Neopentyl Glycol as Active Supporting Media in Shape-Stabilized PCMs
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
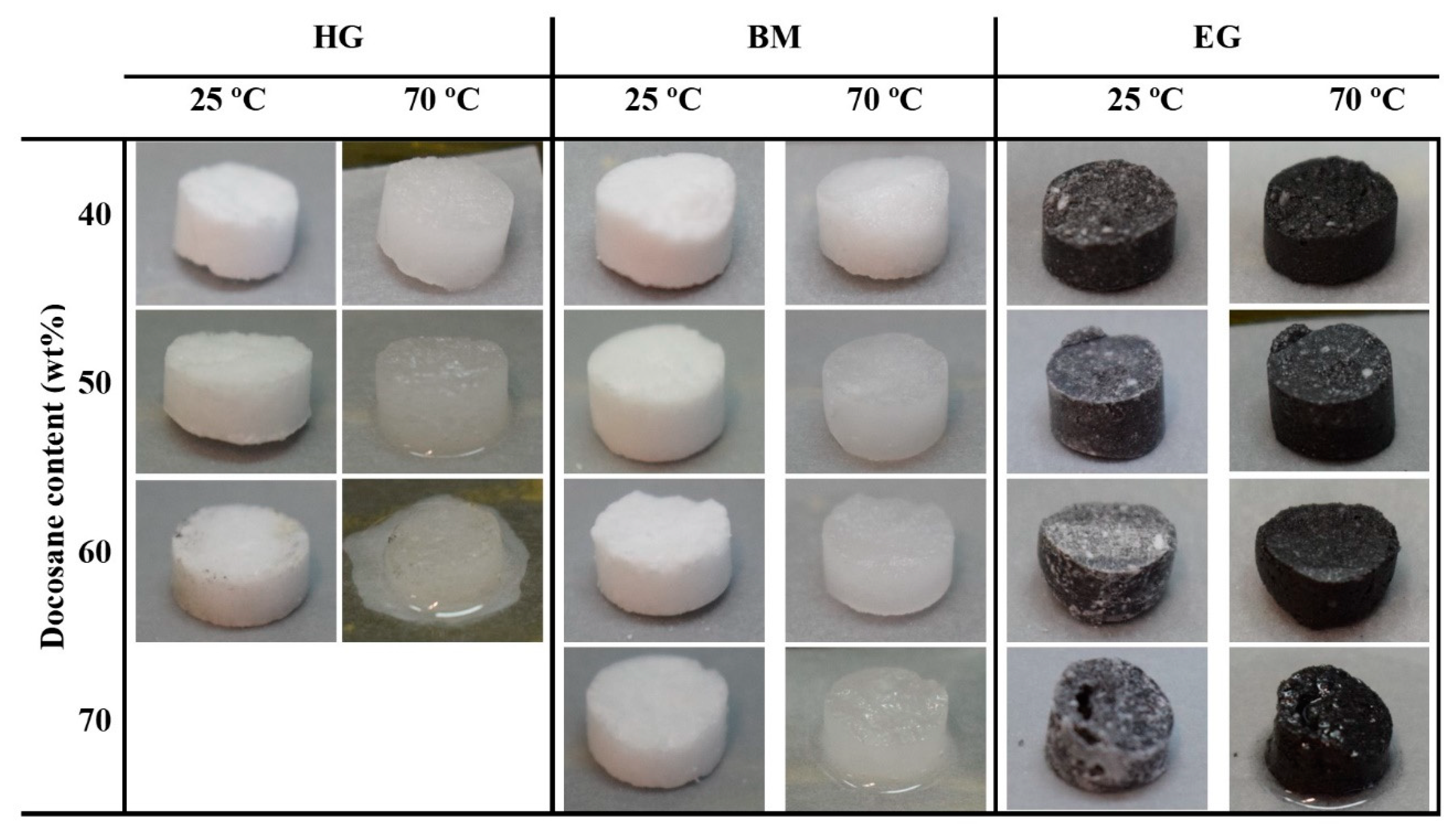
2.2. SSPCM Preparation
2.3. Composite Characterization
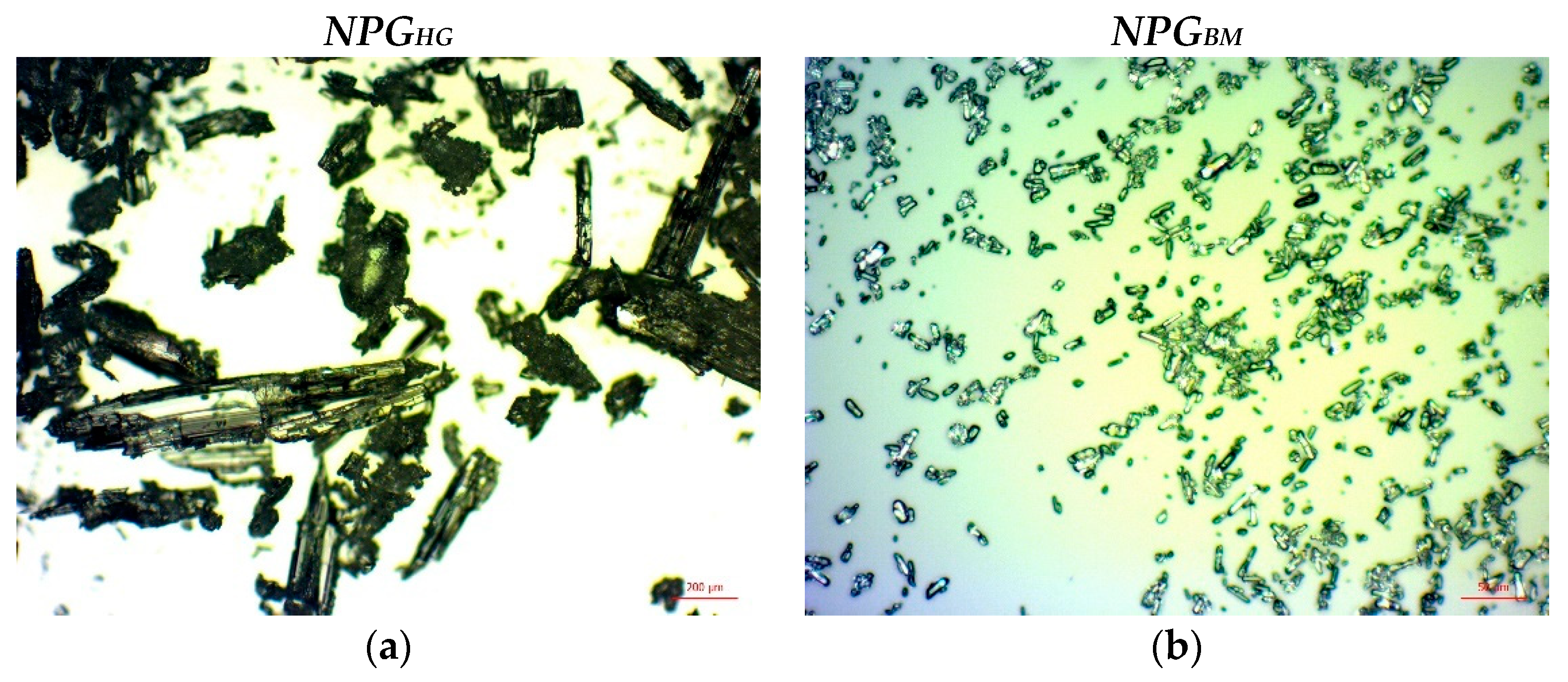
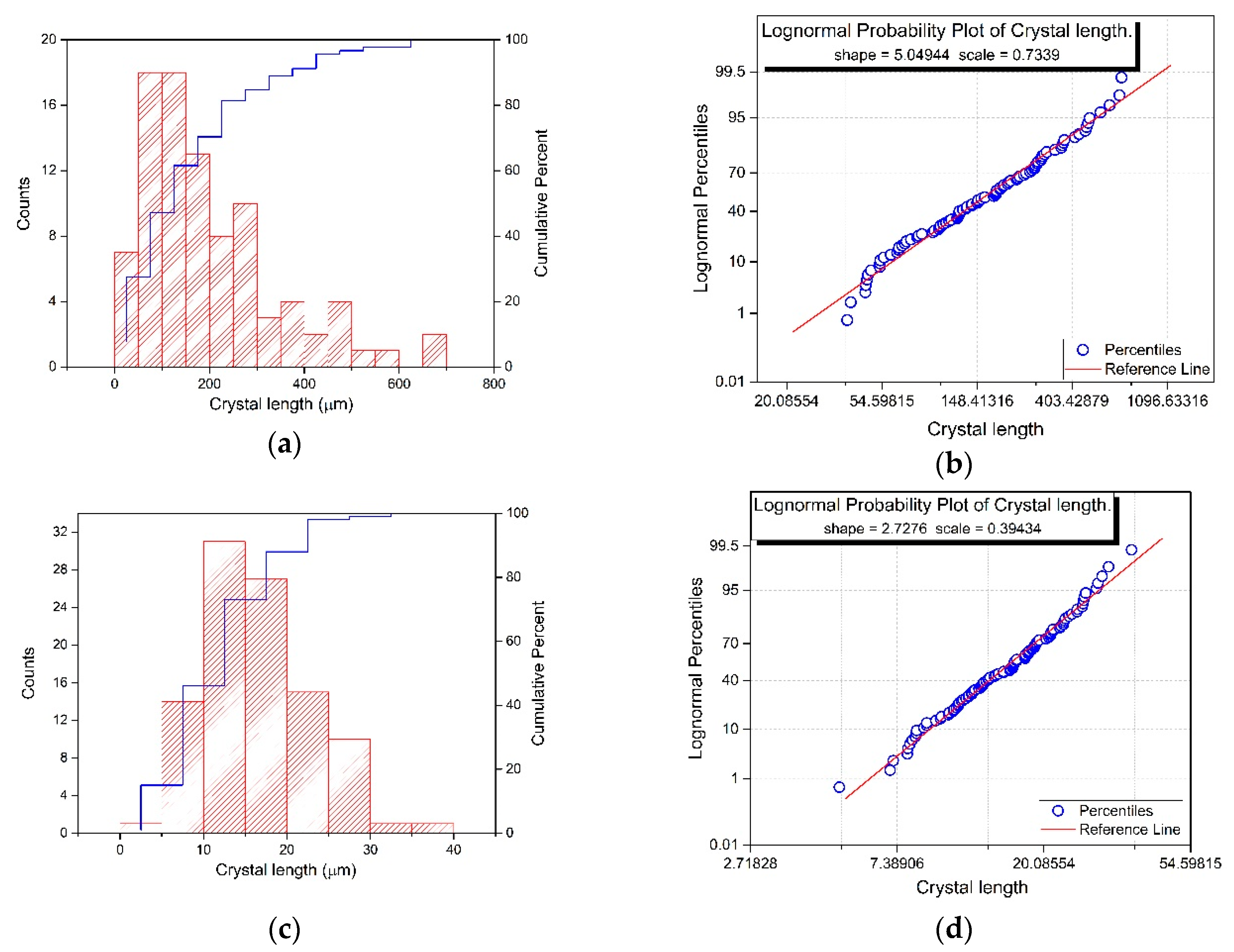
2.3.1. NPG Crystal Size
2.3.2. NPG-Docosane Wettability
2.3.3. Leakage Test
2.3.4. X-ray Diffraction (XRD)
2.3.5. Scanning Electron Microscopy (SEM)
2.3.6. SSPCMs Density
2.3.7. Thermal Degradation of SSPCMs Composites
2.3.8. Thermal Properties
3. Results and Discussion
3.1. NPG Crystal Sizes
3.2. NPG-Docosane Wettability
3.3. Leakage Test
3.4. Inner Morphology
3.5. Composite Density
3.6. Crystallization Properties of NPG/Docosane Composites
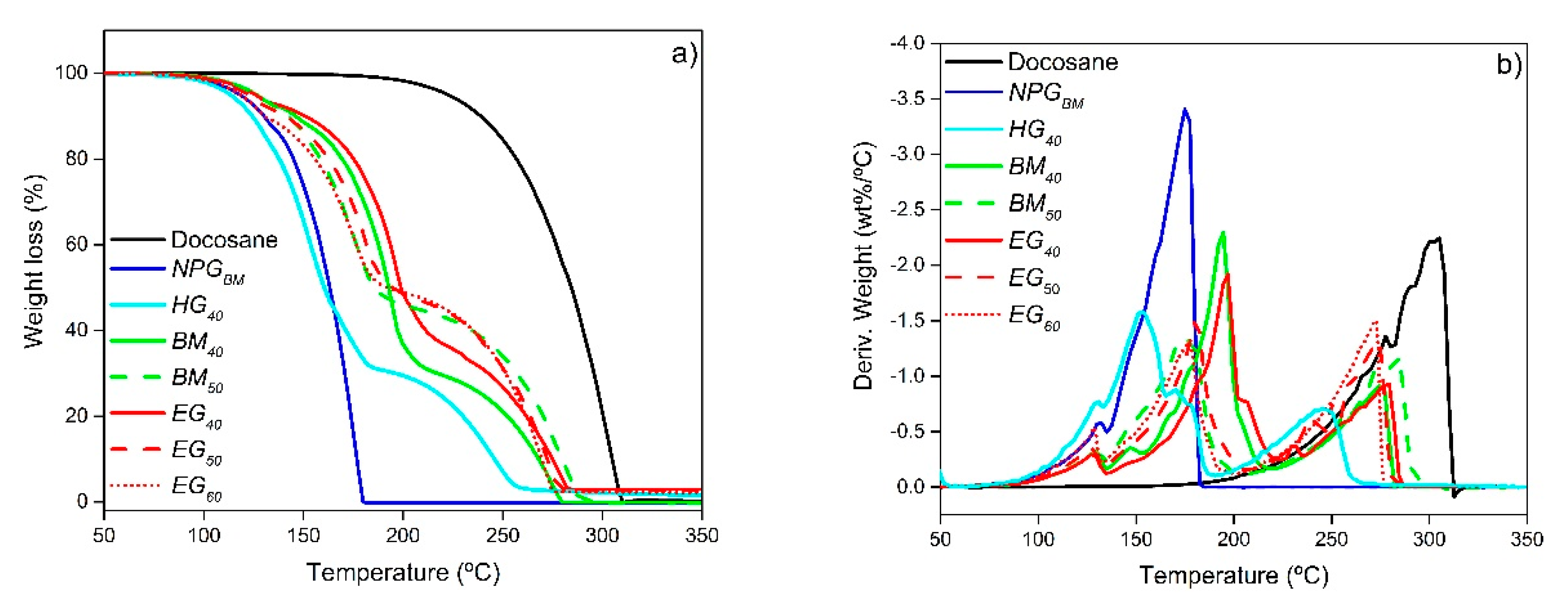
3.7. Thermal Stability
3.8. Thermal Properties
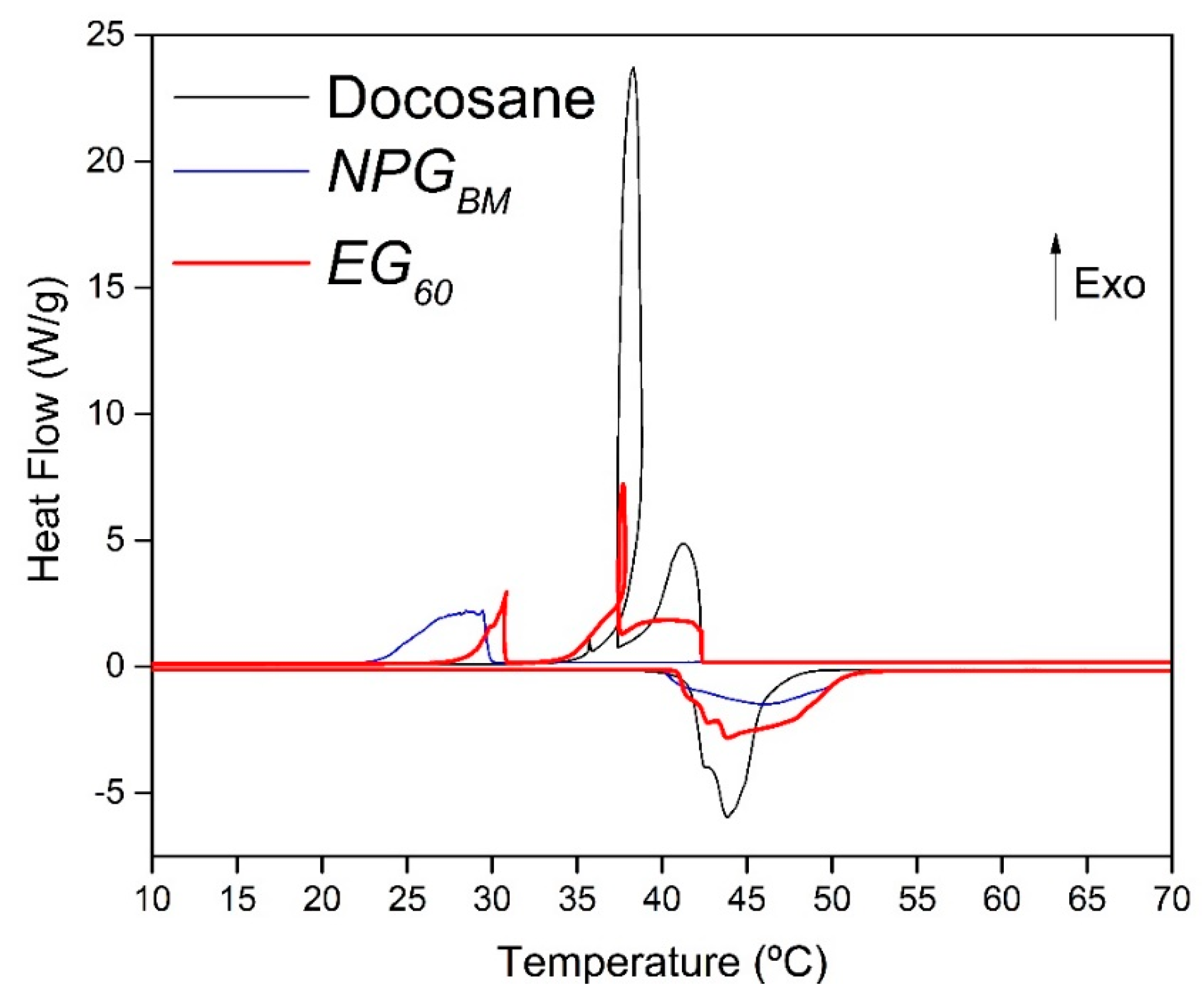
3.8.1. DSC Analysis
3.8.2. Hot Disk
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sarbu, I.; Sebarchievici, C. A comprehensive review of thermal energy storage. Sustainability 2018, 10, 191. [Google Scholar] [CrossRef]
- Sutterlin, W.R. Phase Change Materials, A Brief Comparison of Ice Packs, Salts, Paraffins, and Vegetable-Derived Phase Change Materials; Entropy Solutions LLC: Plymouth, MN, USA, 2014. [Google Scholar]
- Nazir, H.; Batool, M.; Bolivar Osorio, F.J.; Isaza-Ruiz, M.; Xu, X.; Vignarooban, K.; Phelan, P.; Inamuddin; Kannan, A.M. Recent developments in phase change materials for energy storage applications: A review. Int. J. Heat Mass Transf. 2019, 129, 491–523. [Google Scholar] [CrossRef]
- Jamekhorshid, A.; Sadrameli, S.M.; Farid, M. A review of microencapsulation methods of phase change materials (PCMs) as a thermal energy storage (TES) medium. Renew. Sustain. Energy Rev. 2014, 31, 531–542. [Google Scholar] [CrossRef]
- Huang, X.; Zhu, C.; Lin, Y.; Fang, G. Thermal properties and applications of microencapsulated PCM for thermal energy storage: A review. Appl. Therm. Eng. 2019, 147, 841–855. [Google Scholar] [CrossRef]
- Chen, C.; Liu, W.; Wang, Z.; Peng, K.; Pan, W.; Xie, Q. Novel form stable phase change materials based on the composites of polyethylene glycol/polymeric solid-solid phase change material. Sol. Energy Mater. Sol. Cells 2015, 134, 80–88. [Google Scholar] [CrossRef]
- Guo, Q.; Wang, T. Preparation and characterization of sodium sulfate/silica composite as a shape-stabilized phase change material by sol-gel method. Chin. J. Chem. Eng. 2014, 22, 360–364. [Google Scholar] [CrossRef]
- Cheng, W.L.; Zhang, R.M.; Xie, K.; Liu, N.; Wang, J. Heat conduction enhanced shape-stabilized paraffin/HDPE composite PCMs by graphite addition: Preparation and thermal properties. Sol. Energy Mater. Sol. Cells 2010, 94, 1636–1642. [Google Scholar] [CrossRef]
- Sari, A.; Alkan, C.; Karaipekli, A.; Uzun, O. Poly(ethylene glycol)/poly(methyl methacrylate) blends as novel form-stable phase-change materials for thermal energy storage. J. Appl. Polym. Sci. 2010, 116, 929–933. [Google Scholar]
- Umair, M.M.; Zhang, Y.; Iqbal, K.; Zhang, S.; Tang, B. Novel strategies and supporting materials applied to shape-stabilize organic phase change materials for thermal energy storage—A review. Appl. Energy 2019, 235, 846–873. [Google Scholar] [CrossRef]
- Li, M.; Shi, J. Review on micropore grade inorganic porous medium based form stable composite phase change materials: Preparation, performance improvement and effects on the properties of cement mortar. Constr. Build. Mater. 2019, 194, 287–310. [Google Scholar] [CrossRef]
- Tang, B.; Cui, J.; Wang, Y.; Jia, C.; Zhang, S. Facile synthesis and performances of PEG/SiO2 composite form-stable phase change materials. Sol. Energy 2013, 97, 484–492. [Google Scholar] [CrossRef]
- Serrano, A.; Martín del Campo, J.; Peco, N.; Rodriguez, J.F.; Carmona, M. Influence of gelation step for preparing PEG–SiO2 shape-stabilized phase change materials by sol-gel method. J. Sol-Gel Sci. Technol. 2019, 89, 731–742. [Google Scholar] [CrossRef]
- Gao, H.; Wang, J.; Chen, X.; Wang, G.; Huang, X.; Li, A.; Dong, W. Nanoconfinement effects on thermal properties of nanoporous shape-stabilized composite PCMs: A review. Nano Energy 2018, 53, 769–797. [Google Scholar] [CrossRef]
- Fallahi, A.; Guldentops, G.; Tao, M.; Granados-Focil, S.; Van Dessel, S. Review on solid-solid phase change materials for thermal energy storage: Molecular structure and thermal properties. Appl. Therm. Eng. 2017, 127, 1427–1441. [Google Scholar] [CrossRef]
- Chen, K.; Yu, X.; Tian, C.; Wang, J. Preparation and characterization of form-stable paraffin/polyurethane composites as phase change materials for thermal energy storage. Energy Convers. Manag. 2014, 77, 13–21. [Google Scholar] [CrossRef]
- Mishra, A.; Talekar, A.; Chandra, D.; Chien, W.M. Ternary phase diagram calculations of pentaerythritol-pentaglycerine-neopentylglycol system. Thermochim. Acta 2012, 535, 17–26. [Google Scholar] [CrossRef]
- Gunasekara, S.N.; Pan, R.; Chiu, J.N.; Martin, V. Polyols as phase change materials for surplus thermal energy storage. Appl. Energy 2016, 162, 1439–1452. [Google Scholar] [CrossRef]
- Zgardzińska, B.; Gorgol, M. Influence of pressure on the size of free volumes in some waxes. Acta Phys. Pol. A 2014, 125, 816–820. [Google Scholar] [CrossRef]
- Wang, J.; Chen, G.; Jiang, H. Theoretical study on a novel phase change process. Int. J. Energy Res. 1999, 23, 287–294. [Google Scholar] [CrossRef]
- Vélez, C.; Khayet, M.; Ortiz De Zárate, J.M. Temperature-dependent thermal properties of solid/liquid phase change even-numbered n-alkanes: N-Hexadecane, n-octadecane and n-eicosane. Appl. Energy 2015, 143, 383–394. [Google Scholar] [CrossRef]
- Chandra, D.; Day, C.S.; Barrett, C.S. Low- and high-temperature structures of neopentylglycol plastic crystal. Powder Diffr. 1993, 8, 109–117. [Google Scholar] [CrossRef]
- Rueden, C.T.; Schindelin, J.; Hiner, M.C.; DeZonia, B.E.; Walter, A.E.; Arena, E.T.; Eliceiri, K.W. ImageJ2: ImageJ for the next generation of scientific image data. BMC Bioinform. 2017, 18, 529. [Google Scholar] [CrossRef] [PubMed]
- Brugnara, M. Contact Angle Plugin for ImageJ; University of Trento: Trento, Italy, 2010. [Google Scholar]
- Gustafsson, S.E. Transient plane source techniques for thermal conductivity and thermal diffusivity measurements of solid materials. Rev. Sci. Instrum. 1991, 62, 797–804. [Google Scholar] [CrossRef]
- Jannot, Y.; Acem, Z. A quadrupolar complete model of the hot disc. Meas. Sci. Technol. 2007, 18, 1229–1234. [Google Scholar] [CrossRef]
- Arkles, B. Hydrophobicity, Hydrophilicity and Silane Surface Modification; Gelest Inc.: Morrisville, PA, USA, 2015. [Google Scholar]
- Or, D.; Tuller, M. Capillarity; Elsevier: Amsterdam, The Netherlands, 2005. [Google Scholar]
- Schrader, M.E. Young-Dupre Revisited. Langmuir 1995, 11, 3585–3589. [Google Scholar] [CrossRef]
- Packham, D.E. Work of adhesion: Contact angles and contact mechanics. Int. J. Adhes. Adhes. 1996, 16, 121–128. [Google Scholar] [CrossRef]
- Megias-Alguacil, D.; Gauckler, L.J. Capillary and van der Waals forces between uncharged colloidal particles linked by a liquid bridge. Colloid Polym. Sci. 2010, 288, 133–139. [Google Scholar] [CrossRef]
- Leroch, S.; Wendland, M. Influence of capillary bridge formation onto the silica nanoparticle interaction studied by grand canonical Monte Carlo simulations. Langmuir 2013, 29, 12410–12420. [Google Scholar] [CrossRef]
- Neugebauer, R.; Koriath, H.J.; Van der Merwe, A.F.; Müller, M.; Matope, S. Study on Applicability of Adhesive Forces for Micro-Material Handling in production technology. In Proceedings of the ISEM 2011 Proceedings, Stellenbosch, South Africa, 21–23 September 2011. [Google Scholar]
- Zhang, P.; Hu, Y.; Song, L.; Ni, J.; Xing, W.; Wang, J. Effect of expanded graphite on properties of high-density polyethylene/paraffin composite with intumescent flame retardant as a shape-stabilized phase change material. Sol. Energy Mater. Sol. Cells 2010, 94, 360–365. [Google Scholar] [CrossRef]
- Wang, W.; Yang, X.; Fang, Y.; Ding, J.; Yan, J. Preparation and thermal properties of polyethylene glycol/expanded graphite blends for energy storage. Appl. Energy 2009, 86, 1479–1483. [Google Scholar] [CrossRef]
- Kenisarin, M.M.; Kenisarina, K.M. Form-stable phase change materials for thermal energy storage. Renew. Sustain. Energy Rev. 2012, 16, 1999–2040. [Google Scholar] [CrossRef]
- Fu, D.; Su, Y.; Xie, B.; Zhu, H.; Liu, G.; Wang, D. Phase change materials of n-alkane-containing microcapsules: Observation of coexistence of ordered and rotator phases. Phys. Chem. Chem. Phys. 2011, 13, 2021. [Google Scholar] [CrossRef] [PubMed]
- Zannetti, R. The unit cell and space group of 2,2-dimethyl-1,3-propanediol. Acta Crystallogr. 1961, 14, 203–204. [Google Scholar] [CrossRef]
- Mahmoud, A.E.; Wasly, H.S.; Doheim, M.A. Studies of crystallite size and lattice strain in Al-Al2O3 powders by high-energy mechanical milling. J. Eng. Sci. 2014, 42, 1430–1439. [Google Scholar]
- Benson, D.K.; Burrows, R.W.; Webb, J.D. Solid state phase transitions in pentaerythritol and related polyhydric alcohols. Sol. Energy Mater. 1986, 13, 133–152. [Google Scholar] [CrossRef]
- Domanska, U.; Wyrzykowska-Stankiewicz, D. Enthalpies of fusion and solid-solid transition of even-numbered paraffins C22H46, C24H50, C26H54 and C28H58. Thermochim. Acta 1991, 179, 265–271. [Google Scholar] [CrossRef]
- Pielichowska, K.; Pielichowski, K. Phase change materials for thermal energy storage. Prog. Mater. Sci. 2014, 65, 67–123. [Google Scholar] [CrossRef]
- Inaba, H.; Tu, P. Evaluation of thermophysical characteristics on shape-stabilized paraffin as a solid-liquid phase change material. Heat Mass Transf. 1997, 32, 307–312. [Google Scholar] [CrossRef]
- Hong, Y.; Xin-shi, G. Preparation of polyethylene-paraffin compound as a form-stable solid-liquid phase change material. Sol. Energy Mater. Sol. Cells 2000, 64, 37–44. [Google Scholar] [CrossRef]
- Xiao, M.; Feng, B.; Gong, K. Preparation and performance of shape stabilized phase change thermal storage materials with high thermal conductivity. Energy Convers. Manag. 2002, 43, 103–108. [Google Scholar] [CrossRef]
- Sari, A. Form-stable paraffin/high density polyethylene composites as solid-liquid phase change material for thermal energy storage: Preparation and thermal properties. Energy Convers. Manag. 2004, 45, 2033–2042. [Google Scholar] [CrossRef]
- Font, J.; Muntasell, J.; Navarro, J.; Tamarit, J.L.; Lloveras, J. Calorimetric study of the mixtures PE/NPG and PG/NPG. Sol. Energy Mater. 1987, 15, 299–310. [Google Scholar] [CrossRef]
- Sari, A.; Kaygusuz, K. Studies on poly(vinyl chloride)/fatty acid blends as shape-stabilized phase change material for latent heat thermal energy storage. Indian J. Eng. Mater. Sci. 2006, 13, 253–258. [Google Scholar]
- Sari, A.; Kaygusuz, K. Poly(vinyl alcohol)/fatty acid blends for thermal energy storage. Energy Sources Part A Recovery Util. Environ. Eff. 2007, 29, 873–883. [Google Scholar] [CrossRef]
- Li, Y.; Wu, M.; Liu, R.; Huang, Y. Cellulose-based solid-solid phase change materials synthesized in ionic liquid. Sol. Energy Mater. Sol. Cells 2009, 93, 1321–1328. [Google Scholar] [CrossRef]
- Sari, A.; Alkan, C.; Karaipekli, A.; Önal, A. Preparation, characterization and thermal properties of styrene maleic anhydride copolymer (SMA)/fatty acid composites as form stable phase change materials. Energy Convers. Manag. 2008, 49, 373–380. [Google Scholar] [CrossRef]
- Alkan, C.; Sari, A. Fatty acid/poly(methyl methacrylate) (PMMA) blends as form-stable phase change materials for latent heat thermal energy storage. Sol. Energy 2008, 82, 118–124. [Google Scholar] [CrossRef]
- Kaygusuz, K.; Alkan, C.; Sari, A.; Uzun, O. Encapsulated fatty acids in an acrylic resin as shape-stabilized phase change materials for latent heat thermal energy storage. Energy Sources Part A Recovery Util. Environ. Eff. 2008, 30, 1050–1059. [Google Scholar] [CrossRef]
- Peng, S.; Fuchs, A.; Wirtz, R.A. Polymeric phase change composites for thermal energy storage. J. Appl. Polym. Sci. 2004, 93, 1240–1251. [Google Scholar] [CrossRef]
- Afanasov, I.M.; Savchenko, D.V.; Ionov, S.G.; Rusakov, D.A.; Seleznev, A.N.; Avdeev, V.V. Thermal conductivity and mechanical properties of expanded graphite. Inorg. Mater. 2009, 45, 486–490. [Google Scholar] [CrossRef]
- Wang, X.; Guo, Q.; Zhong, Y.; Wei, X.; Liu, L. Heat transfer enhancement of neopentyl glycol using compressed expanded natural graphite for thermal energy storage. Renew. Energy 2013, 51, 241–246. [Google Scholar] [CrossRef]




| CAS Number | Tt (°C) | ΔH (J/g) | ρ25°C (g/cm3) | k30°C (W/m·K) | |
|---|---|---|---|---|---|
| Neopentyl Glycol | 126-30-7 | 48 1 | 126.33 3 | 1.060 | 0.25 [20] |
| Docosane | 629-97-0 | 42–45 2 | 259.14 3 | 0.778 | 0.28 [21] |
| Sample | True Density (g/cm3) | Bulk Density (g/cm3) |
|---|---|---|
| HG40 | 1.014 | 0.878 |
| BM40 | 0.993 | 0.843 |
| BM50 | 0.990 | 0.810 |
| EG40 | 1.013 | 0.894 |
| EG50 | 0.999 | 0.856 |
| EG60 | 0.972 | 0.799 |
| Sample | Heating | Cooling | ||||||
|---|---|---|---|---|---|---|---|---|
| Tonset (°C) | Toffset (°C) | ΔH (J/g) | ΔHth (J/g) | 1st Tonset (°C) | 2nd Tonset (°C) | ΔH (J/g) | ΔHth (J/g) | |
| Docosane | 41.64 | 46.36 | 259.14 | 42.29 | 258.83 | |||
| NPG | 40.08 | 52.31 | 126.33 | 30.01 | 113.96 | |||
| HG40 | 40.50 | 51.24 | 177.04 | 179.45 | 42.14 | 30.38 | 172.94 | 171.91 |
| BM40 | 40.70 | 53.21 | 176.67 | 179.45 | 42.03 | 30.35 | 170.51 | 171.91 |
| BM50 | 40.56 | 48.42 | 190.65 | 192.74 | 42.20 | 30.83 | 187.67 | 186.40 |
| EG40 | 40.75 | 50.41 | 165.40 | 176.30 | 42.30 | 30.66 | 160.70 | 169.06 |
| EG50 | 40.70 | 50.02 | 196.12 | 189.58 | 42.40 | 30.21 | 193.30 | 183.55 |
| EG60 | 40.93 | 51.18 | 210.74 | 202.86 | 42.38 | 30.82 | 206.93 | 198.03 |
| Type of Composite | Composition | PCM (wt%) | Tt (°C) | ΔH (J/g) |
|---|---|---|---|---|
| SSPCM | High-density polyethylene + Pentacosane [43] | 74 | 37.5–54 | 121 |
| SSPCM | High-density polyethylene + Refined Paraffin [44] | 75 | 40–55 | 157 |
| SSPCM | Styrene-butadiene-styrene + Paraffin [45] | 80 | 56–58 | 165.2 |
| Solid-solid Polyurethane + SSPCM | PolyurethanePCM + n-octadecane/n-eicosane [16] | 25 | 20–65 | 141.2 |
| SSPCM | HDPE + P2 Paraffin + 3wt% Expanded graphite [46] | 77 | 45.2–55.7 | 162.2 |
| Eutectic Solid-solid PCM | Pentaglycerine + Neopentylglycol [47] | 70 1 | 44–74 | 117.9 2 |
| SSPCM | Poly(vinyl chloride) + Lauric acid [48] | 50 | 38.8 | 91.6 |
| SSPCM | Poly(vinyl chloride) + Malonic acid [48] | 50 | 49.2 | 99.2 |
| SSPCM | Poly(vinyl alcohol) + Lauric acid [49] | 50 | 39.8 | 96.4 |
| SSPCM | Poly(vinyl alcohol) + Malonic acid [49] | 50 | 50.2 | 105.3 |
| Solid-solid PCM | Cellulose graft poly(ethylene glycol) copolymers [50] | 77.4 | 42.8 | 92 |
| SSPCM | Styrene maleic anhydride copolymer + Malonic acid [51] | 85 | 51.75 | 176.49 |
| SSPCM | Styrene maleic anhydride copolymer + Lauric acid [51] | 85 | 41.48 | 160.83 |
| SSPCM | Poly (methyl methacrylate) + Malonic acid [52] | 80 | 51 | 166.56 |
| SSPCM | Poly (methyl methacrylate) + Lauric acid [52] | 80 | 40.96 | 149.55 |
| SSPCM | Acrylic cationic resin + Malonic acid [53] | 70 | 51.44 | 135.62 |
| Encapsulation + SSPCM | Styrene-ethylene-butylene-styrene + Paraffin IGI 422 [54] | 75 | 44–55 | 189 |
| Sample | k (W/m·K) | α (mm2/s) |
|---|---|---|
| HG40 | 0.277 | 0.221 |
| BM40 | 0.276 | 0.174 |
| BM50 | 0.295 | 0.197 |
| EG40 | 0.433 | 0.332 |
| EG50 | 0.519 | 0.345 |
| EG60 | 0.450 | 0.467 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serrano, A.; Dauvergne, J.-L.; Doppiu, S.; Palomo Del Barrio, E. Neopentyl Glycol as Active Supporting Media in Shape-Stabilized PCMs. Materials 2019, 12, 3169. https://doi.org/10.3390/ma12193169
Serrano A, Dauvergne J-L, Doppiu S, Palomo Del Barrio E. Neopentyl Glycol as Active Supporting Media in Shape-Stabilized PCMs. Materials. 2019; 12(19):3169. https://doi.org/10.3390/ma12193169
Chicago/Turabian StyleSerrano, Angel, Jean-Luc Dauvergne, Stefania Doppiu, and Elena Palomo Del Barrio. 2019. "Neopentyl Glycol as Active Supporting Media in Shape-Stabilized PCMs" Materials 12, no. 19: 3169. https://doi.org/10.3390/ma12193169
APA StyleSerrano, A., Dauvergne, J.-L., Doppiu, S., & Palomo Del Barrio, E. (2019). Neopentyl Glycol as Active Supporting Media in Shape-Stabilized PCMs. Materials, 12(19), 3169. https://doi.org/10.3390/ma12193169





