Preparation and Photocatalytic Properties of CdS and ZnS Nanomaterials Derived from Metal Xanthate
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents Used
2.2. Preparation of ZnS
2.3. Preparation of CdS
2.4. Characterization of the Products
2.5. Photocatalytic Properties
2.6. Equations Used
3. Results and Discussion
3.1. Formation of ZnS and CdS

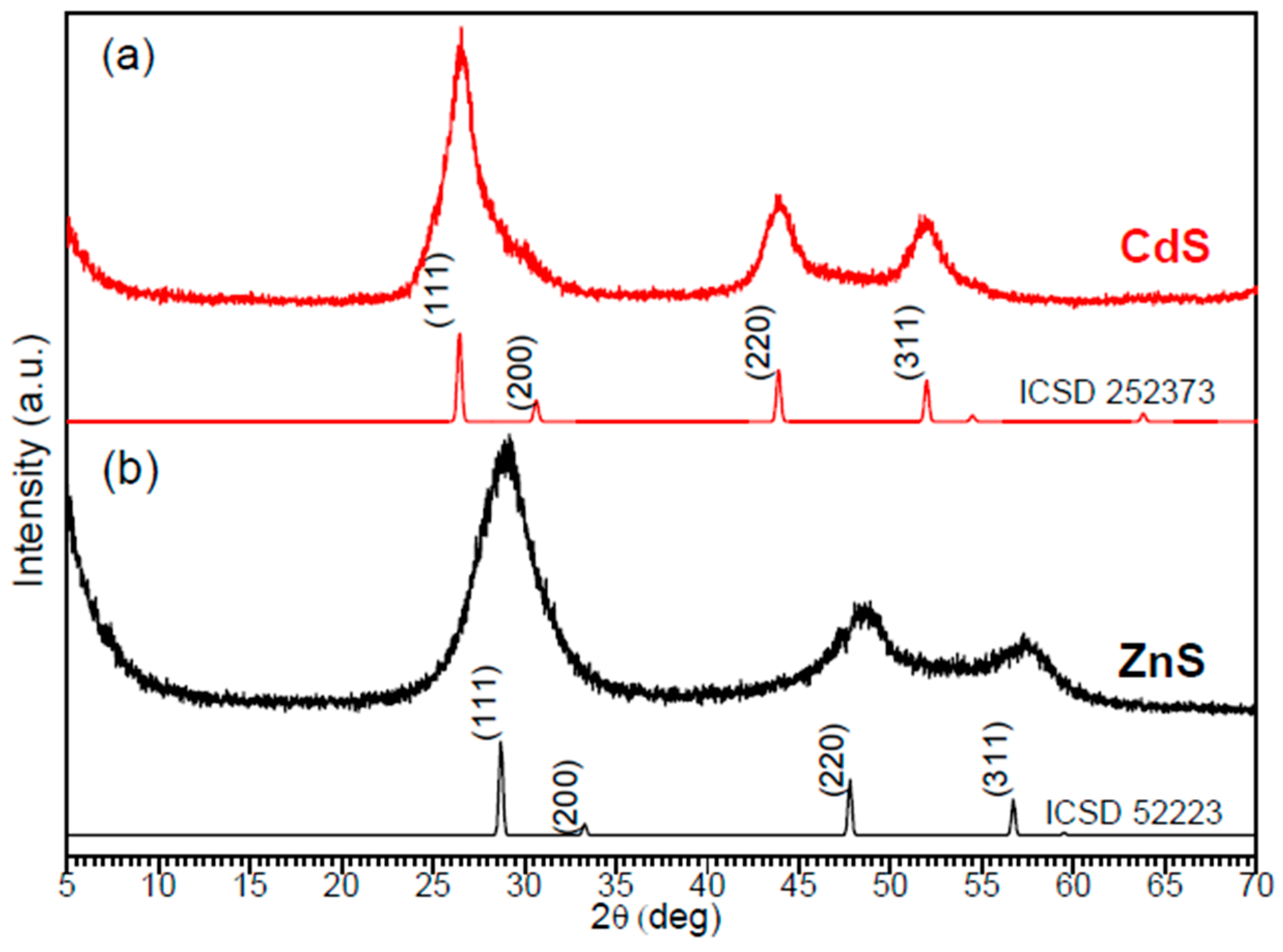
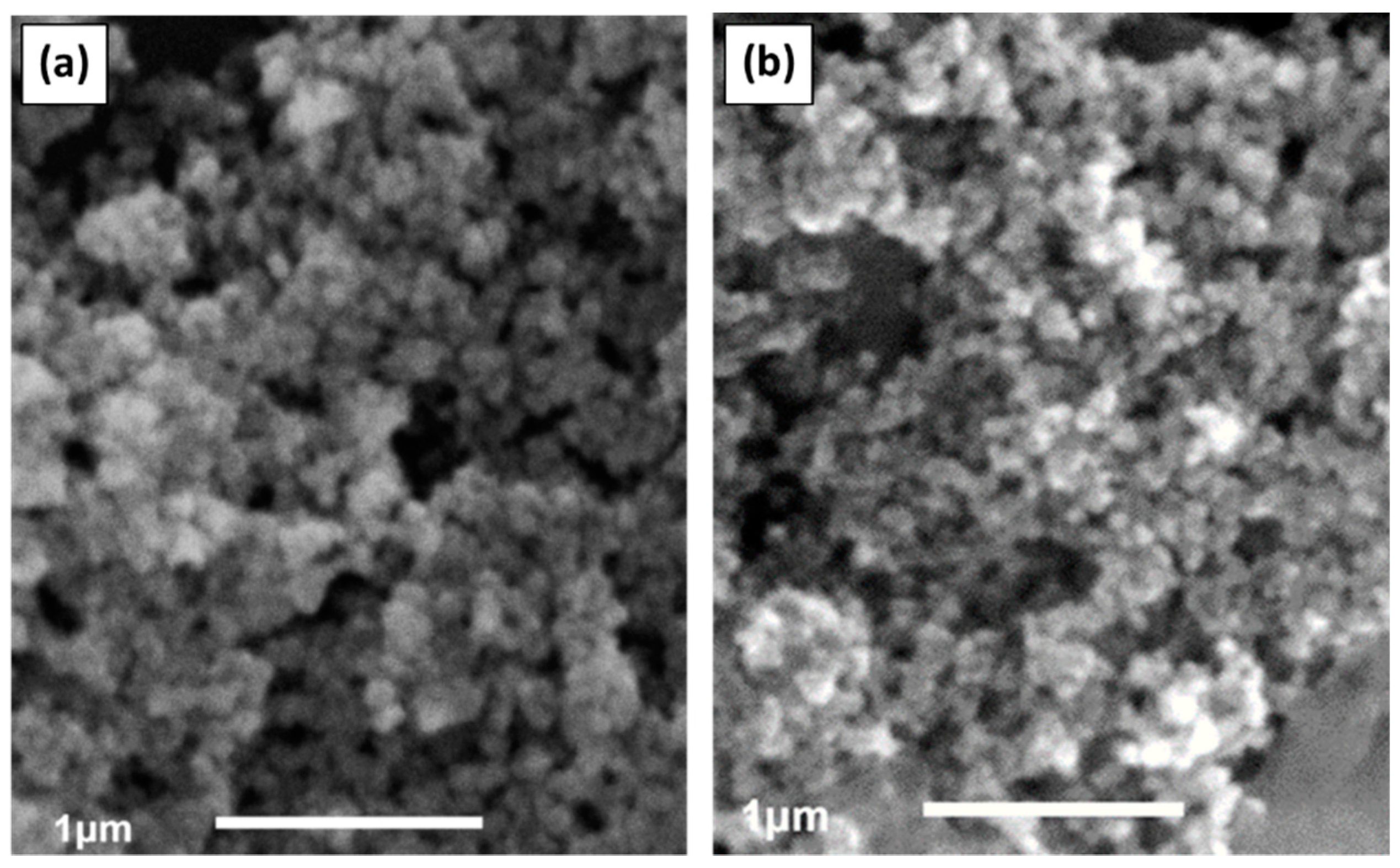
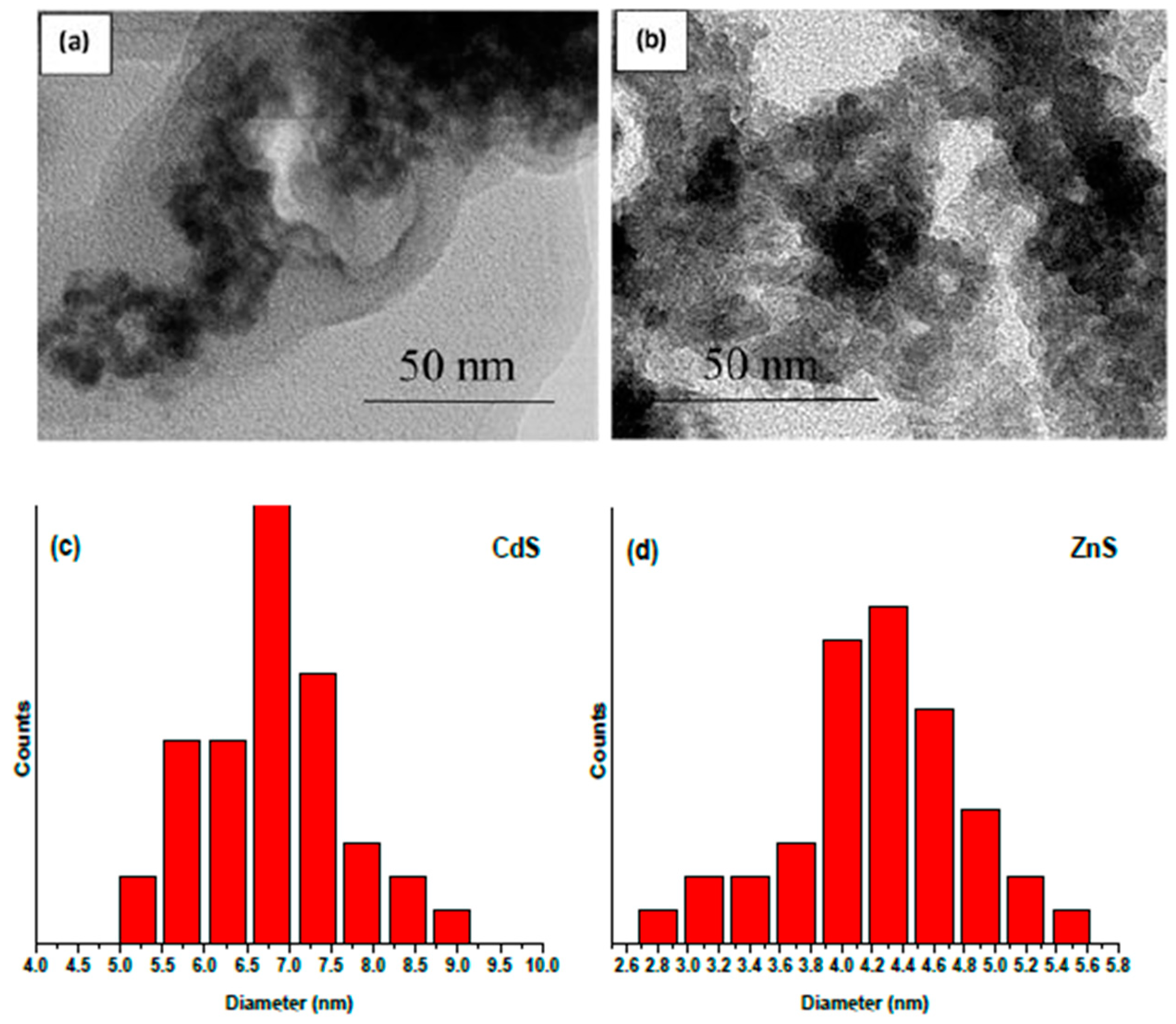
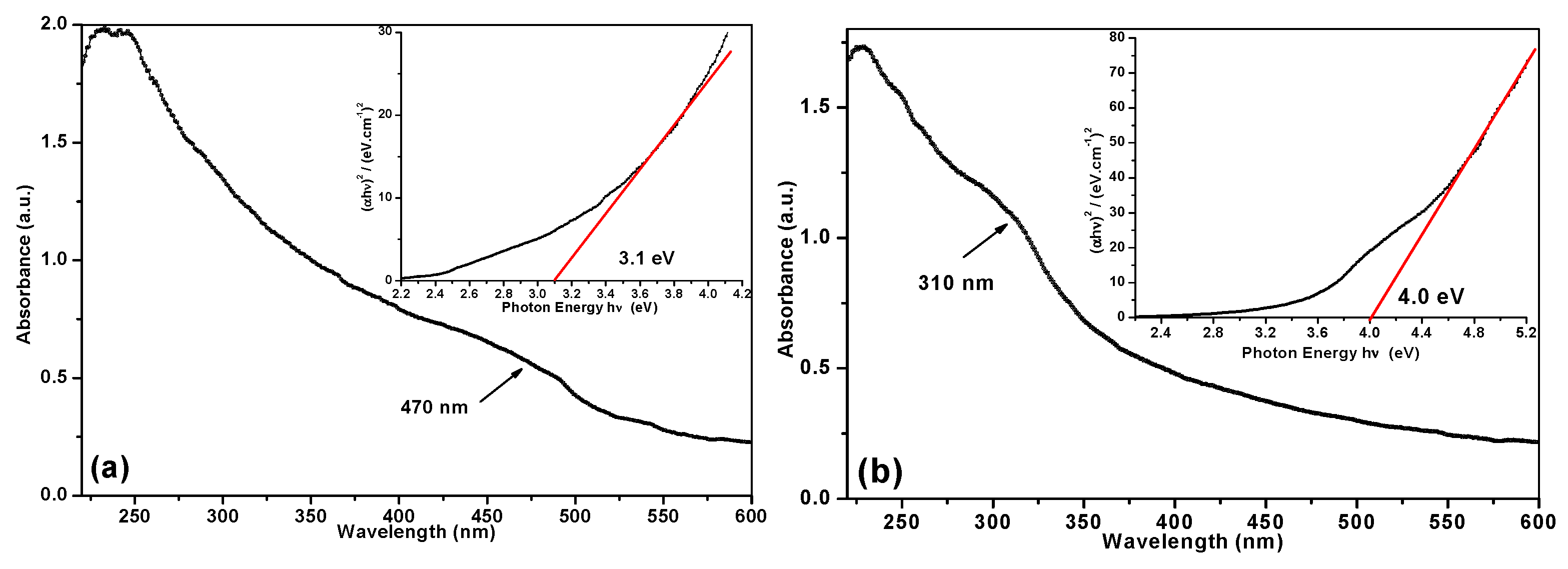
3.2. Characterization of Nanomaterials
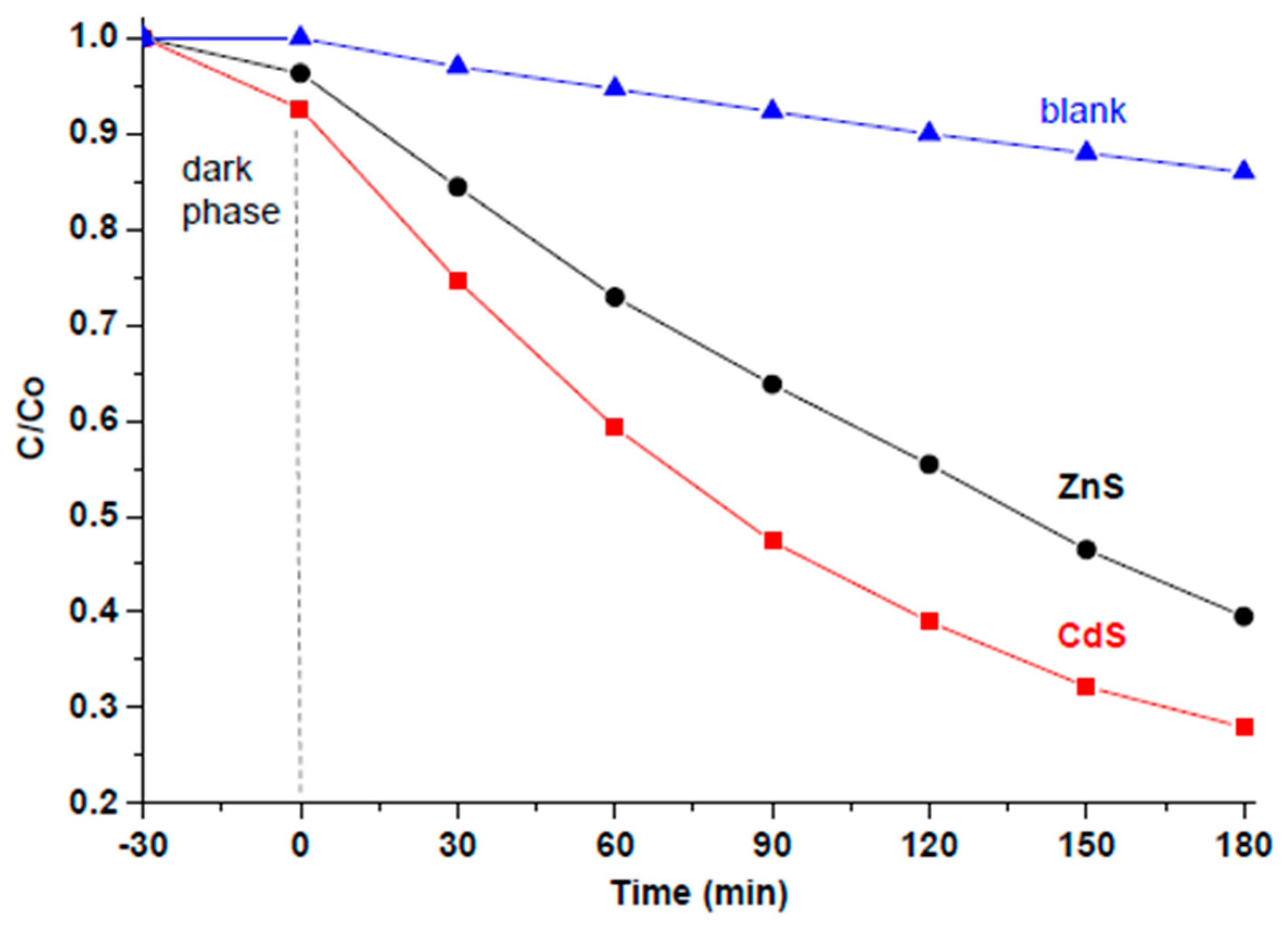
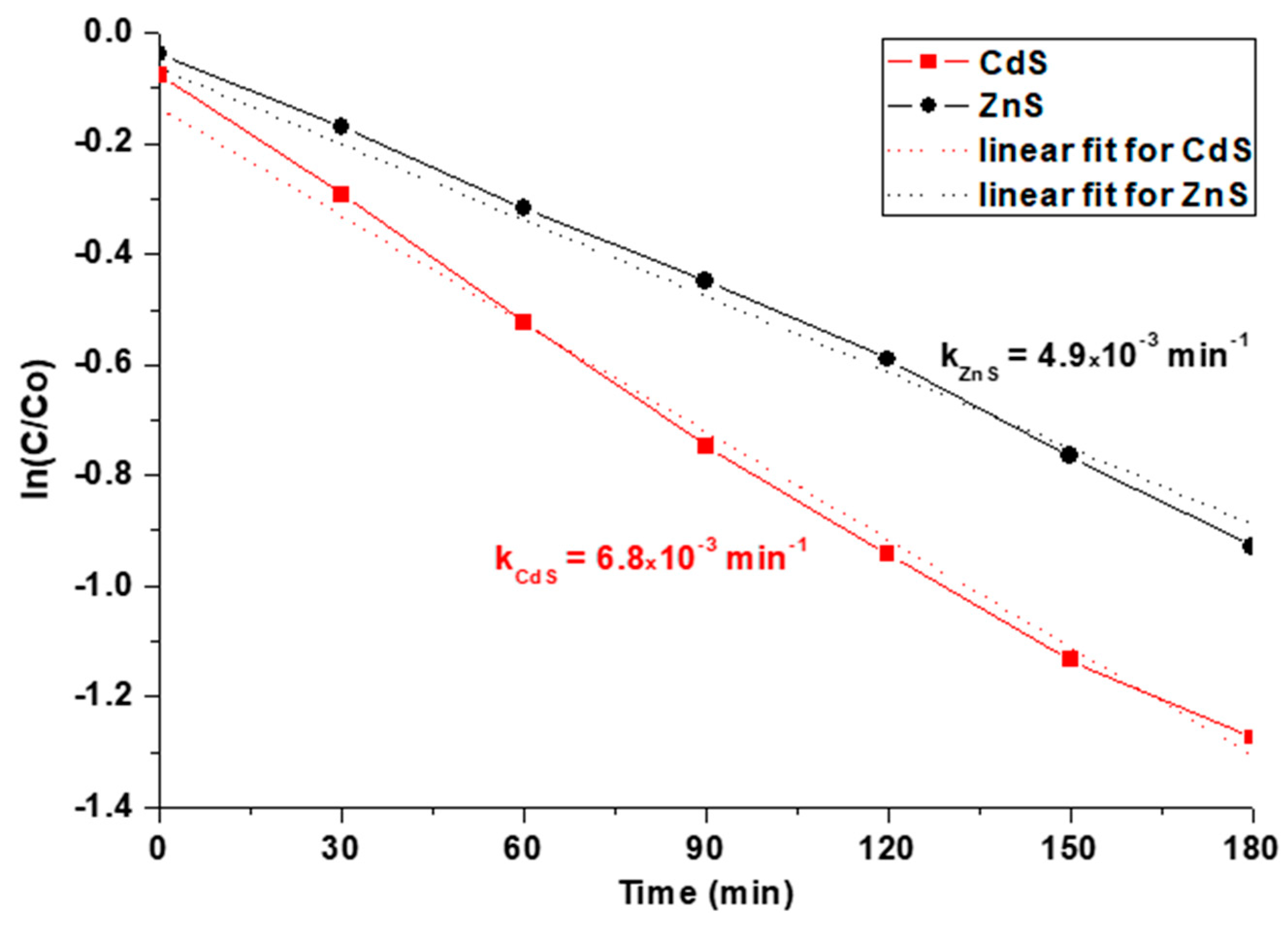
3.3. Photocatalytic Tests
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bera, D.; Qian, L.; Tseng, T.-K.; Holloway, P.H. Quantum dots and their multimodal applications: A review. Materials 2010, 3, 2260–2345. [Google Scholar] [CrossRef]
- Edvinsson, T. Optical quantum confinement and photocatalytic properties in two-, one- and zero-dimensional nanostructures. R. Soc. Open Sci. 2018, 5, 180387. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Anusuyadevi, P.R.; Aymonier, C.; Luque, R.; Marre, S. Nanostructures materials for photocatalysis. Chem. Soc. Rev. 2019, 48, 3868–3902. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.L.; Chang, C.J. Recent Progress on metal sulfide composite nanomaterials for photocatalytic hydrogen production. Catalysts 2019, 9, 457. [Google Scholar] [CrossRef]
- Lee, G.-J.; Wu, J.J. Recent developments in ZnS photocatalysts from synthesis to photocatalytic applications—A review. Powder Technol. 2017, 318, 8–22. [Google Scholar] [CrossRef]
- Cheng, L.; Xiang, Q.; Liao, Y.; Zhang, H. CdS-Based photocatalysts. Energy Environ. Sci. 2018, 11, 1362–1391. [Google Scholar] [CrossRef]
- Lee, H.L.; Issam, A.M.; Belmahi, M.; Assouar, M.B.; Rinnert, H.; Alnot, M. Synthesis and characterizations of bare CdS nanocrystals using chemical precipitation method for photoluminescence application. J. Nanomater. 2009, 2009, 914501. [Google Scholar] [CrossRef]
- Nirmala Jothi, N.S.; Joshi, A.G.; Jerald Vijay, R.; Muthuvinayagam, A.; Sagayaraj, P. Investigation on one-pot hydrothermal synthesis, structural and optical properties of ZnS quantum dots. Mater. Chem. Phys. 2013, 138, 186–191. [Google Scholar] [CrossRef]
- Wageh, S.; Ling, Z.S.; Xu-Rong, X. Growth and optical properties of colloidal ZnS nanoparticles. J. Cryst. Growth 2003, 255, 332–337. [Google Scholar] [CrossRef]
- Tezuka, K.; Takagi, H.; Shan, Y.J.; Imoto, H. Simple synthesis of zinc sulfide and cadmium sulfide under hydrothermal conditions. J. Ceram. Soc. Jpn. 2011, 119, 55–59. [Google Scholar] [CrossRef]
- Andrew, F.P.; Ajibade, P.A. Metal complexes of alkyl-aryl dithiocarbamates: Structural studies, anticancer potentials and applications as precursors for semiconductor nanocrystals. J. Mol. Struct. 2018, 1155, 843–855. [Google Scholar] [CrossRef]
- Onwudiwe, D.C.; Ajibade, P.A. Zn(II), Cd(II) and Hg(II) complexes of N-methyl-N-phenyl dithiocarbamate as single-source precursors for the synthesis of metal sulfide nanoparticles. Mater. Lett. 2011, 65, 3258–3261. [Google Scholar] [CrossRef]
- Onwudiwe, D.C.; Strydom, C.; Oluwafemi, O.S.; Songca, S.P. Effect of temperature on the optical and structural properties of hexadecylamine capped ZnS nanoparticles using Zinc(II) N-ethyl-N-phenyldithiocarbamate as single source precursor. Mater. Res. Bull. 2012, 47, 4445–4451. [Google Scholar] [CrossRef]
- Pawar, A.S.; Mlowe, S.; Garje, S.S.; Akerman, M.P.; Revaprasadu, N. Zinc thiosemicarbazone complexes: Single source precursors for alkylamine capped ZnS nanoparticles. Inorg. Chim. Acta 2017, 463, 7–13. [Google Scholar] [CrossRef]
- Pawar, A.S.; Masikane, S.C.; Mlowe, S.; Garje, S.S.; Revaprasadu, N. Preparation of CdS nanoparticles from thiosemicarbazone complexes: Morphological influence of chlorido and iodido ligands. Eur. J. Inorg. Chem. 2016, 2016, 366–372. [Google Scholar] [CrossRef]
- Zhang, Z.; Lim, W.P.; Wong, C.T.; Xu, H.; Yin, F.; Chin, W.S. From metal thiobenzoates to metal sulfide nanocrystals: An experimental and theoretical investigation. Nanomaterials 2012, 2, 113–133. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yin, S.; Yang, S. Glutathione as both ligand and sulfur source for the synthesis of full-color luminescent water-soluble CdS nanocrystals. Mater. Lett. 2017, 196, 260–263. [Google Scholar] [CrossRef]
- Tong, H.; Zhu, Y.-J. Synthesis of CdS nanocrystals based on low-temperature thermolysis of one single-source organometallic precursor. Nanotechnology 2006, 17, 845–851. [Google Scholar] [CrossRef]
- Sun, S.; Han, Q.; Wu, X.; Zhu, J.; Wang, X. The facile synthesis of PbS cubes and Bi2S3 nanoflowers from molecular precursors at room temperature. Mater. Lett. 2011, 65, 3344–3347. [Google Scholar] [CrossRef]
- Han, Q.; Zhao, J.; Wu, L.; Zhu, J.; Wang, X. Synthesis of CdS multipods from cadmium xanthate in ethylenediamine solution. Particuology 2015, 19, 45–52. [Google Scholar] [CrossRef]
- Nair, P.S.; Radhakrishnan, T.; Revaprasadu, N.; Kolawole, G.; O’Brien, P. Cadmium ethylxanthate: A novel single-source precursor for the preparation of CdS nanoparticles. J. Mater. Chem. 2002, 12, 2722–2725. [Google Scholar] [CrossRef]
- Han, Q.; Sun, Y.; Wang, X.; Chen, L.; Yang, X.; Lu, L. Controllable synthesis of Bi2S3 hierarchical nanostructures: Effect of addition method on structures. J. Alloy. Compd. 2009, 481, 520–525. [Google Scholar] [CrossRef]
- Sun, Y.; Han, Q.; Lu, J.; Yang, X.; Lu, L.; Wang, X. Preparation of uniform Bi2S3 nanoribbons at a low temperature. Mater. Lett. 2008, 62, 3730–3732. [Google Scholar] [CrossRef]
- Singhal, A.; Dutta, D.P.; Tyagi, A.K.; Mobin, S.M.; Mathur, P.; Lieberwirth, I. Palladium(II)/allylpalladium(II) complexes with xanthate ligands: Single-source precursors for the generation of palladium sulfide nanocrystals. J. Organomet. Chem. 2007, 692, 5285–5294. [Google Scholar] [CrossRef]
- Pradhan, N.; Efrima, S. Single-Precursor, One-pot versatile synthesis under near ambient conditions of tunable, single and dual band fluorescing metal sulfide nanoparticles. J. Am. Chem. Soc. 2003, 125, 2050–2051. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, N.; Katz, B.; Efrima, S. Synthesis of high-quality metal sulfide nanoparticles from alkyl xanthate single precursors in alkylamine solvents. J. Phys. Chem. B 2003, 107, 13843–13854. [Google Scholar] [CrossRef]
- Mintcheva, N.; Gicheva, G.; Panayotova, M.; Kulinich, S.A. Zinc xanthates as molecular precursors for formation of ZnS nanoparticles. Unpublished work.
- Mintcheva, N.; Aljulaih, A.A.; Wunderlich, W.; Kulinich, S.A.; Iwamori, S. Laser-ablated ZnO nanoparticles and their photocatalytic activity towards organic pollutants. Materials 2018, 11, 1127. [Google Scholar] [CrossRef] [PubMed]
- Tauc, J.; Grigorovici, R.; Vancu, A. Optical properties and electronic structure of amorphous germanium. Phys. Status Solidi 1966, 15, 627–637. [Google Scholar] [CrossRef]
- Sodium Ethyl Xanthate; Public Report; Australian Government Publishing Service: Canberra, Australia, 1995; pp. 5–16. ISBN 0644352833.
- Khan, G.A.; Gabrielova, L.I.; Vlasova, N.S. Flotation Reagents and Their Application; Nedra: Moscow, Russia, 1986; pp. 38–46. (In Russian) [Google Scholar]
- Falcon, H.; Tartaj, P.; Vaquero, F.; Navarro, R.M.; Fierro, J.L.G.; Bolletta, J.P.; de Paoli, J.M.; Carbonio, R.E.; Fernandez-Diaz, M.T.; Alonso, J.A. Straightforward high-pressure synthesis and characterization of indium-based thiospinels: Photocatalytic potential for hydrogen production. Eur. J. Inorg. Chem. 2016, 10, 1558–1565. [Google Scholar] [CrossRef]
- Jamieson, J.C.; Demarest, H.H., Jr. A note on the compression of cubic ZnS. J. Phys. Chem. Solids 1980, 41, 963–962. [Google Scholar] [CrossRef]
- Sillen, L.G.; Martell, A.E. Stability Constants of Metal-Ion Complexes. In Lange’s Handbook; The Chemical Society: London, UK, 1964; pp. 8.6–8.11. Available online: http://www.wiredchemist.com/chemistry/data/solubility-product-constants (accessed on 23 July 2019).
- Rossetti, R.; Hull, R.; Gibson, J.M.; Brus, L.E. Excited electronic states and optical spectra of ZnS and CdS crystallites in the 15 to 50 A size range: Evolution from molecular to bulk semiconducting properties. J. Chem. Phys. 1985, 82, 552–559. [Google Scholar] [CrossRef]
- Kumbhojkar, N.; Nikesh, V.V.; Kshirsagar, A.; Mahamunia, S. Photophysical properties of ZnS nanoclusters. J. Appl. Phys. 2000, 88, 6260–6264. [Google Scholar] [CrossRef]
- Gaur, R.; Jeevanandam, P. Evolution of different morphologies of CdS nanoparticles by thermal decomposition of bis (thiourea) cadmium chloride in various solvents. J. Nanopart. Res. 2015, 17, 156–168. [Google Scholar] [CrossRef]
- Zulmajdi, S.L.N.; Ajak, S.N.F.H.; Hobley, J.; Duraman, N.; Harunsani, M.H.; Yasin, H.M.; Nur, M.; Usman, A. Kinetics of photocatalytic degradation of methylene blue in aqueous dispersions of TiO2 nanoparticles under UV-LED irradiation. Am. J. Nanomater. 2017, 5, 1–6. [Google Scholar] [CrossRef]
- Stoyanova, M.; Christoskova, S. Catalytic degradation of methylene blue in aqueous solutions over Ni- and Co- oxide systems. Cent. Eur. J. Chem. 2011, 9, 1000–1007. [Google Scholar] [CrossRef]
- Soltani, N.; Saion, E.; Hussein, M.Z.; Erfani, M.; Abedini, A.; Bahmanrokh, G.; Navasery, M.; Vaziri, P. Visible light-induced degradation of methylene blue in the presence of photocatalytic ZnS and CdS panoparticles. Int. J. Mol. Sci. 2012, 13, 12242–12258. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Huang, B.; Li, Z.; Lou, Z.; Wang, Z.; Dai, Y.; Whangbo, M.-H. Synthesis and characterization of ZnS with controlled amount of S vacancies for photocatalytic H2 production under visible light. Sci. Rep. 2015, 5, 8544. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mintcheva, N.; Gicheva, G.; Panayotova, M.; Wunderlich, W.; Kuchmizhak, A.A.; Kulinich, S.A. Preparation and Photocatalytic Properties of CdS and ZnS Nanomaterials Derived from Metal Xanthate. Materials 2019, 12, 3313. https://doi.org/10.3390/ma12203313
Mintcheva N, Gicheva G, Panayotova M, Wunderlich W, Kuchmizhak AA, Kulinich SA. Preparation and Photocatalytic Properties of CdS and ZnS Nanomaterials Derived from Metal Xanthate. Materials. 2019; 12(20):3313. https://doi.org/10.3390/ma12203313
Chicago/Turabian StyleMintcheva, Neli, Gospodinka Gicheva, Marinela Panayotova, Wilfried Wunderlich, Aleksandr A. Kuchmizhak, and Sergei A. Kulinich. 2019. "Preparation and Photocatalytic Properties of CdS and ZnS Nanomaterials Derived from Metal Xanthate" Materials 12, no. 20: 3313. https://doi.org/10.3390/ma12203313
APA StyleMintcheva, N., Gicheva, G., Panayotova, M., Wunderlich, W., Kuchmizhak, A. A., & Kulinich, S. A. (2019). Preparation and Photocatalytic Properties of CdS and ZnS Nanomaterials Derived from Metal Xanthate. Materials, 12(20), 3313. https://doi.org/10.3390/ma12203313








