Synthesis of Novel Phenyl Porous Organic Polymers and Their Excellent Visible Light Photocatalytic Performance on Antibiotics
Abstract
:1. Introduction
2. Material and Methods
3. Results
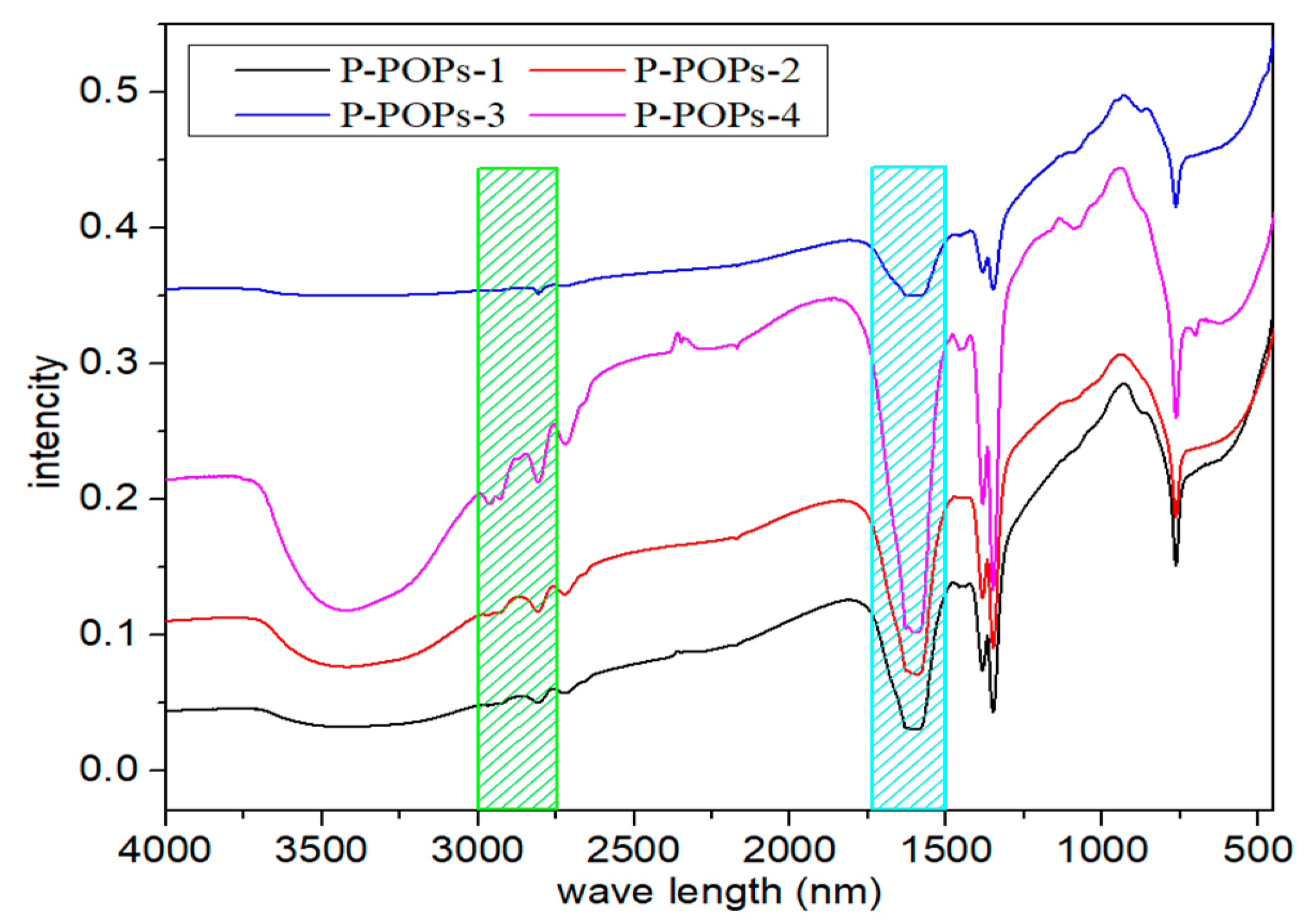
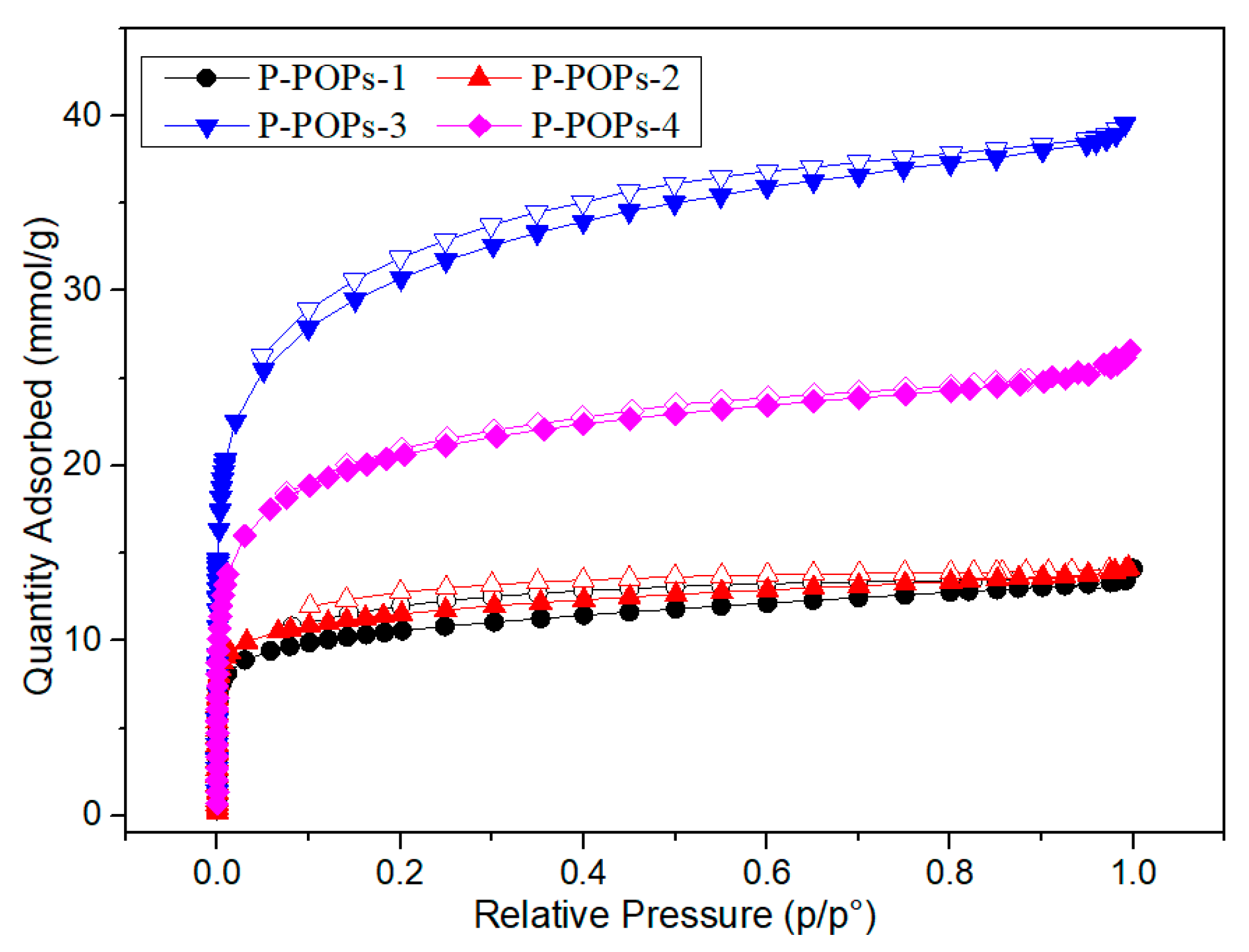
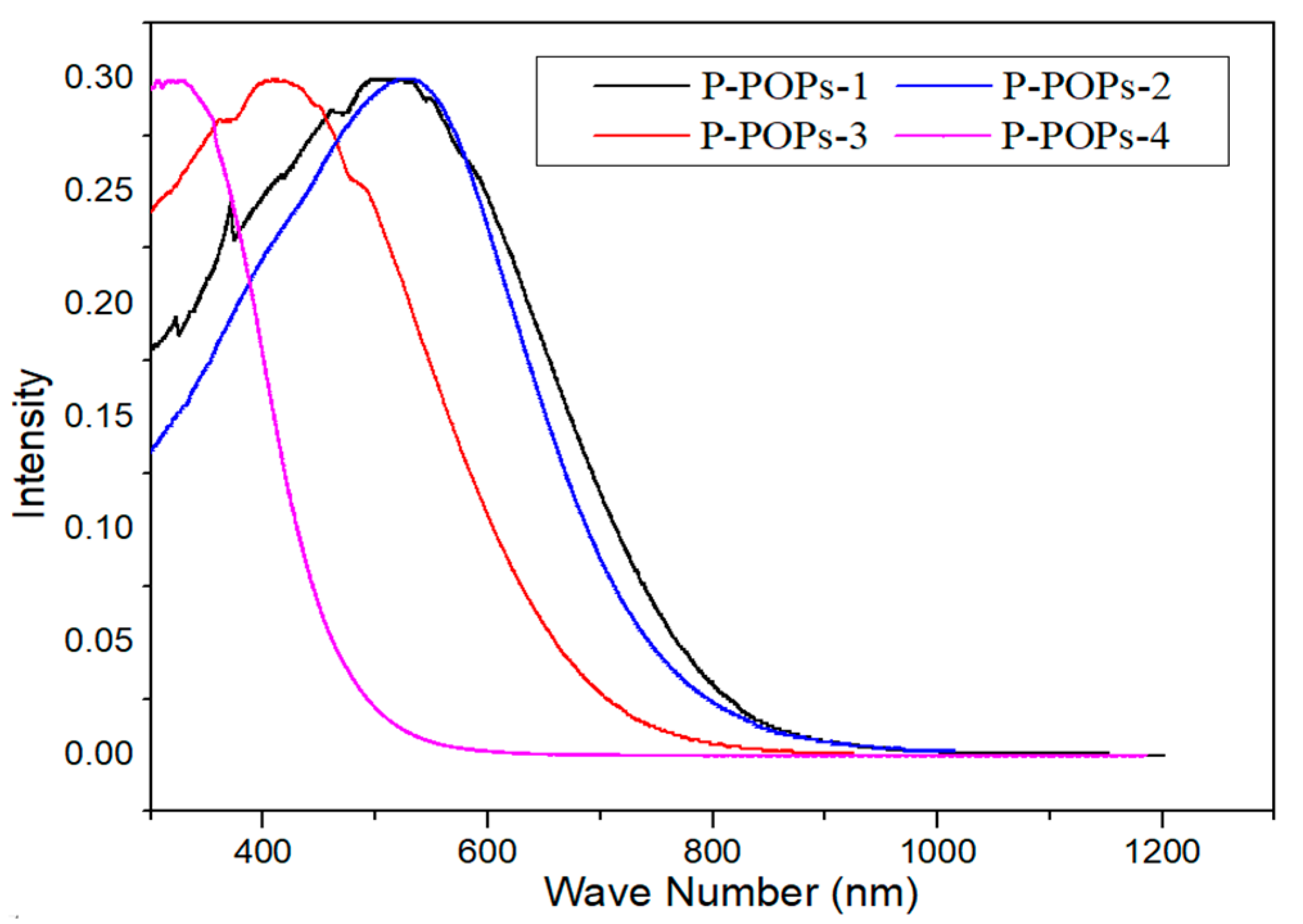
3.1. Material Characterization
3.2. Activity of Catalysts
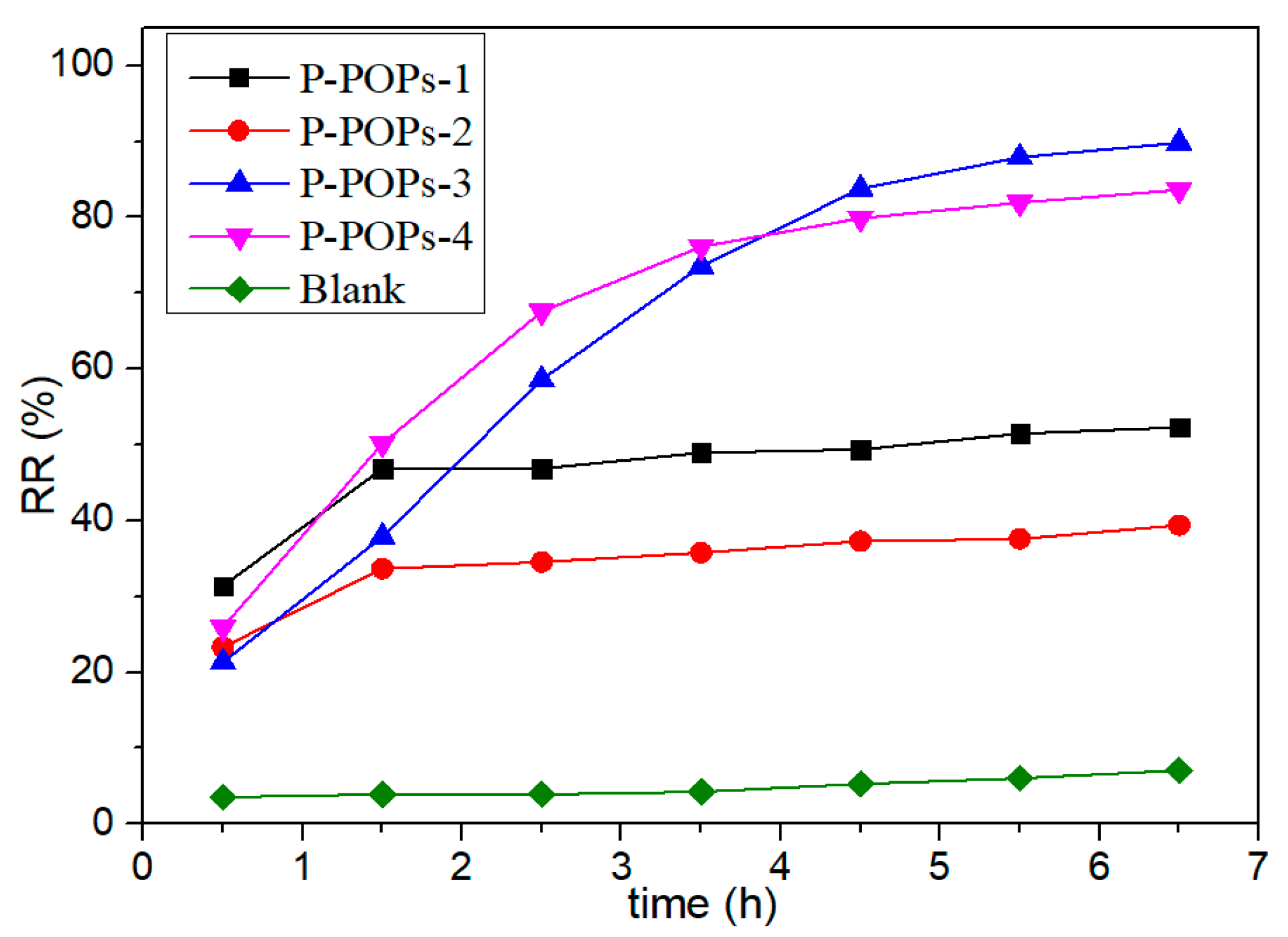
3.2.1. The Effect of Different Catalysts
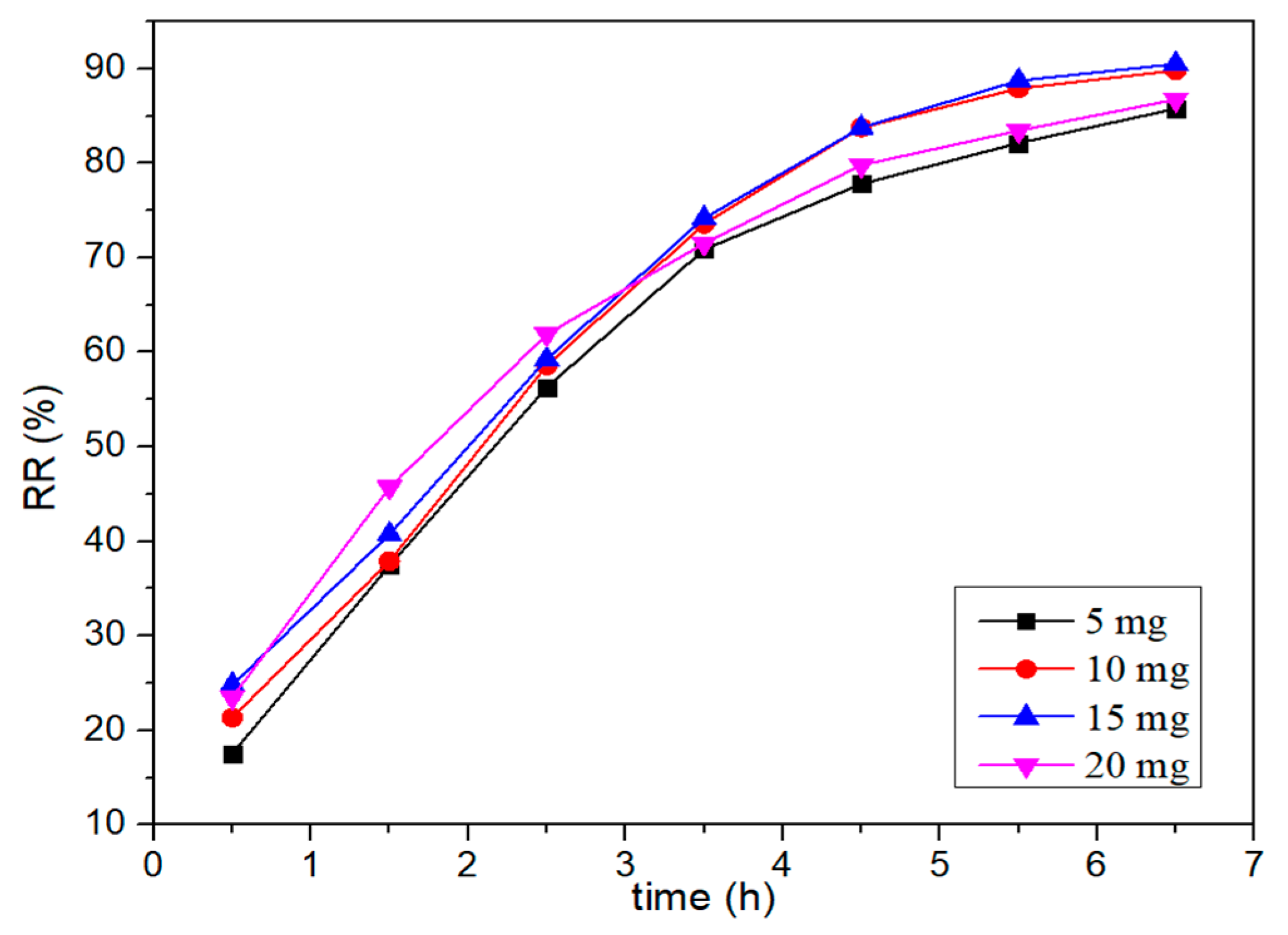
3.2.2. The Effect of the Catalyst Amount
3.2.3. The Effect of Initial Tetracycline Concentration
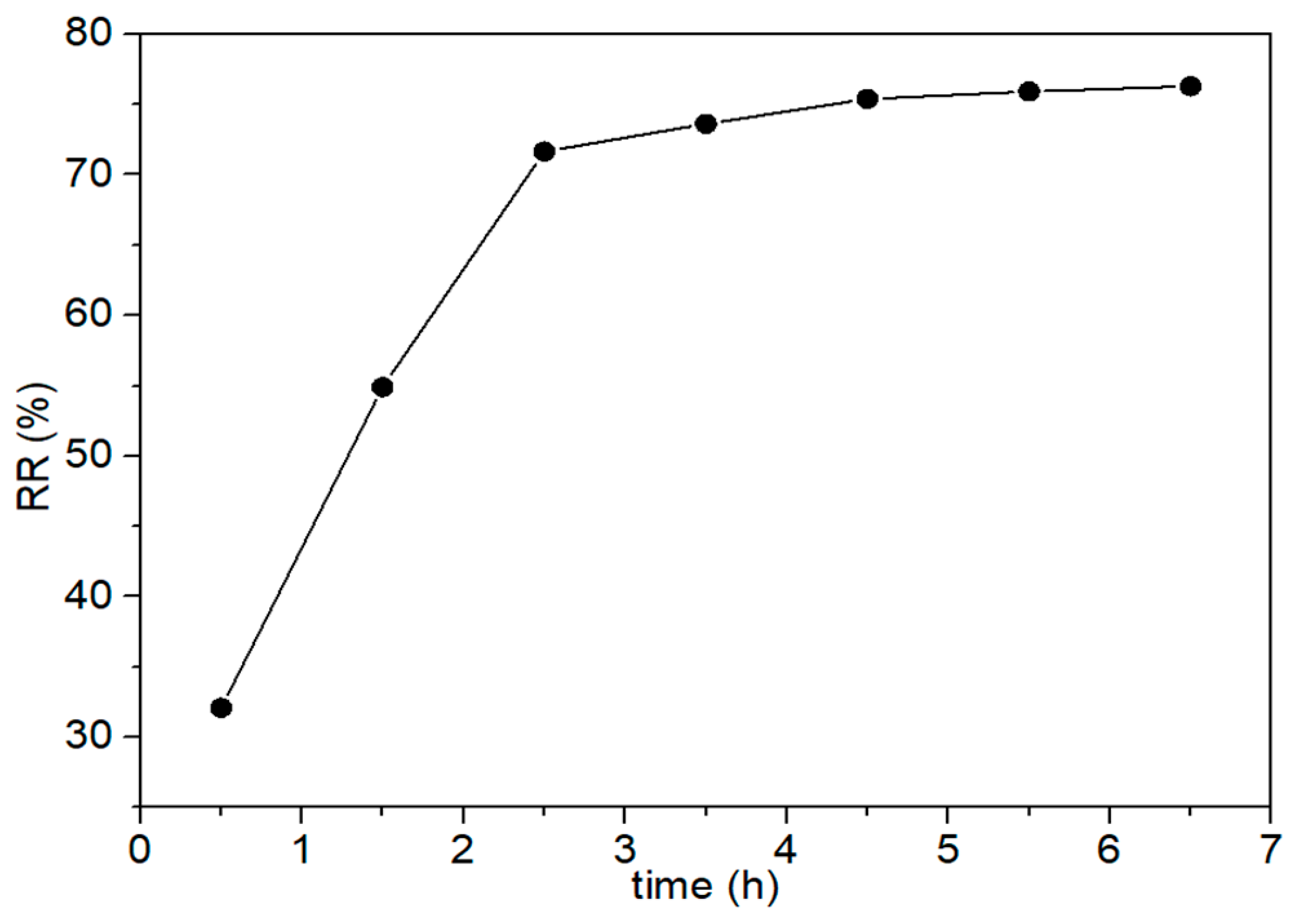
3.3. Different Substrates
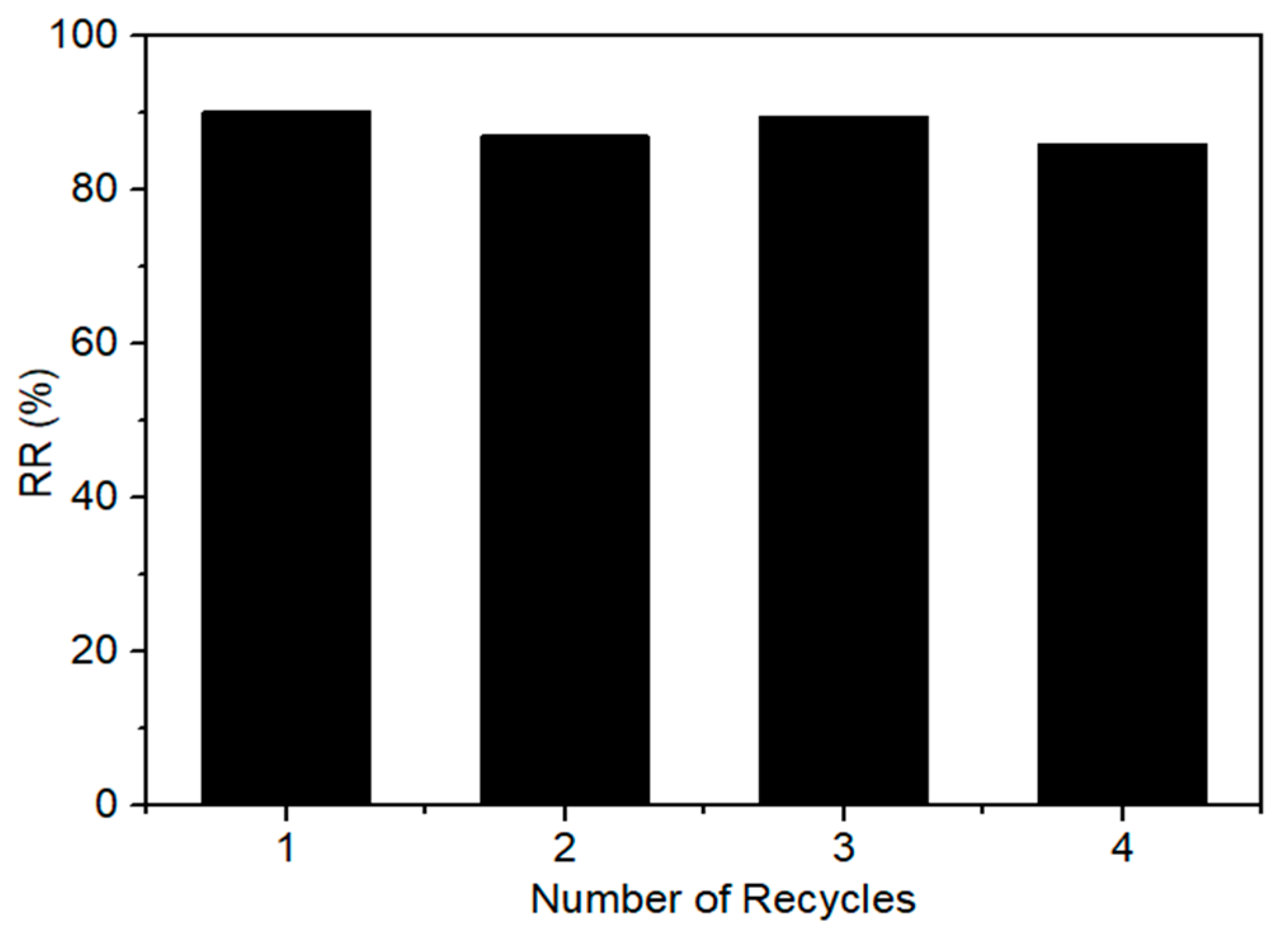
3.4. Catalyst Recyclability
4. Conclusion
Author Contributions
Funding
Conflicts of Interest
References
- Richardson, B.J.; Lam, P.K.S.; Martin, M. Emerging chemicals of concern: Pharmaceuticals and personal care products (PPCPs) in Asia, with particular reference to Southern China. Mar. Pollut. Bull. 2005, 50, 913–920. [Google Scholar] [CrossRef]
- Sarmah, A.K.; Meyer, M.T.; Boxall, A.B. A global perspective on the use, sales, exposure pathways, occurrence, fate and effects of veterinary antibiotics (VAs) in the environment. Chamosphere 2006, 65, 725–759. [Google Scholar] [CrossRef]
- Cabello, F.C. Heavy use of prophylactic in aquaculture: A growing problem for human and animal health and for the environment. Environ. Microbiol. 2006, 8, 1137–1144. [Google Scholar] [CrossRef]
- Park, K.; Kwak, I.S. Disrupting effects of antibiotic sulfathiazole on developmental process during sensitive life-cycle stage of Chironomus riparius. Chemosphere 2018, 190, 25–34. [Google Scholar] [CrossRef]
- Al-Gheethi, A.A.S.; Ismail, N. Biodegradation of Pharmaceutical Wastes in Treated Sewage Effluents by Bacillus subtilis, 1556WTNC. Environ. Process. 2014, 1, 459–481. [Google Scholar] [CrossRef]
- Li, B.; Zhang, T. Biodegradation and adsorption of antibiotics in the activated sludge process. Environ. Sci. Technol. 2010, 44, 3468–3473. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Guan, W.; Zhang, G.; Ye, L.; Zhou, Y.; Zhang, X. TiO2/Fe2O3/CNTs magnetic photocatalyst: A fast and convenient synthesis and visible-light-driven photocatalytic degradation of tetracycline. Micro Nano Lett. 2013, 8, 749–752. [Google Scholar] [CrossRef]
- Zhao, J.; Chen, C.; Ma, W. Photocatalytic degradation of organic pollutants under visible light irradiation. Top. Catal. 2005, 35, 269–278. [Google Scholar] [CrossRef]
- Wang, T.; Wei, Q.; Jiang, D.; Chen, L.; Li, D.; Meng, S.; Chen, M. Synthesis of redox-mediator-free direct Z-scheme AgI/WO3 nanocomposite photocatalysts for the degradation of tetracycline with enhanced photocatalytic activity. Chem. Eng. J. 2016, 300, 280–290. [Google Scholar] [CrossRef]
- Li, D.; Shi, W. Recent developments in visible-light photocatalytic degradation of antibiotics. Chin. J. Catal. 2016, 37, 792–799. [Google Scholar] [CrossRef]
- Zhang, N.; Liu, S.; Fu, X.; Xu, Y. Fabrication of coenocytic Pd@CdS nanocomposite as a visible light photocatalyst for selective transformation under mild conditions. J. Mater. Chem. 2012, 22, 5042–5052. [Google Scholar] [CrossRef]
- Gerrity, D.; Mayer, B.; Ryu, H.; Crittenden, J.; Abbaszadegan, M. A comparison of pilot-scale photocatalysis and enhanced coagulation for disinfection byproduct mitigation. Water Res. 2009, 43, 1597–1610. [Google Scholar] [CrossRef] [PubMed]
- Yasmina, M.; Mourad, K.; Mohammed, S.H.; Khaoula, C. Treatment heterogeneous photocatalysis; factors influencing the photocatalytic degradation by TiO2. Energy Procedia 2014, 50, 559–566. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemial photocatalysis of water at a semconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Neppolian, B.; Choi, H.C.; Sakthivel, S.; Arabindoo, B.; Murugesan, V. Solar/UV-induced photocatalytic degradation of three commercial textile dyes. J. Hazard. Mater. 2002, 89, 303–317. [Google Scholar] [CrossRef]
- Tang, Y.; Liu, X.; Ma, C.; Zhou, M.; Huo, P.; Yu, L.; Yan, Y. Enhanced photocatalytic degradation of tetracycline antibiotics by reduced graphene oxide–CdS/ZnS heterostructure photocatalysts. New J. Chem. 2015, 39, 5150–5160. [Google Scholar] [CrossRef]
- Zhang, G.; Zhang, X.; Wu, Y.; Shi, W.; Guan, W. Rapid microwave-assisted synthesis of Bi2O3 tubes and photocatalytic properties for antibiotics. Micro Nano Lett. 2013, 8, 177–180. [Google Scholar] [CrossRef]
- Xue, J.; Ma, S.; Zhou, Y.; Zhang, Z.; Jiang, P. Synthesis of Ag/ZnO/C plasmonic photocatalyst with enhanced adsorption capacity and photocatalytic activity to antibiotics. RSC Adv. 2015, 5, 18832–18840. [Google Scholar] [CrossRef]
- Sun, J.X.; Yuan, Y.P.; Qiu, L.G.; Jiang, X.; Xie, A.J.; Shen, Y.H.; Zhu, J.F. Fabrication of composite photocatalyst g-C3N4-ZnO and enhancement of photocatalytic activity under visible light. Dalton Trans. 2012, 41, 6756–6763. [Google Scholar] [CrossRef]
- Shen, J.; Steinbach, R.; Tobin, J.M.; Nakata, M.M.; Bower, M.; McCoustra, M.R.; Vilela, F. Photoactive and metal-free polyamide-based polymers for water and wastewater treatment under visible light irradiation. Appl. Catal. B: Environ. 2016, 193, 226–233. [Google Scholar] [CrossRef]
- Zhang, Y.; Riduan, S.N. Functional porous organic polymers for heterogeneous catalysis. Chem. Soc. Rev. 2012, 41, 2083–2094. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Dai, Z.; Liu, X.; Sheng, N.; Deng, F.; Meng, X.; Xiao, F.S. Highly efficient heterogeneous hydroformylation over Rh-metalated porous organic polymers: Synergistic effect of high ligand concentration and flexible framework. J. Am. Chem. Soc. 2015, 137, 5204–5209. [Google Scholar] [CrossRef]
- Li, B.; Guan, Z.; Wang, W.; Yang, X.; Hu, J.; Tan, B.; Li, T. Highly dispersed Pd catalyst locked in knitting aryl network polymers for suzuki–miyaura coupling reactions of Aryl chlorides in aqueous media. Adv. Mater. 2012, 24, 3390–3395. [Google Scholar] [CrossRef] [PubMed]
- Song, K.; Liu, P.; Wang, J.; Pang, L.; Chen, J.; Hussain, I.; Li, T. Controlled synthesis of uniform palladium nanoparticles on novel micro-porous carbon as a recyclable heterogeneous catalyst for the Heck reaction. Dalton Trans. 2015, 44, 13906–13913. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Song, K.; Li, T.; Tan, B. Palladium catalyst coordinated in knitting N-heterocyclic carbene porous polymers for efficient Suzuki-Miyaura coupling reactions. J. Mater. Chem. A 2015, 3, 1272–1278. [Google Scholar] [CrossRef]
- Dawson, R.; Cooper, A.I.; Adams, D.J. Nanoporous organic polymer networks. Prog. Polym. Sci. 2012, 37, 530–563. [Google Scholar] [CrossRef]
- Li, R.; Wang, Z.J.; Wang, L.; Ma, B.C.; Ghasimi, S.; Lu, H.; Zhang, K.A. Photocatalytic selective bromination of electron-rich aromatic compounds using microporous organic polymers with visible light. Acs Catal. 2016, 6, 1113–1121. [Google Scholar] [CrossRef]
- Wang, C.A.; Li, Y.W.; Cheng, X.L.; Zhang, J.P.; Han, Y.F. Eosin Y dye-based porous organic polymers for highly efficient heterogeneous photocatalytic dehydrogenative coupling reaction. RSC Adv. 2017, 7, 408–414. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, C.; Shu, Y.; Jiang, S.; Xia, Q.; Chen, L.; Tan, B. Layered microporous polymers by solvent knitting method. Sci. Adv. 2017, 3, e1602610. [Google Scholar] [CrossRef]
- Rozyyev, V.; Thirion, D.; Ullah, R.; Lee, J.; Jung, M.; Oh, H.; Yavuz, C.T. High-capacity methane storage in flexible alkane-linked porous aromatic network polymers. Nat. Energy 2019, 4, 604–611. [Google Scholar] [CrossRef]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, X.; Liu, J.; Liu, Z.; Deng, Y.; Nie, W.; Zhang, L.; Xie, Z.; Chen, L.; Zhou, A. Synthesis of Novel Phenyl Porous Organic Polymers and Their Excellent Visible Light Photocatalytic Performance on Antibiotics. Materials 2019, 12, 3296. https://doi.org/10.3390/ma12203296
Gao X, Liu J, Liu Z, Deng Y, Nie W, Zhang L, Xie Z, Chen L, Zhou A. Synthesis of Novel Phenyl Porous Organic Polymers and Their Excellent Visible Light Photocatalytic Performance on Antibiotics. Materials. 2019; 12(20):3296. https://doi.org/10.3390/ma12203296
Chicago/Turabian StyleGao, Xiang, Jiao Liu, Zhaopeng Liu, Yuehua Deng, Wenjie Nie, Lei Zhang, Zufeng Xie, Leyuan Chen, and Anning Zhou. 2019. "Synthesis of Novel Phenyl Porous Organic Polymers and Their Excellent Visible Light Photocatalytic Performance on Antibiotics" Materials 12, no. 20: 3296. https://doi.org/10.3390/ma12203296
APA StyleGao, X., Liu, J., Liu, Z., Deng, Y., Nie, W., Zhang, L., Xie, Z., Chen, L., & Zhou, A. (2019). Synthesis of Novel Phenyl Porous Organic Polymers and Their Excellent Visible Light Photocatalytic Performance on Antibiotics. Materials, 12(20), 3296. https://doi.org/10.3390/ma12203296



