batteries. J. Mater. Chem. 2010, 20, 1506. [Google Scholar] [CrossRef]
- Huang, G.; Xu, S.; Lu, S.; Li, L.; Sun, H. Porous polyhedral and fusiform Co3O4 anode materials for high-performance lithium-ion batteries. Electrochim. Acta 2014, 135, 420–427. [Google Scholar] [CrossRef]
- Nassar, M.Y. Size-controlled synthesis of CoCO3 and Co3O4 nanoparticles by free-surfactant hydrothermal method. Mater. Lett. 2013, 94, 112–115. [Google Scholar] [CrossRef]
- Barceloux, D.G.; Barceloux, D. Cobalt. J. Clin. Toxicol. 1999, 37, 201–216. [Google Scholar] [CrossRef] [PubMed]
- Sha, L.; Kai-hua, X. Preparation of nano-crystalline Co powder from CoCO3. Int. J. Refract. Metals Hard Mater. 2009, 27, 61–65. [Google Scholar] [CrossRef]
- Xiong, Z.; Shao, G.; Shi, X.; Duan, X.; Yan, L. Ultrafine hardmetals prepared by WC–10wt.%Co composite powder. Int. J. Refract. Metals Hard Mater. 2008, 26, 242–250. [Google Scholar] [CrossRef]
- Jing, X.; Song, S.; Wang, J.; Ge, L.; Jamil, S.; Liu, Q.; Mann, T.; He, Y.; Zhang, M.; Wei, H.; et al. Solvothermal synthesis of morphology controllable CoCO3 and their conversion to Co3O4 for catalytic application. Powder Technol. 2012, 217, 624–628. [Google Scholar] [CrossRef]
- Xue, P.; Guo, X.Y.; Tian, Q.H. Preparation of Metal Cobalt Powder by Co-Precipitation and Heat Decomposition Method. Adv. Mater. Res. 2011, 228, 1129–1134. [Google Scholar] [CrossRef]
- Mostafa, M.G.; Matsumoto, A.; Wase, K.; Kishi, Y. Simulation of chemicalpHenomena in the Co(NO3)2–Na2CO3–H2O system based on a thermodynamic model. Hydrometallurgy 2000, 57, 97–108. [Google Scholar] [CrossRef]
- Shao, X.; Zhang, T.; Wen, Z. One Step Fabrication of Co3O4-PPy Cathode for Lithium-O2Batteries. Chin. J. Chem. 2017, 35, 35–40. [Google Scholar] [CrossRef]
- Darbar, D.; Anilkumar, M.R.; Rajagopalan, V.; Indranil, B.; Hendry, I.E.; Ramakrishnappaf, T.; Ezemag, F.I.; Joseh, R.; Reddy, M.V. Studies on spinel cobaltites, MCo2O4 (M = Mn, Zn, Fe, Ni and Co) and their functional properties. Ceram. Int. 2018, 44, 4630–4639. [Google Scholar] [CrossRef]
- Zhao, Z.; Wang, Z.; Denis, D.K.; Sun, X.; Zhang, J.; Hou, L.; Zhang, X.; Yuan, C. Intrinsic lithium storage mechanisms and superior electrochemical behaviors of monodispersed hierarchical CoCO3 sub-microspheroids as a competitive anode towards Li-ion batteries. Electrochim. Acta 2019, 307, 20–29. [Google Scholar] [CrossRef]
- Li, H.Y.; Tseng, C.M.; Yang, C.H.; Lee, T.C.; Su, Y.C.; Hsiehet, C.T.; Chang, J.K. Eco-Efficient Synthesis of Highly Porous CoCO3 Anodes from Supercritical CO2 for Li+ and Na+ Storage. ChemSusChem 2017, 10, 2464–2472. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Zhang, M.; Liu, Y.; Hua, X.Y.; Guo, H.T.; Yang, G. Efficient construction of a CoCO3/graphene composite anode material for lithium-ion batteries by stirring solvothermal reaction. Ceram. Int. 2018, 44, 3718–3725. [Google Scholar] [CrossRef]
- Huang, C.; Jiao, G.H.; Xiao, F.X.; Yi, P.; Zhang, W.Z. Study on the preparation of microncobalt tetroxide by precipitation. Hydrometallurgy 2019, 38, 243–248. [Google Scholar] [CrossRef]
- Putnis, A.; Prieto, M.; Fernandez-Diaz, L. Fluid supersaturation and crystallization in porous media. Geol. Mag. 2009, 132, 1–13. [Google Scholar] [CrossRef]
- Martins, M.A.; Santos, C.; Almeida, M.M.; Costa, M.E. Hydroxyapatite micro- and nanoparticles: nucleation and growth mechanisms in the presence of citrate species. J. Colloid Interface Sci. 2008, 318, 210–216. [Google Scholar] [CrossRef]
- Determination of Apparent Density—Part 1: Funnel Method; ISO 3923-1:2018; German Institute for Standardization: Berlin, Germany, 2018.
- Yang, Y.; Xu, S.; Xie, M.; He, Y.; Huang, G.; Yang, Y. Growth mechanisms for spherical mixed hydroxide agglomerates prepared by co-precipitation method: A case of Ni1/3Co1/3Mn1/3(OH)2. J. Alloys Compd. 2015, 619, 846–853. [Google Scholar] [CrossRef]
- Liiri, M.; Koiranen, T.; Aittamaa, J. Secondary nucleation due to crystal–impeller and crystal–vessel collisions by population balances in CFD-modelling. J. Cryst. Growth 2002, 237, 2188–2193. [Google Scholar] [CrossRef]
- Chou, K.-S.; Chen, C.-C. The critical conditions for secondary nucleation of silica colloids in a batch Stöber growth process. Ceram. Int. 2008, 34, 1623–1627. [Google Scholar] [CrossRef]
- Frawley, P.J.; Mitchell, N.A.; Ó’Ciardhá, C.T.; Hutton, K.W. The effects of supersaturation, temperature, agitation and seed surface area on the secondary nucleation of paracetamol in ethanol solutions. Chem. Eng. Sci. 2012, 75, 183–197. [Google Scholar] [CrossRef]
- Maskara, A.; Smith, D.M. Agglomeration during the Drying of Fine Silica Powders, Part II: The Role of Particle Solubility. J. Am. Ceram. Soc. 1997, 80, 1715–1722. [Google Scholar] [CrossRef]




© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, M.; Xi, X.; Li, S.; Li, C.; Nie, Z.; Xu, K. Preparation of Small-Particle and High-Density Cobalt Carbonate Using a Continuous Carbonate Precipitation Method and Evaluation of Its Growth Mechanism. Materials 2019, 12, 3394. https://doi.org/10.3390/ma12203394
Guo M, Xi X, Li S, Li C, Nie Z, Xu K. Preparation of Small-Particle and High-Density Cobalt Carbonate Using a Continuous Carbonate Precipitation Method and Evaluation of Its Growth Mechanism. Materials. 2019; 12(20):3394. https://doi.org/10.3390/ma12203394
Chicago/Turabian StyleGuo, Miaomiao, Xiaoli Xi, Sen Li, Chenwei Li, Zuoren Nie, and Kaihua Xu. 2019. "Preparation of Small-Particle and High-Density Cobalt Carbonate Using a Continuous Carbonate Precipitation Method and Evaluation of Its Growth Mechanism" Materials 12, no. 20: 3394. https://doi.org/10.3390/ma12203394
APA StyleGuo, M., Xi, X., Li, S., Li, C., Nie, Z., & Xu, K. (2019). Preparation of Small-Particle and High-Density Cobalt Carbonate Using a Continuous Carbonate Precipitation Method and Evaluation of Its Growth Mechanism. Materials, 12(20), 3394. https://doi.org/10.3390/ma12203394