Interfacial Reactions and Mechanical Properties Studies of C-Coated and C/B4C Duplex-Coated SiC Fiber-Reinforced Ti2AlNb Composites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Characterization
3. Results and Discussion
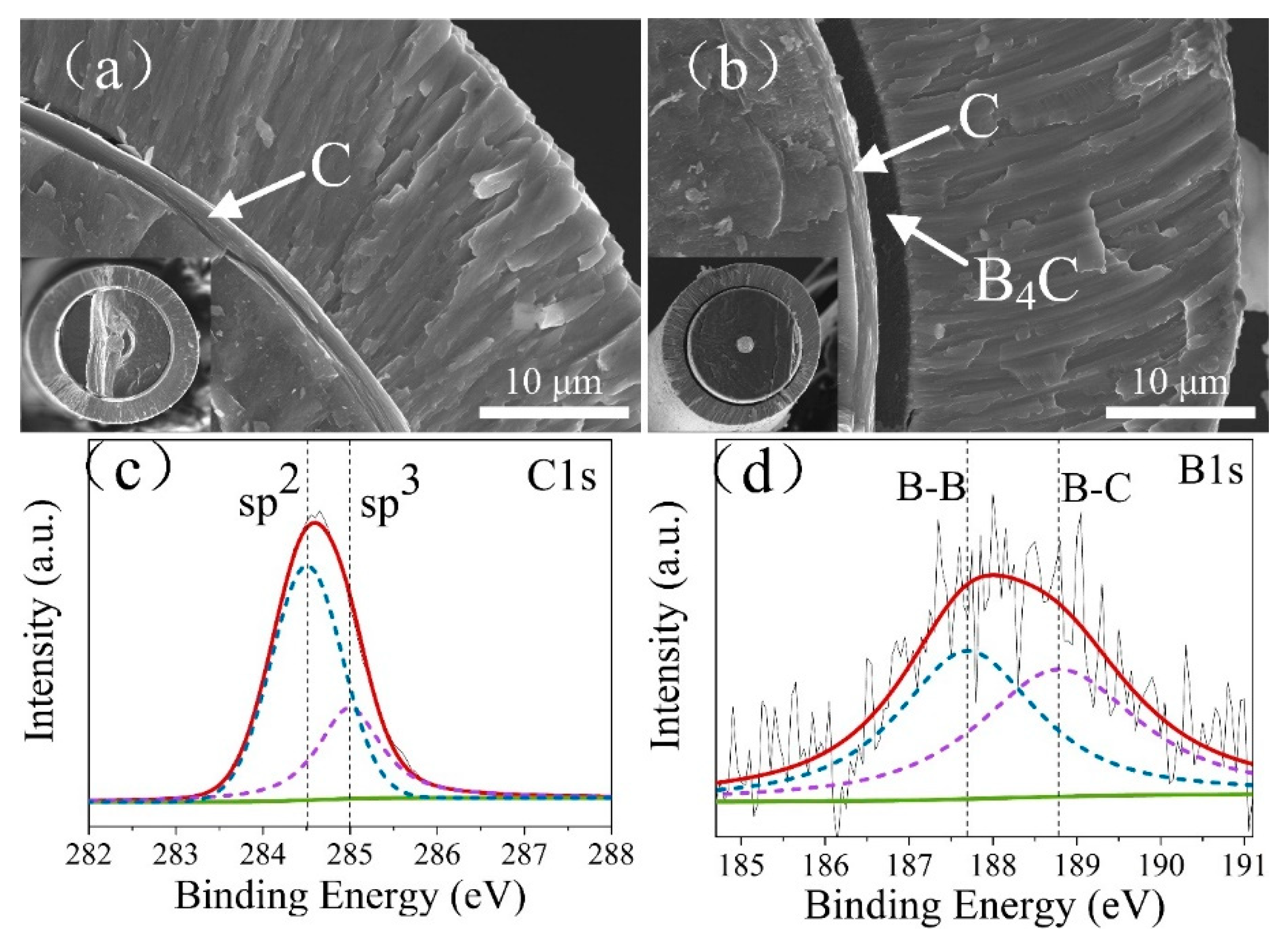
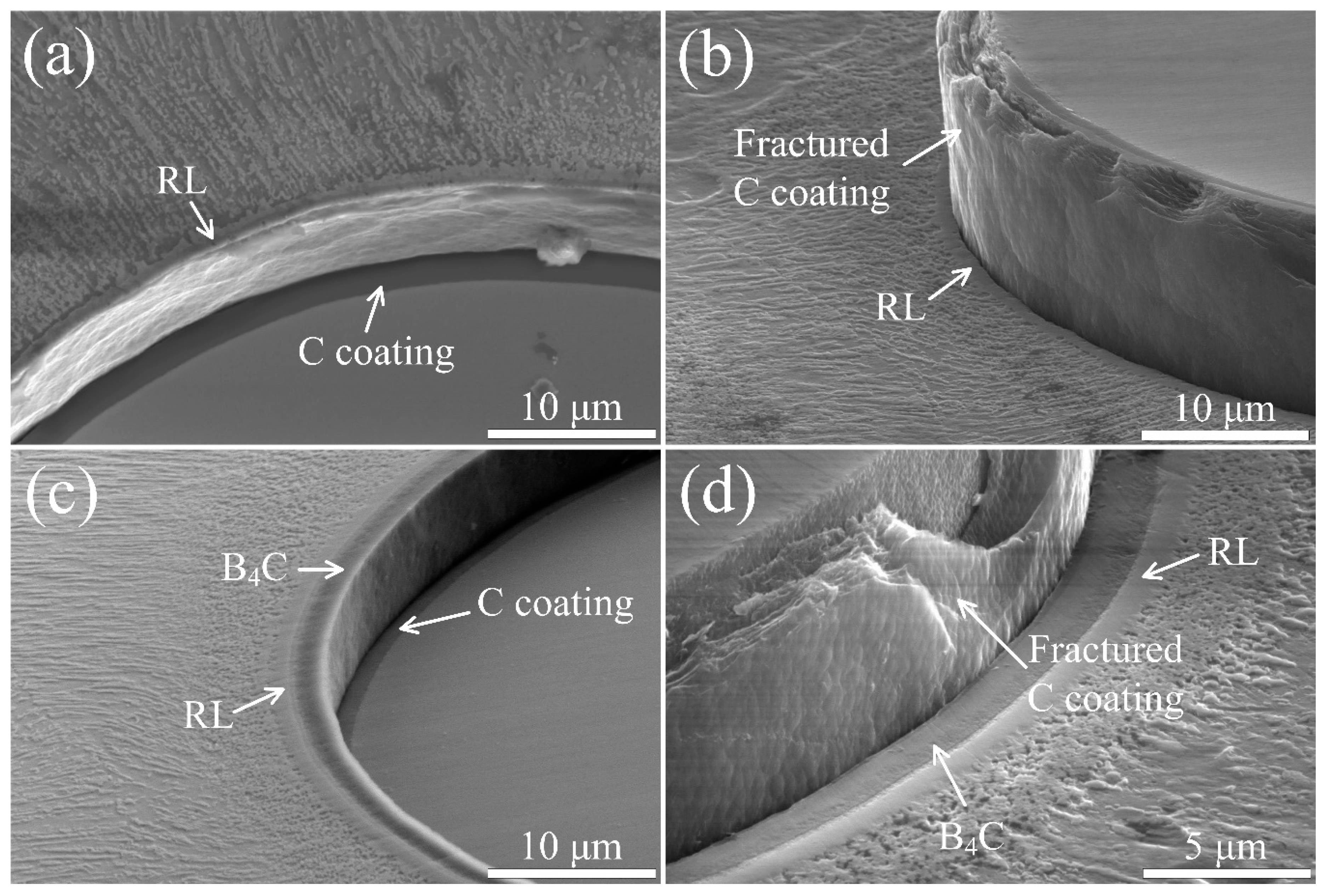
3.1. Microstructure of C and C/B4C Coatings
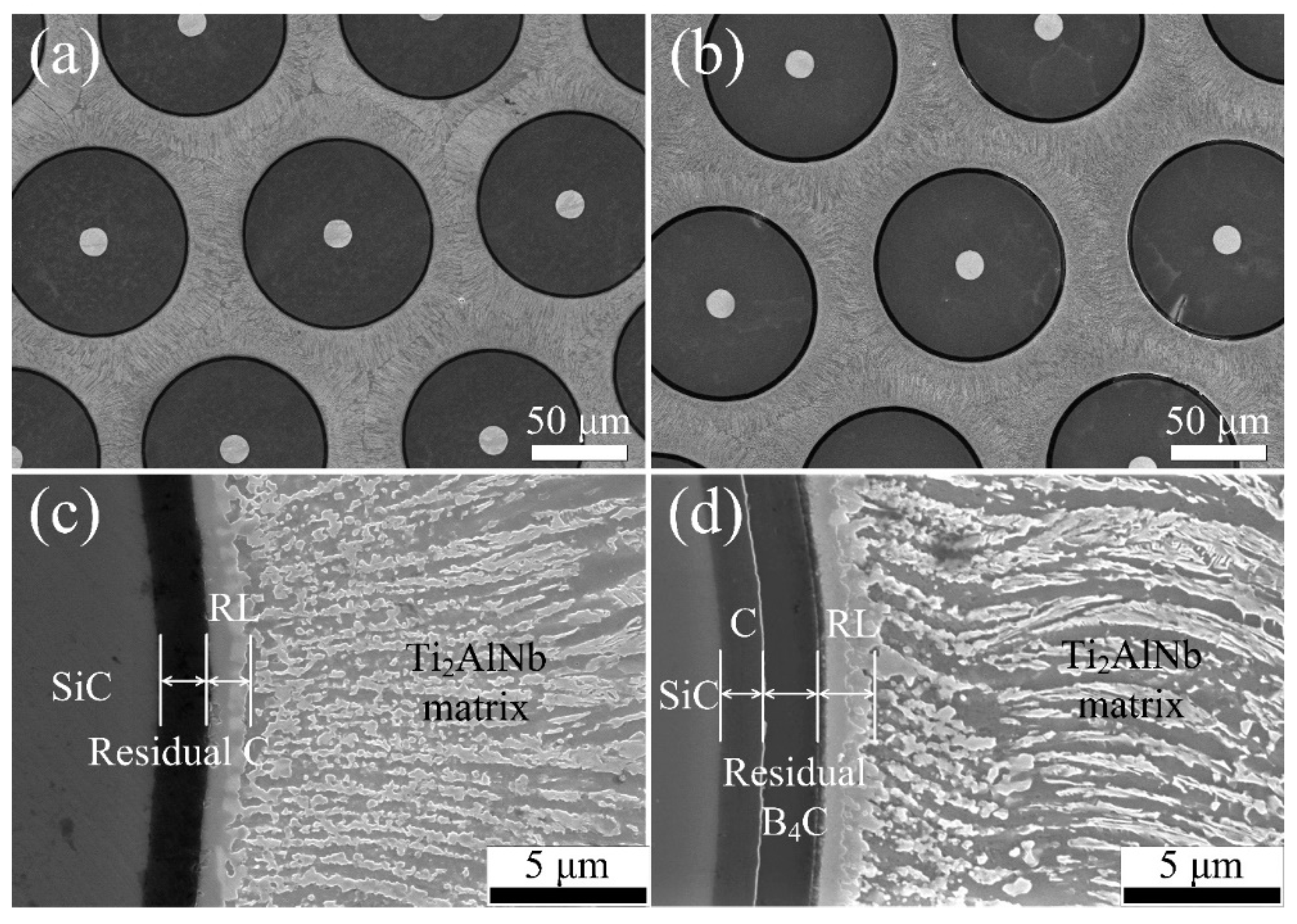
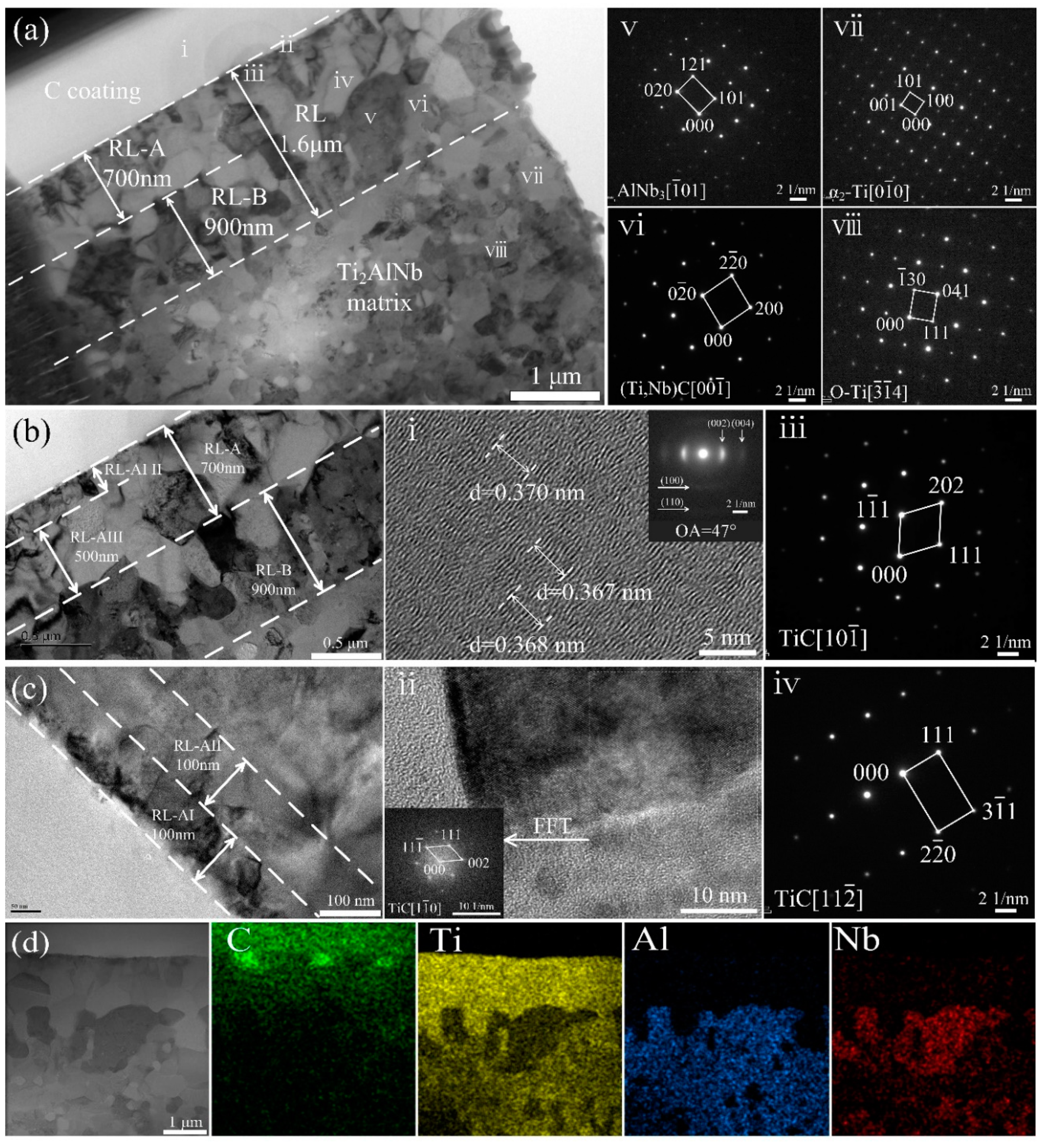
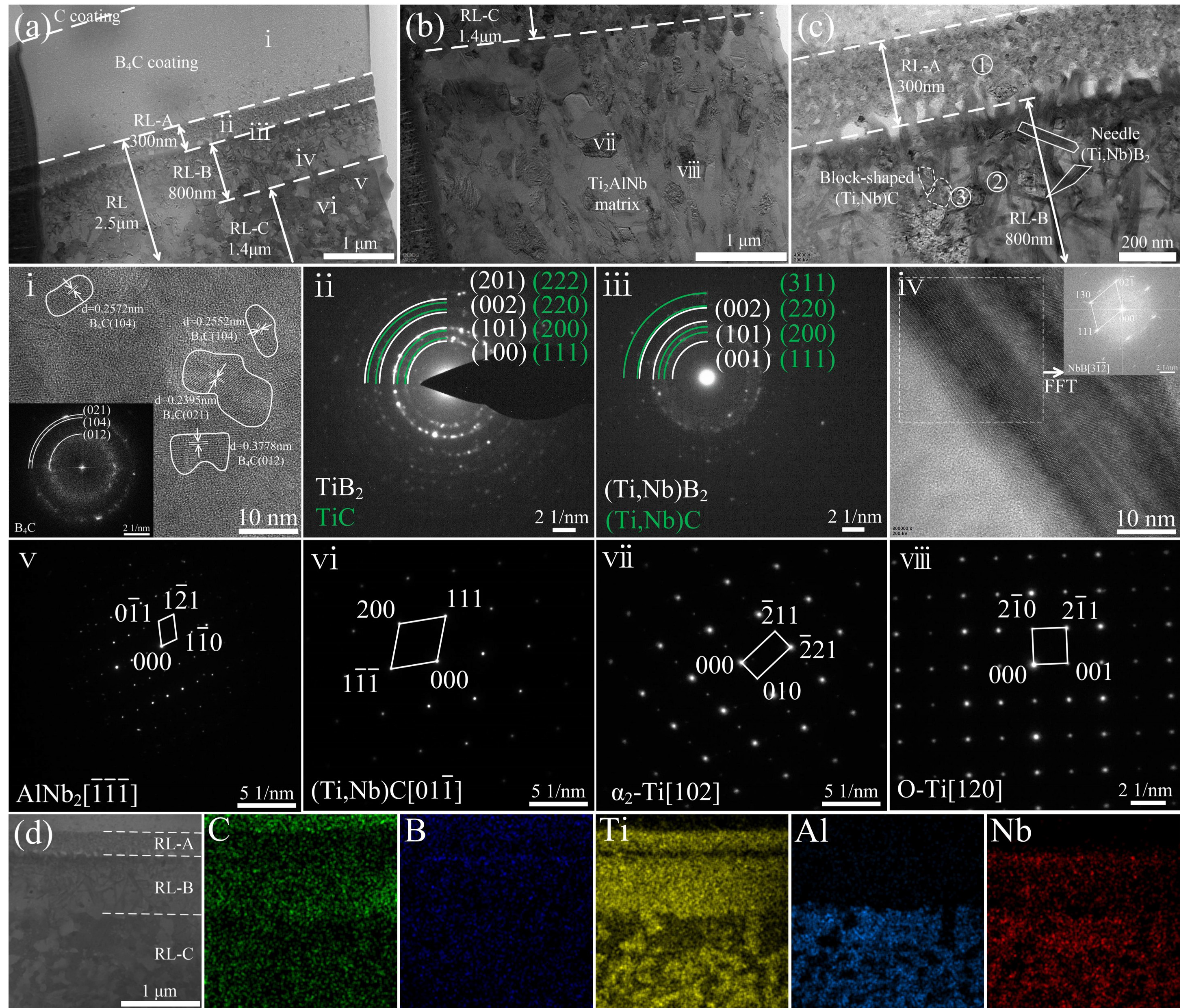
3.2. Interfacial Reactions in the SiCf/C/Ti2AlNb and SiCf/C/B4C/Ti2AlNb
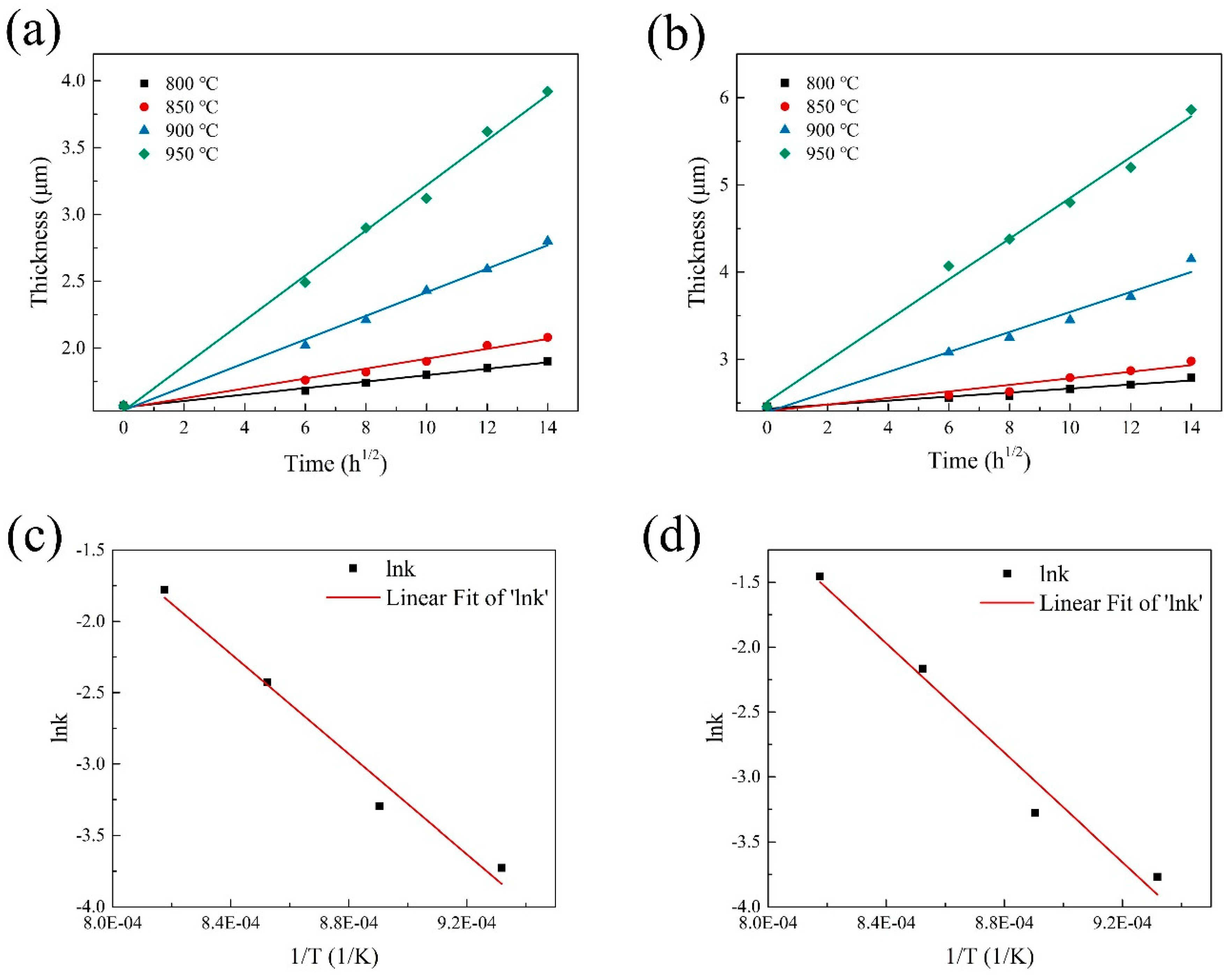
3.3. Reaction Kinetics of SiCf/C/Ti2AlNb and SiCf/C/B4C/Ti2AlNb
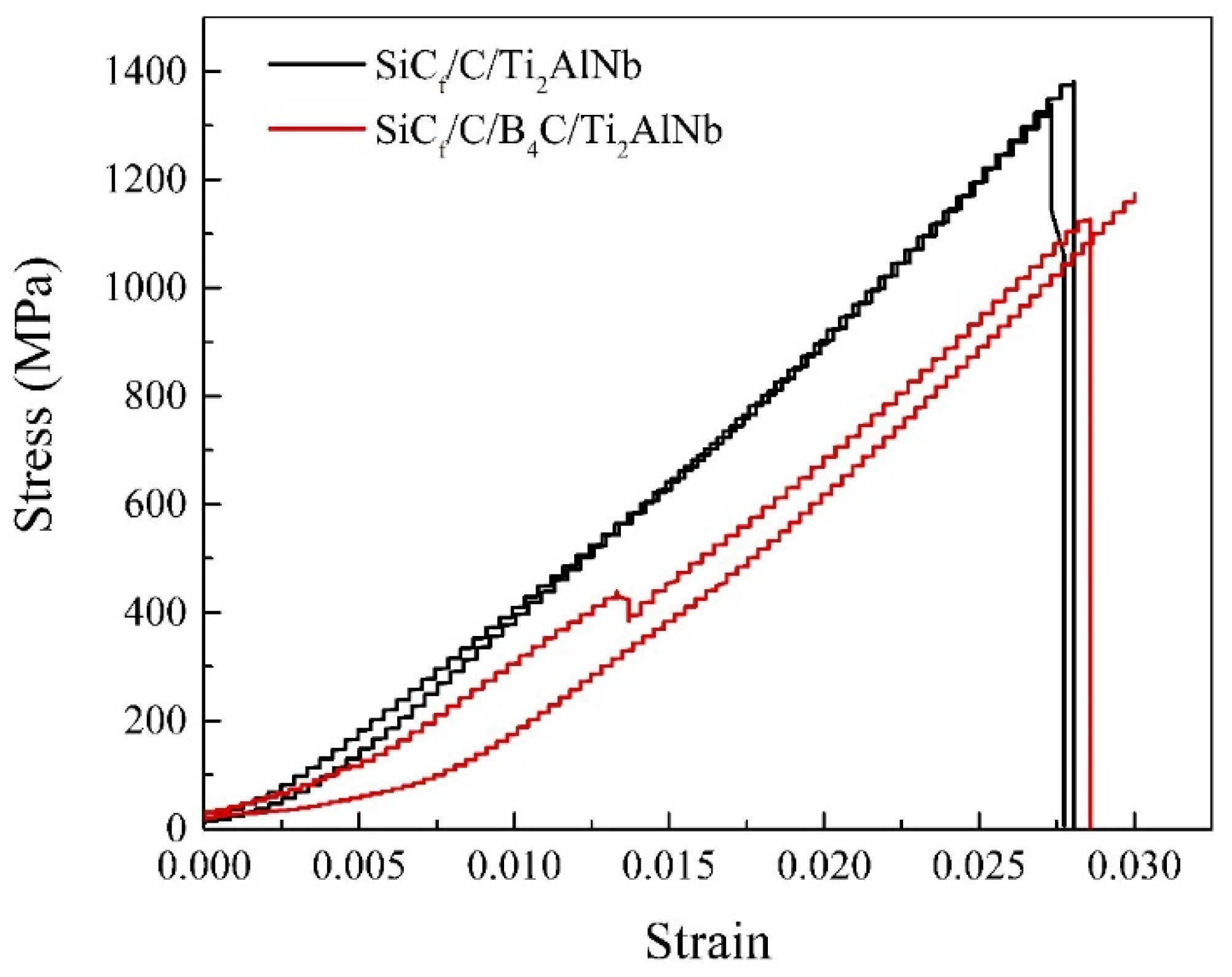
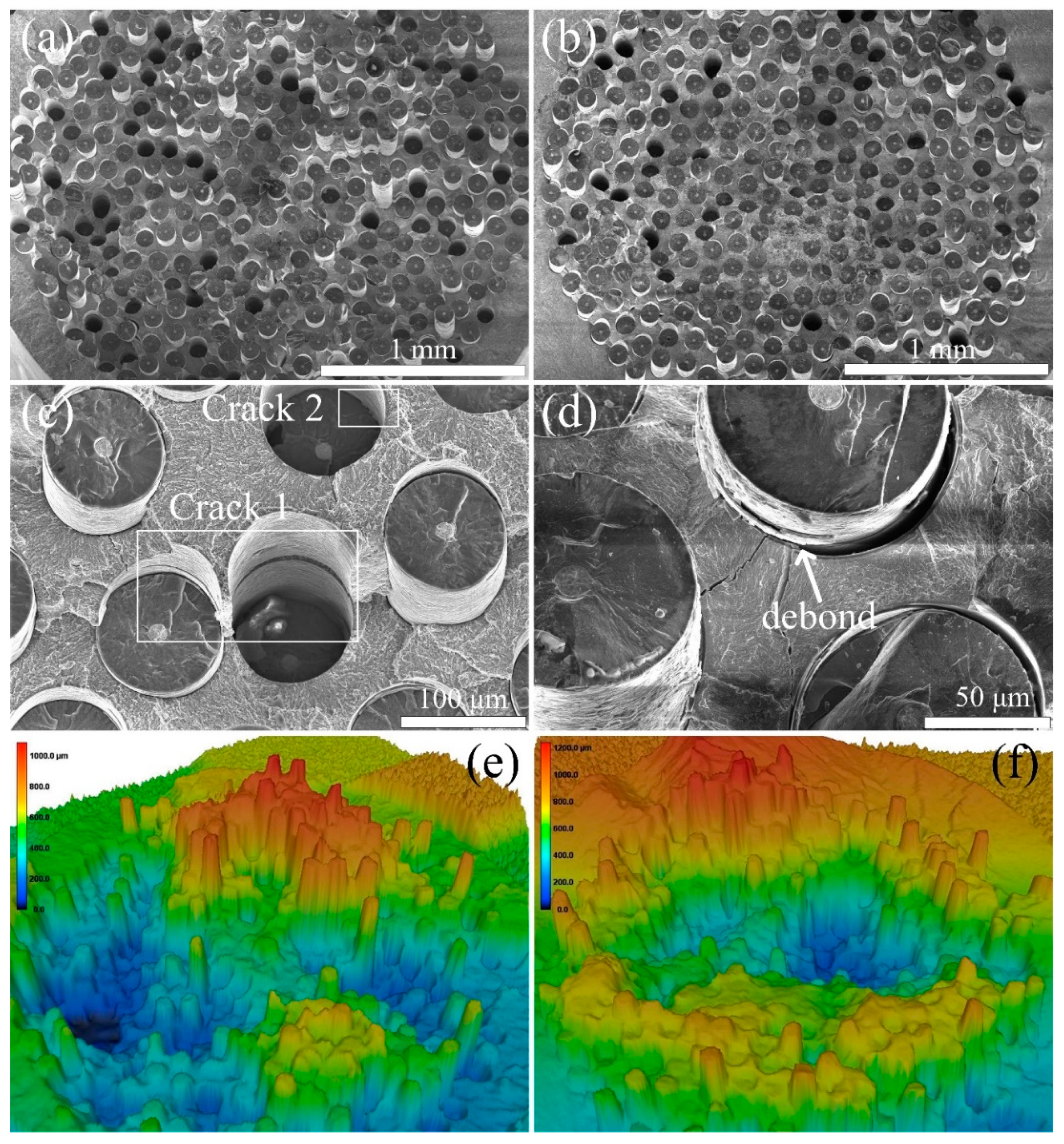
3.4. Interfacial Strength and Tensile Strength
4. Conclusions
- (1)
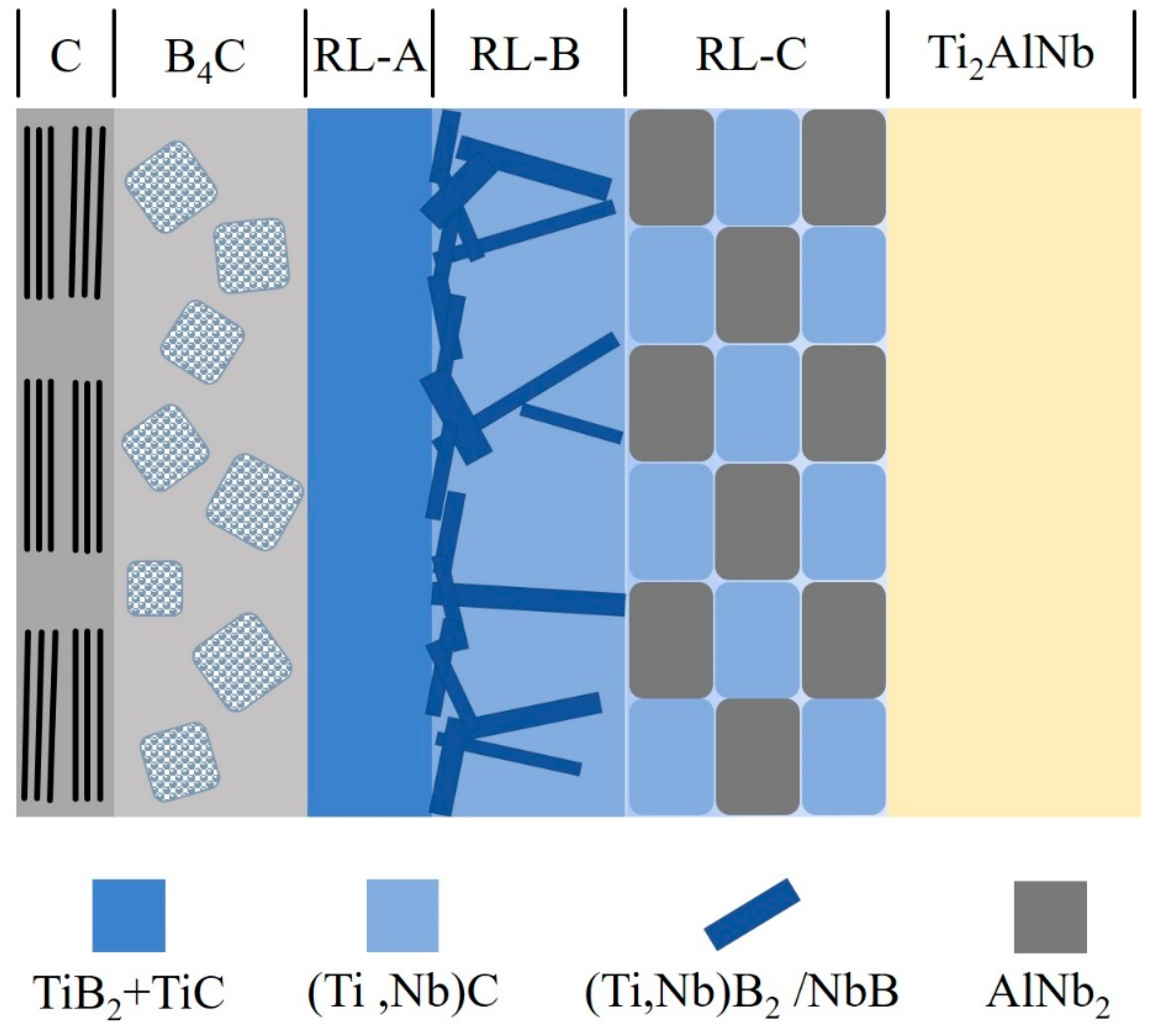
- C-coated and C/B4C duplex-coated SiC fiber-reinforced Ti2AlNb composites were fabricated and the interfacial reaction products of both composites were identified. The reaction products sequence was the different-sized TiC and the coarse-grained (Ti,Nb)C+AlNb3 for the SiCf/C/Ti2AlNb; and the fine-grained TiB2+TiC, needle-shaped (Ti,Nb)B2/NbB +(Ti,Nb)C, and coarse-grained (Ti,Nb)C+AlNb2 for the SiCf/C/B4C/Ti2AlNb. When the interfacial reaction process of two composites was dominated by the diffusion-controlled, the SiCf/C/B4C/Ti2AlNb had a higher diffusion activation energy Q due to the facilitated elemental diffusion by the needle-shaped borides, making the frequency factor k0 much larger than SiCf/C/Ti2AlNb, which resulted in a thicker RL in SiCf/C/B4C/Ti2AlNb under the same consolidating conditions.
- (2)
- The SiCf/C/Ti2AlNb with a thinner RL exhibited a higher interface strength than SiCf/C/B4C/Ti2AlNb when the interfacial debonding occurred between pyrolytic carbon and the amorphous polycrystalline structure of B4C. The SiCf/C/Ti2AlNb with a higher interface strength exhibited a more serious step-like fracture than SiCf/C/B4C/Ti2AlNb and thus contributed to a higher tensile strength of the SiCf/C/Ti2AlNb.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Larsen, J.M.; Russ, S.M.; Jones, J.W. An evaluation of fiber-reinforced titanium matrix composites for advanced high-temperature aerospace applications. Met. Mater. Trans. A 1995, 26, 3211–3223. [Google Scholar] [CrossRef]
- Guo, Z.X.; Derby, B. Solid-state fabrication and interfaces of fiber reinforced metal matrix composites. Prog. Mater. Sci. 1995, 39, 411–495. [Google Scholar] [CrossRef]
- Leyens, C.; Kocian, F.; Hausmann, J.; Kaysser, W.A. Materials and design concepts for high performance compressor components. Aerosp. Sci. Technol. 2003, 7, 201–210. [Google Scholar] [CrossRef]
- Singerman, S.; Jackson, J. Titanium Metal Matrix Composites for Aerospace Applications. Superalloys 1996, 579–586. [Google Scholar]
- Mall, S. Titanium Matrix Composites: Mechanical Behavior; CRC Press: Lancaster, PA, USA, 1997. [Google Scholar]
- Breuer, J.; Wilger, T.; Friesel, M.; Herzig, C. Interstitial and substitutional diffusion of metallic solutes in Ti3Al. Intermetallics 1999, 7, 381–388. [Google Scholar] [CrossRef]
- Lütjering, G.; Williams, J.C. Titanium, 2nd ed.; Springer Science & Business Media: Berlin, Germany, 2007. [Google Scholar]
- Banerjee, D.; Gogia, A.K.; Nandi, T.K.; Joshi, V.A. A new ordered orthorhombic phase in a Ti3AlNb alloy. Acta Metall. 1988, 36, 871–882. [Google Scholar] [CrossRef]
- Banerjee, D. The intermetallic Ti2AlNb. Prog. Mater. Sci. 1997, 42, 135–158. [Google Scholar] [CrossRef]
- Kumpfert, J. Intermetallic Alloys Based on Orthorhombic Titanium Aluminide. Adv. Eng. Mater. 2001, 3, 851. [Google Scholar] [CrossRef]
- Ward-Close, C.; Minor, R.; Doorbar, P. Intermetallic-matrix composites—A review. Intermetallics 1996, 4, 217–229. [Google Scholar] [CrossRef]
- Brunet, A.; Valle, R.; Vassel, A. Intermetallic TiAl-based matrix composites: Investigation of the chemical and mechanical compatibility of a protective coating adapted to an alumina fiber. Acta Mater. 2000, 48, 4763–4774. [Google Scholar] [CrossRef]
- Smith, P.R.; Graves, J.A.; Rhodes, C. Comparison of orthorhombic and alpha-two titanium aluminides as matrices for continuous SiC-reinforced composites. Met. Mater. Trans. A 1994, 25, 1267–1283. [Google Scholar] [CrossRef]
- Luo, X.; Wang, Y.Q.; Yang, Y.Q.; Zhang, M.X.; Huang, B.; Liu, S.; Jin, N. Effect of C/Mo duplex coating on the interface and tensile strength of SiCf/Ti-21Al-29Nb composites. J. Alloys Compd. 2017, 721, 653–660. [Google Scholar] [CrossRef]
- Yang, Y.; Zhu, Y.; Ma, Z.; Chen, Y.; Yang, Y. Formation of interfacial reaction products in SCS-6 SiC/Ti2AlNb composites. Scr. Mater. 2004, 51, 385–389. [Google Scholar] [CrossRef]
- Wu, M.; Zhang, K.; Huang, H.; Wang, M.; Li, H.; Zhang, S.; Wen, M. Interfacial reactions in SiCf/C/Ti17 composites dominated by texture of carbon coatings. Carbon 2017, 124, 238–249. [Google Scholar] [CrossRef]
- Dudek, H.J.; Borath, R.; Leucht, R.; Kaysser, W.A. Transmission electron microscopy of the fiber-matrix interface in SiC-SCS-6-fiber-reinforced IMI834 alloys. J. Mater. Sci. 1997, 32, 5355–5362. [Google Scholar] [CrossRef]
- Gentile, M.; Xiao, P.; Abram, T. Palladium interaction with silicon carbide. J. Nucl. Mater. 2015, 462, 100–107. [Google Scholar] [CrossRef]
- Kieschke, R.; Clyne, T. Development of a diffusion barrier for SiC monofilaments in titanium. Mater. Sci. Eng. A 1991, 135, 145–149. [Google Scholar] [CrossRef]
- Choy, K.L. Functionally graded coatings on SiC fibers for protection in Ti-based metal matrix composites. Scr. Mater. 1996, 34, 1753–1758. [Google Scholar] [CrossRef]
- Choy, K.L.; Durodola, J.F.; Derby, B.; Ruiz, C. Effect of TiB2, TiC and TiN protective coatings on tensile strength and fracture behaviour of SiC monofilament fibers. Composites 1995, 26, 531–539. [Google Scholar] [CrossRef]
- Luo, X.; Yang, Y.; Sun, Q.; Yu, Y.; Huang, B.; Chen, Y. Effect of Cu/Mo duplex coating on the interface and property of SiCf/Ti6Al4V composite. Mater. Sci. Eng. A 2012, 535, 6–11. [Google Scholar] [CrossRef]
- XRD and TG-DSC Analysis of the Silicon Carbide-Palladium Reaction. Available online: https://www.researchgate.net/publication/265595117_XRD_and_TG-DSC_Analysis_of_the_Silicon_Carbide-Palladium_Reaction (accessed on 25 September 2019).
- Fu, Y.; Shi, N.; Zhang, D.; Yang, R. Effect of C coating on the interfacial microstructure and properties of SiC fiber-reinforced Ti matrix composites. Mater. Sci. Eng. A 2006, 426, 278–282. [Google Scholar] [CrossRef]
- Huang, B.; Yang, Y.; Luo, H.; Yuan, M. Effects of the coating system and interfacial region thickness on the thermal residual stresses in SiCf/Ti–6Al–4V composites. Mater. Des. 2009, 30, 718–722. [Google Scholar] [CrossRef]
- Zhang, W.; Yang, Y.; Zhao, G.; Feng, Z.; Huang, B.; Luo, X.; Li, M.; Chen, Y.; Yang, Y. Interfacial reaction studies of B4C-coated and C-coated SiC fiber reinforced Ti–43Al–9V composites. Intermetallics 2014, 50, 14–19. [Google Scholar] [CrossRef]
- Wang, M.-J.; Huang, H.; Li, H.; Zhang, S.-M.; Wen, M.; Song, C.; Pan, F. Microstructure and interfacial strength of SiC fiber-reinforced Ti17 alloy composites with different consolidation temperatures. Rare Met. 2018, 37, 759–768. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, M.; Wen, M.; Wu, M.; Wang, Q.; Huang, H. Interfacial reactions and matrix microstructure evolution in SiCf/Ti composites dominated by primary structure of Ti matrix. Ceram. Int. 2019, 45, 17767–17774. [Google Scholar] [CrossRef]
- Sun, Q.; Luo, X.; Yang, Y.; Feng, G.; Zhao, G.; Huang, B.; Yang, Y. A review on the research progress of push-out method in testing interfacial properties of SiC fiber-reinforced titanium matrix composites. Compos. Interfaces 2015, 22, 367–386. [Google Scholar] [CrossRef]
- Díaz, J.; Paolicelli, G.; Ferrer, S.; Comin, F. Separation of the sp3 and sp2 components in the C1 s photoemission spectra of amorphous carbon films. Phys. Rev. B 1996, 54, 8064–8069. [Google Scholar] [CrossRef]
- Mc Evoy, N.; Peltekis, N.; Kumar, S.; Rezvani, E.; Nolan, H.; Keeley, G.P.; Blau, W.J.; Duesberg, G.S. Synthesis and analysis of thin conducting pyrolytic carbon films. Carbon 2012, 50, 1216–1226. [Google Scholar] [CrossRef]
- Cao, X.; Shang, L.; Liang, Y.; Lu, Z.; Zhang, G.; Xue, Q. The effect of tribo-chemical reactions of mating materials on tribological behaviors of the B4C film in various relative humidity environments. Ceram. Int. 2019, 45, 4581–4589. [Google Scholar] [CrossRef]
- Bourrat, X.; Trouvat, B.; Limousin, G.; Vignoles, G.; Doux, F. Pyrocarbon anisotropy as measured by electron diffraction and polarized light. J. Mater. Res. 2000, 15, 92–101. [Google Scholar] [CrossRef]
- Reznik, B.; Hüttinger, K. On the terminology for pyrolytic carbon. Carbon 2002, 40, 621–624. [Google Scholar] [CrossRef]
- Langeʼs Handbook of Chemistry. Available online: https://www.researchgate.net/publication/249343603_Langes_Handbook_of_Chemistry (accessed on 17 September 2019).
- Lihrmann, J.-M. Thermodynamics of the Al2O3–Al4C3 system: I. Thermochemical functions of Al oxide, carbide and oxycarbides between 298 and 2100K. J. Eur. Ceram. Soc. 2008, 28, 633–642. [Google Scholar] [CrossRef]
- Hansen, M.; Kamen, E.L.; Kessler, H.D.; McPherson, D.J. Systems Titanium-Molybdenum and Titanium-Columbium. JOM 1951, 3, 881–888. [Google Scholar] [CrossRef]
- Yang, Y.; Dudek, H.; Kumpfert, J.; Yang, Y. Interfacial reaction and stability of SCS-6 SiC/Ti–25Al–10Nb–3V–1Mo composites. Mater. Sci. Eng. A 1998, 246, 213–220. [Google Scholar] [CrossRef]
- Yang, Y.Q.; Dudek, H.J.; Kumpfert, J. TEM investigations of the fiber/matrix interface in SCS-6 SiC/Ti–25Al–10Nb–3V–1Mo composites. Compos. Part A: Appl. Sci. Manuf. 1998, 29, 1235–1241. [Google Scholar] [CrossRef]
- Mei, H.; Bai, Q.; Sun, Y.; Li, H.; Wang, H.; Cheng, L. The effect of heat treatment on the strength and toughness of carbon fiber/silicon carbide composites with different pyrolytic carbon interphase thicknesses. Carbon 2013, 57, 288–297. [Google Scholar] [CrossRef]
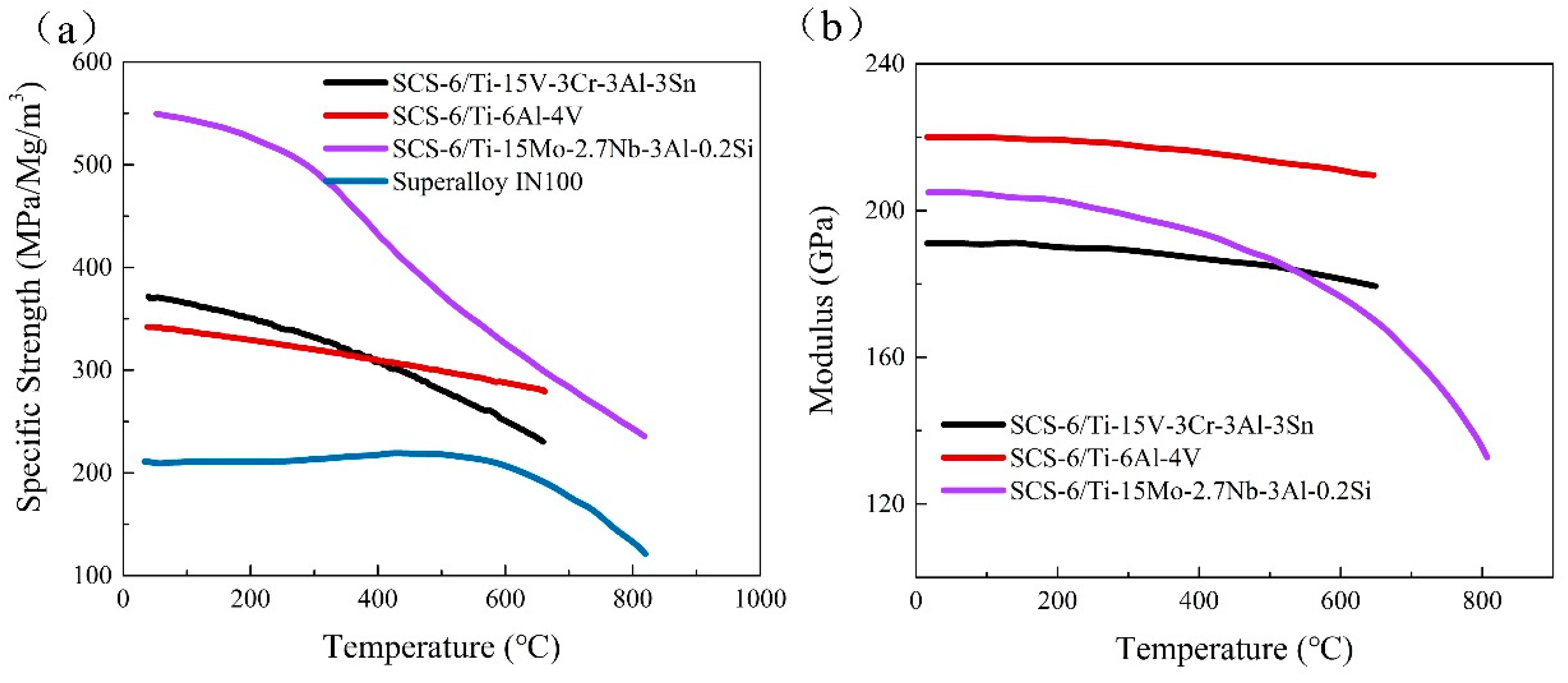

| Properties (Direction of Fibers) | Conventional Ti MMC | Ti Aluminide Ti MMC | Superalloys |
|---|---|---|---|
| Density, g/cm3 | 4.04 | 4.18 | 8.3 |
| Stiffness, GPa | 200 | 242 | 207 |
| Max use temperature, ℃ | 538 | 760 | 1090 |
| CTE, °C−1 × 10-6 | 8.91 | 9.18 | 13.0 |
| Position | C | Ti | Al | Nb |
|---|---|---|---|---|
| a (ⅱ) | 80.15 | 19.85 | - | - |
| a (ⅳ) | 76.54 | 23.46 | - | - |
| a (ⅴ) | 17.23 | 16.29 | 18.63 | 47.85 |
| a (ⅵ) | 59.75 | 33.85 | 0.40 | 6.00 |
| No. | Interfacial Reaction Equation | ΔrG/kJ mol−1 |
|---|---|---|
| 1 | 2Ti + B4C = 2TiB2 + C | −230.5 |
| 2 | Ti + 2B = TiB2 | −258.7 |
| 3 | Ti + B = TiB | −155.6 |
| 4 | Ti + B4C = TiC + 4B | −111.9 |
| 5 | Ti + C = TiC | −170.3 |
| 6 | TiB2 + Ti = 2TiB | −52.4 |
| 7 | 2Nb + B4C = 2NbB2 + C | −133.7 |
| 8 | Nb + 2B = NbB2 | −162.9 |
| 9 | Nb + B = NbB | −107.7 |
| 10 | Nb + C = NbC | −134 |
| 11 | 4Al + 3C = Al4C3 | −138 |
| Position | B | C | Ti | Al | Nb |
|---|---|---|---|---|---|
| Spot 1 (RL-A) | 65.14 | 14.10 | 20.76 | - | - |
| Spot 2 (fine needle in RL-B) | 62.25 | 9.50 | 13.88 | 0.23 | 14.13 |
| Spot 3 (block in RL-B) | 40.21 | 28.27 | 27.38 | 0.23 | 3.91 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, S.; Wang, M.; Wen, M.; Chen, J.; Li, H.; Xie, C.; Fan, W.; Wang, Q.; Huang, H. Interfacial Reactions and Mechanical Properties Studies of C-Coated and C/B4C Duplex-Coated SiC Fiber-Reinforced Ti2AlNb Composites. Materials 2019, 12, 3257. https://doi.org/10.3390/ma12193257
Zhang S, Wang M, Wen M, Chen J, Li H, Xie C, Fan W, Wang Q, Huang H. Interfacial Reactions and Mechanical Properties Studies of C-Coated and C/B4C Duplex-Coated SiC Fiber-Reinforced Ti2AlNb Composites. Materials. 2019; 12(19):3257. https://doi.org/10.3390/ma12193257
Chicago/Turabian StyleZhang, Shuming, Minjuan Wang, Mao Wen, Jianhong Chen, Hu Li, Chuan Xie, Wangtengfei Fan, Qingfeng Wang, and Hao Huang. 2019. "Interfacial Reactions and Mechanical Properties Studies of C-Coated and C/B4C Duplex-Coated SiC Fiber-Reinforced Ti2AlNb Composites" Materials 12, no. 19: 3257. https://doi.org/10.3390/ma12193257
APA StyleZhang, S., Wang, M., Wen, M., Chen, J., Li, H., Xie, C., Fan, W., Wang, Q., & Huang, H. (2019). Interfacial Reactions and Mechanical Properties Studies of C-Coated and C/B4C Duplex-Coated SiC Fiber-Reinforced Ti2AlNb Composites. Materials, 12(19), 3257. https://doi.org/10.3390/ma12193257




