Designing and Fabricating Ordered Mesoporous Metal Oxides for CO2 Catalytic Conversion: A Review and Prospect
Abstract
1. Introduction
2. Types of Ordered Mesoporous Materials
2.1. Ordered Mesoporous Non-Metal Oxide Materials
2.2. Ordered Mesoporous Metal Oxide Materials
3. Synthetic Method of Ordered Mesoporous Metal Oxides
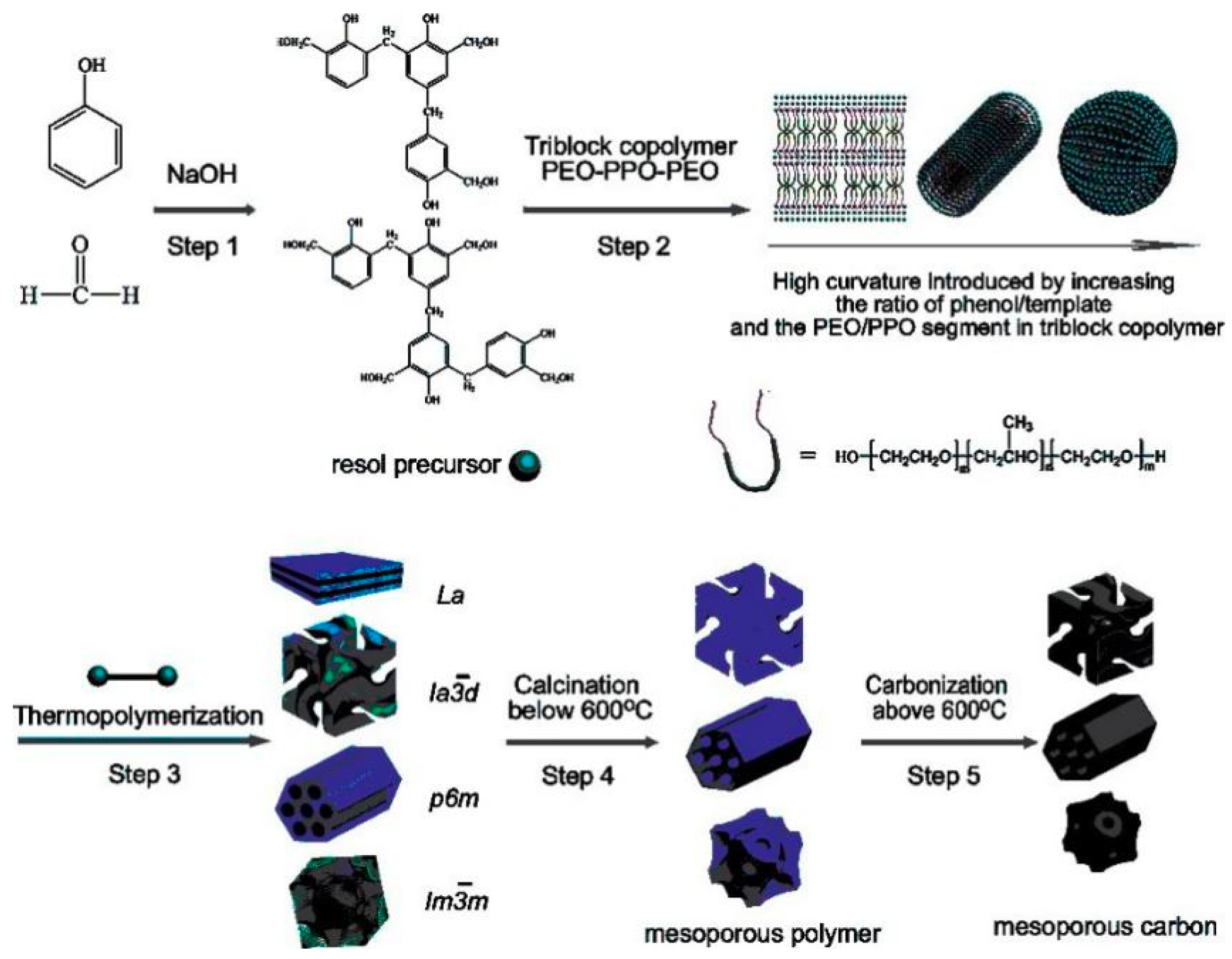
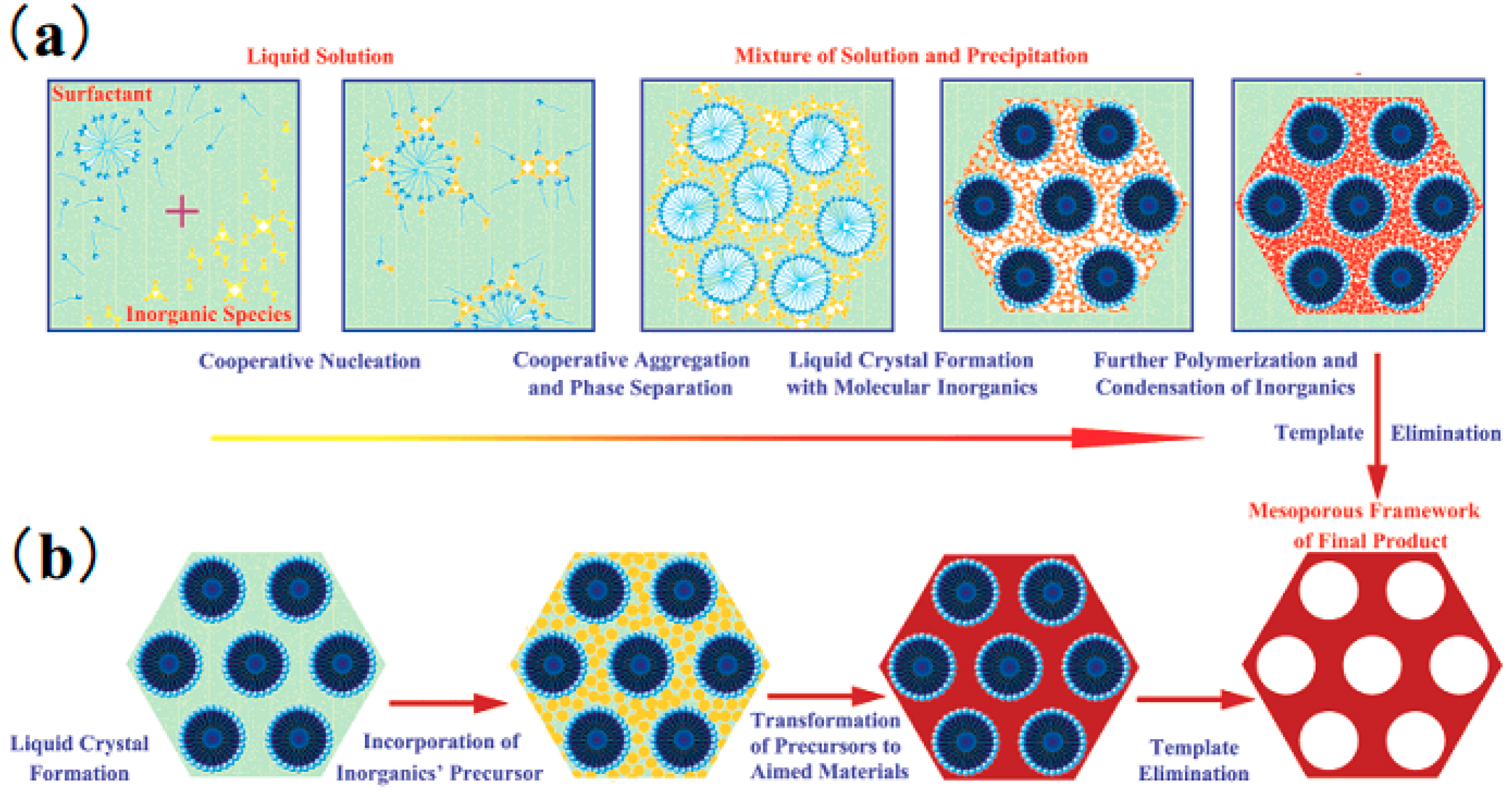
3.1. Soft Template
3.1.1. Type of Soft Template Agent
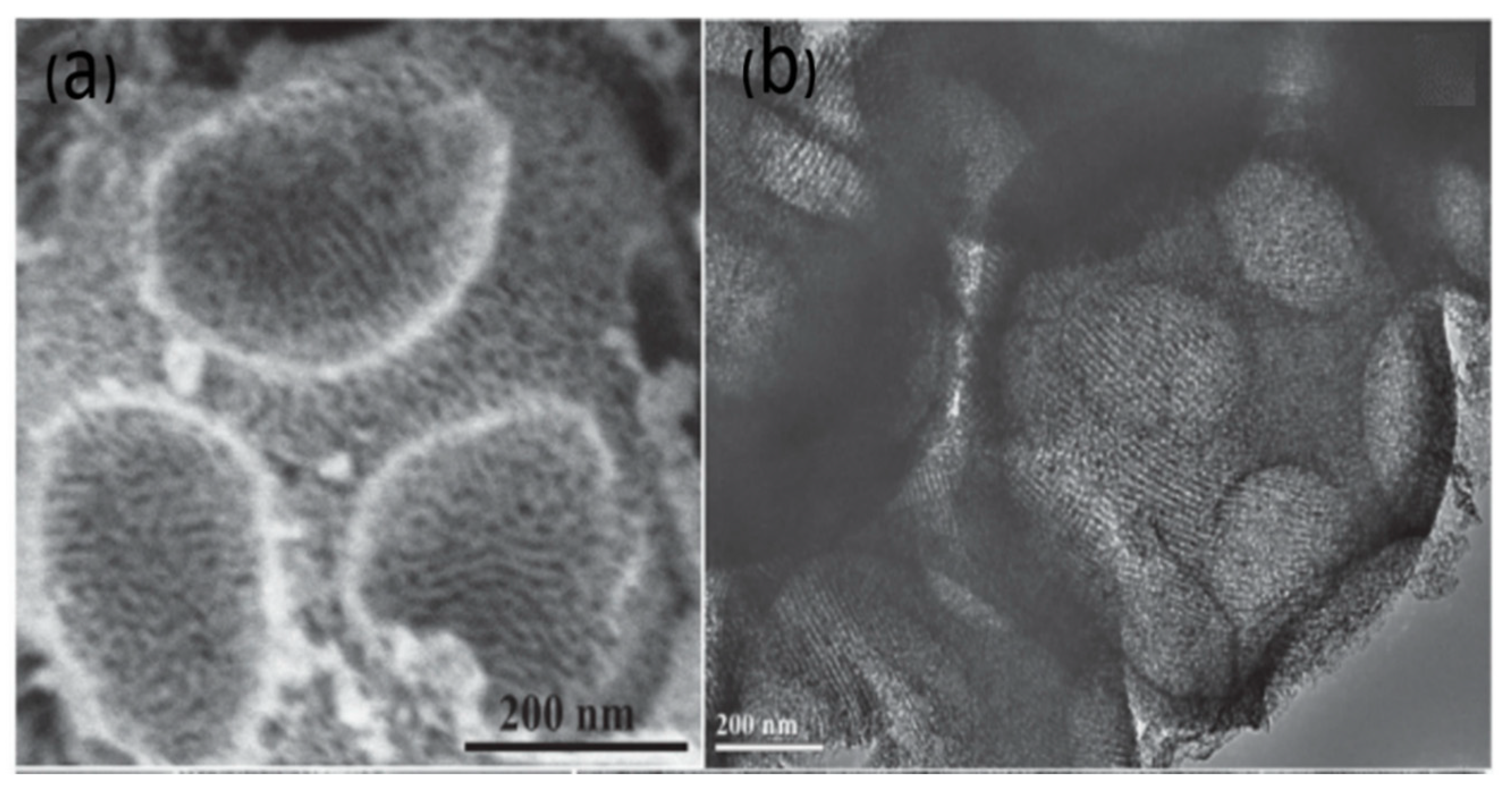
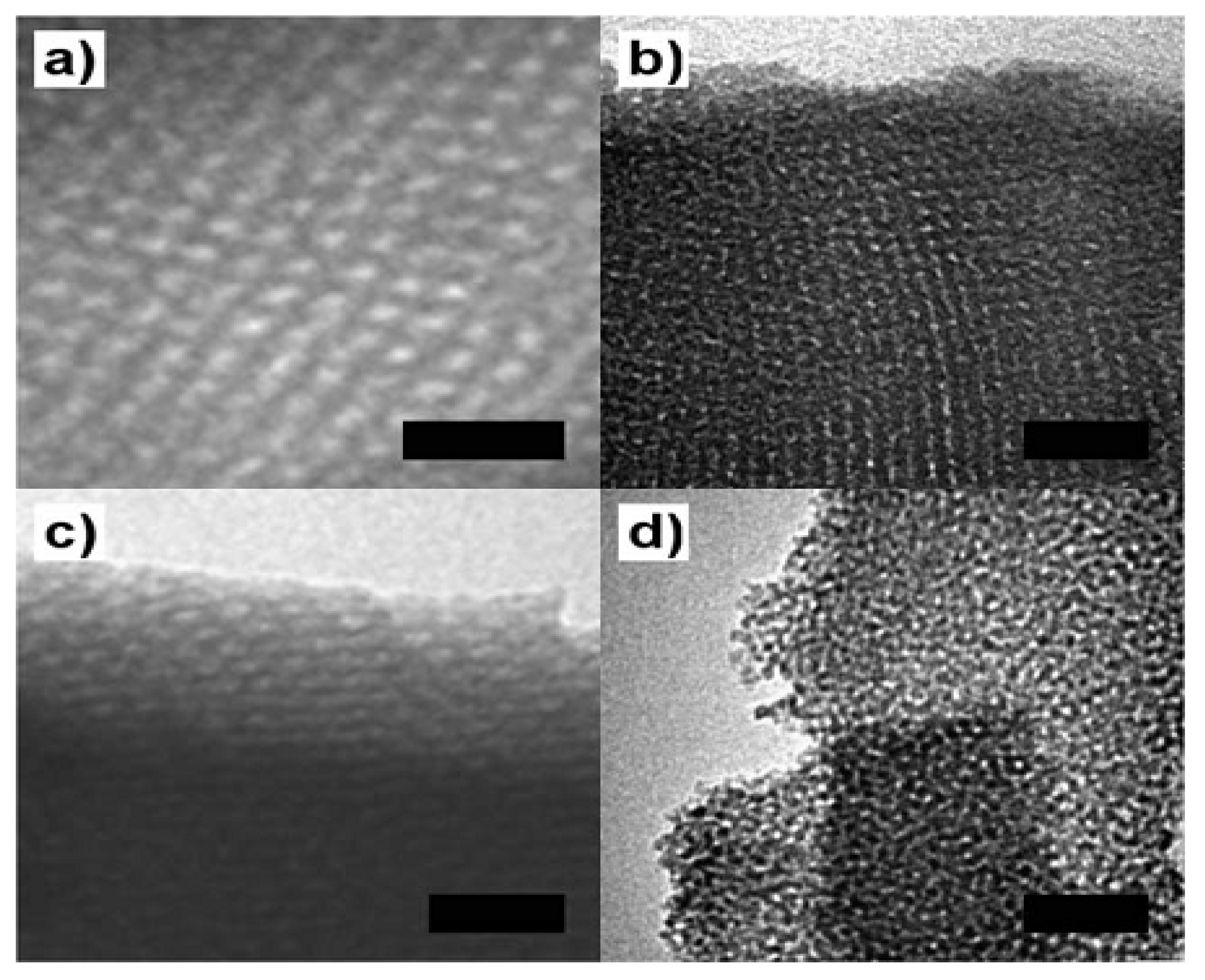
3.2. Hard Template
3.2.1. Mesoporous Silica as a Template
3.2.2. Mesoporous Carbon as a Template
4. Catalytic Application of Mesoporous Metal Oxides in Catalytic Conversion of CO2
4.1. CO2 Hydrogenation to Methanol
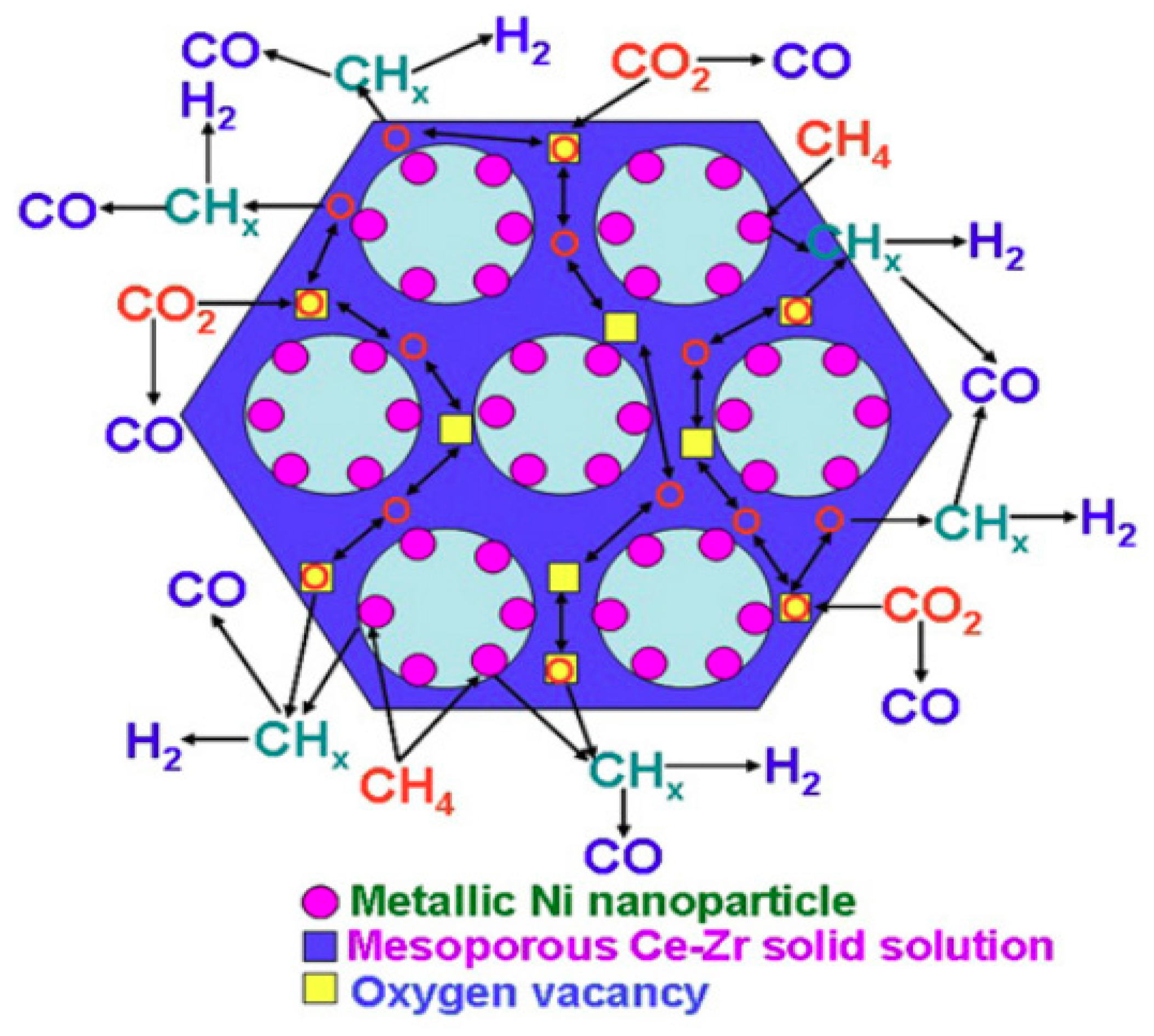
4.2. CO2 Reforming of Methane (CRM)
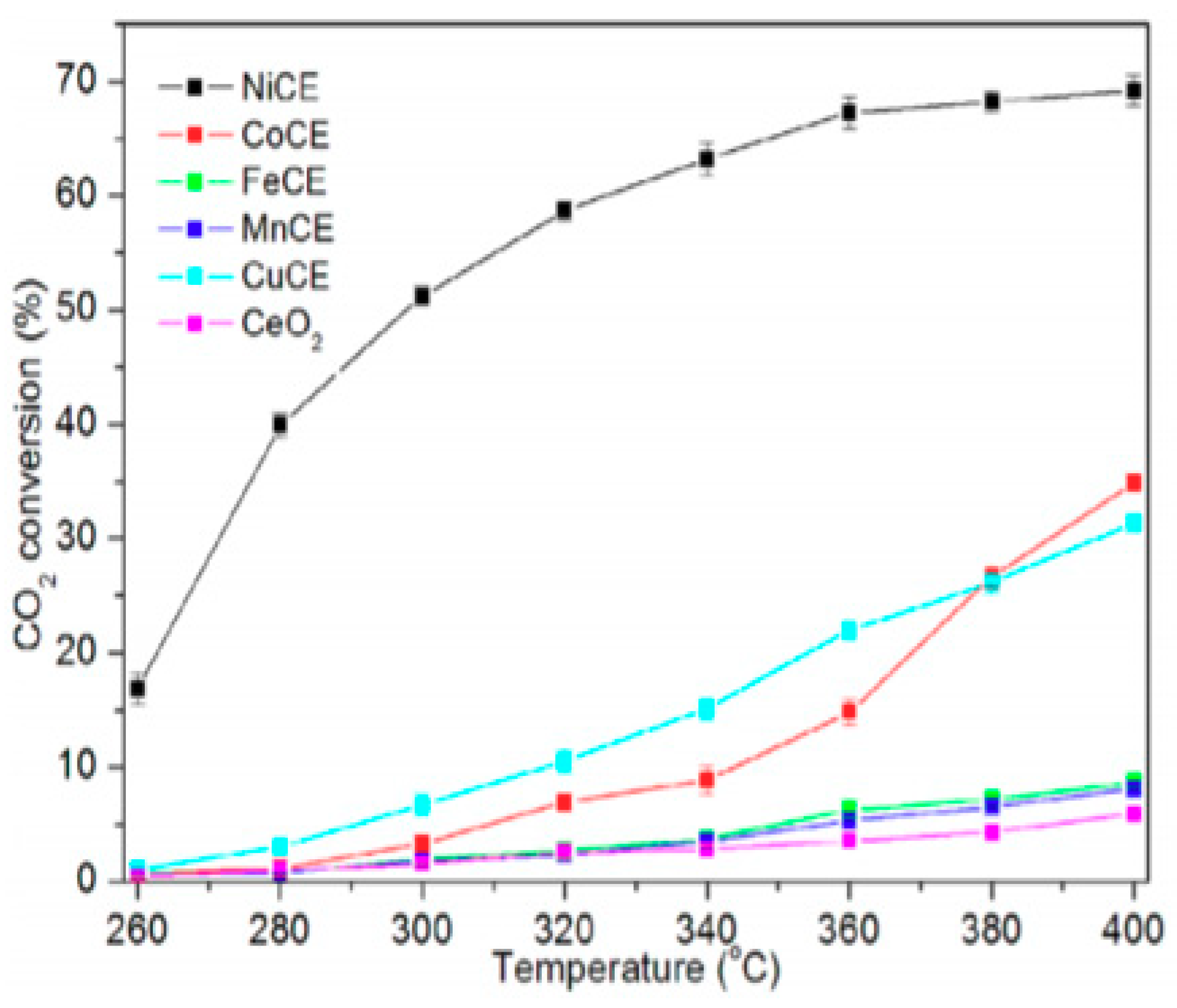
4.3. CO2 Methanation
4.4. Synthesis of Dimethyl Ether (DME)
4.5. CO2 Reverse Water Gas Shift (RWGS) Reaction
5. Conclusions and Perspective
Author Contributions
Funding
Conflicts of Interest
References
- Kresge, C.T.; Leonowicz, M.E.; Roth, W.J.; Vartuli, J.C.; Beck, J.S. Ordered mesoporous molecular sieves synthedized by a liquid-crystal template mechanism. Nature 1992, 359, 710–712. [Google Scholar] [CrossRef]
- Gondal, M.A.; Dastageer, M.A.; Oloore, L.E.; Baig, U.; Rashid, S.G. Enhanced photo-catalytic activity of ordered mesoporous indium oxide nanocrystals in the conversion of CO2 into methanol. J. Environ. Sci. Health A 2017, 52, 785–793. [Google Scholar] [CrossRef] [PubMed]
- Younas, M.; Kong, L.L.; Bashir, M.J.K.; Nadeem, H.; Shehzad, A.; Sethupathi, S. Recent Advancements, Fundamental Challenges, and Opportunities in Catalytic Methanation of CO2. Energy Fuel 2016, 30, 8815–8831. [Google Scholar] [CrossRef]
- Satthawong, R.; Koizumi, N.; Song, C.; Prasassarakich, P. Comparative Study on CO2 Hydrogenation to Higher Hydrocarbons over Fe-Based Bimetallic Catalysts. Top. Catal. 2014, 57, 588–594. [Google Scholar] [CrossRef]
- Fechete, I.; Wang, Y.; Vedrine, J.C. The Past, Present and future of heterogeneous catalysis. Catal. Today 2012, 189, 2–27. [Google Scholar] [CrossRef]
- Iver, S.; Astrid, B.; Ester, G.; Kenny, S.; Søren, P.; Søren, D.; Anna, C.; Jacobsen, C.J.H. Carbon Nanotube Templated Growth of Mesoporous Zeolite Single Crystals. Chem. Mater. 2001, 13, 4416–4418. [Google Scholar] [CrossRef]
- Janssen, A.H.; Koster, A.J.; de Jong, K.P. Three-Dimensional Transmission Electron Microscopic Observations of Mesopores in Dealuminated Zeolite Y Supported by NWO under grant 98037 The research of AJK has been made possible by a fellowship of the Royal Netherlands Academy of Arts and Sciences. Angew. Chem. Int. Edit. 2001, 40, 1102–1104. [Google Scholar] [CrossRef]
- Lai, C.Y.; Brian, G.T.; Dusan, M.J.; Ksenija, J.; Xu, S.; Srdija, J.; Victor, S.L. A mesoporous silica nanosphere-based carrier system with chemically removable CdS nanoparticle caps for stimuli-responsive controlled release of neurotransmitters and drug molecules. J. Am. Chem. Soc. 2003, 125, 4451–4459. [Google Scholar] [CrossRef]
- Radu, D.R.; Lai, C.Y.; Jeftinija, K.; Rowe, E.W.; Jeftinija, S.; Lin, V.S. A polyamidoamine dendrimer-capped mesoporous silica nanosphere-based gene transfection reagent. J. Am. Chem. Soc. 2004, 126, 13216–13217. [Google Scholar] [CrossRef]
- Vallet-Regi, M.; Rámila, A.; Real, R.P.D.; Pérez-Pariente, J. A new property of MCM-41: Drug delivery system. Chem. Mater. 2001, 13, 308–311. [Google Scholar] [CrossRef]
- Vartuli, J.C.; Schmitt, K.D.; Kresge, C.T.; Roth, W.J.; Leonowicz, M.E.; Mccullen, S.B.; Hellring, S.D.; Beck, J.S.; Schlenker, J.L. Effect of Surfactant/Silica Molar Ratios on the Formation of Mesoporous Molecular Sieves: Inorganic Mimicry of Surfactant Liquid-Crystal Phases and Mechanistic Implications. Chem. Mater. 1994, 6, 2317–2326. [Google Scholar] [CrossRef]
- Yanagisawa, T.; Harayama, M.; Kuroda, K.; Kato, C. Organic derivatives of layered polysilicates III Reaction of magadiite and kenyaite with allydimethylchlorosilane. Solid State Ion. 1990, 42, 15–19. [Google Scholar] [CrossRef]
- Wei, J.; Sun, Z.; Luo, W.; Li, Y.; Elzatahry, A.A.; Al-Enizi, A.M.; Deng, Y.; Zhao, D. New Insight into the Synthesis of Large-Pore Ordered Mesoporous Materials. J. Am. Chem. Soc. 2017, 139, 1706–1713. [Google Scholar] [CrossRef]
- Goscianska, J.; Pietrzak, R.; Matos, J. Catalytic performance of ordered mesoporous carbons modified with lanthanides in dry methane reforming. Catal. Today 2018, 301, 204–216. [Google Scholar] [CrossRef]
- Kumar, A.V.N.; Li, Y.; Yin, S.; Li, C.; Xue, H.; Xu, Y.; Li, X.; Wang, H.; Wang, L. Mesoporous Co3O4 Nanobundle Electrocatalysts. Chem.-Asian J. 2018, 13, 2093–2100. [Google Scholar] [CrossRef] [PubMed]
- Yalikun, N.; Mamat, X.; Li, Y.; Hu, X.; Wagberg, T.; Dong, Y.; Hu, G. Synthesis of an iron-nitrogen co-doped ordered mesoporous carbon-silicon nanocomposite as an enhanced electrochemical sensor for sensitive and selective determination of chloramphenicol. Colloid Surf. B-Biointerfaces 2018, 172, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Xiao, P.W.; Zhao, L.; Sui, Z.Y.; Xu, M.Y.; Han, B.H. Direct synthesis of ordered mesoporous hydrothermal carbon materials via a modified soft-templating method. Microporous Mesoporous Mater. 2017, 253, 215–222. [Google Scholar] [CrossRef]
- Han, J.; Wang, T.Y.; Li, T.T.; Yu, H.; Yang, Y.; Dong, X.T. Enhanced NOx Gas Sensing Properties of Ordered Mesoporous WO3/ZnO Prepared by Electroless Plating. Adv. Mater. Interfaces 2018, 5, 1701167. [Google Scholar] [CrossRef]
- Wang, W.; Gong, J. Methanation of carbon dioxide: An overview. Front. Chem. Sci. Eng. 2011, 5, 2–10. [Google Scholar] [CrossRef]
- Riener, K.; Haslinger, S.; Raba, A.; Högerl, M.P.; Cokoja, M.; Herrmann, W.A.; Kühn, F.E. Chemistry of iron N-heterocyclic carbene complexes: Syntheses, structures, reactivities, and catalytic applications. Chem. Rev. 2014, 45, 5215–5272. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.J.; Larrazabal, G.O.; Perez-Ramirez, J. ChemInform Abstract: Towards Sustainable Fuels and Chemicals Through the Electrochemical Reduction of CO2: Lessons from Water Electrolysis. Green Chem. 2015, 17, 5114–5130. [Google Scholar] [CrossRef]
- Cokoja, M.; Bruckmeier, C.; Rieger, B.; Herrmann, W.A.; Kuehn, F.E. Transformation of Carbon Dioxide with Homogeneous Transition-Metal Catalysts: A Molecular Solution to a Global Challenge. Angew. Chem. Int. Edit. 2011, 50, 8510–8537. [Google Scholar] [CrossRef] [PubMed]
- Yu, R.; Zhen, M.; Bruce, P.G. Ordered mesoporous metal oxides: Synthesis and applications. Cheminform 2012, 41, 4909–4927. [Google Scholar] [CrossRef]
- Tozuka, Y.; Oguchi, T.; Yamamoto, K. Adsorption and entrapment of salicylamide molecules into the mesoporous structure of folded sheets mesoporous material (FSM-16). Pharm. Res. 2003, 20, 926–930. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.Y.; Feng, J.L.; Huo, Q.S.; Melosh, N.W.; Stucky, G.D. Triblock Copolymer Syntheses of Mesoporous Silica with Periodic 50 to 300 Angstrom. Pores Sci. 1998, 279, 548–552. [Google Scholar] [CrossRef]
- El-Safty, S.A. Monolithic Nanostructured Silicate Family Templated by Lyotropic Liquid-Crystalline Nonionic Surfactant Mesophases. Chem. Mater. 2003, 15, 2892–2902. [Google Scholar] [CrossRef]
- Nandiyanto, A.B.D.; Kim, S.-G.; Iskandar, F.; Okuyama, K. Synthesis of spherical mesoporous silica nanoparticles with nanometer-size controllable pores and outer diameters. Microporous Mesoporous Mater. 2009, 120, 447–453. [Google Scholar] [CrossRef]
- Wei, H.; Chen, H.; Fu, N.; Chen, J.; Lan, G.; Qian, W.; Liu, Y.; Lin, H.; Han, S. Excellent electrochemical properties and large CO2 capture of nitrogen-doped activated porous carbon synthesised from waste longan shells. Electrochim. Acta 2017, 231, 403–411. [Google Scholar] [CrossRef]
- Xiang, S.; Yang, X.; Lin, X.; Chang, C.; Que, H.; Li, M. Nitrogen and sulfur co-doped polyurethane-based porous carbon materials as supercapacitors exhibit excellent electrochemical performance. J. Solid State Electrochem. 2017, 21, 1457–1465. [Google Scholar] [CrossRef]
- Meng, Y.; Gu, D.; Zhang, F.; Shi, Y.; Cheng, L.; Feng, D.; Wu, Z.; Chen, Z.; Wan, Y.; Andreas, S. A Family of Highly Ordered Mesoporous Polymer Resin and Carbon Structures from Organic−Organic Self-Assembly. Chem. Mater. 2006, 18, 4447–4464. [Google Scholar] [CrossRef]
- Budhiraju, V.S.; Kumar, R.; Sharma, A.; Sivakumar, S. Structurally stable hollow mesoporous graphitized carbon nanofibers embedded with NiMoO4 nanoparticles for high performance asymmetric supercapacitors. Electrochim. Acta 2017, 238, 337–348. [Google Scholar] [CrossRef]
- Fu, X.; Liu, S.M.; Zhu, D.L.; Xu, Y.B.; Yan, X.Y. A facile and novel route for the direct synthesis superparamagnetic ordered mesoporous carbon. Mater. Lett. 2019, 234, 269–271. [Google Scholar] [CrossRef]
- Ryong Ryoo, S.H.J.; Jun, S. Synthesis of Highly Ordered Carbon Molecular Sieves via Template-Mediated Structural Transformation. J. Phys. Chem. B 1999, 103, 7743–7746. [Google Scholar] [CrossRef]
- Juarez, J.M.; Costa, M.G.; Anunziata, O.A. Direct synthesis of ordered mesoporous carbon applied in hydrogen storage. J. Porous Mater. 2018, 25, 1359–1363. [Google Scholar] [CrossRef]
- Zhao, Y.; Dong, F.; Han, W.; Zhao, H.; Tang, Z. The synergistic catalytic effect between graphene oxide and three-dimensional ordered mesoporous Co3O4 nanoparticles for low-temperature CO oxidation. Microporous Mesoporous Mater. 2019, 273, 1–9. [Google Scholar] [CrossRef]
- Liu, S.; Wang, Z.; Zhao, H.; Fei, T.; Zhang, T. Ordered mesoporous Co3O4 for high-performance toluene sensing. Sens. Actuators B Chem. 2014, 197, 342–349. [Google Scholar] [CrossRef]
- Li, M.; Fu, X.; Peng, L.; Bai, L.; Wu, S.; Kan, Q.; Guan, J. Synthesis of Three-Dimensional-Ordered Mesoporous Cobalt Oxides for Selective Oxidation of Benzyl Alcohol. ChemistrySelect 2017, 2, 9486–9489. [Google Scholar] [CrossRef]
- Tang, Q.; Angelome, P.C.; Soler-Illia, G.J.A.A.; Mueller, M. Formation of ordered mesostructured TiO2 thin films: A soft coarse-grained simulation study. Phys. Chem. Chem. Phys. 2017, 19, 28249–28262. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lan, K.; Bagabas, A.A.; Zhang, P.; Gao, W.; Wang, J.; Sun, Z.; Fan, J.; Elzatahry, A.A.; Zhao, D. Ordered Macro/Mesoporous TiO2 Hollow Microspheres with Highly Crystalline Thin Shells for High-Efficiency Photoconversion. Small 2016, 12, 860–867. [Google Scholar] [CrossRef]
- Ma, J.; Ren, Y.; Zhou, X.; Liu, L.; Zhu, Y.; Cheng, X.; Xu, P.; Li, X.; Deng, Y.; Zhao, D. Pt Nanoparticles Sensitized Ordered Mesoporous WO3 Semiconductor: Gas Sensing Performance and Mechanism Study. Adv. Funct. Mater. 2018, 28. [Google Scholar] [CrossRef]
- Zhan, S.; Zhang, H.; Zhang, Y.; Shi, Q.; Li, Y.; Li, X. Efficient NH3-SCR removal of NOx with highly ordered mesoporous WO3(chi)-CeO2 at low temperatures. Appl. Catal. B Environ. 2017, 203, 199–209. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Jia, C.; Wang, C.; Xiao, F.; Zhao, N.; Sun, N.; Wei, W.; Sun, Y. Ordered mesoporous CoO-NiO-Al2O3 bimetallic catalysts with dual confinement effects for CO2 reforming of CH4. Catal. Today 2017, 281, 241–249. [Google Scholar] [CrossRef]
- Xiao, Y.; Zheng, X.; Chen, X.; Jiang, L.; Zheng, Y. Synthesis of Mg-Doped Ordered Mesoporous Pd-Al2O3 with Different Basicity for CO, NO, and HC Elimination. Ind. Eng. Chem. Res. 2017, 56, 1687–1695. [Google Scholar] [CrossRef]
- Liu, F.; Zheng, X.; Chen, J.; Zheng, Y.; Jiang, L. Controlling the synthesis and application of nanocrystalline spherical and ordered mesoporous alumina with high thermal stability. RSC Adv. 2015, 5, 93917–93925. [Google Scholar] [CrossRef]
- Haneda, M.; Takamura, K.; Doi, Y.; Bion, N.; Vivier, L. Synthesis of ordered porous zirconia containing sulfate ions and evaluation of its surface acidic properties. J. Mater. Sci. 2017, 52, 5835–5845. [Google Scholar] [CrossRef]
- Yuan, Q.; Li, L.-L.; Lu, S.-L.; Duan, H.-H.; Li, Z.-X.; Zhu, Y.-X.; Yan, C.-H. Facile Synthesis of Zr-Based Functional Materials with Highly Ordered Mesoporous Structures. J. Phys. Chem. C 2009, 113, 4117–4124. [Google Scholar] [CrossRef]
- Yuan, Q.; Liu, Y.; Li, L.-L.; Li, Z.-X.; Fang, C.-J.; Duan, W.-T.; Li, X.-G.; Yan, C.-H. Highly ordered mesoporous titania-zirconia photocatalyst for applications in degradation of rhodamine-B and hydrogen evolution. Microporous Mesoporous Mater. 2009, 124, 169–178. [Google Scholar] [CrossRef]
- Li, C.; Li, Z.; Park, S.B.; Hong, G.; Park, J.S.; Oh, H.Y.; Kim, J.M. Ordered Mesoporous Cu-Co-CeO2 Catalyst for Water-Gas Shift Reaction at High Temperature. J. Nanosci. Nanotechnol. 2017, 17, 8149–8152. [Google Scholar] [CrossRef]
- Li, K.; Zhao, Y.; Song, C.; Guo, X. Magnetic ordered mesoporous Fe3O4/CeO2 composites with synergy of adsorption and Fenton catalysis. Appl. Surf. Sci. 2017, 425, 526–534. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, Y.; Zhou, Y.; Zhang, Z.; Xue, J.; Xu, Y.; Zhang, C.; Sheng, X.; Kui, N. Nanocasting synthesis of an ordered mesoporous CeO2-supported Pt nanocatalyst with enhanced catalytic performance for the reduction of 4-nitrophenol. RSC Adv. 2016, 6, 730–739. [Google Scholar] [CrossRef]
- Dong, Z.; Liu, S. In2O3-decorated ordered mesoporous NiO for enhanced NO2 sensing at room temperature. J. Mater. Sci. Mater. Electron. 2018, 29, 2645–2653. [Google Scholar] [CrossRef]
- Wang, H.; Guo, W.; Jiang, Z.; Yang, R.; Jiang, Z.; Pan, Y.; Shangguan, W. New insight into the enhanced activity of ordered mesoporous nickel oxide in formaldehyde catalytic oxidation reactions. J. Catal. 2018, 361, 370–383. [Google Scholar] [CrossRef]
- Liu, H.; Du, X.; Xing, X.; Wang, G.; Qiao, S.Z. Highly ordered mesoporous Cr2O3 materials with enhanced performance for gas sensors and lithium ion batteries. Chem. Commun. 2012, 48, 865–867. [Google Scholar] [CrossRef] [PubMed]
- Tueysuez, H.; Weidenthaler, C.; Grewe, T.; Salabas, E.L.; Romero, M.J.B.; Schueth, F. A Crystal Structure Analysis and Magnetic Investigation on Highly Ordered Mesoporous Cr2O3. Inorg. Chem. 2012, 51, 11745–11752. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Zhao, Y.; Li, Y.; Hu, F.; Zhang, L.; Chen, W. Vacuum-assisted hard-templating impregnation fabrication of three-dimensional ordered mesoporous samarium oxide. J. Porous Mater. 2016, 23, 1591–1595. [Google Scholar] [CrossRef]
- Xue, P.; Yang, X.; Lai, X.; Xia, W.; Li, P.; Fang, J. Controlling synthesis and gas-sensing properties of ordered mesoporous In2O3-reduced graphene oxide (rGO) nanocomposite. Sci. Bull. 2015, 60, 1348–1354. [Google Scholar] [CrossRef]
- Zhao, R.; Wang, L.; Chai, Z.-F.; Shi, W.-Q. Synthesis of ordered mesoporous uranium dioxide by a nanocasting route. Radiochim. Acta 2016, 104, 549–553. [Google Scholar] [CrossRef]
- Antonelli, D.M.; Ying, J.Y. Synthesis of Hexagonally Packed Mesoporous TiO2 by a Modified Sol–Gel Method. Angew. Chem. Int. Edit. 1995, 34, 2014–2017. [Google Scholar] [CrossRef]
- Chen, D.; Zou, L.; Li, S.; Zheng, F. Nanospherical like reduced graphene oxide decorated TiO2 nanoparticles: An advanced catalyst for the hydrogen evolution reaction. Sci Rep. 2016, 6, 20335. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, M.C.; He, H.; Li, H. Effect of heat treatment on the crystalline structure and hydrophilic properties of TiO2 porous thin films. J. Sol-Gel Sci. Technol. 2016, 80, 881–892. [Google Scholar] [CrossRef]
- Hu, M.Z.; Shi, D.; Blom, D.A. Nanostructured Mesoporous Silica Wires with Intrawire Lamellae via Evaporation-Induced Self-Assembly in Space-Confined Channels. J. Nanomater. 2014, 2014, 1–8. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, Q.; Yang, X.; Deng, Y. One-step synthesis of in-situ N-doped ordered mesoporous titania for enhanced gas sensing performance. Microporous Mesoporous Mater. 2018, 270, 75–81. [Google Scholar] [CrossRef]
- Signoretto, M.; Breda, A.; Somma, F.; Pinna, F.; Cruciani, G. Mesoporous sulphated zirconia by liquid-crystal templating method. Microporous Mesoporous Mater. 2006, 91, 23–32. [Google Scholar] [CrossRef]
- Sang, X.; Zhang, L.; Wang, H.; He, D.; Deng, L.; Huang, S.; Wang, J.; Luo, Y. Influence of synthetic parameters on structural and catalytic properties of sulfated zirconia nanoparticles prepared by employing sulfate-containing anion surfactants via one-step route. Powder Technol. 2014, 253, 590–595. [Google Scholar] [CrossRef]
- Reddy, J.S.; Sayari, A. Nanoporous zirconium oxide prepared using the supramolecular templating approach. Catal. Lett. 1996, 38, 219–223. [Google Scholar] [CrossRef]
- Knowles, J.A.; Hudson, M.J. ChemInform Abstract: Preparation and Characterization of Mesoporous, High Surface Area Zirconium(IV) Oxides. Cheminform 1996, 27, 89–95. [Google Scholar] [CrossRef]
- Zelcer, A.; Soler-Illia, G.J.A.A. One-step preparation of UV transparent highly ordered mesoporous zirconia thin films. J. Mater. Chem. C 2013, 1, 1359–1367. [Google Scholar] [CrossRef]
- Khalack, J.; Ashrit, P.V. Tunable pseudogaps in electrochromic WO3 inverted opal photonic crystals. Appl. Phys. Lett. 2006, 89, 211112. [Google Scholar] [CrossRef]
- Ciesla, U.; Demuth, D.; Leon, R.; Petroff, P.; Stucky, G.; Unger, K. Surfactant Controlled Preparation of Mesostructured Transition-Metal Oxide Compounds. J. Chem. Soc. 1994, 11, 1387–1388. [Google Scholar] [CrossRef]
- Zhu, K.; He, H.; Xie, S.; Zhang, X.; Zhou, W.; Jin, S.; Yue, B. Crystalline WO3 nanowires synthesized by templating method. Chem. Phys. Lett. 2012, 377, 317–321. [Google Scholar] [CrossRef]
- Cui, X.; Zhang, H.; Dong, X.; Chen, H.; Zhang, L.; Guo, L.; Shi, J. Electrochemical catalytic activity for the hydrogen oxidation of mesoporous WO3 and WO3/C composites. J. Mater. Chem. 2008, 18, 3575–3580. [Google Scholar] [CrossRef]
- Shi, J. On the synergetic catalytic effect in heterogeneous nanocomposite catalysts. Chem. Rev. 2013, 113, 2139–2181. [Google Scholar] [CrossRef]
- Rossinyol, E.; Prim, A.; Pellicer, E.; Arbiol, J.; Hernández-Ramírez, F.; Peiró, F.; Cornet, A.; Morante, J.R.; Solovyov, L.A.; Tian, B. Synthesis and Characterization of Chromium-Doped Mesoporous Tungsten Oxide for Gas Sensing Applications. Adv. Funct. Mater. 2010, 17, 1801–1806. [Google Scholar] [CrossRef]
- Feng, W.; Wu, G.; Gao, G. Ordered mesoporous WO3 film with outstanding gasochromic properties. J. Mater. Chem. A 2013, 2, 585–590. [Google Scholar] [CrossRef]
- Bagshaw, S.A.; Pinnavaia, T.J. Mesoporous Alumina Molecular Sieves. Angew. Chem. Int. Ed. 1996, 35, 1102–1105. [Google Scholar] [CrossRef]
- Neirinck, B.; Vleugels, J.; Fransaer, J.; Van Der, B.O. Process for Producing Sintered Porous Materials. U.S. Patent US20100233009 A2, 27 September 2011. [Google Scholar]
- Zhu, X.D.; Kong, Q.Q.; Feng, W.; Wu, Y.T.; Zhu, R.R.; Pei, L.X. Preparation and Photocatalytic Activity of TiO2 with Different Crystal Structures. J. Synth. Cryst. 2017, 46, 616–620, 633. [Google Scholar] [CrossRef]
- Yuan, Q.; Yin, A.-X.; Luo, C.; Sun, L.-D.; Zhang, Y.-W.; Duan, W.-T.; Liu, H.-C.; Yan, C.-H. Facile synthesis for ordered mesoporous gamma-aluminas with high thermal stability. J. Am. Chem. Soc. 2008, 130, 3465–3472. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Zhou, T.; Louis, B.; Wang, Q. Hydrothermal Fabrication of High Specific Surface Area Mesoporous MgO with Excellent CO2 Adsorption Potential at Intermediate Temperatures. Catalysts 2017, 7, 116. [Google Scholar] [CrossRef]
- Locus, R.; Verboekend, D.; D’Halluin, M.; Dusselier, M.; Liao, Y.; Nuttens, N.; Jaumann, T.; Oswald, S.; Mafra, L.; Giebeler, L. Synthetic & Catalytic Potential of Amorphous Mesoporous Aluminosilicates Prepared by Post-Synthetic Aluminations of Silica in Aqueous Media. Chemcatchem 2017, 10, 1385–1397. [Google Scholar] [CrossRef]
- Serrano, D.; Coronado, J.; Delapenao’Shea, V.; Pizarro, P.; Botas, J. Advances in the design of ordered mesoporous materials for low-carbon catalytic hydrogen production. J. Mater. Chem. A 2013, 1, 12016–12027. [Google Scholar] [CrossRef]
- Pal, N.; Bhaumik, A. Soft templating strategies for the synthesis of mesoporous materials: Inorganic, organic-inorganic hybrid and purely organic solids. Adv. Colloid Interface 2013, 44, 21–41. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Zhao, D. On the controllable soft-templating approach to mesoporous silicates. Chem. Rev. 2007, 107, 2821–2860. [Google Scholar] [CrossRef] [PubMed]
- Teng, H.N.; Shen, T.; Hou, Y.X.; Chen, Y.; Kou, X.Y.; Wang, X.Y. The phase behaviors of cationic and anionic aqueous mixtures and the usage as templates for synthesis of mesoporous materials. Glass Phys. Chem. 2016, 42, 554–560. [Google Scholar] [CrossRef]
- He, W.C.; Wang, J.G.; Chen, T.H. Synthesis of silica particles with lamellar and wormhole-like bi-modal mesopores using anionic surfactant as the template. Sci. China Chem. 2009, 52, 1423–1426. [Google Scholar] [CrossRef]
- Danumah, C.; Vaudreuil, S.; Bonneviot, L.; Bousmina, M.; Giasson, S.; Kaliaguine, S. Synthesis of macrostructured MCM-48 molecular sieves. Microporous Mesoporous Mater. 2001, 44, 241–247. [Google Scholar] [CrossRef]
- Yada, M.; Kitamura, H.; Masato Machida, a.; Kijima, T. Biomimetic Surface Patterns of Layered Aluminum Oxide Mesophases Templated by Mixed Surfactant Assemblies. Langmuir 1997, 13, 5252–5257. [Google Scholar] [CrossRef]
- Cabrera, S.; Haskouri, J.E.; Alamo, J.; Beltrán, A.; Beltrán, D.; Mendioroz, S.; Marcos, M.D.; Amorós, P. Surfactant-Assisted Synthesis of Mesoporous Alumina Showing Continuously Adjustable Pore Sizes. Adv. Mater. 1999, 11, 379–381. [Google Scholar] [CrossRef]
- Vaudry, F.; Khodabandeh, S.; Davis, M.E. Synthesis of Pure Alumina Mesoporous Materials. Chem. Mater. 1996, 8, 1451–1464. [Google Scholar] [CrossRef]
- Hartmann, S.; Sachse, A.; Galarneau, A. Challenges and Strategies in the Synthesis of Mesoporous Alumina Powders and Hierarchical Alumina Monoliths. Materials 2012, 5, 336–349. [Google Scholar] [CrossRef]
- Holland, B.T.; Isbester, P.K.; Blanford, C.F.; And, E.J.M.; Stein, A. Synthesis of Ordered Aluminophosphate and Galloaluminophosphate Mesoporous Materials with Anion-Exchange Properties Utilizing Polyoxometalate Cluster/Surfactant Salts as Precursors. J. Am. Chem. Soc. 1997, 119, 6796–6803. [Google Scholar] [CrossRef]
- Tran, C.C.H.; Damas, C.; Santos-Pena, J. Capacitor behavior in neutral electrolytes of ordered mesoporous manganese oxide obtained from oxidation of perfluorinated alkenes by soft template CTAMnO(4). Electrochim. Acta 2018, 272, 108–118. [Google Scholar] [CrossRef]
- Zhao, W.; He, X.; Peng, Y.; Zhang, H.; Sun, D.; Wang, X. Preparation of mesoporous TiO2 with enhanced photocatalytic activity towards tannery wastewater degradation. Water Sci. Technol. 2017, 75, 1494–1499. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Zhao, D.; David, I.M.; Bradley, F.C.; Galen, D.S. Block Copolymer Templating Syntheses of Mesoporous Metal Oxides with Large Ordering Lengths and Semicrystalline Framework. Chem. Mater. 1999, 11, 2813–2826. [Google Scholar] [CrossRef]
- Zhao, D.; Huo, Q.; Feng, J.; Chmelka, B.F.; Stucky, G.D. Nonionic Triblock and Star Diblock Copolymer and Oligomeric Surfactant Syntheses of Highly Ordered, Hydrothermally Stable, Mesoporous Silica Structures. J. Am. Chem. Soc. 1998, 136, 6024–6036. [Google Scholar] [CrossRef]
- Bagshaw, S.A.; Prouzet, E.; Pinnavaia, T.J. Templating of mesoporous molecular sieves by nonionic polyethylene oxide surfactants. Science 1995, 269, 1242–1244. [Google Scholar] [CrossRef]
- Liu, J.; Aksay, I.A.; Choi, D.; Wang, D.; Yang, Z. Nanocomposite of Graphene and Metal Oxide Materials. U.S. Patent US8257867 B2, 2012. [Google Scholar]
- Shan, Z.; Jansen, J.C.; Zhou, W.; Maschmeyer, T. Al-TUD-1, stable mesoporous aluminas with high surface areas. Appl. Catal. A Gen. 2003, 254, 339–343. [Google Scholar] [CrossRef]
- Wang, T.; Meng, X.; Li, P.; Ouyang, S.; Chang, K.; Liu, G.; Mei, Z.; Ye, J. Photoreduction of CO2 over the well-crystallized ordered mesoporous TiO2 with the confined space effect. Nano Energy 2014, 9, 50–60. [Google Scholar] [CrossRef]
- Luo, W.; Wei, J.; Deng, Y.-H.; Li, Y.-H.; Wang, L.-J.; Zhao, T.; Jiang, W. Progress on the Fabrication of Ordered Mesoporous Materials with Large Pores by Using Novel Amphiphilic Block Copolymers as Templates. J. Inorg. Mater. 2017, 32, 1–10. [Google Scholar] [CrossRef]
- Wang, W.Z.; Han, Z.; Xia, A.N.; Xie, X.M. Progress on synthesis of mesoporous metal oxides by hard-template method. Appl. Chem. Ind. 2016, 45, 1134–1139. [Google Scholar] [CrossRef]
- Yang, H.F.; Zhao, D.Y. Synthesis of replica mesostructures by the nanocasting strategy. J. Mater. Chem. 2005, 15, 1217–1231. [Google Scholar] [CrossRef]
- Kleitz, F.; Choi, S.H.; Ryoo, R. Cubic Ia3d large mesoporous silica: Synthesis and replication to platinum nanowires, carbon nanorods and carbon nanotubes. Chem. Commun. 2003, 17, 2136–2137. [Google Scholar] [CrossRef]
- Ding, C.; Ma, Y.; Lai, X.; Yang, Q.; Xue, P.; Hu, F.; Geng, W. Ordered Large-Pore Mesoporous Cr2O3 with Ultrathin Frameworks for Formaldehyde Sensing. ACS Appl. Mater. Interfaces 2017, 9, 18170–18177. [Google Scholar] [CrossRef] [PubMed]
- González-Prior, J.; López-Fonseca, R.; Gutiérrez-Ortiz, J.I.; Rivas, B.D. Catalytic removal of chlorinated compounds over ordered mesoporous cobalt oxides synthesised by hard-templating. Appl. Catal. B Environ. 2018, 222, 9–17. [Google Scholar] [CrossRef]
- Zhao, J.; Yang, T.; Liu, Y.; Wang, Z.; Li, X.; Sun, Y.; Du, Y.; Li, Y.; Lu, G. Enhancement of NO2 gas sensing response based on ordered mesoporous Fe-doped In2O3. Sens. Actuator B Chem. 2014, 191, 806–812. [Google Scholar] [CrossRef]
- Waitz, T.; Wagner, T.; Sauerwald, T.; Kohl, C.D.; Tiemann, M. Ordered Mesoporous In2O3: Synthesis by Structure Replication and Application as a Methane Gas Sensor. Adv. Funct. Mater. 2009, 19, 653–661. [Google Scholar] [CrossRef]
- Liu, H.; Wang, G.; Liu, J.; Qiao, S.; Ahn, H. Highly ordered mesoporous NiO anode material for lithium ion batteries with an excellent electrochemical performance. J. Mater. Chem. 2011, 21, 3046–3052. [Google Scholar] [CrossRef]
- Ji, P.; Zhang, J.; Chen, F.; Anpo, M. Ordered Mesoporous CeO2 Synthesized by Nanocasting from Cubic Ia3d Mesoporous MCM-48 Silica: Formation, Characterization and Photocatalytic Activity. Mater. Sci. Semicon Proc. 2014, 15, 270–276. [Google Scholar] [CrossRef]
- Villa, K.; Murcia-López, S.; Andreu, T.; Morante, J.R. Mesoporous WO3 photocatalyst for the partial oxidation of methane to methanol using electron scavengers. Appl. Catal. B Environ. 2015, 163, 150–155. [Google Scholar] [CrossRef]
- Su, B.P.; Li, Z.; Park, G.O.; Ji, M.K. Effective Photocatalytic Performance of Ordered Mesoporous Fe2O3-TiO2 Under Visible Light. Top. Catal. 2017, 60, 789–795. [Google Scholar] [CrossRef]
- Zhu, S.; Dong, B.; Yu, Y.; Bu, L.; Deng, J.; Zhou, S. Heterogeneous catalysis of ozone using ordered mesoporous Fe3O4 for degradation of atrazine. Chem. Eng. J. 2017, 328, 527–535. [Google Scholar] [CrossRef]
- Zhi, B.; Ding, H.; Wang, D.; Cao, Y.; Zhang, Y.; Wang, X.; Liu, Y.; Huo, Q. Ordered mesoporous MnO2 as a synergetic adsorbent for effective arsenic(III) removal. J. Mater. Chem. A 2014, 2, 2374–2382. [Google Scholar] [CrossRef]
- Bai, B.; Qiao, Q.; Li, J.; Hao, J. Synthesis of three-dimensional ordered mesoporous MnO2 and its catalytic performance in formaldehyde oxidation. Chin. J. Catal. 2016, 37, 27–31. [Google Scholar] [CrossRef]
- Lin, S.; Liu, R.; Li, N.; Guo, P.; Shi, L. A controlled alkaline treatment of Al-SBA-15: A facile route to adjust the chemical composition and synthesize an ordered mesoporous carbon material, CMK-3, possessing strong pressure resistant capability. New J. Chem. 2017, 41, 7634–7642. [Google Scholar] [CrossRef]
- Laha, S.C.; Ryoo, R. Synthesis of thermally stable mesoporous cerium oxide with nanocrystalline frameworks using mesoporous silica templates. Chem. Commun. 2003, 9, 2138–2139. [Google Scholar] [CrossRef]
- Shang, T.; Lu, Q.S.; Chao, L.M.; Qin, Y.L.; Yun, Y.H.; Yun, G.H. Effects of ordered mesoporous structure and La-doping on the microwave absorbing properties of CoFe2O4. Appl. Surf. Sci. 2018, 434, 234–242. [Google Scholar] [CrossRef]
- Tian, B.; Luan, Z.; Li, M. Low-temperature synthesis of allyl dimethylamine by selective heating under microwave irradiation used for water treatment. Radiat. Phys. Chem. 2005, 73, 328–333. [Google Scholar] [CrossRef]
- Park, Y.K.; Lee, S.; Yoon, S.; Lee, J.W. Scalable Mesoporous Silicon-Carbon Composite Prepared by Stöber Method and Magnesiothermic Reduction for High Power Anode of Lithium-Ion Batteries. J. Nanosci. Nanotechnol. 2017, 17, 8468–8474. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, Y.; He, M.; Wu, P. Synthesis of zeolitic mesoporous titanosilicate using mesoporous carbon as a hard template. Stud. Surf. Sci. Catal. 2007, 165, 523–526. [Google Scholar] [CrossRef]
- Jiao, F.; Shaju, K.M.; Bruce, P.G. Synthesis of nanowire and mesoporous low-temperature LiCoO2 by a post-templating reaction. Cheminform 2010, 36, 6708–6711. [Google Scholar]
- Jiao, F.; Harrison, A.; Jumas, J.C.; Chadwick, A.V.; Kockelmann, W.; Bruce, P.G. Ordered mesoporous Fe2O3 with crystalline walls. J. Am. Chem. Soc. 2006, 128, 5468–5474. [Google Scholar] [CrossRef]
- Polarz, S.; Orlov, A.V.; Schüth, F.; Lu, A.H. Preparation of high-surface-area zinc oxide with ordered porosity, different pore sizes, and nanocrystalline walls. Chem. Eur. J. 2010, 13, 592–597. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Liu, X.; Sun, B.; Zhou, H.; Fu, A.; Wang, Y.; Guo, Y.G.; Guo, P.; Li, H. Carbon materials with hierarchical porosity: Effect of template removal strategy and study on their electrochemical properties. Carbon 2018, 130, 690–691. [Google Scholar] [CrossRef]
- Shi, Y.; Wan, Y.; Zhai, Y.; Liu, R.; Meng, Y.; Tu, B.; Zhao, D. Ordered Mesoporous SiOC and SiCN Ceramics from Atmosphere-Assisted in Situ Transformation. Chem. Mater. 2007, 19, 1761–1771. [Google Scholar] [CrossRef]
- He, Z.; Zhang, G.; Chen, Y.; Xie, Y.; Zhu, T.; Guo, H.; Chen, Y. The effect of activation methods on the electrochemical performance of ordered mesoporous carbon for supercapacitor applications. J. Mater. Sci. 2017, 52, 2422–2434. [Google Scholar] [CrossRef]
- Enterría, M.; Suárez-García, F.; Martínez-Alonso, A.; Tascón, J. Synthesis of ordered micro–mesoporous carbons by activation of SBA-15 carbon replicas. Microporous Mesoporous Mater. 2012, 151, 390–396. [Google Scholar] [CrossRef]
- Suk, B.Y.; Jeong, Y.K.; Jong-Sung, Y.; Kamil, P.G.; Mietek, J. Fabrication and Characterization of Mesostructured Silica, HUM-1, and Its Ordered Mesoporous Carbon Replica. Ind. Eng. Chem. Res. 2005, 44, 4316–4322. [Google Scholar] [CrossRef]
- Centi, G.; Quadrelli, E.A.; Perathoner, S. Catalysis for CO2 conversion: A key technology for rapid introduction of renewable energy in the value chain of chemical industries. Energy Environ. Sci. 2013, 6, 1711–1731. [Google Scholar] [CrossRef]
- Catizzone, E.; Bonura, G.; Migliori, M.; Frusteri, F.; Giordano, G. CO2 Recycling to Dimethyl Ether: State-of-the-Art and Perspectives. Molecules 2017, 23, 31. [Google Scholar] [CrossRef]
- Sankaranarayanan, S.; Srinivasan, K. Carbon dioxide-A potential raw material for the production of fuel, fuel additives and bio-derived chemicals. J. Org. Chem. 2012, 51, 1252–1262. [Google Scholar] [CrossRef]
- Olah, G.A.; Goeppert, A.; Czaun, M. Single Step Bi-reforming and Oxidative Bi-reforming of Methane (Natural Gas) with Steam and Carbon Dioxide to Metgas (CO-2H2) for Methanol Synthesis: Self-Sufficient Effective and Exclusive Oxygenation of Methane to Methanol with Oxygen. J. Am. Chem. Soc. 2015, 137, 8720–8729. [Google Scholar] [CrossRef]
- Wambach, J.; Baiker, A.; Wokaun, A. CO2 hydrogenation over metal/zirconia catalysts. Phys. Chem. Chem. Phys. 1999, 1, 5071–5080. [Google Scholar] [CrossRef]
- Dagle, R.A.; Platon, A.; Palo, D.R.; Datye, A.K.; Vohs, J.M.; Wang, Y. PdZnAl catalysts for the reactions of water-gas-shift, methanol steam reforming, and reverse-water-gas-shift. Appl. Catal. A Gen. 2008, 342, 63–68. [Google Scholar] [CrossRef]
- Gondal, M.A.; Dastageer, M.A.; Rashid, S.G.; Zubair, S.M.; Ali, M.A.; Anjum, D.H.; Lienhard, J.H.; Mckinley, G.H.; Varanasi, K. Plasmon resonance enhanced photocatalysis under visible light with Au/Cu-TiO2 nanoparticles: Removal Cr (VI) from water as a case of study. Sci. Adv. Mater. 2013, 5, 2007–2014. [Google Scholar] [CrossRef]
- Richardson, P.L.; Perdigoto, M.L.N.; Wang, W.; Lopes, R.J.G. Manganese- and copper-doped titania nanocomposites for the photocatalytic reduction of carbon dioxide into methanol. Appl. Catal. B Environ. 2012, 126, 200–207. [Google Scholar] [CrossRef]
- Bulushev, D.A.; Ross, J.R.H. Catalysis for conversion of biomass to fuels via pyrolysis and gasification: A review. Catal. Today 2011, 171, 1–13. [Google Scholar] [CrossRef]
- Teramura, K.; Tanaka, T.; Ishikawa, H.; Kohno, Y.; Funabiki, T. Photocatalytic Reduction of CO2 to CO in the Presence of H2 or CH4 as a Reductant over MgO. J. Phys. Chem. B 2004, 108, 346–354. [Google Scholar] [CrossRef]
- Xu, L.; Wang, F.; Chen, M.; Zhang, J.; Yuan, K.; Wang, L.; Wu, K.; Xu, G.; Chen, W. Carbon Dioxide Reforming of Methane over Cobalt-Nickel Bimetal-Doped Ordered Mesoporous Alumina Catalysts with Advanced Catalytic Performances. Chemcatchem 2016, 8, 2536–2548. [Google Scholar] [CrossRef]
- Zhang, Z.; Hu, X.; Li, J.; Gao, G.; Dong, D.; Westerhof, R.; Hu, S.; Xiang, Y.; Wang, Y. Steam reforming of acetic acid over Ni/Al2O3 catalysts: Correlation of nickel loading with properties and catalytic behaviors of the catalysts. Fuel 2018, 217, 389–403. [Google Scholar] [CrossRef]
- Xu, L.; Wang, F.; Chen, M.; Fan, X.; Yang, H.; Nie, D.; Qi, L. Alkaline-promoted Co-Ni bimetal ordered mesoporous catalysts with enhanced coke-resistant performance toward CO2 reforming of CH4. J. CO2 Util. 2017, 18, 1–14. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, X.; Chen, M.; Qi, L.; Nie, D.; Ma, Y. Facilely fabricating Mg, Ca modified Co based ordered mesoporous catalysts for CO2 reforming of CH4: The effects of basic modifiers. Int. J. Hydrogen Energy 2016, 41, 17348–17360. [Google Scholar] [CrossRef]
- Xu, L.; Chou, H.S. Mesoporous nanocrystalline ceriaezirconia solid solutions supported nickel based catalysts for CO2 reforming of CH4. Int. J. Hydrogen Energy 2012, 37, 18001–18020. [Google Scholar] [CrossRef]
- Xu, L.; Miao, Z.; Song, H.; Chen, W.; Chou, L. Significant roles of mesostructure and basic modifier for ordered mesoporous Ni/CaO–Al2O3 catalyst towards CO2 reforming of CH4. Catal. Sci. Technol. 2014, 4, 1759–1770. [Google Scholar] [CrossRef]
- Xu, L.; Song, H.; Chou, L. One-Pot Synthesis of Ordered Mesoporous NiO–CaO–Al2O3 Composite Oxides for Catalyzing CO2 Reforming of CH4. ACS Catal. 2012, 2, 1331–1342. [Google Scholar] [CrossRef]
- Kim, H.-J.; Yang, E.-H.; Noh, Y.S.; Hong, G.H.; Park, J.I.; Shin, S.A.; Lee, K.-Y.; Moon, D.J. Studies on the steam CO2 reforming of methane over ordered mesoporous nickel-magnesium-alumina catalysts. Res. Chem. Intermed. 2018, 44, 1131–1148. [Google Scholar] [CrossRef]
- Zhang, C.; Hu, X.; Zhang, Z.; Hu, X.; Zhang, Z.; Zhang, L.J.; Dong, D.H.; Gao, G.G.; Westerhof, R.; Shatir, S. Steam reforming of acetic acid over Ni/Al2O3 catalyst: Correlation of calcination temperature with the interaction of nickel and alumina. Fuel 2018, 227, 307–324. [Google Scholar] [CrossRef]
- Inui, T.; Takeguchi, T. Effective conversion of carbon dioxide and hydrogen to hydrocarbons. Catal. Today 1991, 10, 95–106. [Google Scholar] [CrossRef]
- Xu, L.; Wang, F.; Chen, M.; Zhang, J.; Yuan, K.; Wang, L.; Wu, K.; Xu, G.; Chen, W. CO2 methanation over a Ni based ordered mesoporous catalyst for the production of synthetic natural gas. RSC Adv. 2016, 6, 28489–28499. [Google Scholar] [CrossRef]
- Perkas, N.; Amirian, G.; Zhong, Z.; Teo, J.; Gofer, Y.; Gedanken, A. Methanation of Carbon Dioxide on Ni Catalysts on Mesoporous ZrO2 Doped with Rare Earth Oxides. Catal. Lett. 2009, 130, 455–462. [Google Scholar] [CrossRef]
- Liu, Q.; Zhong, Z.; Gu, F. CO methanation on ordered mesoporous Ni-Cr-Al catalysts: Effects of the catalyst structure and Cr promoter on the catalytic. J. Catal. 2016, 337, 221–232. [Google Scholar] [CrossRef]
- Wang, N.; Shen, K.; Huang, L. Facile Route for Synthesizing Ordered Mesoporous Ni-Ce-Al Oxide Materials and Their Catalytic Performance for Methane Dry Reforming to Hydrogen and Syngas. ACS Catal. 2013, 3, 1638–1651. [Google Scholar] [CrossRef]
- Shin, H.H.; Lu, L.; Yang, Z. Cobalt Catalysts Decorated with Platinum Atoms Supported on Barium Zirconate Provide Enhanced Activity and Selectivity for CO2 Methanation. ACS Catal. 2016, 6, 2811–2818. [Google Scholar] [CrossRef]
- Xu, J.; Su, X.; Duan, H.; Hou, B.; Lin, Q.; Liu, X.; Pan, X.; Pei, G.; Geng, H.; Huang, Y. Influence of pretreatment temperature on catalytic performance of rutile TiO2-supported ruthenium catalyst in CO2 methanation. J. Catal. 2016, 333, 227–237. [Google Scholar] [CrossRef]
- Frontera, P.; Macario, A.; Ferraro, M.; Antonucci, P. Supported Catalysts for CO2 Methanation: A Review. Catalysts 2017, 7, 59. [Google Scholar] [CrossRef]
- Xu, L.; Lian, X.; Chen, M.; Cui, Y.; Wang, F.; Li, W.; Huang, B. CO2 methanation over Co-Ni bimetal-doped ordered mesoporous Al2O3 catalysts with enhanced low-temperature activities. Int. J. Hydrogen Energy 2018, 43, 17172–17184. [Google Scholar] [CrossRef]
- Xu, L.; Yang, H.; Chen, M.; Wang, F.; Nie, D.; Qi, L.; Lian, X.; Chen, H.; Wu, M. CO2 methanation over Ca doped ordered mesoporous Ni-Al composite oxide catalysts: The promoting effect of basic modifier. J. CO2 Util. 2017, 21, 200–210. [Google Scholar] [CrossRef]
- Xu, L.; Wang, F.; Chen, M.; Yang, H.; Nie, D.; Qi, L.; Lian, X. Alkaline-promoted Ni based ordered mesoporous catalysts with enhanced low-temperature catalytic activity toward CO2 methanation. RSC Adv. 2017, 7, 18199–18210. [Google Scholar] [CrossRef]
- Xu, L.; Wang, F.; Chen, M.; Nie, D.; Lian, X.; Lu, Z.; Chen, H.; Zhang, K.; Ge, P. CO2 methanation over rare earth doped Ni based mesoporous catalysts with intensified low-temperature activity. Int. J. Hydrogen Energy 2017, 42, 15523–15539. [Google Scholar] [CrossRef]
- Liu, Q.; Bian, B.; Fan, J.; Yang, J. Cobalt doped Ni based ordered mesoporous catalysts for CO2 methanation with enhanced catalytic performance. Int. J. Hydrogen Energy 2018, 43, 4893–4901. [Google Scholar] [CrossRef]
- Qiao, J.; Liu, Y.; Hong, F.; Zhang, J. ChemInform Abstract: A Review of Catalysts for the Electroreduction of Carbon Dioxide to Produce Low-Carbon Fuels. Chem. Soc. Rev. 2013, 43, 631–675. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Xu, C.H.; Zhao, H.Y.; Wang, Y.X.; Liu, J.; Liu, J.Y. Methanol synthesis from CO2 hydrogenation over Cu/γ-Al2O3 catalysts modified by ZnO, ZrO2 and MgO. J. Ind. Eng. Chem. 2015, 28, 261–267. [Google Scholar] [CrossRef]
- Wang, S.; Mao, D.; Guo, X.; Wu, G.; Lu, G. Dimethyl ether synthesis via CO2 hydrogenation over CuO-TiO2-ZrO2/HZSM-5 bifunctional catalysts. Catal. Commun. 2009, 10, 1367–1370. [Google Scholar] [CrossRef]
- Vesborg, P.C.K.; Chorkendorff, I.; Knudsen, I.; Balmes, O.; Nerlov, J.; Molenbroek, A.M.; Clausen, B.S.; Helveg, S. Transient behavior of Cu/ZnO-based methanol synthesis catalysts. J. Catal. 2009, 262, 65–72. [Google Scholar] [CrossRef]
- Akarmazyan, S.S.; Panagiotopoulou, P.; Kambolis, A.; Papadopoulou, C.; Kondarides, D.I. Methanol dehydration to dimethylether over Al2O3 catalysts. Appl. Catal. B Environ. 2014, 145, 136–148. [Google Scholar] [CrossRef]
- Li, Z.; Li, J.; Dai, M.; Liu, Y.; Han, D.; Wu, J. The effect of preparation method of the Cu–La2O3-ZrO2 /γ-Al2O3 hybrid catalysts on one-step synthesis of dimethyl ether from syngas. Fuel 2014, 121, 173–177. [Google Scholar] [CrossRef]
- Ham, H.; Kim, J.; Cho, S.J.; Choi, J.H.; Dong, J.M.; Bae, J.W. Enhanced Stability of Spatially Confined Copper Nanoparticles in an Ordered Mesoporous Alumina for Dimethyl Ether Synthesis from Syngas. ACS Catal. 2016, 6, 5629–5640. [Google Scholar] [CrossRef]
- Luan, C.; Zhang, A.; Huang, W.; Yin, L. Catalytic Dehydration of Methanol to Dimethyl Ether Over Mesoporous gamma-Al2O3 Energy Sources Part A-Recovery. Util. Environ. Eff. 2015, 37, 2285–2292. [Google Scholar] [CrossRef]
- Daza, Y.A.; Kuhn, J.N. CO2 conversion by reverse water gas shift catalysis: Comparison of catalysts, mechanisms and their consequences for CO2 conversion to liquid fuels. RSC Adv. 2016, 6, 49675–49691. [Google Scholar] [CrossRef]
- Zhang, X.; Zhu, X.; Lin, L.; Yao, S.; Zhang, M.; Liu, X.; Wang, X.; Li, Y.; Shi, C.; Ma, D. Highly dispersed Copper over β-Mo2C as Efficient and Stable Catalysts for RWGS Reaction. ACS Catal. 2016, 7, 912–918. [Google Scholar] [CrossRef]
- Gonçalves, R.V.; Vono, L.L.R.; Wojcieszak, R.; Dias, C.S.B.; Wender, H.; Teixeira-Neto, E.; Rossi, L.M. Selective hydrogenation of CO2 into CO on a highly dispersed nickel catalyst obtained by magnetron sputtering deposition: A step towards liquid fuels. Appl. Catal. B Environ. 2017, 209, 240–246. [Google Scholar] [CrossRef]
- Yang, X.; Su, X.; Chen, X.; Duan, H.; Liang, B.; Liu, Q.; Liu, X.; Ren, Y.; Huang, Y.; Zhang, T. Promotion effects of potassium on the activity and selectivity of Pt/zeolite catalysts for reverse water gas shift reaction. Appl. Catal. B Environ. 2017, 216, 95–105. [Google Scholar] [CrossRef]
- Liang, B.; Duan, H.; Su, X.; Chen, X.; Huang, Y.; Chen, X.; Jose Delgado, J.; Zhang, T. Promoting role of potassium in the reverse water gas shift reaction on Pt/mullite catalyst. Catal. Today 2017, 281, 319–326. [Google Scholar] [CrossRef]
- Wu, H.C.; Chang, Y.C.; Wu, J.H.; Lin, J.H.; Lin, I.K.; Chen, C.S. Methanation of CO2 and reverse water gas shift reactions on Ni/SiO2 catalysts: The influence of particle size on selectivity and reaction pathway. Catal. Sci. Technol. 2015, 5, 4154–4163. [Google Scholar] [CrossRef]
- Wang, L.C.; Khazaneh, M.T.; Widmann, D.; Behm, R.J. TAP reactor studies of the oxidizing capability of CO2 on a Au/CeO2 catalyst-A first step toward identifying a redox mechanism in the Reverse Water-Gas Shift reaction. J. Catal. 2013, 302, 20–30. [Google Scholar] [CrossRef]
- Dai, B.; Cao, S.; Xie, H.; Zhou, G.; Chen, S. Reduction of CO2 to CO via reverse water-gas shift reaction over CeO2 catalyst. Korean J. Chem. Eng. 2018, 35, 421–427. [Google Scholar] [CrossRef]
- Zhang, T.; Qu, R.Y.; Su, W.K.; Li, J.H. A novel Ce-Ta mixed oxide catalyst for the selective catalytic reduction of NOx with NH3. Appl. Catal. B Environ. 2015, 176, 338–346. [Google Scholar] [CrossRef]
- Zhou, G.L.; Lan, H.; Yang, X.Q.; Du, Q.X.; Xie, H.M.; Fu, M. Effects of the structure of Ce-Cu catalysts on the catalytic combustion of toluene in air. Ceram. Int. 2013, 39, 3677–3683. [Google Scholar] [CrossRef]
- Wang, L.H.; Liu, H. Mesoporous Co-CeO2 catalyst prepared by colloidal solution combustion method for reverse water-gas shift reaction. Catal. Today 2018, 316, 155–161. [Google Scholar] [CrossRef]
- Dai, B.; Zhou, G.; Ge, S.; Xie, H.; Jiao, Z.; Zhang, G.; Xiong, K. CO2 Reverse Water-Gas Shift Reaction on Mesoporous M-CeO2 Catalysts. Can. J. Chem. Eng. 2017, 95, 634–642. [Google Scholar] [CrossRef]
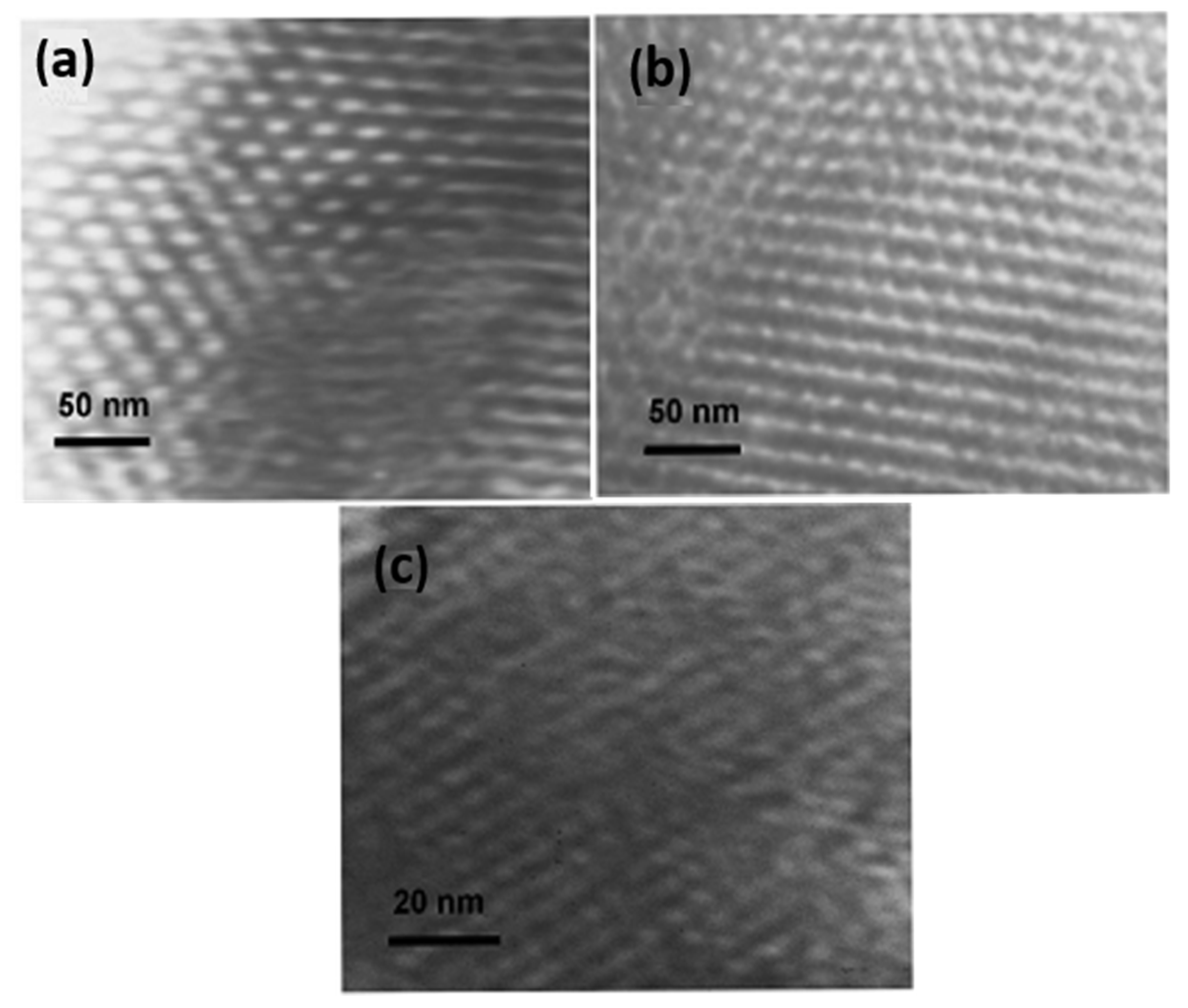
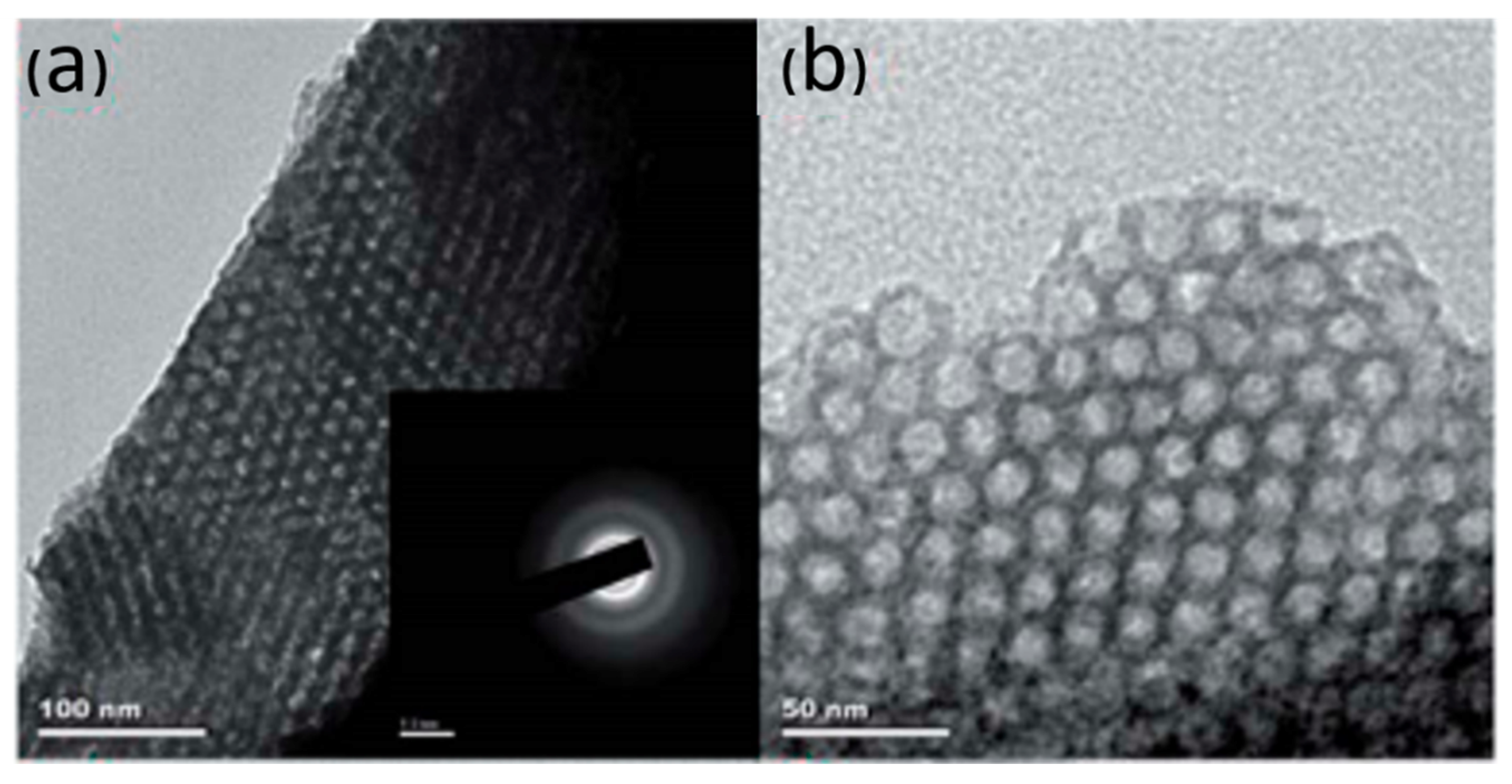
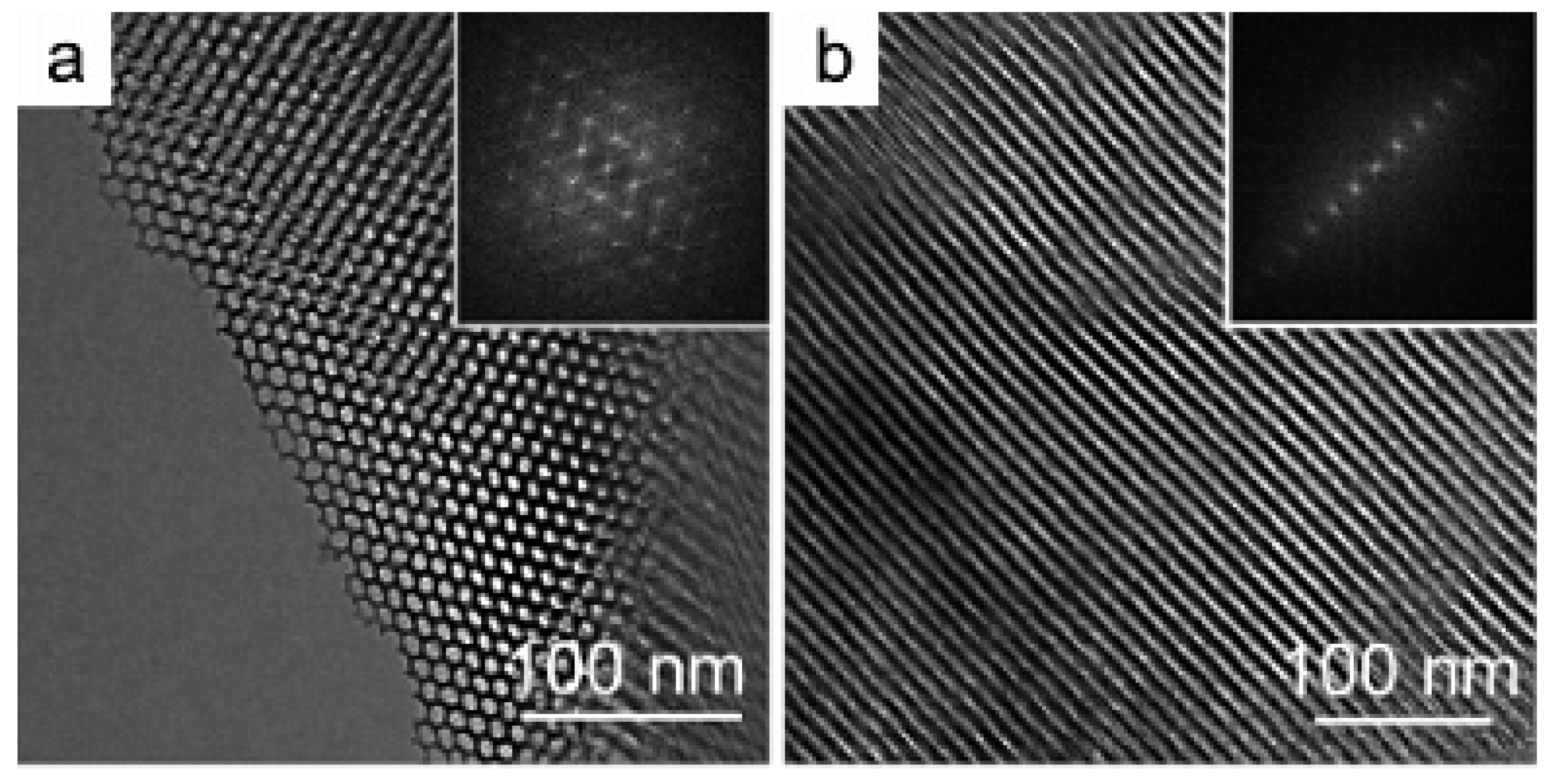

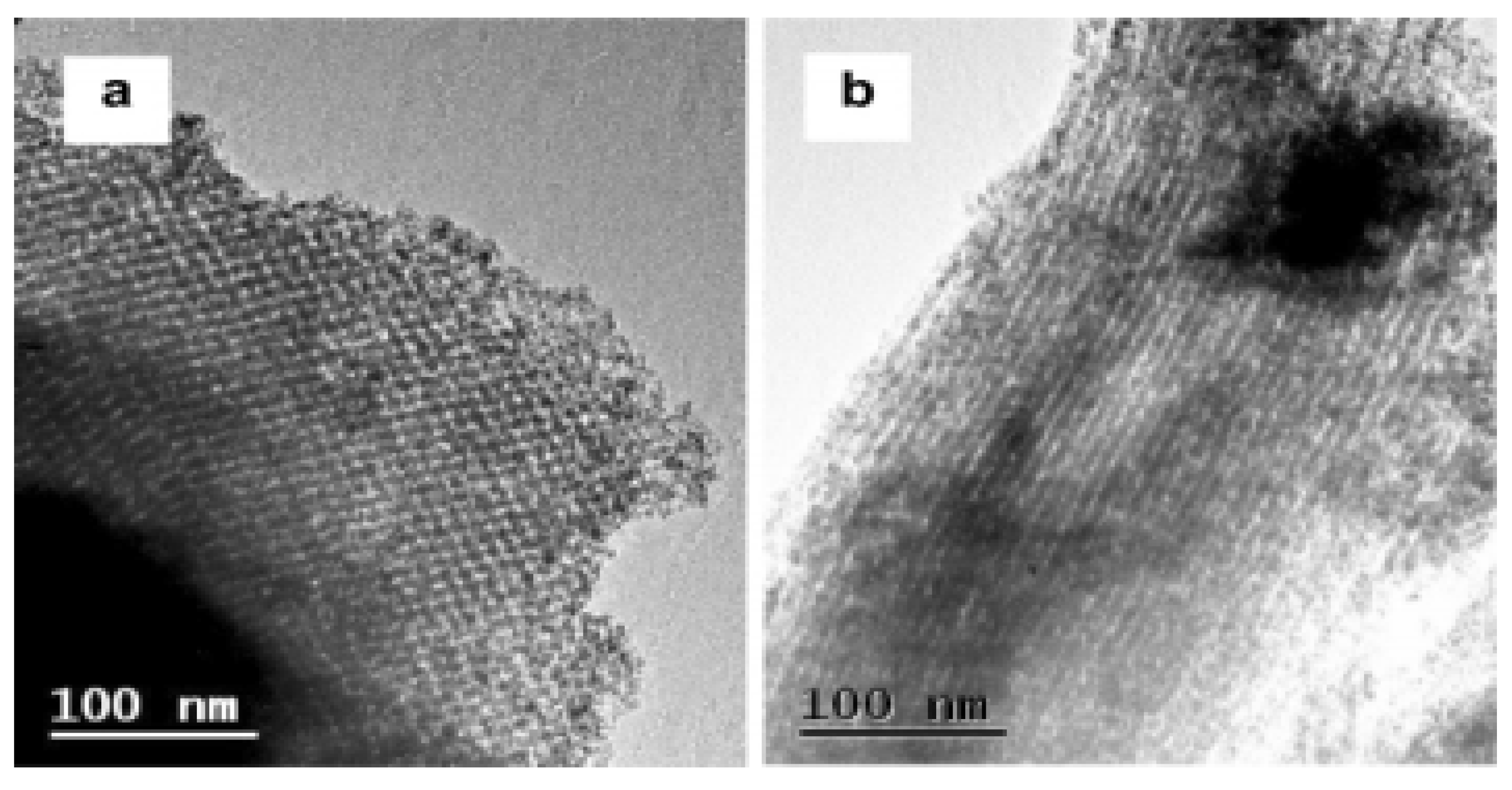

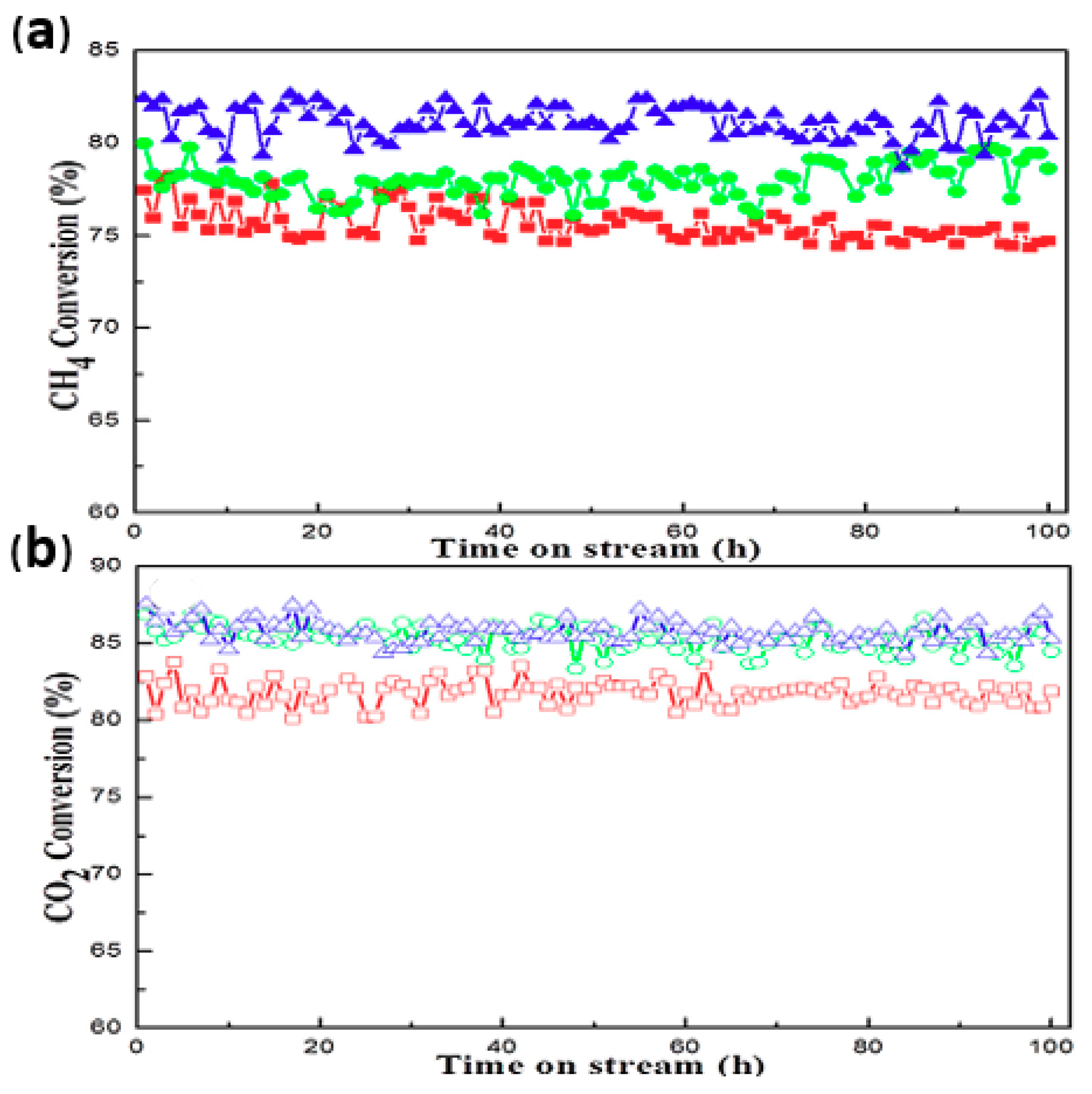
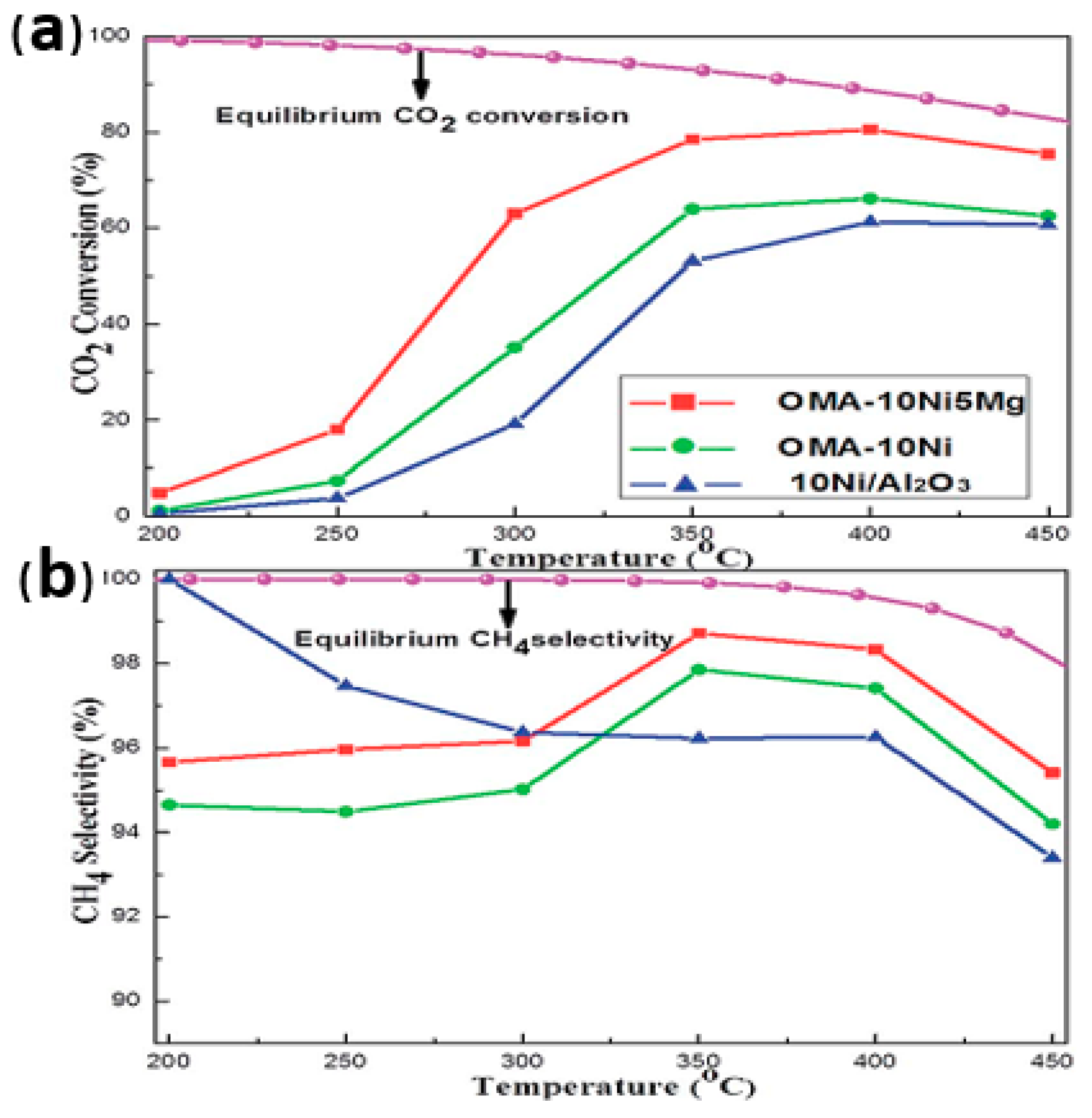
| Template | Surfactant Formula | Pore Size/(nm) | Surface Area/(m2·g−1) |
|---|---|---|---|
| Tergitol 15-S-9 | C11-15[PEO]9 | 3.3 | 490 |
| Tergitol 15-S-12 | C11-15[PEO]12 | 3.5 | 425 |
| Triton X-114 | C8Ph[PEO]8 | 3.6 | 445 |
| Pluronic 64L | [PEO]13[PPO]30[PEO]13 | 2.4 | 430 |
| Pluronic P123 | [PEO]20[PPO]69[PEO]20 | 10.3 | 487 |
| Caproic | C5COOH | 2.1 | 530 |
| Lauric acid | C11COOH | 1.9 | 710 |
| Stearic acid | C17COOH | 2.1 | 700 |
| CTMABr | C16N(CH3)4Br | 10 | 407 |
| CTMAB+palmitic acid | C16N(CH3)4Br +C15COOH | 2.7 | 810 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, Y.; Lian, X.; Xu, L.; Chen, M.; Yang, B.; Wu, C.-e.; Li, W.; Huang, B.; Hu, X. Designing and Fabricating Ordered Mesoporous Metal Oxides for CO2 Catalytic Conversion: A Review and Prospect. Materials 2019, 12, 276. https://doi.org/10.3390/ma12020276
Cui Y, Lian X, Xu L, Chen M, Yang B, Wu C-e, Li W, Huang B, Hu X. Designing and Fabricating Ordered Mesoporous Metal Oxides for CO2 Catalytic Conversion: A Review and Prospect. Materials. 2019; 12(2):276. https://doi.org/10.3390/ma12020276
Chicago/Turabian StyleCui, Yan, Xinbo Lian, Leilei Xu, Mindong Chen, Bo Yang, Cai-e Wu, Wenjing Li, Bingbo Huang, and Xun Hu. 2019. "Designing and Fabricating Ordered Mesoporous Metal Oxides for CO2 Catalytic Conversion: A Review and Prospect" Materials 12, no. 2: 276. https://doi.org/10.3390/ma12020276
APA StyleCui, Y., Lian, X., Xu, L., Chen, M., Yang, B., Wu, C.-e., Li, W., Huang, B., & Hu, X. (2019). Designing and Fabricating Ordered Mesoporous Metal Oxides for CO2 Catalytic Conversion: A Review and Prospect. Materials, 12(2), 276. https://doi.org/10.3390/ma12020276






