The Effects of Silica on the Properties of Vitreous Enamels
Abstract
:1. Introduction
2. Experimental
2.1. Sample Preparation
2.2. XRD, Density and Dilatometric Analysis
2.3. Procedures for the Impact Resistance and Corrosion Test
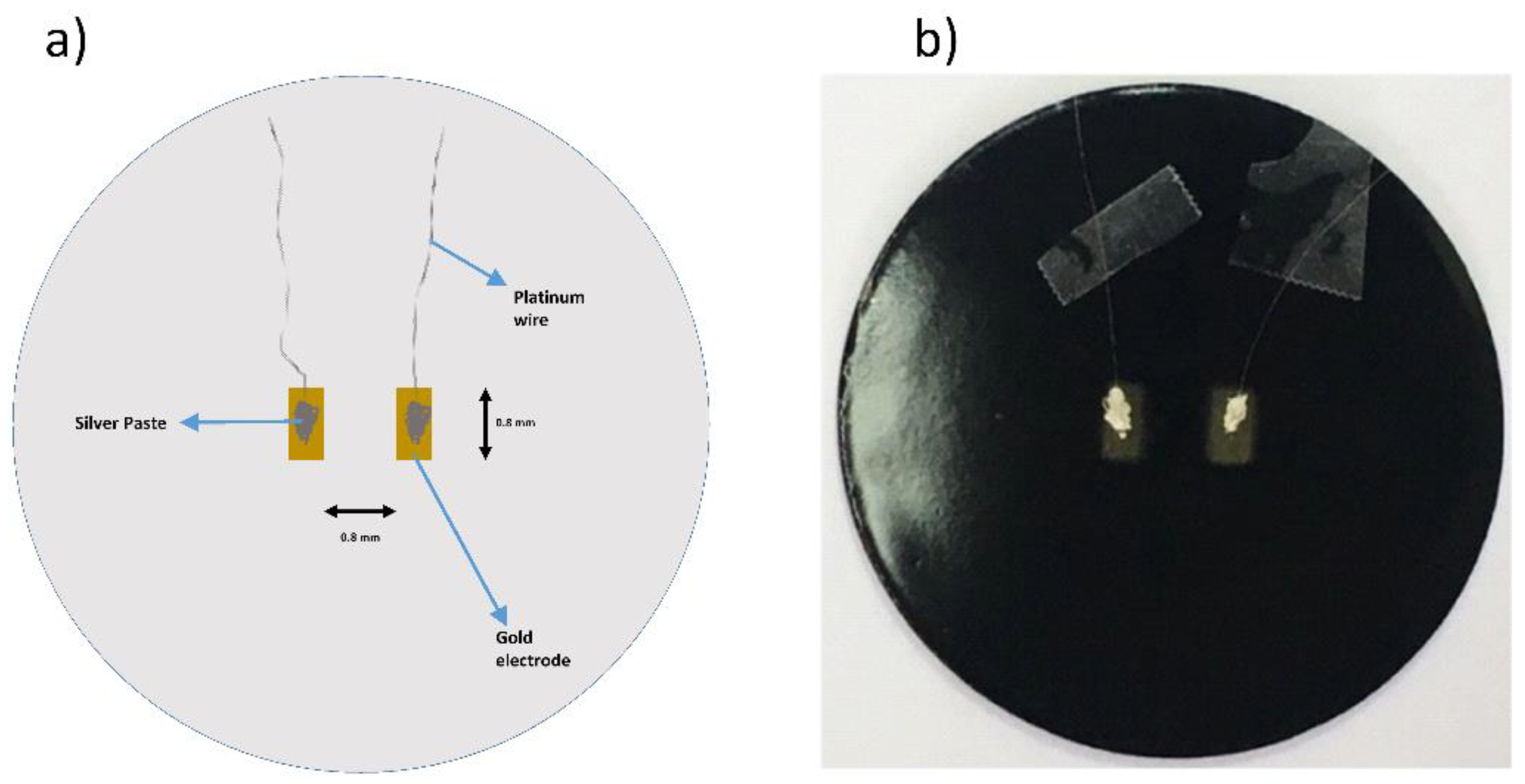
2.4. Electrical Measurements
3. Results
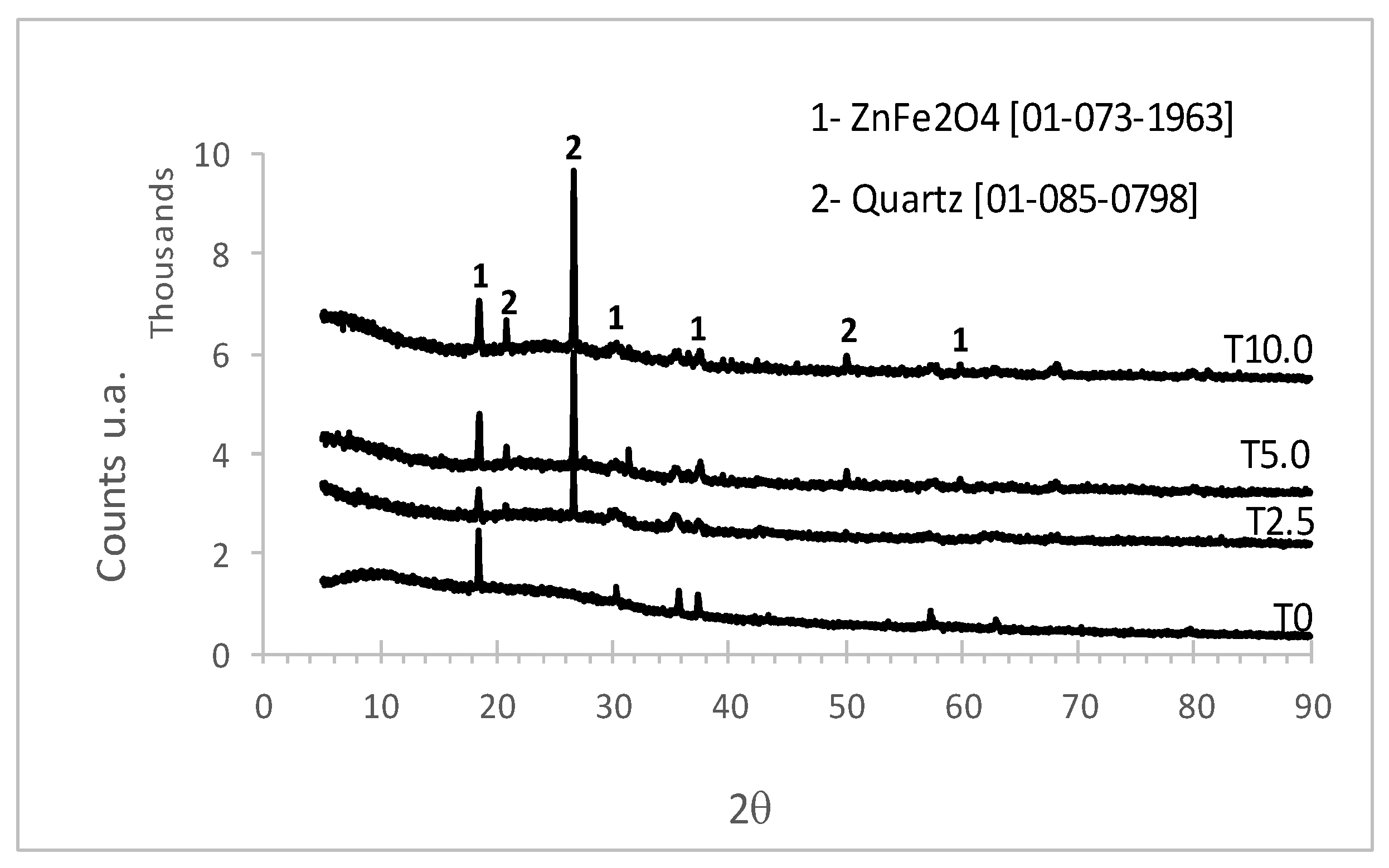
3.1. XRD
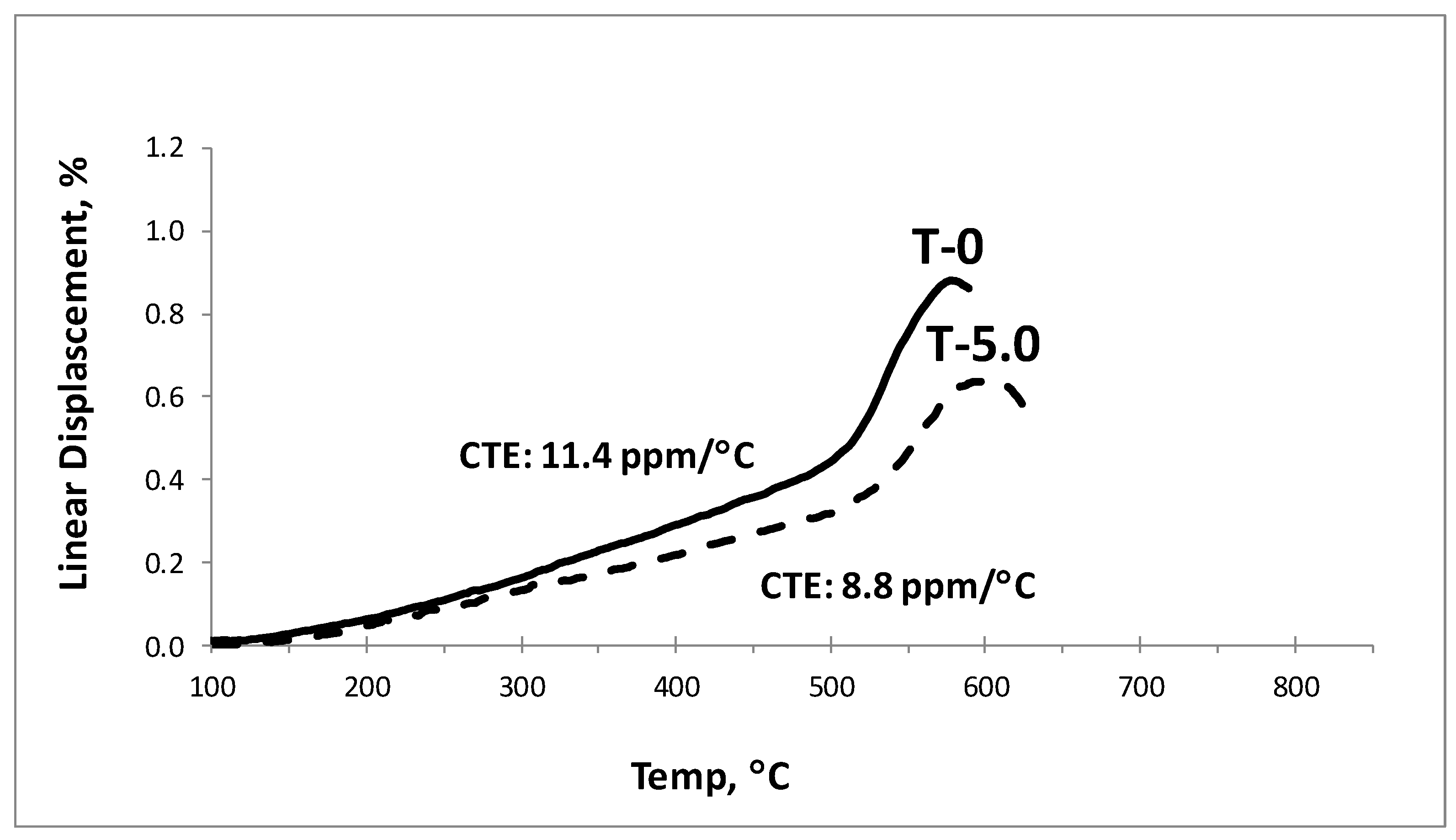
3.2. Density and Dilatometric Analysis
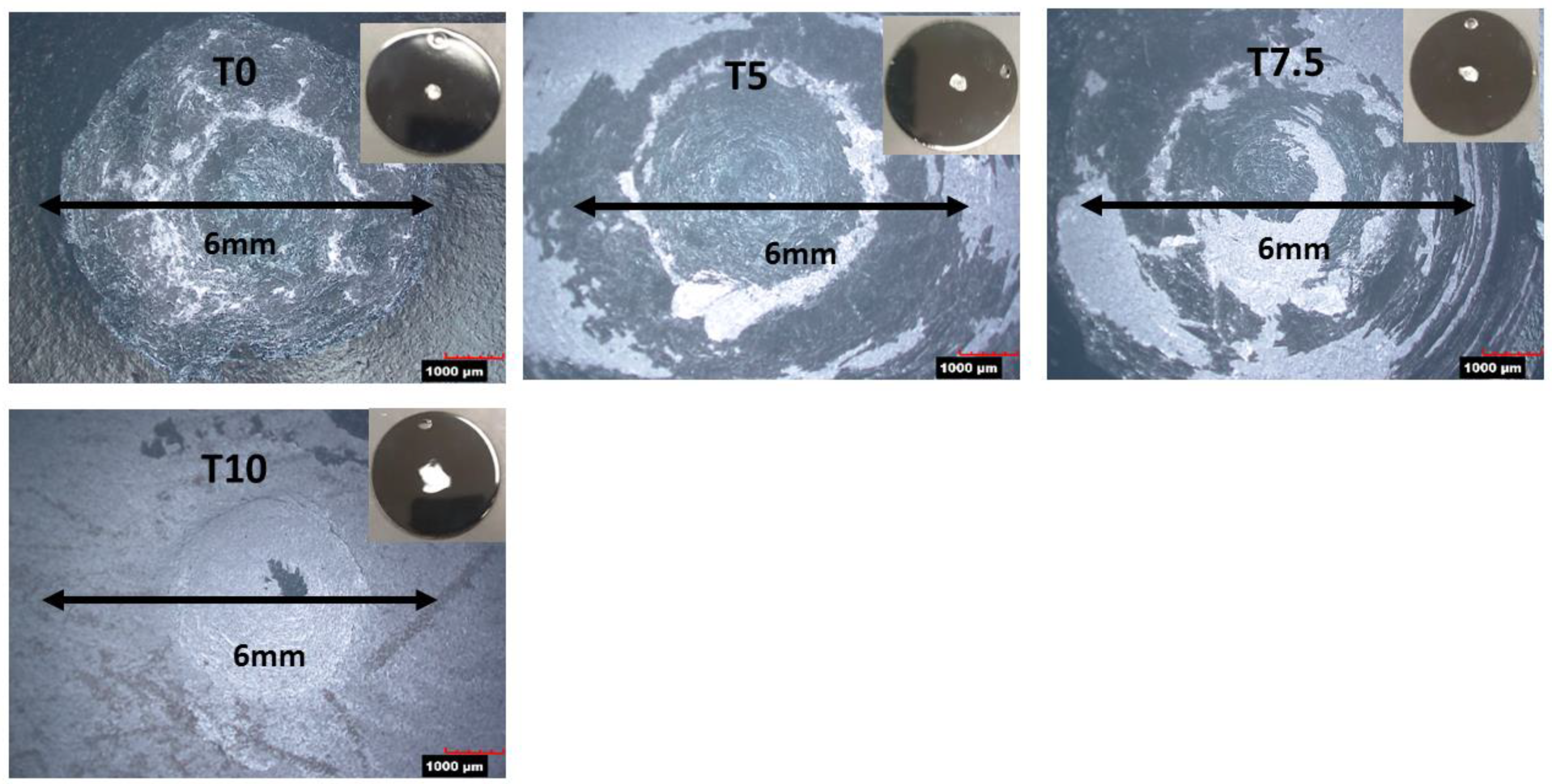
3.3. Effect of Silica on the Impact Resistance
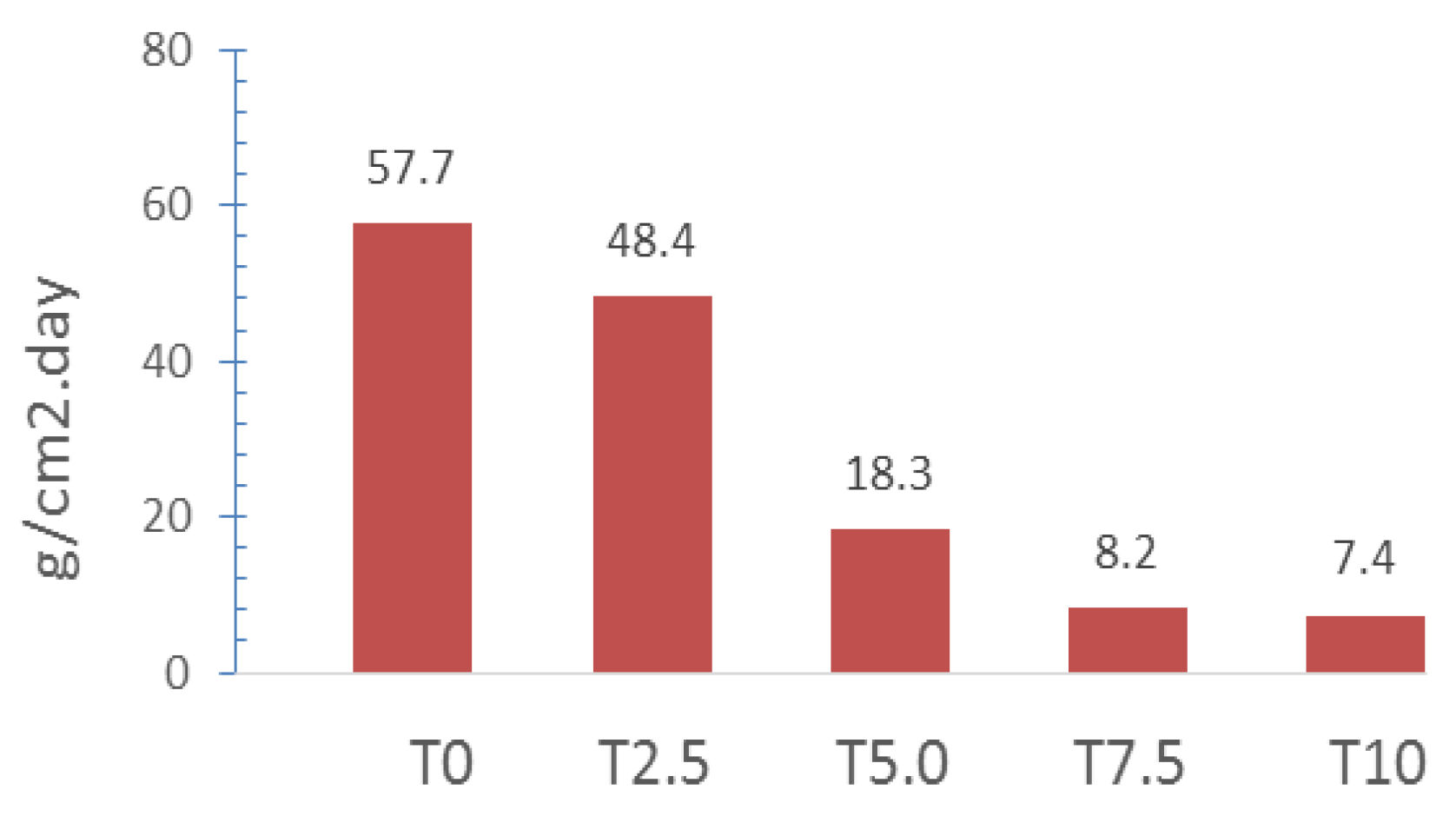
3.4. Effect of Silica on the Corrosion Resistance
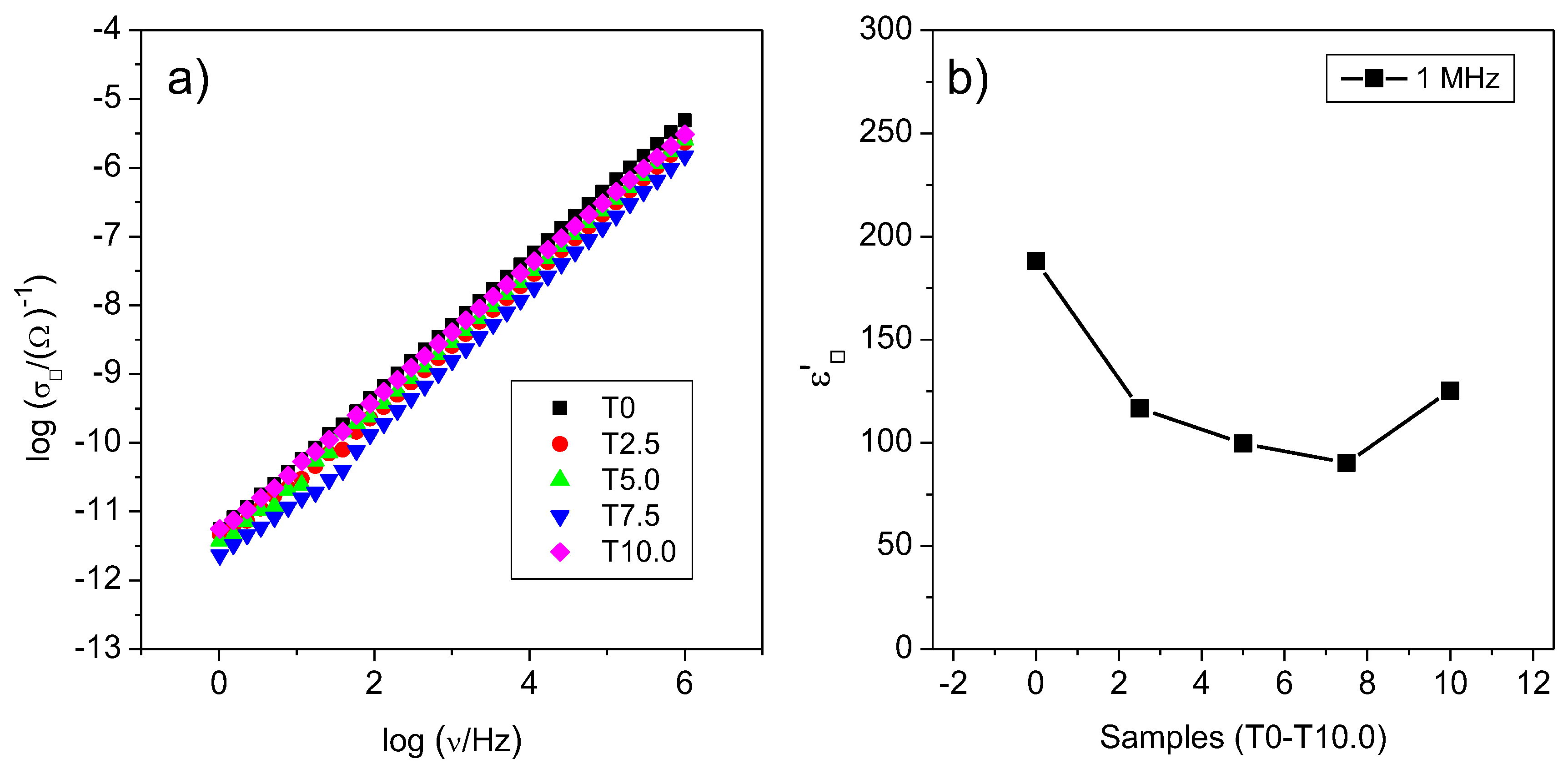
3.5. Effect of Silica on the Electrical Properties
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jones, T.J.; Berard, M.F. Ceramics, Industrial Processing and Testing, 2nd ed.; Iowa University Press: Iowa City, IA, USA, 1985; Volume 9. [Google Scholar]
- Min’ko, N.I.; Matveeva, T.A. Glass enamels for steel and cast—Iron articles. Glass Ceram. 1999, 56, 358–363. [Google Scholar] [CrossRef]
- Conde, A.; Macaya, D.; Damborenea, J. Corrosion of enameled steel by domestic cleaning fluids. Br. Corros. J. 2000, 35, 279. [Google Scholar] [CrossRef]
- Clark, D.E.; Dilmore, M.F.; Ethridge, E.C.; Hench, L.L. A durability evaluation of soda lime silica glasses. J. Am. Ceram. Soc. 1976, 59, 62. [Google Scholar] [CrossRef]
- Goetcheus, D.R. Porcelain enamels as a protective coating for hot water tanks. J. Am. Ceram. Soc. 2006, 25, 164–168. [Google Scholar] [CrossRef]
- Porcelain Enamel Institute. Properties of Porcelain Enamels: Resistance and Corrosion; Data Bulletin PEI 503; PEI: Alpharetta, GA, USA, 2006. [Google Scholar]
- Alikina, I.B.; Sirootinskii, A.A. Corrosion resistance of non-ground enamel coatings. Glass Ceram. 1984, 41, 337–340. [Google Scholar] [CrossRef]
- Kassem, A.S.; Mostafa, M.Z.; Abadir, M.F.; El-Sherbiny, S.A. Hot water acid resistant enamels. Mater. Corros. 2010, 61, 58–63. [Google Scholar] [CrossRef]
- Doremus, R.H. Glass Science; Wiley: New York, NY, USA, 1973; p. 245. [Google Scholar]
- Amin, S.K.; Ahmed, M.M.; El−Sayed, S.K.; Abadir, M.F. Effect of Silica Content on the Acid Resistance of Enamels. Adv. Nat. Appl. Sci. 2013, 7, 300–306. [Google Scholar]
- Yan, D.; Reis, S.T.; Tao, X.; Chen, G.; Brow, R.K.; Koenigstein, M.L. Effect of chemically reactive enamel coating on bonding strength at steel/mortar interface. Construct. Build. Mater. 2012, 28, 512–518. [Google Scholar] [CrossRef]
- Dielectric Analyzers for the Electrical Characterisation of Materials or Components in the Frequency Domain. Available online: http://www.novocontrol.de/html/index_analyzer.htm (accessed on 8 January 2019).
- Mogus-Milankovic, A.; Reis, S.T.; Day, D.E. Electrical properties of phosphate glasses. Mater. Sci. Eng. 2009, 2, 1. [Google Scholar] [CrossRef]
| Elements | C | Mn | P | S | Si | Cu | Ni | Cr | Al | N |
|---|---|---|---|---|---|---|---|---|---|---|
| wt% | 0.04 | 0.18 | 0.01 | 0.005 | 0.013 | 0.015 | 0.01 | 0.03 | 0.04 | 0.04 |
| Oxides | SiO2 | B2O3 | Na2O | Al2O3 | CaO | TiO2 | MnO | Fe2O3 | CoO | NiO | CuO | ZnO | ZrO2 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| wt% | 53.9 | 12.5 | 12.7 | 1.0 | 3.8 | 1.6 | 3.9 | 1.3 | 1.3 | 2.6 | 1.4 | 2.5 | 0.5 |
| Sample ID | Glass Frit (wt%) | Silica Addition (wt%) |
|---|---|---|
| T0 | 100 | 0 |
| T2.5 | 97.5 | 2.5 |
| T5.0 | 95.0 | 5.0 |
| T7.5 | 92.5 | 7.5 |
| T10.0 | 90.0 | 10.0 |
| Sample ID | Density (g/cm3) | Tg (°C) | Ts (°C) | CTE (200 to 400) (ppm/°C) |
|---|---|---|---|---|
| T0 | 2.56 ± 0.02 | 511 | 578 | 11.4 |
| T2.5 | 2.63 ± 0.01 | 514 | 592 | 8.8 |
| T5.0 | 2.64 ± 0.02 | 530 | 603 | 8.3 |
| T7.5 | 2.62 ± 0.02 | 531 | 604 | 7.3 |
| T10.0 | 2.63 ± 0.02 | 535 | 606 | 7.4 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reis, S.T.; Koenigstein, M.; Fan, L.; Chen, G.; Pavić, L.; Moguš-Milanković, A. The Effects of Silica on the Properties of Vitreous Enamels. Materials 2019, 12, 248. https://doi.org/10.3390/ma12020248
Reis ST, Koenigstein M, Fan L, Chen G, Pavić L, Moguš-Milanković A. The Effects of Silica on the Properties of Vitreous Enamels. Materials. 2019; 12(2):248. https://doi.org/10.3390/ma12020248
Chicago/Turabian StyleReis, Signo T., Mike Koenigstein, Liang Fan, Genda Chen, Luka Pavić, and Andrea Moguš-Milanković. 2019. "The Effects of Silica on the Properties of Vitreous Enamels" Materials 12, no. 2: 248. https://doi.org/10.3390/ma12020248
APA StyleReis, S. T., Koenigstein, M., Fan, L., Chen, G., Pavić, L., & Moguš-Milanković, A. (2019). The Effects of Silica on the Properties of Vitreous Enamels. Materials, 12(2), 248. https://doi.org/10.3390/ma12020248








