Setting Characteristics, Mechanical Properties and Microstructure of Cement Pastes Containing Accelerators Mixed with Superabsorbent Polymers (SAPs): An NMR Study Combined with Additional Methods
Abstract
1. Introduction
2. Materials and Experiment
2.1. Materials
2.2. Nuclear Magnetic Resonance Tests
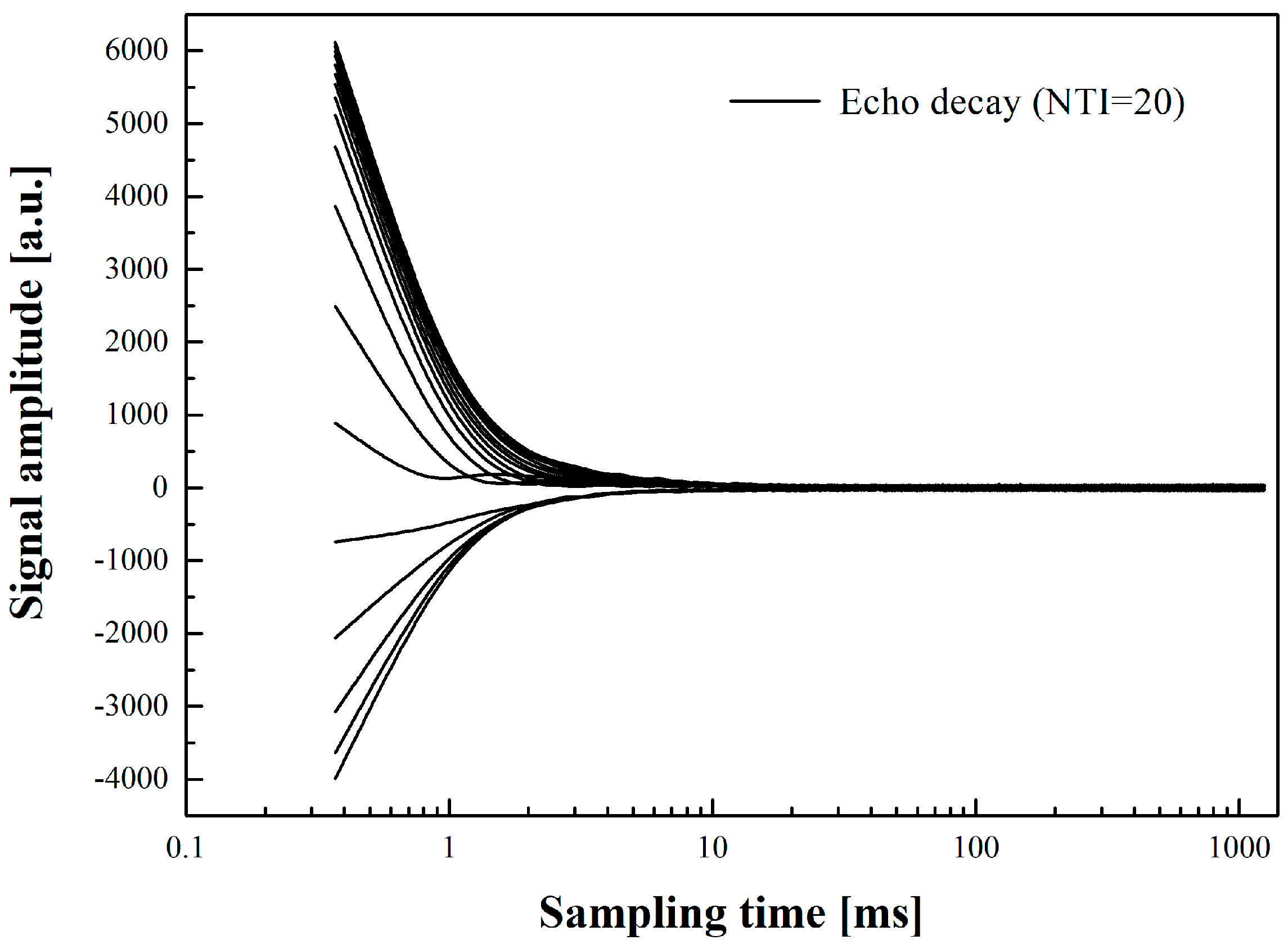
2.2.1. Transverse Relaxation Time
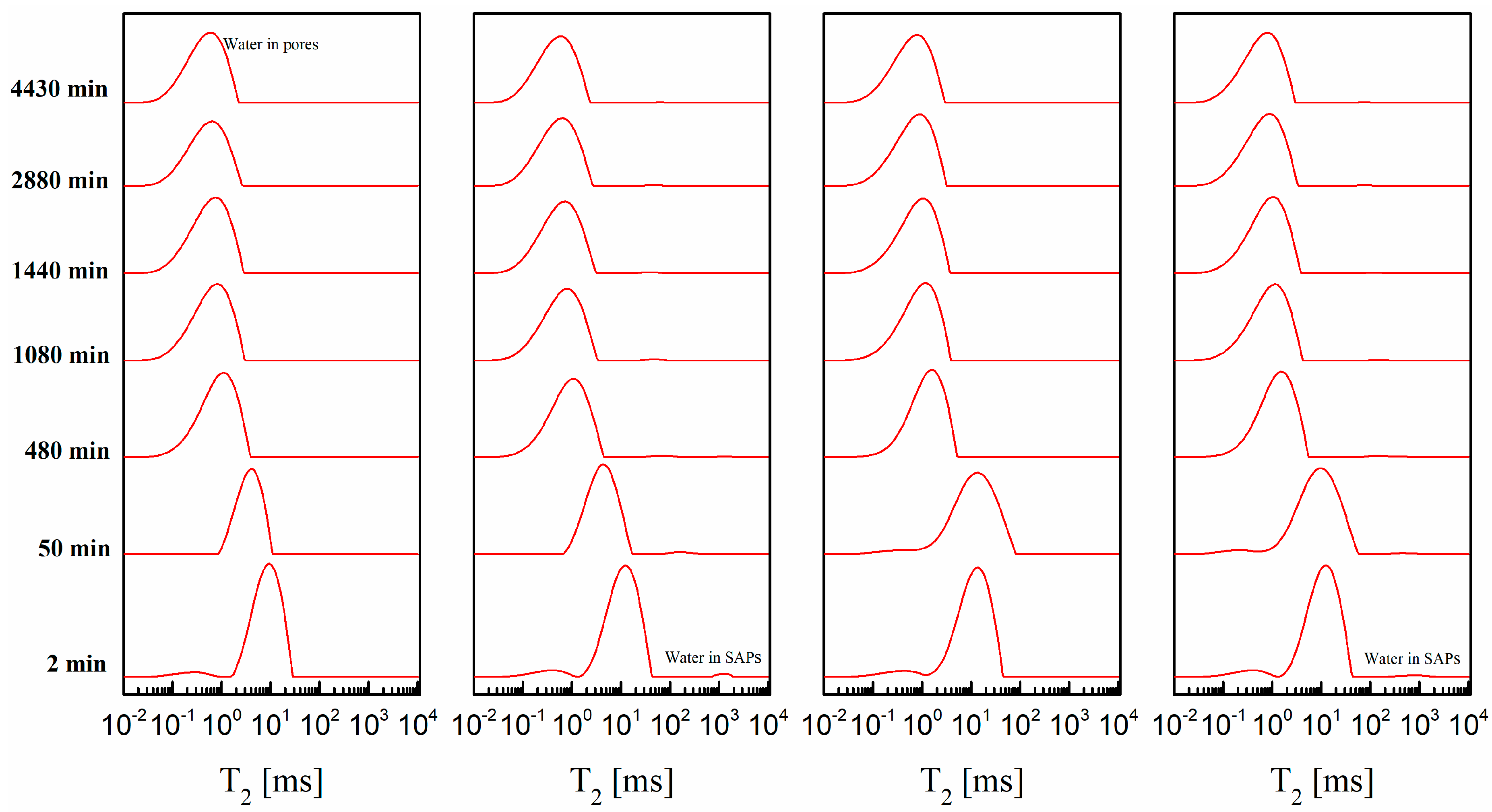
2.2.2. Two-Dimensional T1-T2 Maps
2.3. Mercury Intrusion Porosimetry
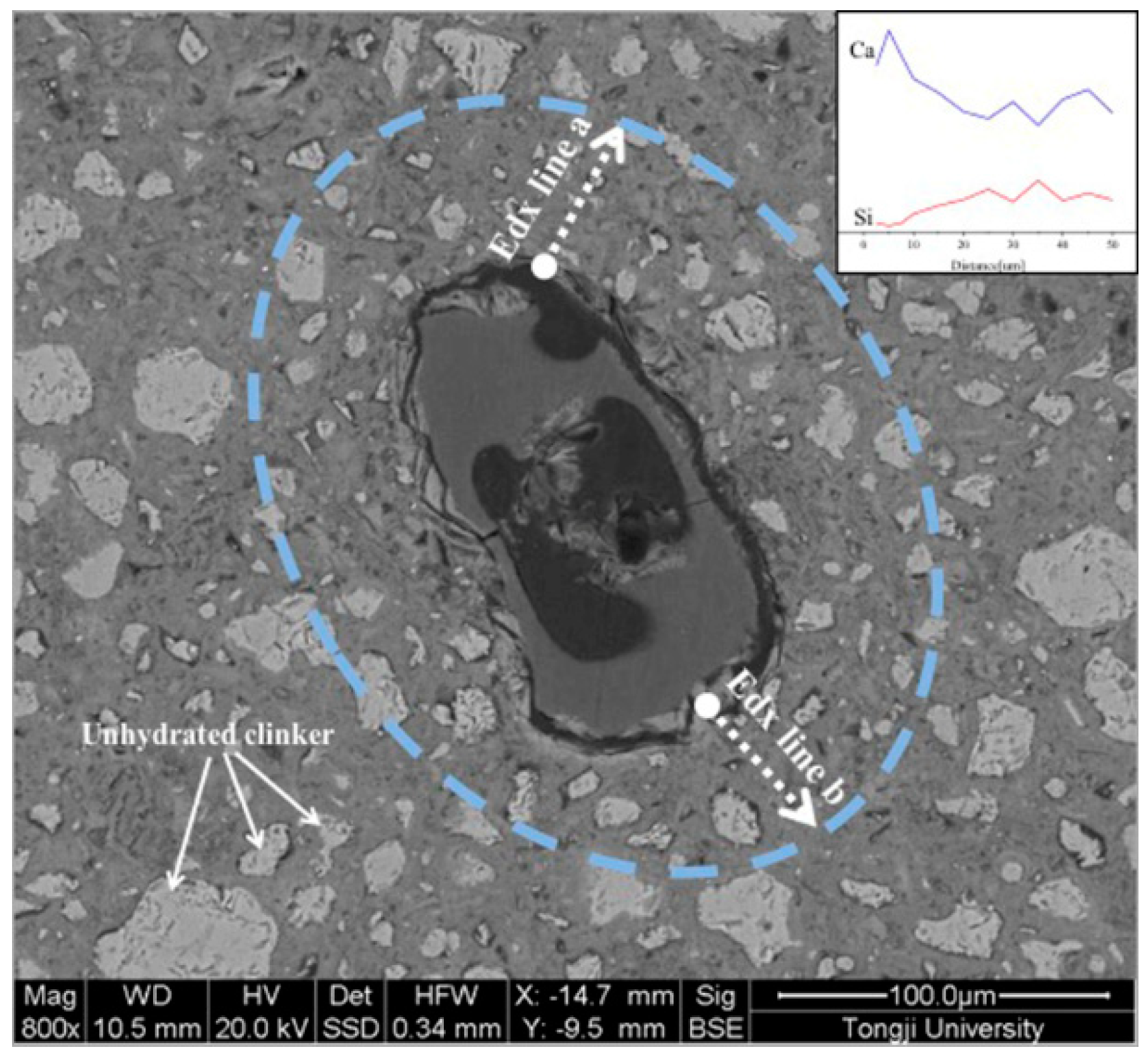
2.4. Back-Scattered Electron Imaging and EDX Analysis
3. Results and Analysis
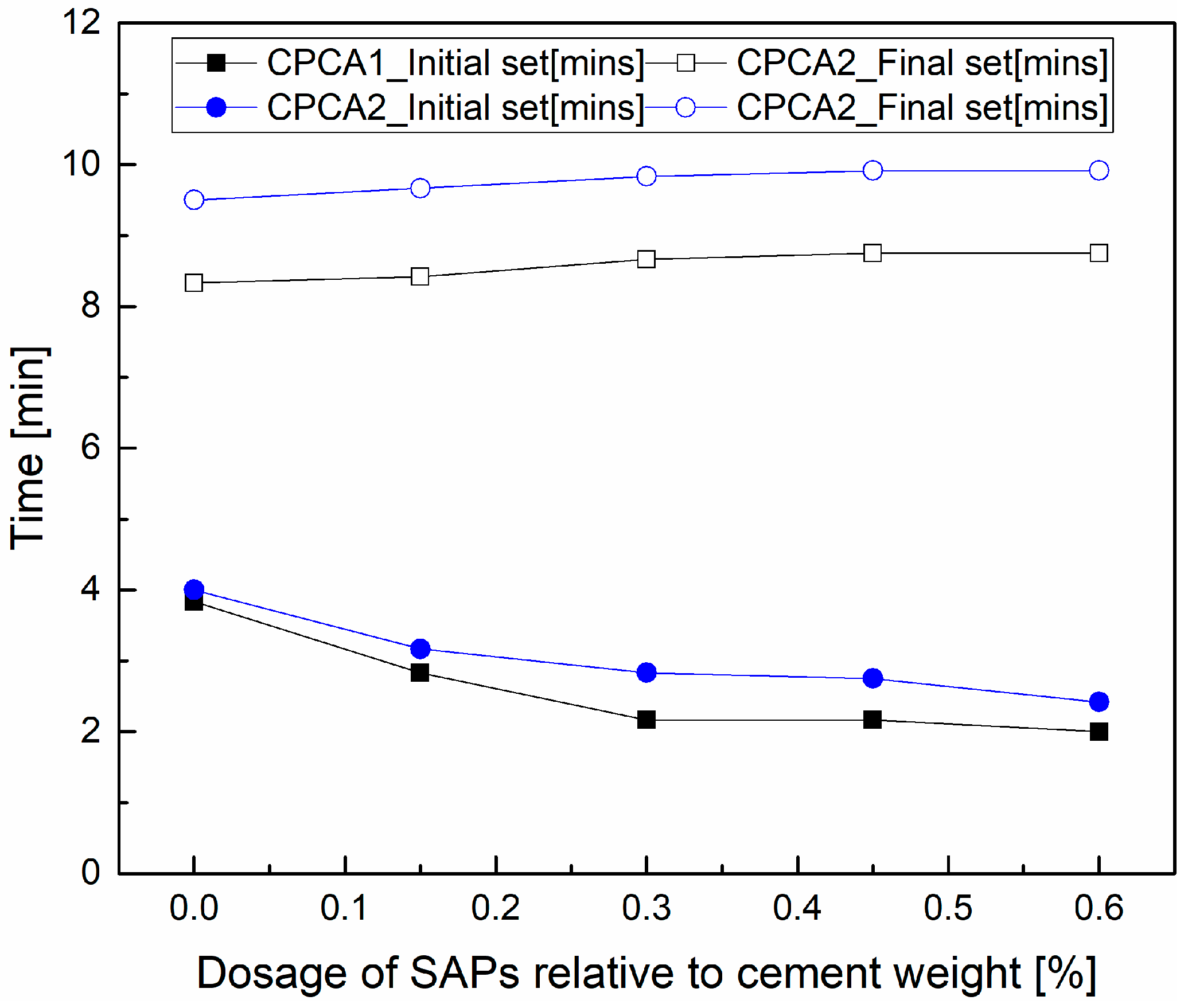
3.1. Setting Time
3.2. Compressive Strength
3.3. Evolution of the Microstructure
3.4. 2D T1-T2 Maps
3.5. Pore Structure
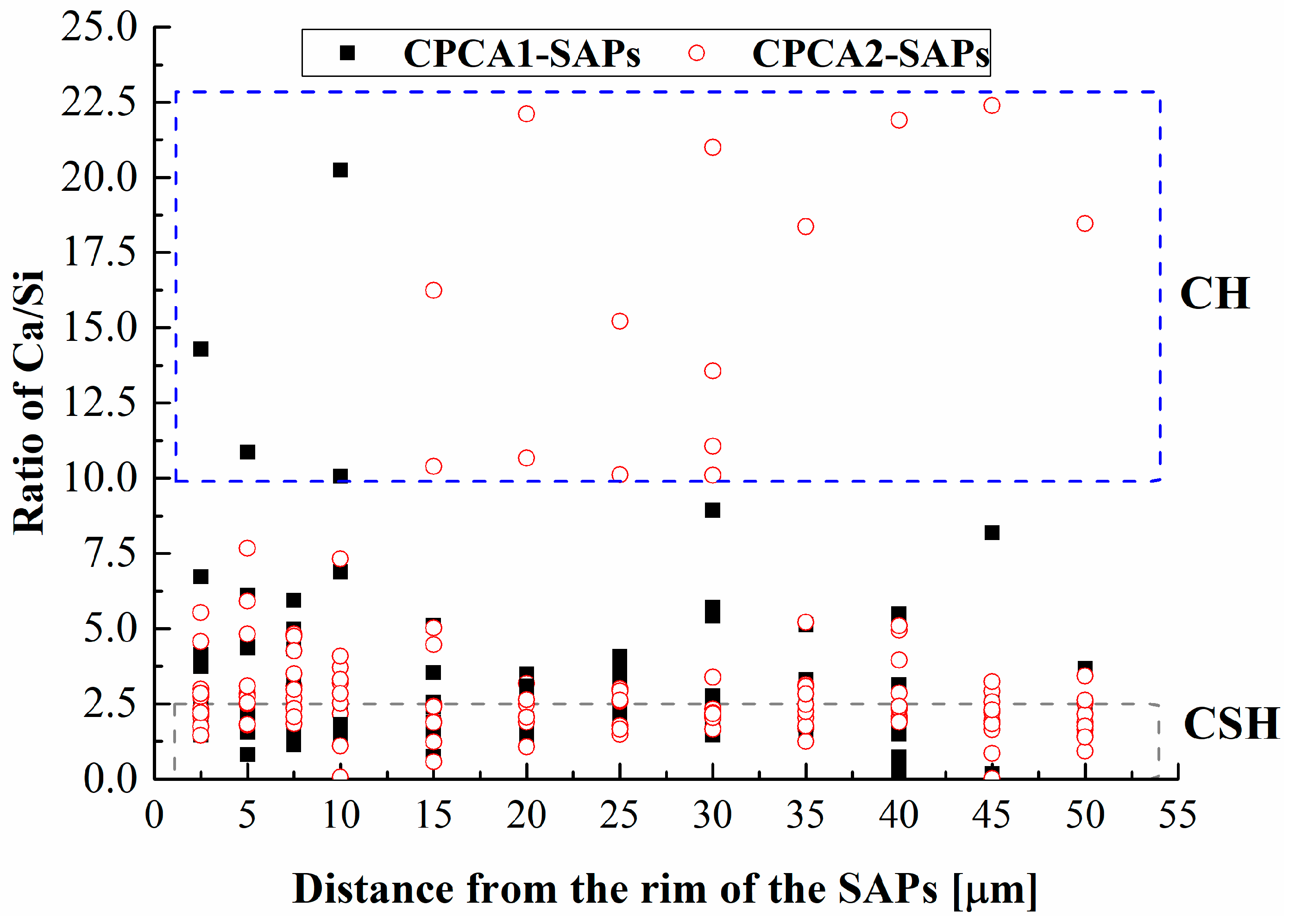
3.6. Back-Scattered Electron and EDX Analysis
4. Conclusions
- (1)
- The addition of SAPs could reduce the initial setting time of cement pastes containing accelerators. In comparison with the SAPs-free samples, the cement paste containing alkali accelerators with SAPs has a lower compressive strength in the later stage, while the CPCA2-SAPs sample has a higher compressive strength during 28 days.
- (2)
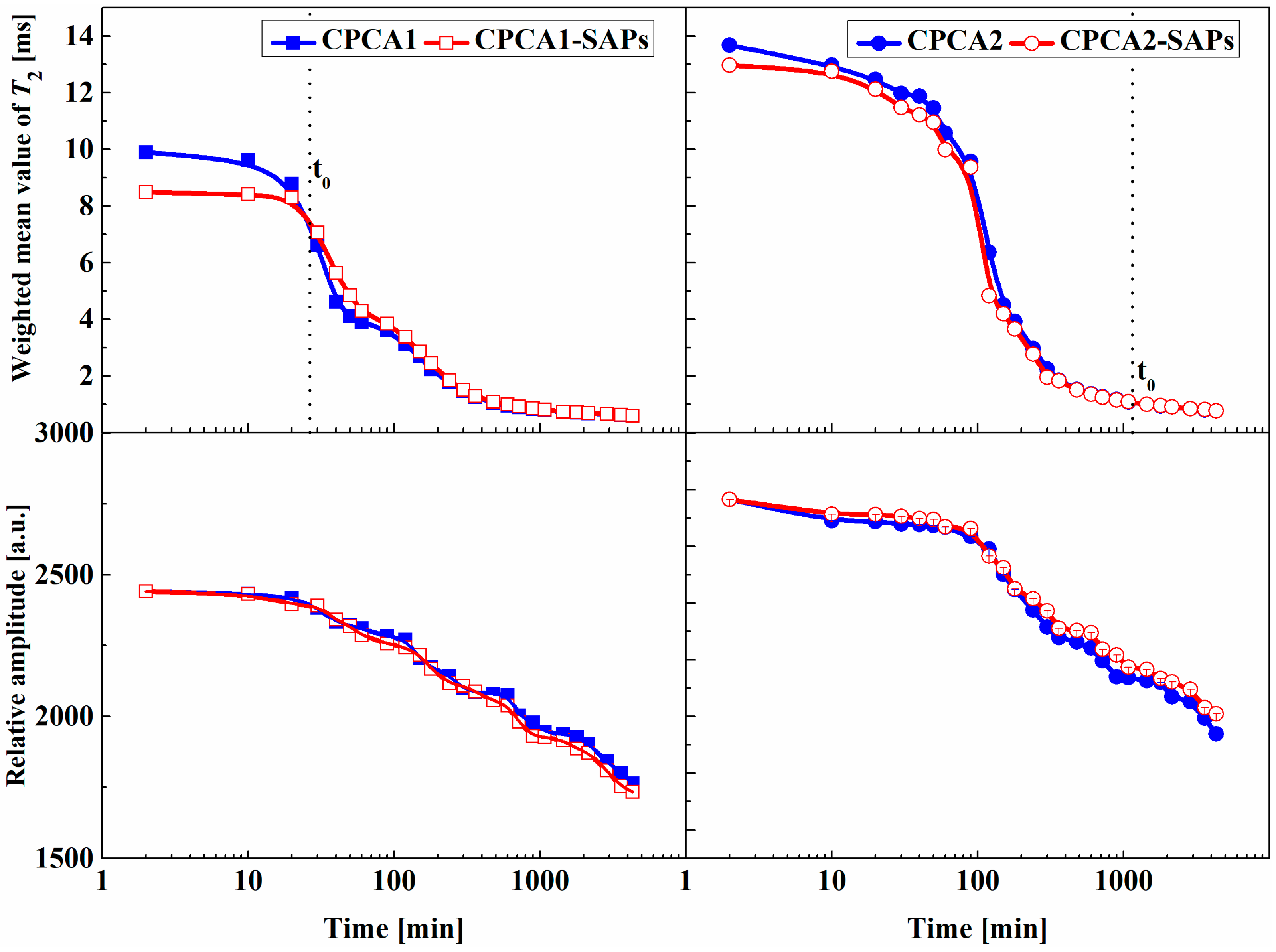
- The smaller T2 value implies a denser structure is formed which accounts for the shortened initial setting time. Compared with the SAP-free samples, the SAPs could decrease the total relative amplitude of the cement paste containing an alkali accelerator but increase it when the alkaline-free accelerator was selected.
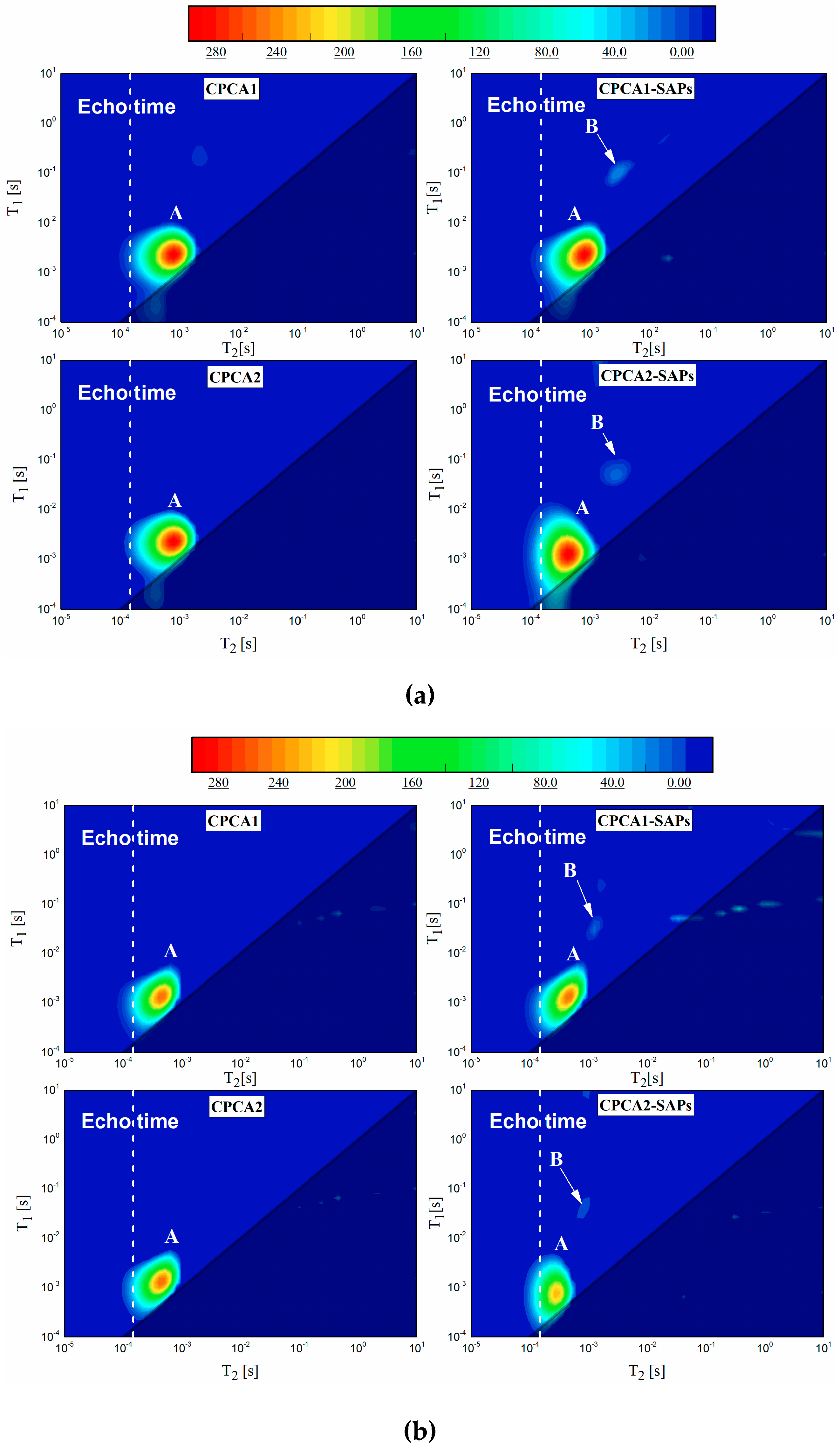
- (3)
- T1-T2 maps indicated that measurable water was composed of two fractions discerned as water in pores and water in SAPs. The extension of water in pores to the limit of the T1 resolution (10−4 s) which suggested that SAPs in cement paste containing alkaline-free accelerator helps to form a denser structure.
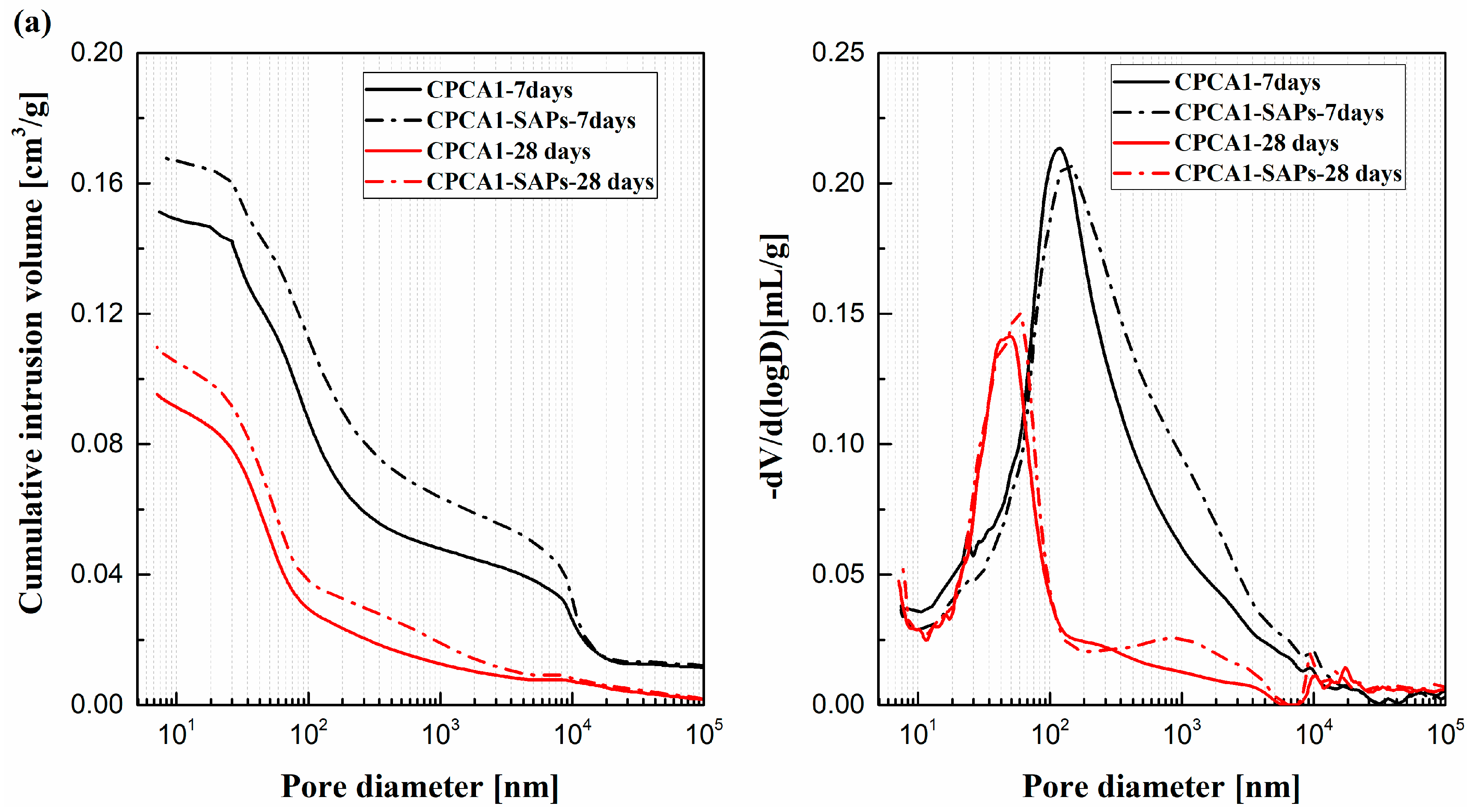
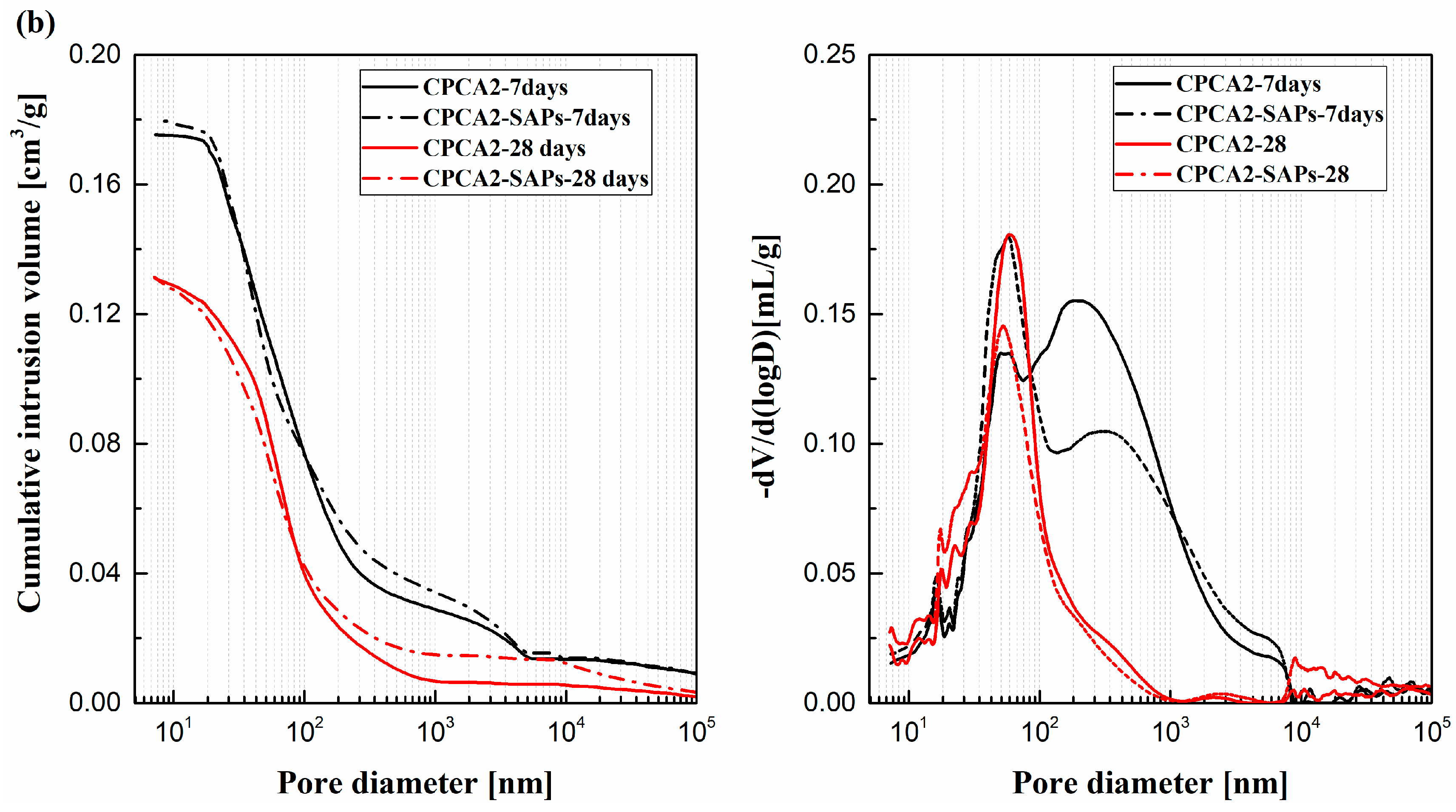
- (4)
- The cement paste containing accelerator with SAPs had a higher porosity compared to the sample lacking SAPs. For the alkaline-free accelerator cement paste. The peak in the pore size distribution of the sample with SAPs was also shifted to smaller pore size ranges, in contrast with the SAPs-free samples.
- (5)
- The voids of the SAPs are large in the alkaline-accelerator containing cement pastes, and in the alkali-free accelerator containing sample, the original SAP reservoirs are filled with CH. The EDX results of Ca/Si ratio indicated that the range of the curing effect caused by the SAPs in the cement paste with alkali-free accelerator was larger than that of the SAPs in the alkali accelerator containing cement paste.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Belie, N.D.; Grosse, C.U.; Kurz, J.; Reinhardt, H.W. Ultrasound monitoring of the influence of different accelerating admixtures and cement types for shotcrete on setting and hardening behavior. Cem. Concr. Res. 2005, 35, 2087–2094. [Google Scholar] [CrossRef]
- Holter, K.G.; Geving, S. Moisture Transport through Sprayed Concrete Tunnel Linings. Rock Mech. Rock Eng. 2016, 49, 243–272. [Google Scholar] [CrossRef]
- Min, T.B.; Cho, I.S.; Park, W.J.; Choi, H.K.; Lee, H.S. Experimental study on the development of compressive strength of early concrete age using calcium-based hardening accelerator and high early strength cement. Constr. Build. Mater. 2014, 64, 208–214. [Google Scholar] [CrossRef]
- Salvador, R.P.; Cavalaro, S.H.P.; Cincotto, M.A.; Figueiredo, A.D.D. Parameters controlling early age hydration of cement pastes containing accelerators for sprayed concrete. Cem. Concr. Res. 2016, 89, 230–248. [Google Scholar] [CrossRef]
- Snashall, H.T. Cementitious Mixes. South African Patent Application ZA9100876 A, 24 December 1991. [Google Scholar]
- Jensen, O.M.; Hansen, P.F. Water-Entrained Cement-Based Materials. PCT Patent Application WO01/02317A1, 11 January 2001. [Google Scholar]
- Mechtcherine, V.; Reinhardt, H.W. Application of Super Absorbent Polymers (SAP) in Concrete Construction; Rilem State-of-the-Art Report; Springer: Berlin, Germany, 2012. [Google Scholar]
- Jensen, O.M. Use of superabsorbent polymers in construction materials. In Proceedings of the 1st International Conference on Microstructure Related Durability of Cementitious Composites, Nanjing, China, 13–15 October 2008; pp. 757–764. [Google Scholar]
- Jolin, M.; Beaupré, D. Understanding wet-mix shotcrete: Mix design, specification, and placement. Shotcrete 2003, 1, 6–12. [Google Scholar]
- Olawuyi, B.J.; Boshoff, W.P. Influence of SAP content and curing age on air void distribution of high performance concrete using 3D volume analysis. Constr. Build. Mater. 2017, 135, 580–589. [Google Scholar] [CrossRef]
- Chinese Construction Industry Standards. Standards for Test Method of Flash Setting Admixtures for Shotcrete; JGJ/T 477-2005; Chinese Construction Industry Standards: Beijing, China, 2005. [Google Scholar]
- Chinese Construction Industry Standards. Standards for Test Method of Basic Properties of Construction Mortar; JGJ/T 70-2009; Chinese Construction Industry Standards: Beijing, China, 2009. [Google Scholar]
- Nestle, N.; Kühn, A.; Friedemann, K.; Horch, C.; Stallmach, F.; Herth, G. Water balance and pore structure development in cementitious materials in internal curing with modified superabsorbent polymer studied by NMR. Microporous Mesoporous Mater. 2009, 125, 51–57. [Google Scholar] [CrossRef]
- Ji, Y.L.; Sun, Z.P.; Yang, X.; Li, C.J.; Tang, X.B. Assessment and mechanism study of bleeding process in cement paste by 1H low-field NMR. Constr. Build. Mater. 2015, 100, 255–261. [Google Scholar] [CrossRef]
- Vogt, S. Nuclear Magnetic Resonance Resonance Studies of Biological and Biogeochemical Processes. Ph.D. Thesis, Montana State University, Bozeman, MT, USA, 2013. [Google Scholar]
- Nathan, H.W.; Magnus, R.; Petrik, G.; Stanley, J.M.; Magnus, N. Obtaining T1-T2 distribution functions from 1-dimensional T1 and T2 measurements: The pseudo 2-D relaxation model. J. Magn. Reson. 2016, 269, 186–195. [Google Scholar]
- Hürlimann, M.D.; Venkataramanan, L. Quantitative Measurement of Two-Dimensional Distribution Functions of Diffusion and Relaxation in Grossly Inhomogeneous Fields. J. Magn. Reson. 2002, 157, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Shi, H.S.; Xu, L.L.; Ye, G.; Schutter, G.D. Microstructural characterization of ITZ in blended cement concretes and its relation to transport properties. Cem. Concr. Res. 2016, 79, 243–256. [Google Scholar] [CrossRef]
- Gajewicz, A.M.; Gartner, E.; Kang, K.; Mcdonald, P.J.; Yermakou, V. A 1H NMR relaxometry investigation of gel-pore drying shrinkage in cement pastes. Cem. Concr. Res. 2016, 86, 12–19. [Google Scholar] [CrossRef]
- Jonsson, B.; Wennerstrom, H.; Nonat, A.; Cabane, B. Onset of cohesion in cement paste. Langmuir 2004, 20, 6702–6709. [Google Scholar] [CrossRef] [PubMed]
- Szeląg, M. Development of cracking patterns in modified cement matrix with microsilica. Materials 2018, 11, 1928. [Google Scholar] [CrossRef] [PubMed]
- Ali, P.; Seyed, M.F.; Alireza, K.; Payam, H. Improving the performance of cement-based composites containing superabsorbent polymers by utilization of nano-SiO2 particles. Mater. Des. 2012, 42, 94–101. [Google Scholar]
- Snoeck, D.; Velasco, L.F.; Mignon, A.; Vlierberghe, S.V.; Dubruel, P.; Lodewyckx, P.; Belie, N.D. The effects of superabsorbent polymers on the microstructure of cementitious materials studied by means of sorption experiments. Cem. Concr. Res. 2015, 77, 26–35. [Google Scholar] [CrossRef]
- Sandra, G.; Anne, A.; Nicolas, R.; Paul, B. Changes in portlandite morphology with solvent composition: Atomistic simulations and experiment. Cem. Concr. Res. 2011, 41, 1330–1338. [Google Scholar]
- Erdem, S.; Dawson, A.R.; Thom, N.H. Influence of the micro- and nano scale local mechanical properties of the interfacial transition zone on impact behavior of concrete made with different aggregates. Cem. Concr. Res. 2012, 42, 447–458. [Google Scholar] [CrossRef]


| SiO2 | Al2O3 | Fe2O3 | CaO | MgO | SO3 | K2O | Specific Weight (g/cm3) | Blaine Specific Surface Area (m2/kg) | Initial Setting Time (min) | |
|---|---|---|---|---|---|---|---|---|---|---|
| Cement | 22.9% | 2.47% | 0.28% | 64.6% | 2.82% | 3.20% | 0.44% | 3.08 | 396 | 105 |
| Sample Names | Cement | Water | Sands | Alkaline Accelerator (A1) | Alkaline-Free Accelerator (A2) | SAPs |
|---|---|---|---|---|---|---|
| CPCA1 | 1 | 0.45 | - | 0.04 | - | - |
| CPCA1-SAPs | 1 | 0.45 | - | 0.04 | - | 0.0030 |
| CPCA2 | 1 | 0.45 | - | - | 0.07 | - |
| CPCA2-SAPs | 1 | 0.45 | - | - | 0.07 | 0.0030 |
| M-CPCA1 | 1 | 0.45 | 1.5 | 0.04 | - | - |
| M-CPCA1-SAPs | 1 | 0.45 | 1.5 | 0.04 | - | 0.0030 |
| M-CPCA2 | 1 | 0.45 | 1.5 | - | 0.07 | - |
| M-CPCA2-SAPs | 1 | 0.45 | 1.5 | - | 0.07 | 0.0030 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ji, Y.; Sun, Z.; Chen, C.; Pel, L.; Barakat, A. Setting Characteristics, Mechanical Properties and Microstructure of Cement Pastes Containing Accelerators Mixed with Superabsorbent Polymers (SAPs): An NMR Study Combined with Additional Methods. Materials 2019, 12, 315. https://doi.org/10.3390/ma12020315
Ji Y, Sun Z, Chen C, Pel L, Barakat A. Setting Characteristics, Mechanical Properties and Microstructure of Cement Pastes Containing Accelerators Mixed with Superabsorbent Polymers (SAPs): An NMR Study Combined with Additional Methods. Materials. 2019; 12(2):315. https://doi.org/10.3390/ma12020315
Chicago/Turabian StyleJi, Yanliang, Zhenping Sun, Chao Chen, Leo Pel, and Ahmed Barakat. 2019. "Setting Characteristics, Mechanical Properties and Microstructure of Cement Pastes Containing Accelerators Mixed with Superabsorbent Polymers (SAPs): An NMR Study Combined with Additional Methods" Materials 12, no. 2: 315. https://doi.org/10.3390/ma12020315
APA StyleJi, Y., Sun, Z., Chen, C., Pel, L., & Barakat, A. (2019). Setting Characteristics, Mechanical Properties and Microstructure of Cement Pastes Containing Accelerators Mixed with Superabsorbent Polymers (SAPs): An NMR Study Combined with Additional Methods. Materials, 12(2), 315. https://doi.org/10.3390/ma12020315




