Inhibitive Properties of Benzyldimethyldodecylammonium Chloride on Microbial Corrosion of 304 Stainless Steel in a Desulfovibrio desulfuricans-Inoculated Medium
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Bacteria and Culture Medium
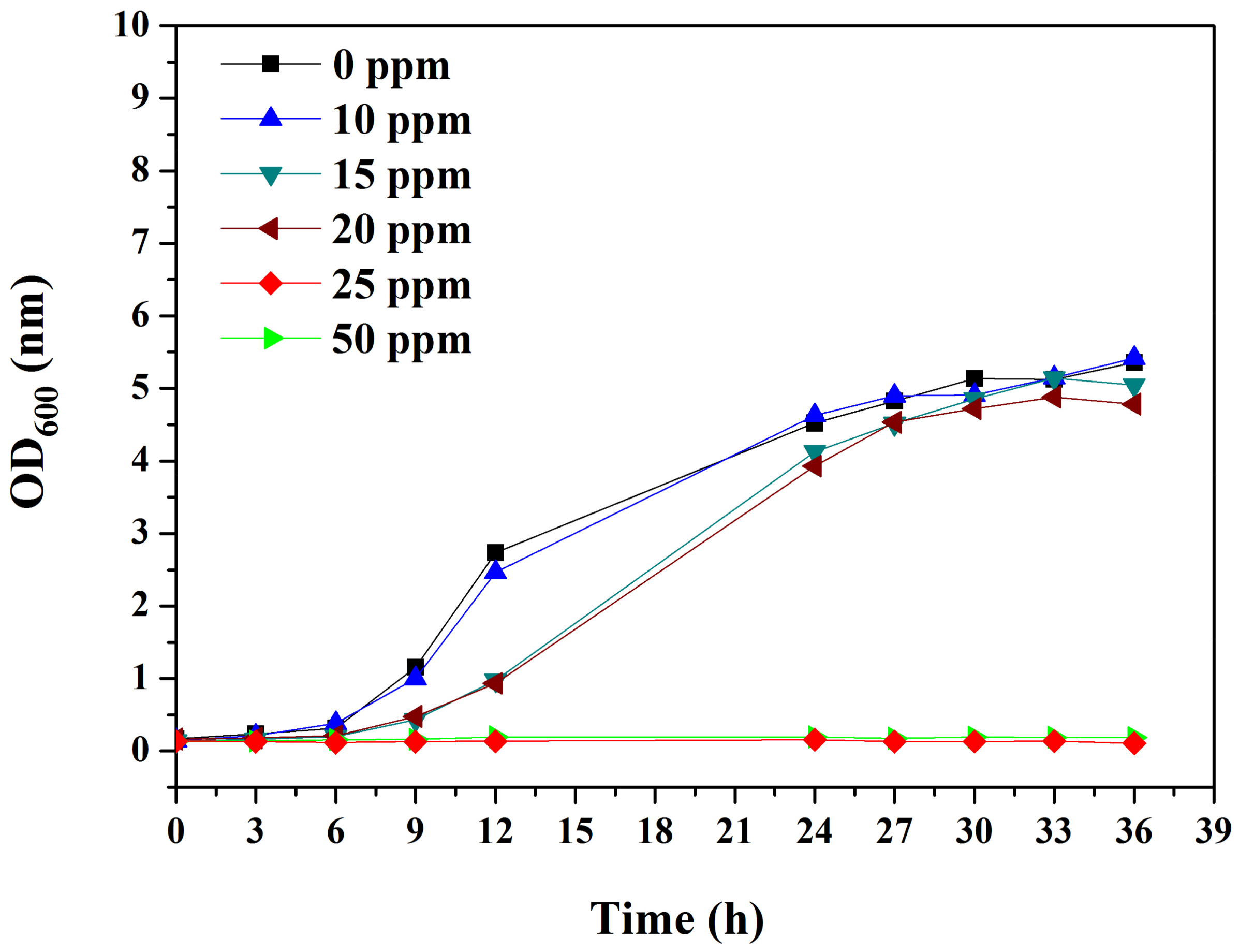
2.3. Bactericidal Assay of BDMDAC
2.4. Electrochemical Measurements
2.5. Surface Characterization
2.6. CLSM
3. Results and Discussion
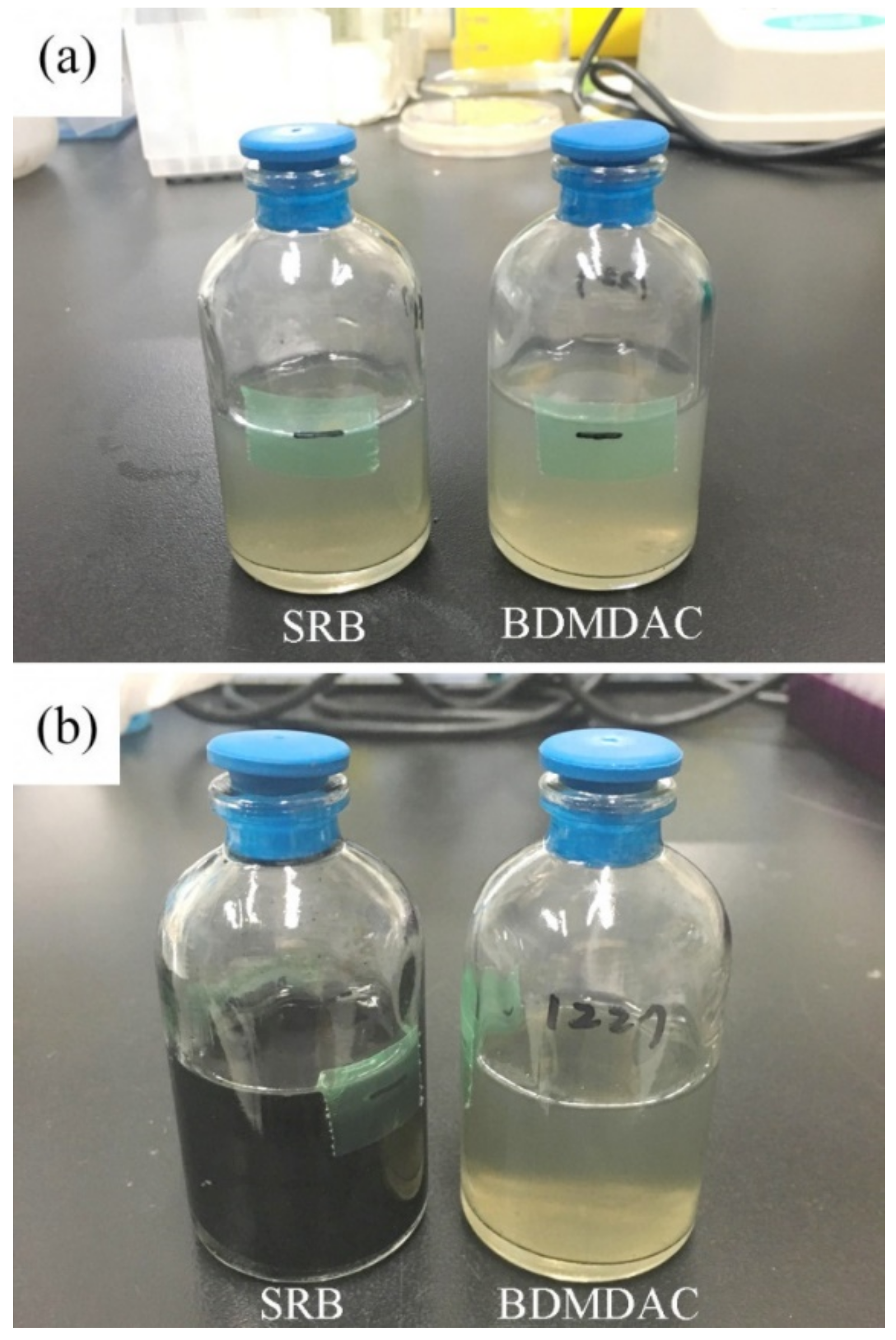
3.1. Bactericidal Assay of BDMDAC
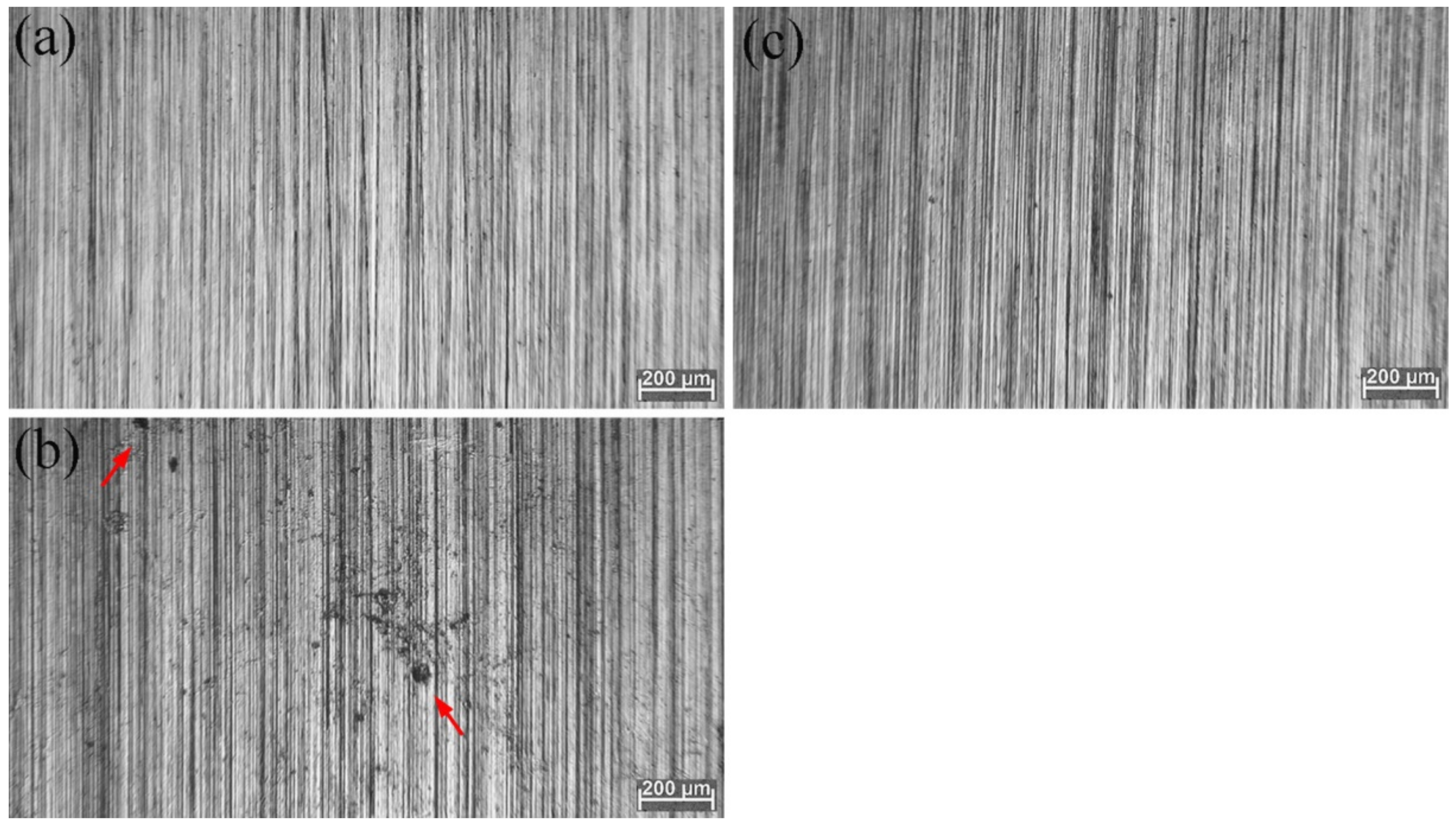
3.2. SEM
3.3. CLSM
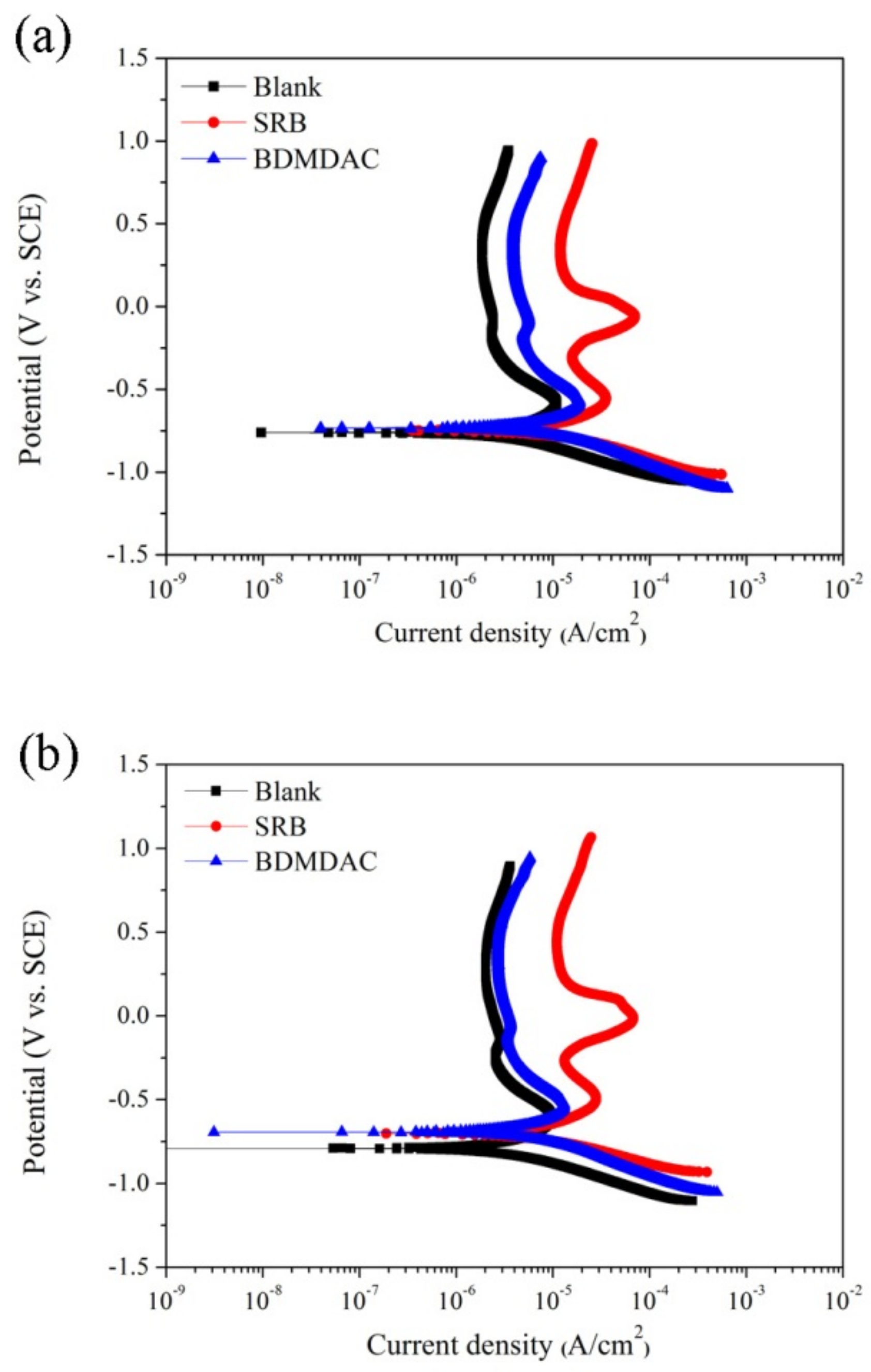
3.4. Potentiodynamic Polarization
3.5. Limitations of This Study
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sorkhabi, H.A.; Haghighi, M.M.; Zarrini, G.; Javaherdashti, R. Corrosion behavior of carbon steel in the presence of two novel iron-oxidizing bacteria isolated from sewage treatment plants. Biodegradation 2012, 23, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Lv, L.; Yuan, S.; Zheng, Y.; Liang, B.; Pehkonen, S.O. Surface modification of mild steel with thermally cured antibacterial poly(vinylbenzyl chloride)–polyaniline bilayers for effective protection against sulfate reducing bacteria induced corrosion. Ind. Eng. Chem. Res. 2014, 53, 12363–12378. [Google Scholar] [CrossRef]
- Venzlaff, H.; Enning, D.D.; Srinivasan, J.; Mayrhofer, K.J.J.; Hassel, A.W.; Widdel, F.; Stratmanna, M. Accelerated cathodic reaction in microbial corrosion of iron due to direct electron uptake by sulfate-reducing bacteria. Corros. Sci. 2013, 66, 88–96. [Google Scholar] [CrossRef]
- Enning, D.; Venzlaff, H.; Garrelfs, J.; Dinh, H.; Meyer, V.; Mayrhofer, K.; Hasse, A.W.; Stratmann, M.; Widdel, F. Marine sulfate-reducing bacteria cause serious corrosion of iron under electroconductive biogenic mineral crust. Environ. Microbiol. 2012, 14, 1772–1787. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Wang, K.; Sun, C.; Wang, F.; Li, X.; Yang, J.; Yu, C. The effects of sulfate reducing bacteria on corrosion of carbon steel Q235 under simulated disbonded coating by using electrochemical impedance spectroscopy. Corros. Sci. 2011, 53, 1554–1562. [Google Scholar] [CrossRef]
- Bao, Q.; Zhang, D.; Lv, D.; Wang, P. Effects of two main metabolites of sulphate-reducing bacteria on the corrosion of Q235 steels in 3.5 wt.% NaCl media. Corros. Sci. 2012, 65, 405–413. [Google Scholar] [CrossRef]
- Liu, H.; Fu, C.; Gu, T.; Zhang, G.; Lv, Y.; Wang, H.; Liu, H. Corrosion behavior of carbon steel in the presence of sulfate reducing bacteria and iron oxidizing bacteria cultured in oilfield produced water. Corros. Sci. 2015, 100, 484–495. [Google Scholar] [CrossRef]
- Sheng, X.; Ting, Y.P.; Pehkonen, S.O. The influence of sulphate-reducing bacteria biofilm on the corrosion of stainless steel AISI 316. Corros. Sci. 2007, 49, 2159–2176. [Google Scholar] [CrossRef]
- Antony, P.J.; Raman, P.K.S.; Mohanram, R.; Kumar, P.; Raman, R. Influence of thermal aging on sulfate-reducing bacteria (SRB)-influenced corrosion behaviour of 2205 duplex stainless steel. Corros. Sci. 2008, 50, 1858–1864. [Google Scholar] [CrossRef]
- Yuan, S.; Liang, B.; Zhao, Y.; Pehkonen, S.O. Surface chemistry and corrosion behaviour of 304 stainless steel in simulated seawater containing inorganic sulphide and sulphate-reducing bacteria. Corros. Sci. 2013, 74, 353–366. [Google Scholar] [CrossRef]
- Starosvetsky, D.; Khaselev, O.; Starosvetsky, J.; Armon, R.; Yahalom, J. Effect of iron exposure in SRB media on pitting initiation. Corros. Sci. 2000, 42, 345–359. [Google Scholar] [CrossRef]
- Liu, H.; Xu, D.; Yang, K.; Liu, H.; Cheng, Y.F. Corrosion of antibacterial Cu-bearing 316L stainless steels in the presence of sulfate reducing bacteria. Corros. Sci. 2018, 132, 46–55. [Google Scholar] [CrossRef]
- Dong, Z.H.; Wei, S.; Hong, M.R.; Guo, A.Z. Heterogeneous corrosion of mild steel under SRB-biofilm characterised by electrochemical mapping technique. Corros. Sci. 2011, 53, 2978–2987. [Google Scholar] [CrossRef]
- Liu, H.; Cheng, Y.F. Mechanism of microbiologically influenced corrosion of X52 pipeline steel in a wet soil containing sulfate-reduced bacteria. Electrochim. Acta 2017, 253, 368–378. [Google Scholar] [CrossRef]
- Javed, M.A.; Stoddart, P.R.; Wade, S.A. Corrosion of carbon steel by sulphate reducing bacteria: Initial attachment and the role of ferrous ions. Corros. Sci. 2015, 93, 48–57. [Google Scholar] [CrossRef]
- Chen, X.; Wang, G.; Gao, F.; Wang, Y.; He, C. Effects of sulphate-reducing bacteria on crevice corrosion in X70 pipeline steel under disbonded coatings. Corros. Sci. 2015, 101, 1–11. [Google Scholar] [CrossRef]
- Xu, D.; Li, Y.; Gu, T. Mechanistic modeling of biocorrosion caused by biofilms of sulfate reducing bacteria and acid producing bacteria. Bioelectrochemistry 2016, 110, 52–58. [Google Scholar] [CrossRef]
- Lee, W.; Lewandowski, Z.; Nielsen, P.H.; Hamilton, W.A. Role of sulfate-reducing bacteria in corrosion of mild steel: A review. Biofouling 1995, 8, 165–194. [Google Scholar] [CrossRef]
- Sungur, E.I.; Cansever, N.; Cotuk, A. Microbial corrosion of galvanized steel by a freshwater strain of sulphate reducing bacteria (Desulfovibrio sp.). Corros. Sci. 2007, 49, 1097–1109. [Google Scholar] [CrossRef]
- Sungur, E.I.; Cotuk, A. Microbial corrosion of galvanized steel in a simulated recirculating cooling tower system. Corros. Sci. 2010, 52, 161–171. [Google Scholar] [CrossRef]
- Sherar, B.W.A.; Power, I.M.; Keech, P.G.; Mitlin, S.; Southam, G.; Shoesmith, D.W. Characterizing the effect of carbon steel exposure in sulfide containing solutions to microbially induced corrosion. Corros. Sci. 2011, 53, 955–960. [Google Scholar] [CrossRef]
- Beech, I.B.; Cheung, C.W.S. Interactions of exopolymers produced by sulphate-reducing bacteria with metal ions, Int. Biodeterior. Biodegrad. 1995, 35, 59–72. [Google Scholar] [CrossRef]
- Xu, D.; Li, Y.; Song, F.; Gu, T. Laboratory investigation of microbiologically influenced corrosion of C1018 carbon steel by nitrate reducing bacterium Bacillus licheniformis. Corros. Sci. 2013, 77, 385–390. [Google Scholar] [CrossRef]
- Wan, Y.; Zhang, D.; Liu, H.; Li, Y.; Hou, B. Influence of sulphate-reducing bacteria on environmental parameters and marine corrosion behavior of Q235 steel in aerobic conditions. Electrochim. Acta 2010, 55, 1528–1534. [Google Scholar] [CrossRef]
- Hamilton, W.A. Microbially influenced corrosion as a model system for the study of metal microbe interactions: A unifying electron transfer hypothesis. Biofouling 2003, 19, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Gieg, L.M.; Jack, T.R.; Foght, J.M. Biological souring and mitigation in oil reservoirs. Appl. Microbiol. Biotechnol. 2011, 92, 263–282. [Google Scholar] [CrossRef]
- Mitra, S.; Gera, R.; Singh, V.; Khandelwal, S. Comparative toxicity of low dose tributyltin chloride on serum, liver, lung and kidney following subchronic exposure. Food Chem. Toxicol. 2014, 64, 335–343. [Google Scholar] [CrossRef]
- Zhang, C.N.; Zhang, J.L.; Ren, H.T.; Zhou, B.H.; Wu, Q.J.; Sun, P. Effect of tributyltin on antioxidant ability and immune responses of zebrafish (Danio rerio). Ecotoxicol. Environ. Saf. 2017, 138, 1–8. [Google Scholar] [CrossRef]
- Cooke, G.M.; Tryphonas, H.; Pulido, O.; Caldwell, D.; Bondy, G.S.; Forsyth, D. Oral (gavage), in utero and postnatal exposure of Sprague–Dawley rats to low doses of tributyltin chloride. Part 1: Toxicology, histopathology and clinical chemistry. Food Chem. Toxicol. 2004, 42, 211–220. [Google Scholar] [CrossRef]
- Cooke, G.M. Toxicology of tributyltin in mammalian animal models. Immunol. Endocr. Metab. Agents Med. Chem. 2006, 6, 63–71. [Google Scholar] [CrossRef]
- Ferreira, M.; Blanco, L.; Garrido, A.; Vieites, J.M.; Cabado, A.G. In vitro approaches to evaluate toxicity induced by organotin compounds Tributyltin (TBT), Dibutyltin (DBT), and Monobutyltin (MBT) in Neuroblastoma cells. J. Agric Food. Chem. 2013, 61, 4195–4203. [Google Scholar] [CrossRef] [PubMed]
- Antizar-Ladislao, B. Environmental levels, toxicity and human exposure to tributyltin (TBT)-contaminated marine environment. Environ. Int. Rev. 2008, 34, 292–308. [Google Scholar] [CrossRef] [PubMed]
- Araújo, J.F.P.; Podratz, P.L.; Sena, G.C.; Merlo, E.; Lima, L.C.F.; Ayub, J.G.M.; Pereira, A.F.Z.; Silva, A.P.S.; Alves, L.M.; Silva, I.V.; et al. The obesogen tributyltin induces abnormal ovarian adipogenesis in adult female rats. Toxicol. Lett. 2018, 295, 99–114. [Google Scholar] [CrossRef] [PubMed]
- Gerike, P.; Fischer, W.K.; Jasiak, W. Surfactant quaternary ammonium salts in aerobic sewage digestion. Water Res. 1978, 12, 1117–1122. [Google Scholar] [CrossRef]
- Ferreira, C.; Rosmaninho, R.; Simoes, M.; Pereira, M.C.; Bastos, M.M.; Nunes, O.C.; Coelho, M.; Melo, L.F. Biofouling control using microparticles carrying a biocide. Biofouling 2009, 26, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Chang, X.; Yang, F.; Wang, Y.Q.; Wang, F.Y.; Dong, W.W.; Zhao, C.C. Effect of oxidizing and non-oxidizing biocides on biofilm at different substrate levels in the model recirculating cooling water system. World J. Microbiol. Biotechnol. 2011, 27, 2989–2997. [Google Scholar] [CrossRef]
- Tomlinson, E.; Brown, M.R.W.; Davis, S.S. Effect of colloidal association on the measured activity of alkylbenzyldimethylammonium chlorides against Pseudomonas aeruginosa. J. Med. Chem. 1977, 20, 1277–1282. [Google Scholar] [CrossRef]
- Ferreira, C.; Pereira, A.M.; Pereira, M.C.; Melo, L.F.; Simões, M. Physiological changes induced by the quaternary ammonium compound benzyldimethyldodecylammonium chloride on Pseudomonas fluorescens. J. Antimicrob. Chemother. 2011, 66, 1036–1043. [Google Scholar] [CrossRef]
- Angelov, A.; Bratkova, S.; Loukanov, A. Microbial fuel cell based on electroactive sulfate-reducing biofilm. Energy Convers. Manag. 2013, 67, 283–286. [Google Scholar] [CrossRef]
- Ghazy, E.A.; Mahmoud, M.G.; Asker, M.S.; Mahmoud, M.N.; Elsoud, M.M.A.; Sami, M.E.A. Cultivation and detection of sulfate reducing bacteria (SRB) in sea water. J. Am. Sci. 2011, 2, 604–608. [Google Scholar]
- Omran, B.A.; Fatthalah, N.A.; El-Gendy, N.S.; El-Shatoury, E.H.; Abouzeid, M.A. Green biocides against sulphate reducing bacteria and macrofouling organisms. J. Pure Appl. Microbiol. 2013, 7, 2219–2232. [Google Scholar]
- Unsal, T.; Ilhan-Sungur, E.; Arkan, S.; Cansever, N. Effects of Ag and Cu ions on the microbial corrosion of 316 L stainless steel in the presence of Desulfovibrio sp. Bioelectrochemistry 2016, 110, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Zhang, Y.; Cheng, G.; Zhu, W. Pitting corrosion behavior of 316L stainless steel in the media of sulphate-reducing and iron-oxidizing bacteria. Mater. Charact. 2008, 59, 245–255. [Google Scholar] [CrossRef]
- Nan, L.; Xu, D.; Gu, T.; Song, X.; Yang, K. Microbiological influenced corrosion resistance characteristics of a 304 L-Cu stainless steel against Escherichia coli. Mater. Sci. Eng. C 2015, 48, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Zhao, K.; Gu, T.; Raad, I.I. A green biocide enhancer for the treatment of sulfate-reducing bacteria (SRB) biofilms on carbon steel surfaces using glutaraldehyde. Int. Biodeterior. Biodegrad. 2009, 63, 1102–1106. [Google Scholar] [CrossRef]
- Li, H.; Zhou, E.; Ren, Y.; Zhang, D.; Xu, D.; Yang, C.; Feng, H.; Jiang, Z.; Li, X.; Gu, T.; et al. Investigation of microbiologically influenced corrosion of high nitrogen nickel-free stainless steel by Pseudomonas aeruginosa. Corros. Sci. 2016, 111, 811–821. [Google Scholar] [CrossRef]
- Li, H.; Yang, C.; Zhou, E.; Yang, C.; Feng, H.; Jiang, Z.; Xu, D.; Gu, T.; Yang, K. Microbiologically influenced corrosion behavior of S32654 super austenitic stainless steel in the presence of marine Pseudomonas aeruginosa biofilm. J. Mater. Sci. Technol. 2017, 33, 1596–1603. [Google Scholar] [CrossRef]
- Wikiel, A.J.; Datsenko, I.; Vera, M.; Sand, W. Impact of Desulfovibrio alaskensis biofilms on corrosion behaviour of carbon steel in marine environment. Bioelectrochemistry 2014, 97, 52–60. [Google Scholar] [CrossRef]
- Lawrence, J.R.; Neu, T.R. Confocal laser scanning microscopy for analysis of microbial biofilms. In Methods in Enzymology; Doyle, R.J., Ed.; Academic Press: San Diego, CA, USA, 1999; Volume 310, pp. 131–144. [Google Scholar]
- Freire, L.; Carmezim, M.J.; Ferreira, M.G.S.; Montemor, M.F. The electrochemical behaviour of stainless steel AISI 304 in alkaline solutions with different pH in the presence of chlorides. Electrochim. Acta 2011, 56, 5280–5289. [Google Scholar] [CrossRef]
- Wu, K.; Jung, W.-S.; Byeon, J.-W. In-situ monitoring of pitting corrosion on vertically positioned 304 stainless steel by analyzing acoustic-emission energy parameter. Corros. Sci. 2016, 105, 8–16. [Google Scholar] [CrossRef]
- Wang, Y.; Cheng, G.; Wu, W.; Qiao, Q.; Li, Y.; Li, X. Effect of pH and chloride on the micro-mechanism of pitting corrosion for high strength pipeline steel in aerated NaCl solutions. Appl. Surf. Sci. 2015, 349, 746–756. [Google Scholar] [CrossRef]
- Liu, H.; Gu, T.; Zhang, G.; Liu, H.; Cheng, Y.F. Corrosion of X80 pipeline steel under sulfate-reducing bacterium biofilms in simulated CO2-saturated oilfield produced water with carbon source starvation. Corros. Sci. 2018, 136, 47–59. [Google Scholar] [CrossRef]
- Yuan, S.J.; Xu, F.J.; Pehkonen, S.O.; Ting, Y.P.; Neoh, K.G.; Kang, E.T. Grafting of antibacterial polymers on stainless steel via surface-initiated atom transfer radical polymerization for inhibiting biocorrosion by Desulfovibrio desulfuricans. Biotechnol. Bioeng. 2009, 103, 268–281. [Google Scholar] [CrossRef] [PubMed]
- Javaherdashti, R. Microbiologically Influenced Corrosion: An Engineering Insight; Springer: Berlin, Germany, 2008. [Google Scholar]
- Pedersen, K. Subterranean micro-organisms and radioactive waste disposal in Sweden. Eng. Geol. 1999, 52, 163–176. [Google Scholar] [CrossRef]
- Stein, A. A Pratical Manual on Microbiologically Influenced Corrosion; Kobrin, G., Ed.; NACE International: Houston, TX, USA, 1993; Volume 1, pp. 101–112. [Google Scholar]
- Little, B.J.; Wagner, P.A.; Hart, K.R.; Ray, R.L. Spatial relationships between bacteria and localized corrosion. Corrosion 1996, 96, 278–284. [Google Scholar]
- De Romero, M.F.; Parra, J.; Ruiz, R.; Ocando, L.; Bracho, M.; de Rincon, O.T.; Romero, G.; Quintero, A. Cathodic polarization effect on sessile SRB growth and iron protection. Corrosion 2006, 6, 652–656. [Google Scholar]


| Immersion Times | Elements (wt %) | O | S | Cr | Fe | Ni | Si |
|---|---|---|---|---|---|---|---|
| 14 days | Spectrum 1 | 8.39 | 4.67 | 14.66 | 64.57 | 5.42 | 2.29 |
| 28 days | Spectrum 2 | - | - | 19.59 | 71.51 | 8.23 | 0.67 |
| Spectrum 3 | 32.26 | 14.34 | - | 53.40 | - | - | |
| Spectrum 4 | - | - | 19.36 | 72.53 | 8.11 | - |
| Samples | Blank 14 days | SRB 14 days | BDMDAC 14 days | Blank 28 days | SRB 28 days | BDMDAC 28 days |
|---|---|---|---|---|---|---|
| Ecorr (mV vs. SCE) | −755 ± 36 | −733 ± 38 | −758 ± 58 | −751 ± 24 | −765 ± 23 | −764 ± 32 |
| Icorr (μA/cm2) | 1.06 ± 0.06 | 2.39 ± 0.25 | 1.57 ± 0.02 | 0.95 ± 0.05 | 3.06 ± 0.25 | 1.83 ± 0.08 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsu, C.-W.; Chen, T.-E.; Lo, K.-Y.; Lee, Y.-L. Inhibitive Properties of Benzyldimethyldodecylammonium Chloride on Microbial Corrosion of 304 Stainless Steel in a Desulfovibrio desulfuricans-Inoculated Medium. Materials 2019, 12, 307. https://doi.org/10.3390/ma12020307
Hsu C-W, Chen T-E, Lo K-Y, Lee Y-L. Inhibitive Properties of Benzyldimethyldodecylammonium Chloride on Microbial Corrosion of 304 Stainless Steel in a Desulfovibrio desulfuricans-Inoculated Medium. Materials. 2019; 12(2):307. https://doi.org/10.3390/ma12020307
Chicago/Turabian StyleHsu, Chung-Wen, Tzu-En Chen, Kai-Yin Lo, and Yueh-Lien Lee. 2019. "Inhibitive Properties of Benzyldimethyldodecylammonium Chloride on Microbial Corrosion of 304 Stainless Steel in a Desulfovibrio desulfuricans-Inoculated Medium" Materials 12, no. 2: 307. https://doi.org/10.3390/ma12020307
APA StyleHsu, C.-W., Chen, T.-E., Lo, K.-Y., & Lee, Y.-L. (2019). Inhibitive Properties of Benzyldimethyldodecylammonium Chloride on Microbial Corrosion of 304 Stainless Steel in a Desulfovibrio desulfuricans-Inoculated Medium. Materials, 12(2), 307. https://doi.org/10.3390/ma12020307





