The Effect of Ge Addition on the Oxidation of Nb-24Ti-18Si Silicide Based Alloys
Abstract
:1. Introduction
2. Experimental
3. Results
3.1. Oxidation at 800 °C
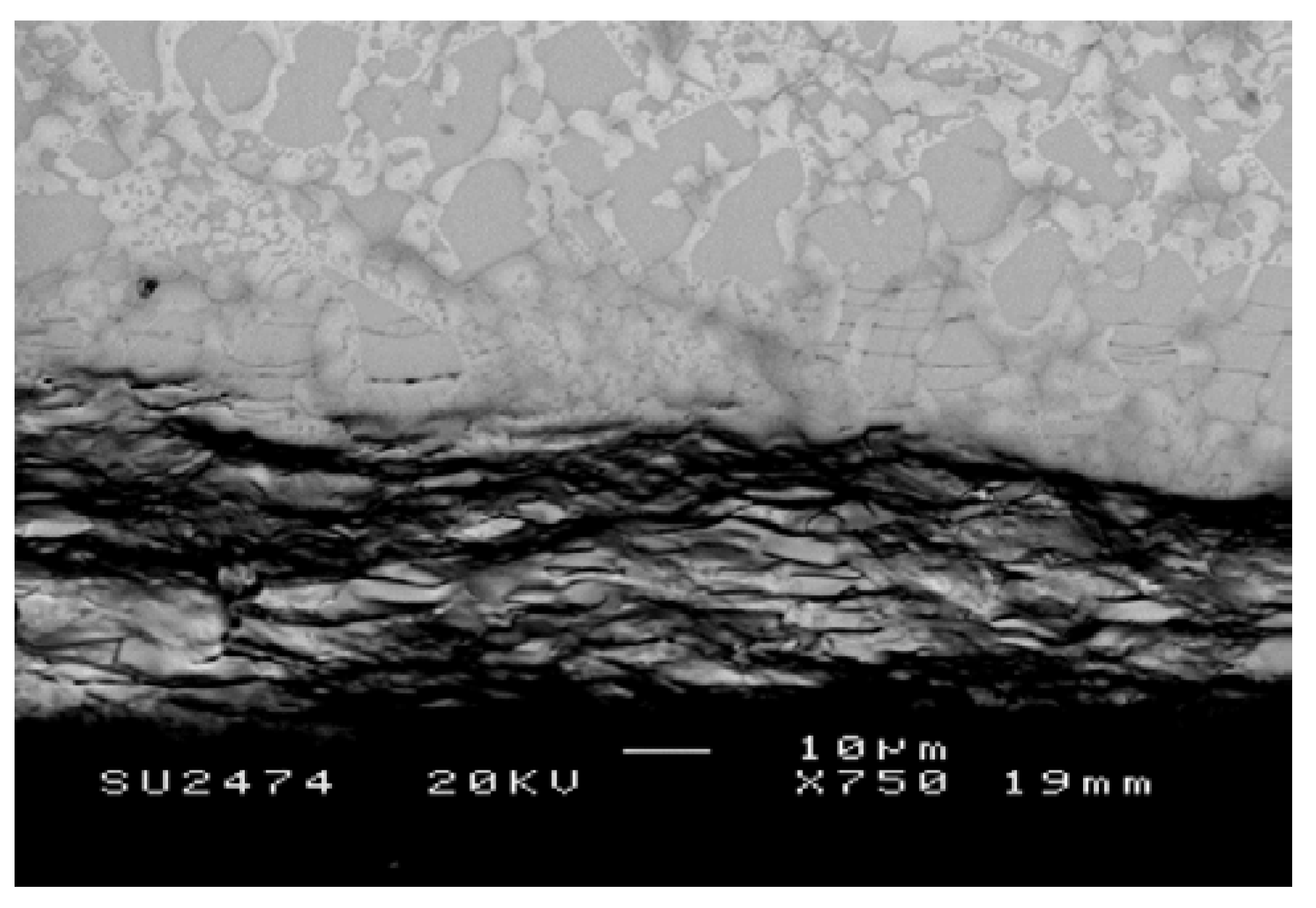
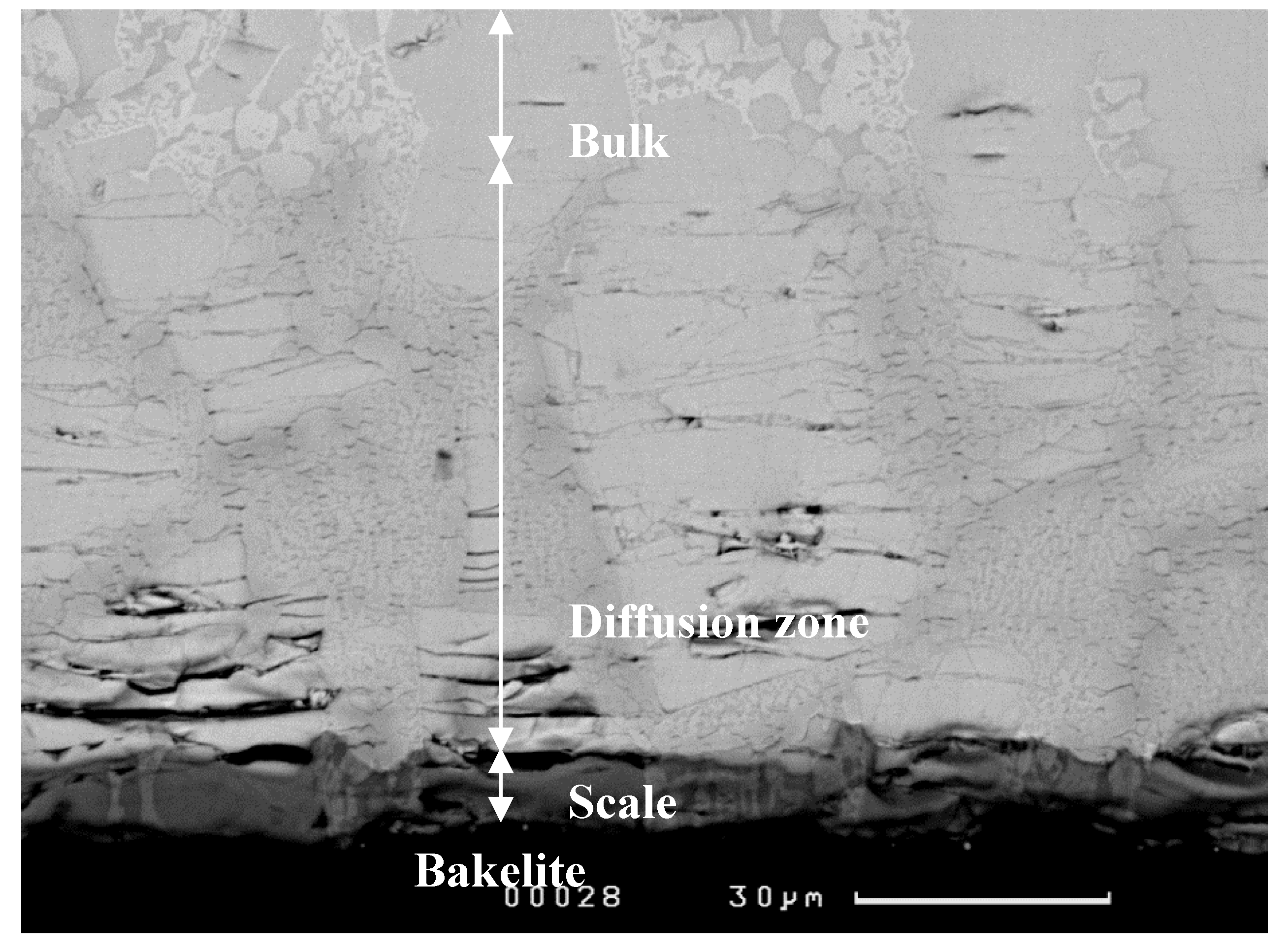
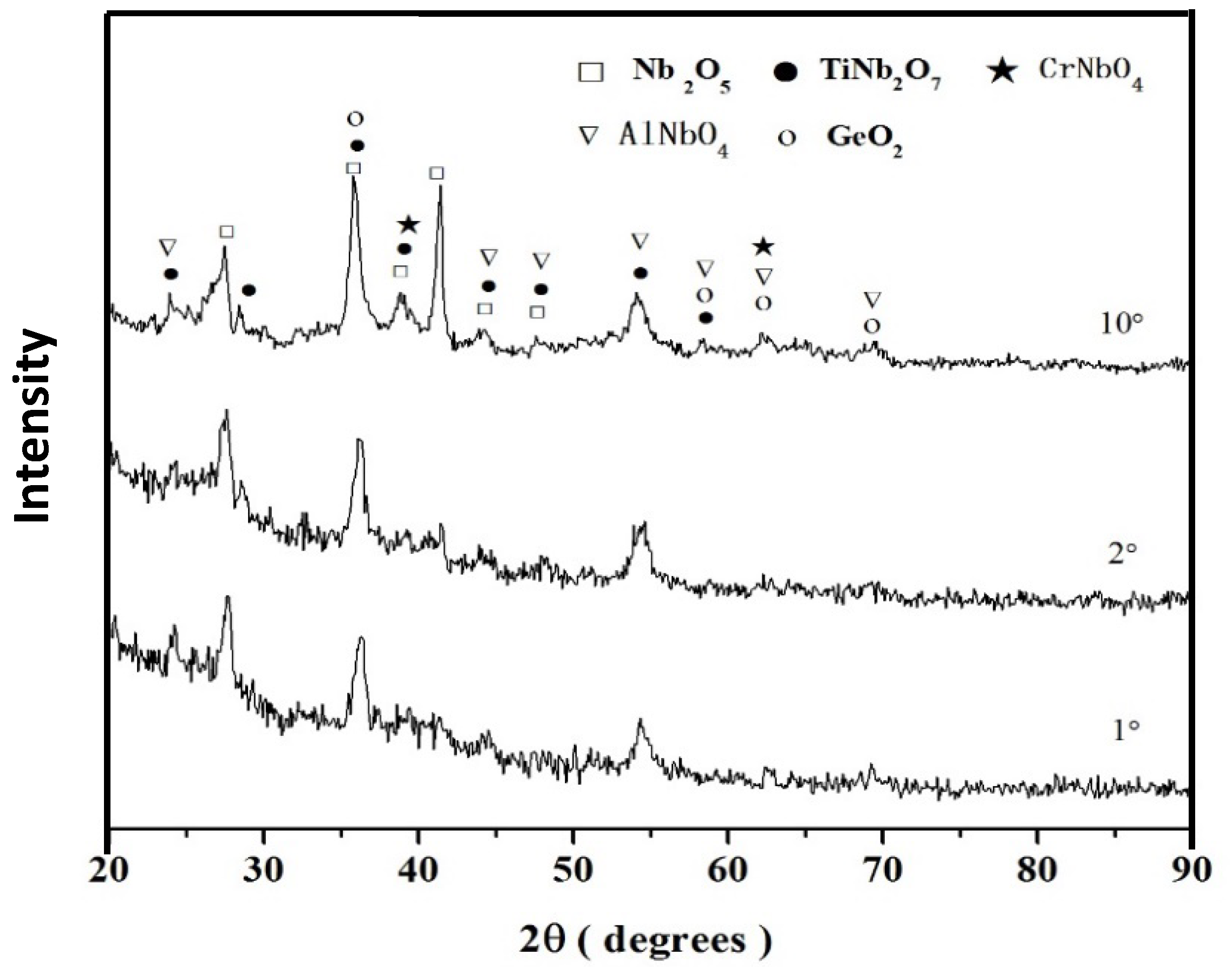
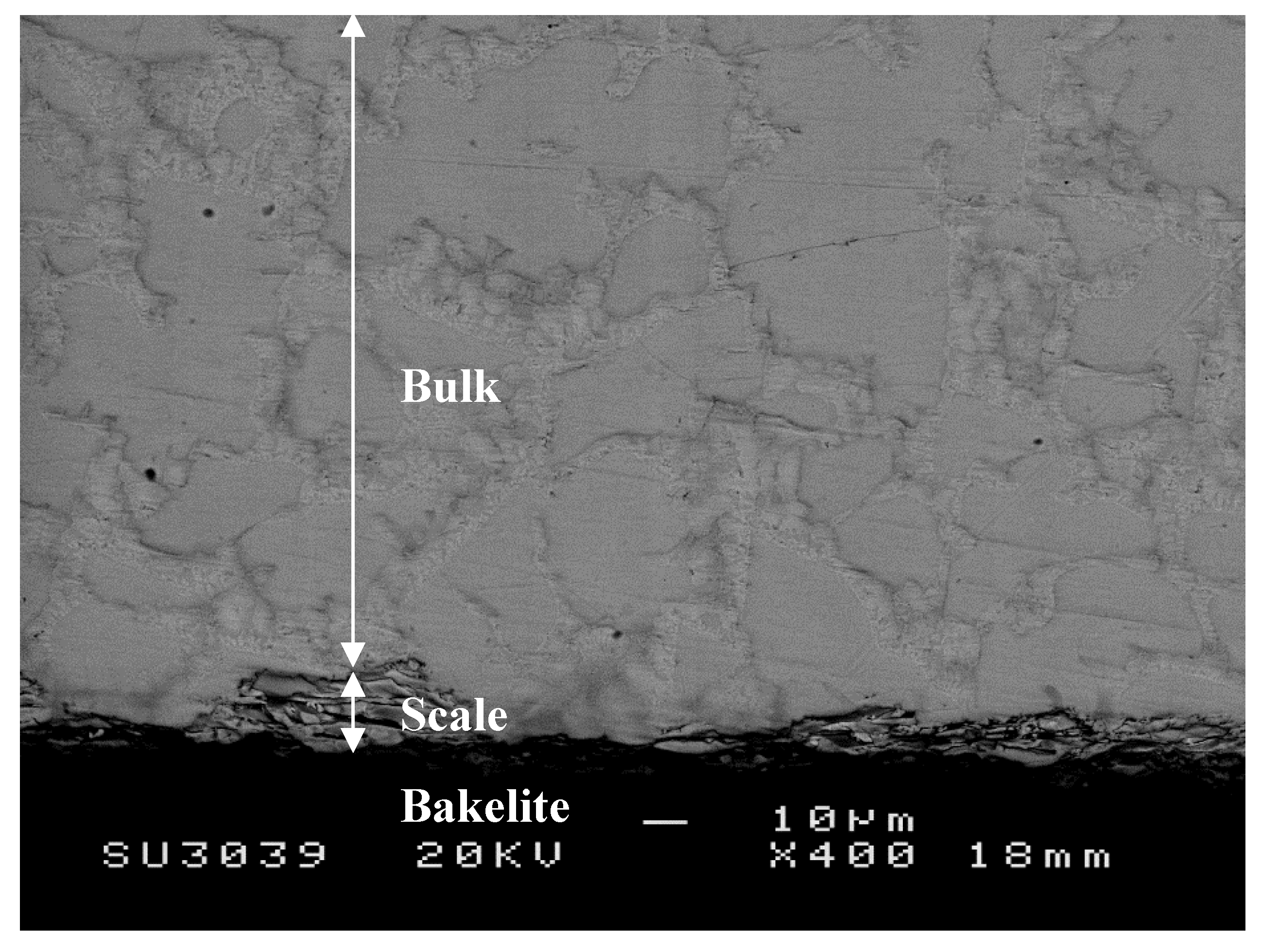
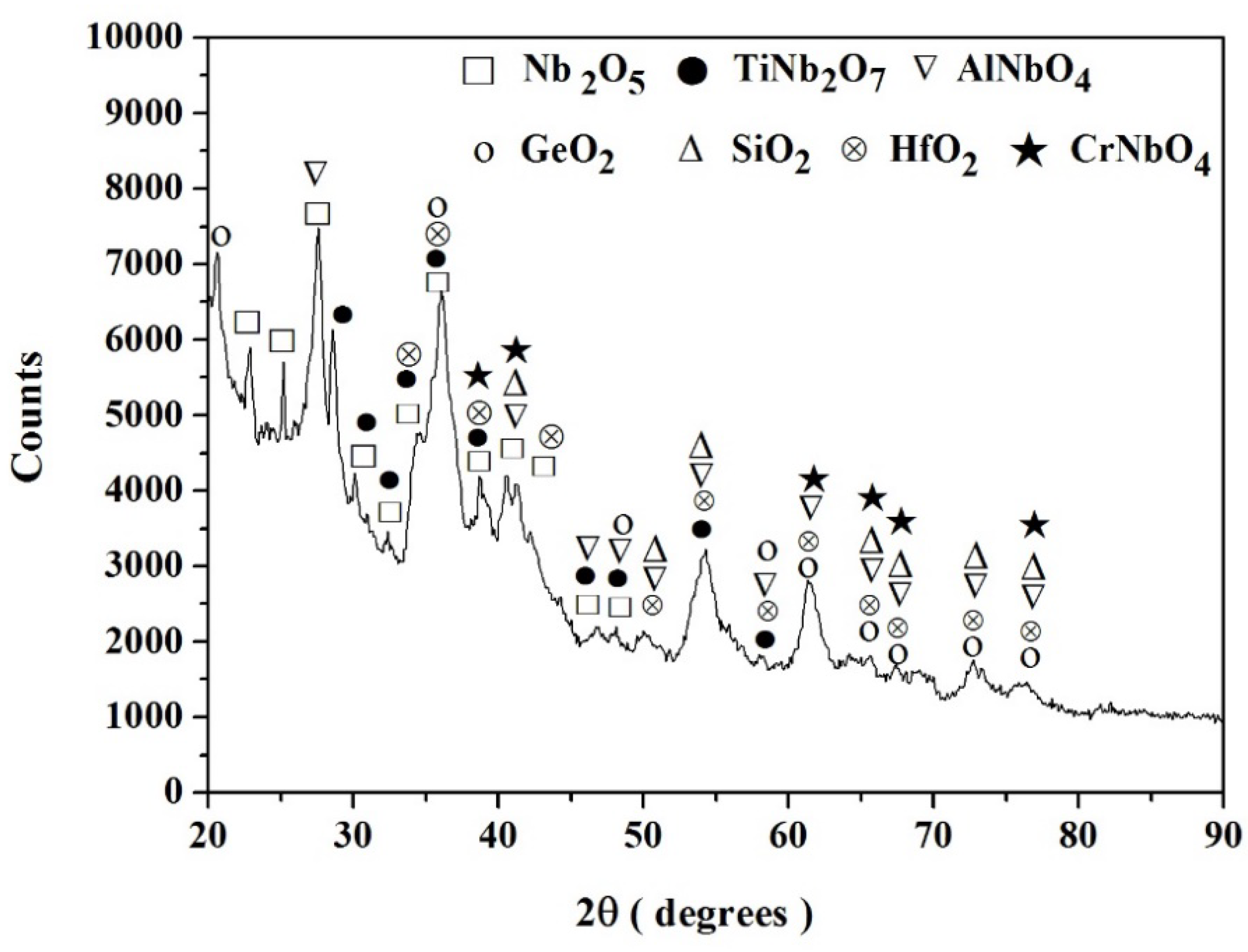
3.1.1. Structure of Scales, Diffusion Zones and Contamination of Phases by Oxygen
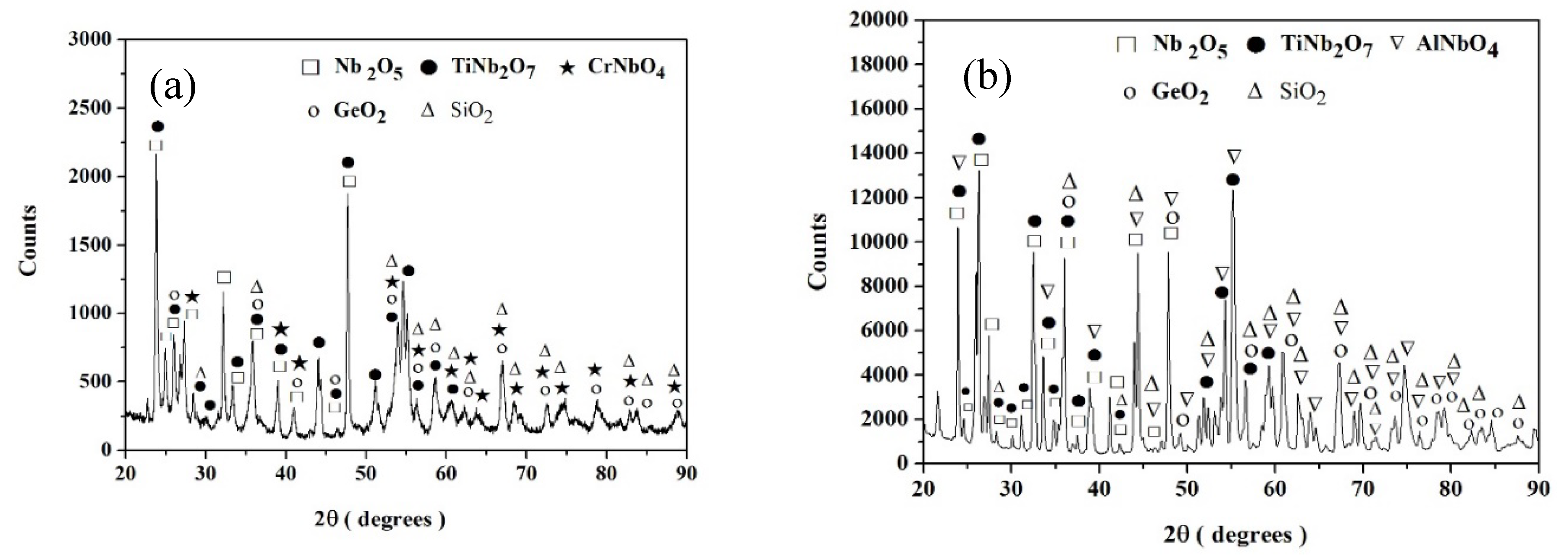
Nb-24Ti-18Si-5Ge-5Cr (alloy ZF4)
Nb-24Ti-18Si-5Ge-5Al (alloy ZF5)
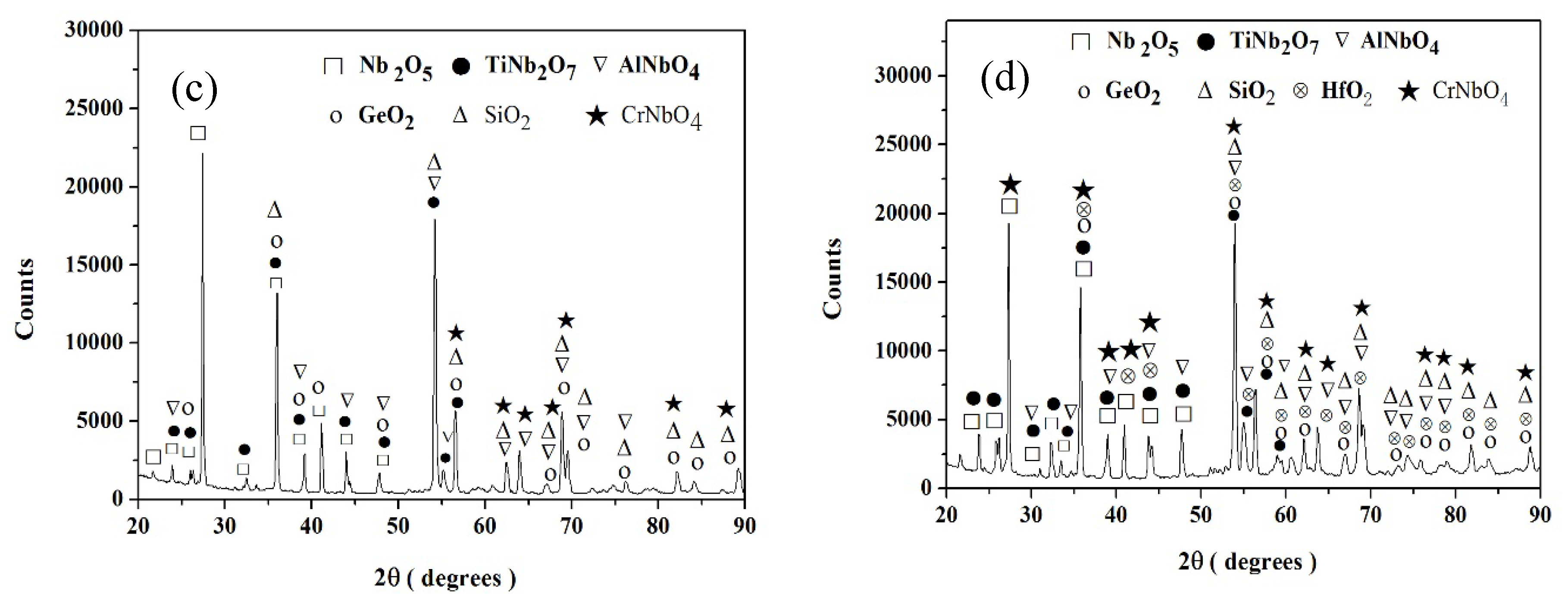
Nb-24Ti-18Si-5Ge-5Cr-5Al (alloy ZF6)
Nb-24Ti-18Si-5Ge-5Cr-5Al-5Hf (alloy ZF9)
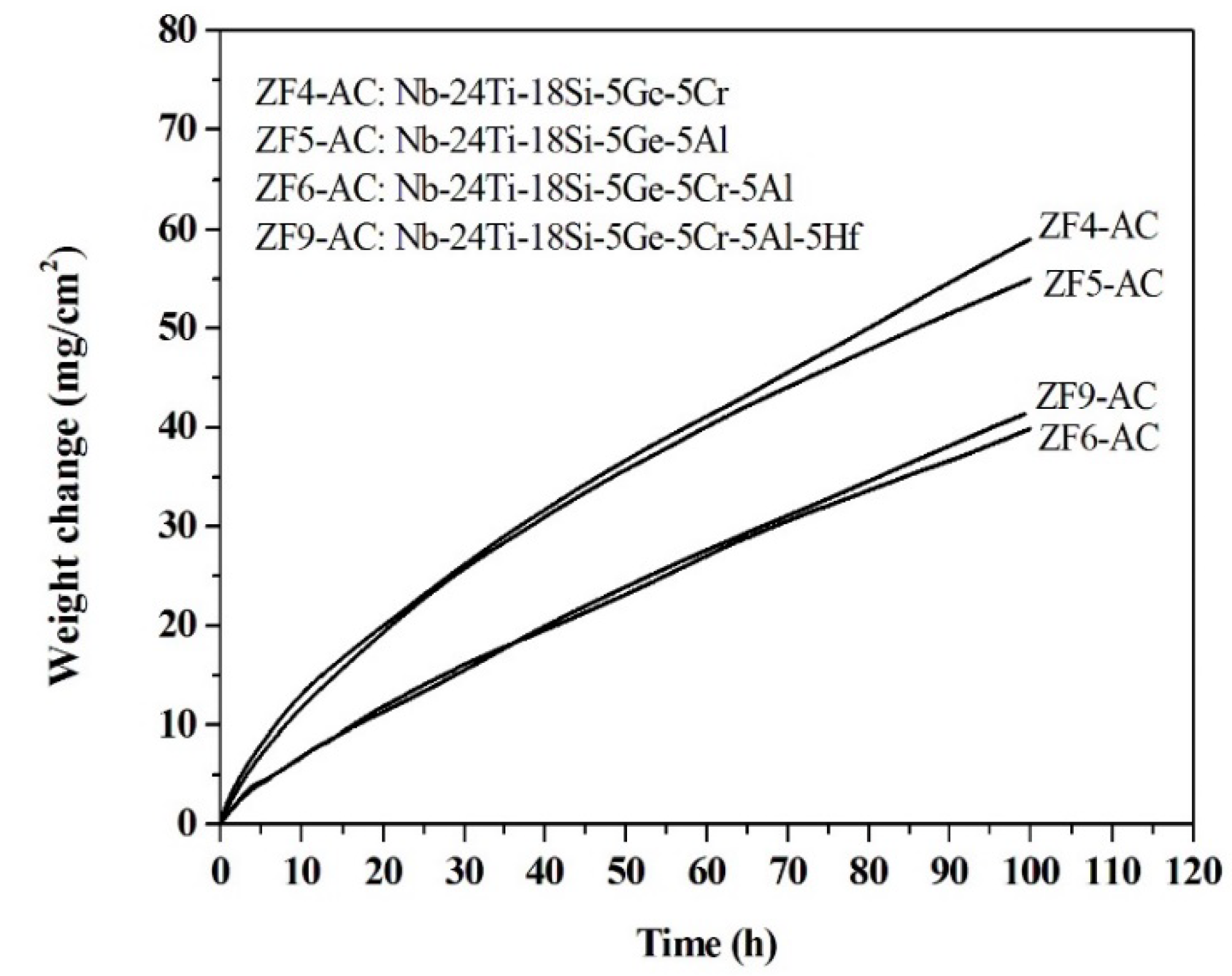
3.2. Oxidation at 1200 °C
4. Discussion
4.1. Oxidation at 800 °C
4.1.1. Pest Oxidation
4.1.2. Oxidation Rates
4.1.3. Role of Microstructure
4.1.4. Scales
4.2. Oxidation at 1200 °C
4.3. Comparison with Refractory Metal HEAs
5. Conclusions
Author Contributions
Funding
Acknowledgements
Conflicts of Interest
References
- Jackson, M.R.; Bewlay, B.P.; Briant, C.L. Creep Resistant Nb Silicide Based Two Phase Composites. U.S. Patent 6447623B1, 10 September 2002. [Google Scholar]
- Jackson, M.R.; Bewlay, B.P.; Zhao, J.-C. Nb Silicide Based Composites Resistant to Low Temperature Pesting. U.S. Patent 6913655B2, 5 July 2005. [Google Scholar]
- Jackson, M.R.; Bewlay, B.P.; Zhao, J.-C. Nb Silicide Based Composites Resistant to High Temperature Oxidation. U.S. Patent 6419765, 16 July 2002. [Google Scholar]
- Tsakiropoulos, P. On Nb silicide based alloys: Alloy design and selection. Materials 2018, 11, 844. [Google Scholar]
- Tsakiropoulos, P. On the alloying and properties of tetragonal Nb5Si3 in Nb-silicide based alloys. Materials 2018, 11, 69. [Google Scholar]
- Tsakiropoulos, P. Alloying and properties of C14-NbCr2 and A15-Nb3X (X = Al, Ge, Si, Sn) in Nb-silicide based alloys. Materials 2018, 11, 395. [Google Scholar]
- Tsakiropoulos, P. On the Nb silicide based alloys: Part I—The bcc Nb solid solution. J Alloys Compd. 2017, 708, 961–971. [Google Scholar]
- Prokoshkin, D.A.; Vasil’eva, E.V. Alloys of Niobium; Samarin, A.M., Ed.; Israel Programme for Scientific Translations: Jerusalem, Israel, 1965; pp. 95–217. [Google Scholar]
- Schutze, M. Protective Oxide Scales and Their Breakdown; Wiley: Hoboken, NJ, USA, 1997. [Google Scholar]
- Birks, N.; Meier, G.H.; Pettit, F.S. Introduction to the High Temperature Oxidation of Metals, 2nd ed.; Cambridge University Press: Cambridge, UK, 2006. [Google Scholar]
- Kingery, W.D.; Bowen, H.K.; Uhlmann, D.R. Introduction to Ceramics; Wiley: New York, NY, USA, 1975; p. 92. [Google Scholar]
- Okamoto, H. Phase Diagrams for Binary Alloys: Desk Handbook; ASM International: Metals Park, OH, USA, 2000. [Google Scholar]
- Meier, G.H.; Pettit, F.S. The oxidation behaviour of intermetallic compounds. Mater. Sci. Eng. A 1992, 153, 548–560. [Google Scholar]
- Tsakiropoulos, P. On Nb silicide based alloys; Part II. J. Alloys Compd. 2018, 748, 569–576. [Google Scholar]
- Kubaschewski, O.; Hopkins, B.E. Oxidation mechanisms of niobium, tantalum, molybdenum and tungsten. J. Less Common Metals 1960, 2, 172–180. [Google Scholar]
- Levin, E.M. Phase equilibria in the system Niobium Pentoxide-Germanium Dioxide. J. Res. Natl. Bur. Stand. A Phys. Chem. 1966, 70, 5–10. [Google Scholar]
- Touloukian, Y.S.; Kirby, P.K.; Taylor, R.E.; Lee, T.Y.R. Thermophysical Properties of Matter; IFI/Plenum: New York, NY, USA, 1977; Volume 13, p. 1354. [Google Scholar]
- Breusov, O.N.; Dremin, A.N. Report on Contract N68171-96-C-9022; US Army: Arlington, VA, USA, 1997.
- Svedberg, R. Oxides associated with the improved air oxidation performance of some niobium intermetallics and alloys. In Properties of High Temperature Alloys; Foroulis, Z.A., Pettit, F.S., Eds.; The Electrochemical Society: Pennington, NJ, USA, 1976; p. 331. [Google Scholar]
- Grill, R. A Gnadenberger, Niobium as mint metal: Production-Properties-Processing. Int. J. Refract. Met. Hard Mater. 2006, 24, 275–282. [Google Scholar]
- Stringer, J.F. High Temperature Corrosion of Aerospace Alloys; AGARD-AG 200; AGARD-NATO: Paris, France, 1975. [Google Scholar]
- Thandorn, T.; Tsakiropoulos, P. Study of the role of B addition on the microstructure of the Nb-24Ti-18Si-8B alloy. Intermetallics 2010, 18, 1033–1038. [Google Scholar]
- Vellios, N.; Tsakiropoulos, P. Study of the role of Fe and Sn additions in the microstructure of Nb-24Ti-18Si-5Cr silicide base alloys. Intermetallics 2010, 18, 1726–1736. [Google Scholar]
- Geng, J.; Tsakiropoulos, P.; Shao, G. A study of the effects of Hf and Sn additions on the microstructure of Nbss/Nb5Si3 based in situ composites. Intermetallics 2007, 15, 69–76. [Google Scholar]
- Geng, J.; Tsakiropoulos, P.; Shao, G. Oxidation of Nb-Si-Cr-Al-Mo in situ composites with Ti and/or Hf additions. Mater. Sci. Eng. A 2006, 441, 26–38. [Google Scholar]
- Geng, J.; Tsakiropoulos, P.; Shao, G. A thermo-gravimetric and microstructural study of the oxidation of Nbss/Nb5Si3 based in situ composites with Sn addition. Intermetallics 2007, 15, 270–281. [Google Scholar]
- Li, Z.; Tsakiropoulos, P. Study of the effect of Ge addition on the microstructure of Nb-18Si in situ composites. Intermetallics 2010, 18, 1072–1078. [Google Scholar]
- Li, Z.; Tsakiropoulos, P. Study of the effect of Ti and Ge in the microstructure of Nb-24Ti-18Si-5Ge in situ composite. Intermetallics 2011, 19, 1291–1297. [Google Scholar]
- Li, Z.; Tsakiropoulos, P. Study of the effects of Cr and Ti additions in the microstructure of Nb-18Si-5Ge based in situ composites. Intermetallics 2012, 26, 18–25. [Google Scholar]
- Li, Z.; Tsakiropoulos, P. The microstructure of Nb-18Si-5Ge-5Al and Nb-24Ti-18Si-5Ge-5Al in situ composites. J. Alloys Compd. 2013, 550, 553–560. [Google Scholar]
- Li, Z.; Tsakiropoulos, P. On the microstructure and hardness of the Nb-24Ti-18Si-5Al-5Cr-5Ge and Nb-24Ti-18Si-5Al-5Cr-5Ge-5Hf (at.%) silicide based alloys. Materials 2019, 12, 2655. [Google Scholar]
- Menon, E.S.K.; Mendiratta, M.G.; Dimiduk, D.M. High temperature oxidation mechanisms in Nb-silicide bearing multicomponent alloys. In Structural Intermetallics; Hemker, K.J., Dimiduk, D.M., Clemens, H., Darolia, R., Inui, H., Larsen, J.M., Sikka, V.K., Thomas, M., Whittenberger, J.D., Eds.; TMS: Warrendale, PA, USA, 2001; pp. 591–600. [Google Scholar]
- He, Y.-R.; Rapp, R.A.; Tortorelli, P.F. Oxidation-resistant Ge doped silicide coating on Cr-Cr2Nb alloys by pack cementation. Mater. Sci. Eng. A 1997, 222, 109–117. [Google Scholar]
- Mueller, A.; Wang, G.; Rapp, R.A.; Courtright, E.L.; Kircher, T.A. Oxidation behaviour of tungsten and germanium alloyed molybdenum disilicide coatings. Mat. Sci. Eng. A 1992, 155, 199–207. [Google Scholar]
- Stergiou, A.; Tsakiropoulos, P. The intermediate and high-temperature oxidation behaviour of (Mo,X)Si2 (X = W, Ta) intermetallic alloys. Intermetallics 1997, 5, 117–126. [Google Scholar]
- Cockeram, B.V. Growth and oxidation resistance of boron-modified and germanium-doped silicide diffusion coatings formed by the halide activated pack cementation method. Surf. Coat. Technol. 1995, 76–77, 20–27. [Google Scholar]
- Su, L.; Jia, L.; Weng, J.; Hong, Z.; Zhou, C.; Zhang, H. Improvement in the oxidation resistance of Nb-Ti-Si-Cr-Al-Hf alloys containing Ge an B. Corros. Sci. 2014, 88, 460–465. [Google Scholar]
- Huang, Q.; Kang, Y.; Song, J.; Qu, S.; Han, Y.; Guo, X. Effects of Ni, Co, B and Ge on the microstructure and mechanical properties of Nb-Ti-Si ternary alloys. Met. Mater. Int. 2014, 20, 475–481. [Google Scholar]
- Chan, K.S.; Davidson, D.L. Improving the fracture toughness of constituent phases and Nb-based in situ composites by a computational alloy design approach. Metal. Mater. Trans. A 2003, 34, 1833–1849. [Google Scholar]
- Kellner, P.; Völkl, R.; Glatzel, U. Microstructural analysis and high temperature creep of Mo-9Si-8B alloys with Al and Ge additions. In Beyond Nickel-Based Superalloys II; Lesley, A., Ed.; ECI Symposium Series; General Electric Global Research: New York, NY, USA, 2016. [Google Scholar]
- Soleimani-Dorcheh, A.; Donner, W.; Galetz, M.C. On ultra-high temperature oxidation of Cr–Cr3Si alloys: Effect of germanium. Mater. Corros. 2014, 65, 1143–1150. [Google Scholar] [CrossRef]
- Hendrik, Y.; Ambardi, P.; Prajitno, D.H. High Temperature Cyclic Oxidation Behaviour Ternary Ni-Al-Ti Alloys doped with Ge at 1200 °C. Rekayasa Mesin 2019, 10, 87–93. [Google Scholar]
- Basuki, E.A.; Prajitno, D.H.; Muhammad, F. Alloys developed for high temperature applications. AIP Conf. Proc. 2017, 1805, 020003. [Google Scholar] [CrossRef]
- Aitken, E.A. Intermetallic Compounds; Westbrook, J.H., Ed.; Wiley: New York, NY, USA, 1967; pp. 491–516. [Google Scholar]
- Argent, B.B.; Phelps, B. The oxidation of niobium-titanium and niobium-molybdenum alloys. J. Less Common Met. 1960, 2, 181–190. [Google Scholar]
- Westbrook, J.H.; Wood, D.L. Pest degradation in beryllides, silicides, aluminides, and related compounds. J. Nucl. Mater. 1964, 12, 208–215. [Google Scholar]
- Zelenitsas, K.; Tsakiropoulos, P. Effect of Al, Cr and Ta additions on the oxidation behaviour of Nb–Ti–Si in situ composites at 800 °C. Mater. Sci. Eng. A 2006, 416, 269–280. [Google Scholar]
- Geng, J.; Tsakiropoulos, P. A Study of the microstructures and oxidation of Nb-Si-Cr-Al-Mo in situ composites with additions of Ti, Hf and Sn. Intermetallics 2007, 15, 382–395. [Google Scholar]
- Xiong, B.W.; Cai, C.C.; Wan, H.; Zheng, Y.H. Effects of Si, W and W–Mo on isothermal oxidation behaviour of Nb/Nb5Si3 in-situ composites at high temperature. J. Alloys Compd. 2009, 486, 330–334. [Google Scholar]
- Geng, J.; Tsakiropoulos, P.; Shao, G. The effects of Ti and Mo additions on the microstructure of Nb-silicide based in situ composites. Intermetallics 2006, 14, 227–235. [Google Scholar]
- Goldschmidt, H.J. An X-ray investigation of Systems between niobium pentoxide and certain additional oxides. Metallurgia 1960, 62, 211–241. [Google Scholar]
- Chan, K.S. Cyclic oxidation response of multiphase niobium-based alloys. Metall. Mater. Trans. A 2004, 35, 589–597. [Google Scholar]
- Khanna, A.S. Introduction to High Temperature Oxidation and Corrosion; ASM: Materials Park, OH, USA, 2002. [Google Scholar]
- Subramanian, P.R.; Mendiratta, M.G.; Dimiduk, D.M.; Stucke, M.A. Advanced intermetallic alloys—Beyond gamma titanium aluminides. Mater. Sci. Eng. A 1997, 239–240, 1–13. [Google Scholar]
- Yao, D.; Cai, R.; Zhou, C.; Sha, J.; Jiang, H. Experimental study and modelling of high temperature oxidation of Nb-base in situ composites. Corros. Sci. 2009, 51, 364–370. [Google Scholar]
- Tsakiropoulos, P. Alloying and hardness of eutectics with Nbss and Nb5Si3 in Nb-silicide based alloys. Materials 2018, 11, 592. [Google Scholar]
- Ghadyani, M.; Utton, C.; Tsakiropoulos, P. Microstructures and isothermal oxidation of the alumina scale forming Nb1.7Si2.4Ti2.4Al3Hf0.5 and Nb1.3Si2.4Ti2.4Al3.5Hf0.4 alloys. Materials 2019, 12, 222. [Google Scholar]
- Hernández-Negrete, O.; Tsakiropoulos, P. On the microstructure and isothermal oxidation at 800, 1200 and 1300 °C of the Al-25.5Nb-6Cr-0.5Hf (at.%) alloy. Materials 2019, 12, 2531. [Google Scholar]
- Zelenitsas, K.; Tsakiropoulos, P. Study of the role of Cr and Al additions in the microstructure of Nb-Ti-Si in situ composites. Intermetallics 2005, 13, 1079–1095. [Google Scholar]
- Nelson, J.; Ghadyani, M.; Utton, C.; Tsakiropoulos, P. A study of the effect of Al, Cr, Hf and Ti additions on the microstructure and oxidation of Nb-24Ti-18Si silicide based alloys. Materials 2018, 11, 1579. [Google Scholar]
- Senkov, O.N.; Senkova, S.V.; Dimiduk, D.M.; Woodward, C.; Miracle, D.B. Oxidation behaviour of a refractory NbCrMo0.5Ta0.5TiZr alloy. J. Mater. Sci. 2012, 47, 6522–6534. [Google Scholar]
- Liu, C.M.; Wang, H.M.; Zhang, S.Q.; Tang, H.B.; Zhang, A.L. Microstructure and oxidation behaviour of new refractory high entropy alloys. J. Alloys Compd. 2014, 583, 762–769. [Google Scholar]

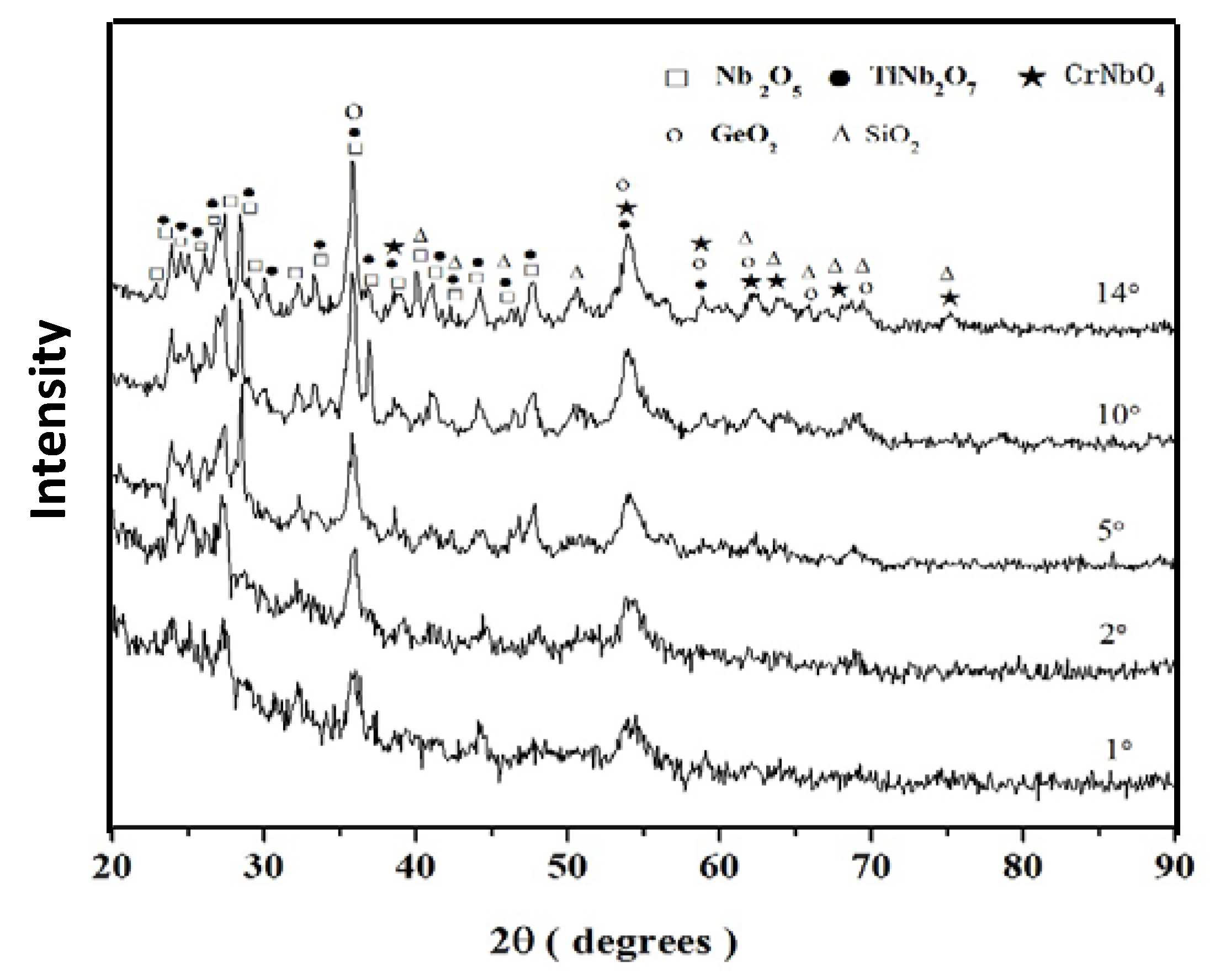
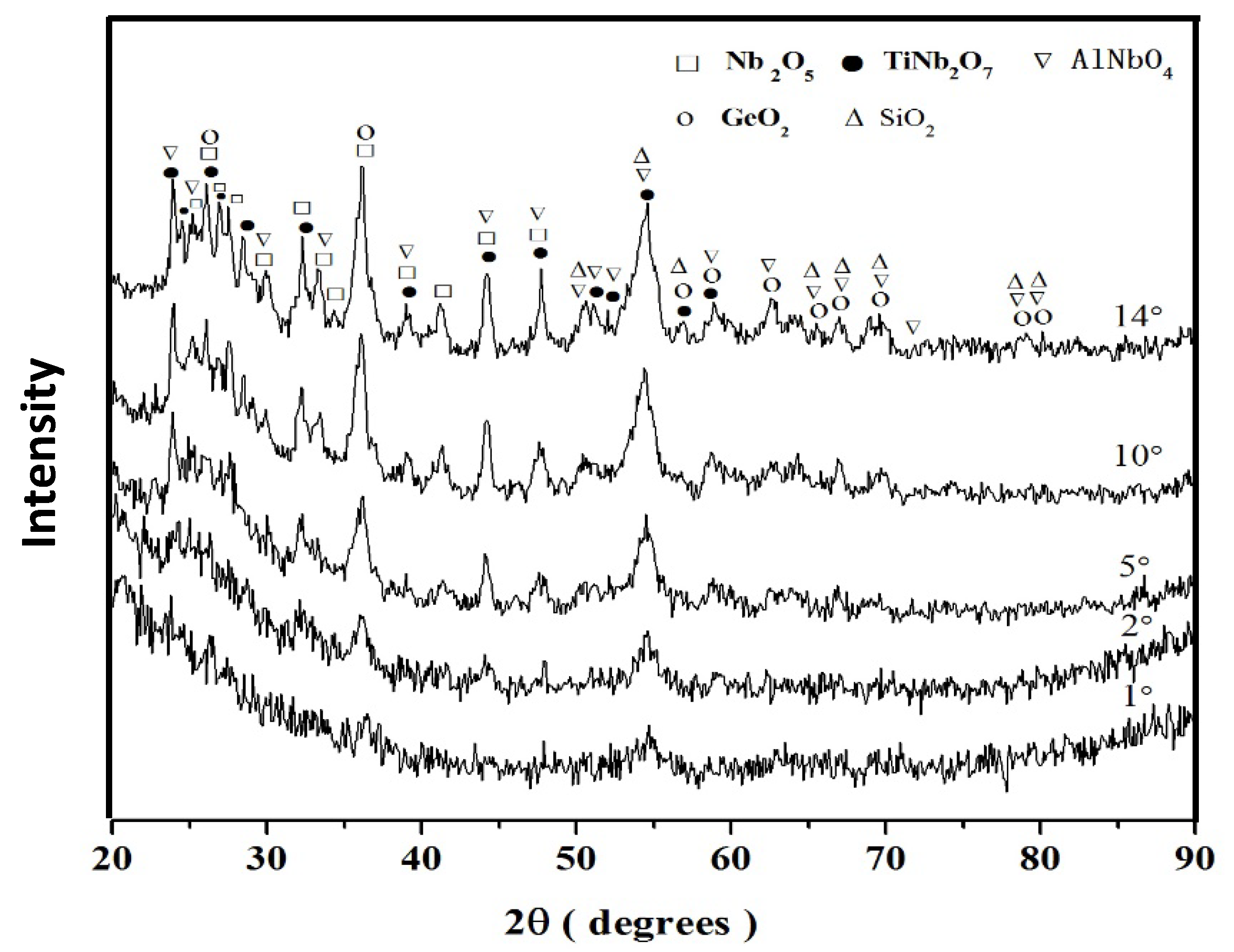
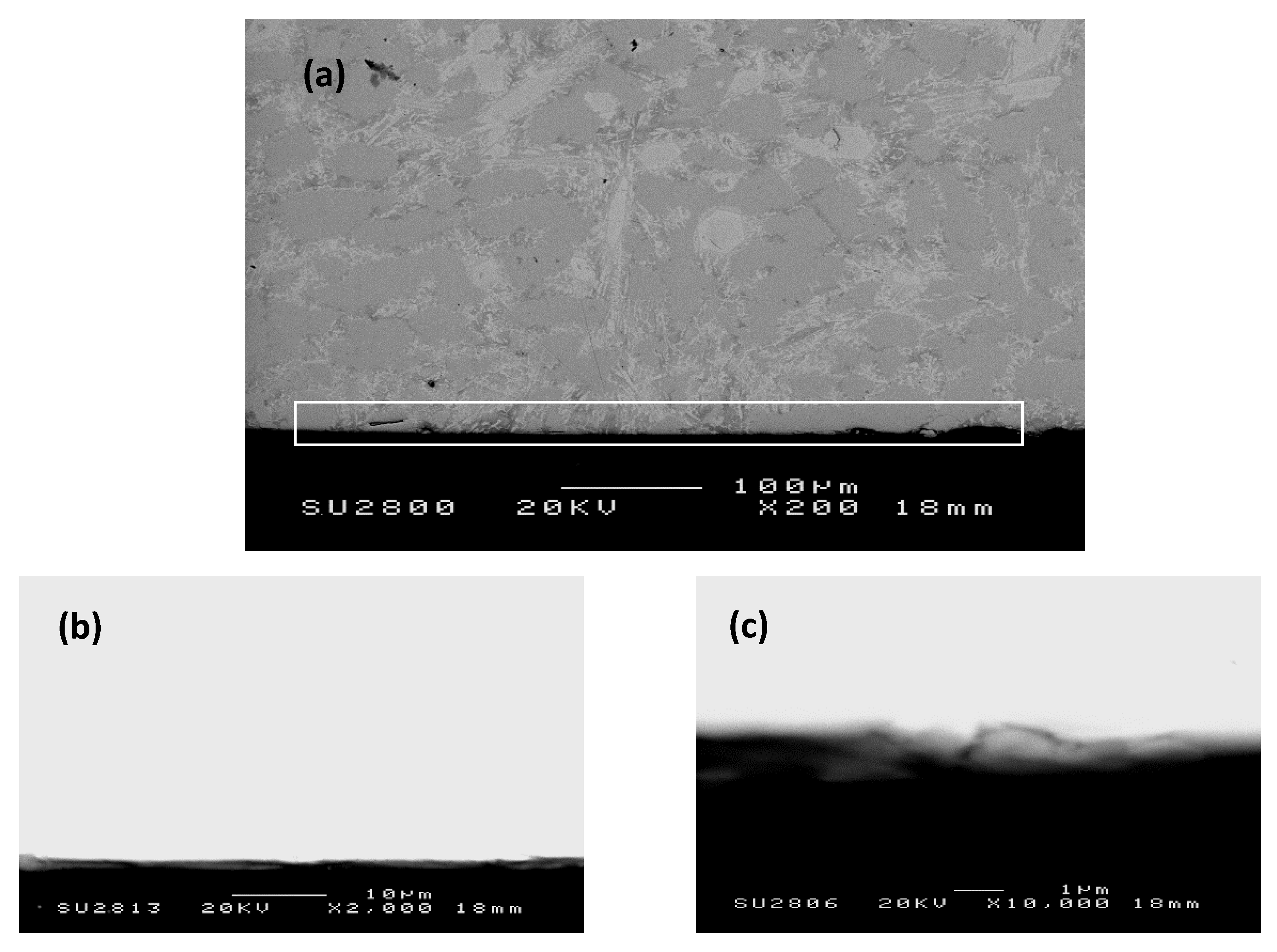

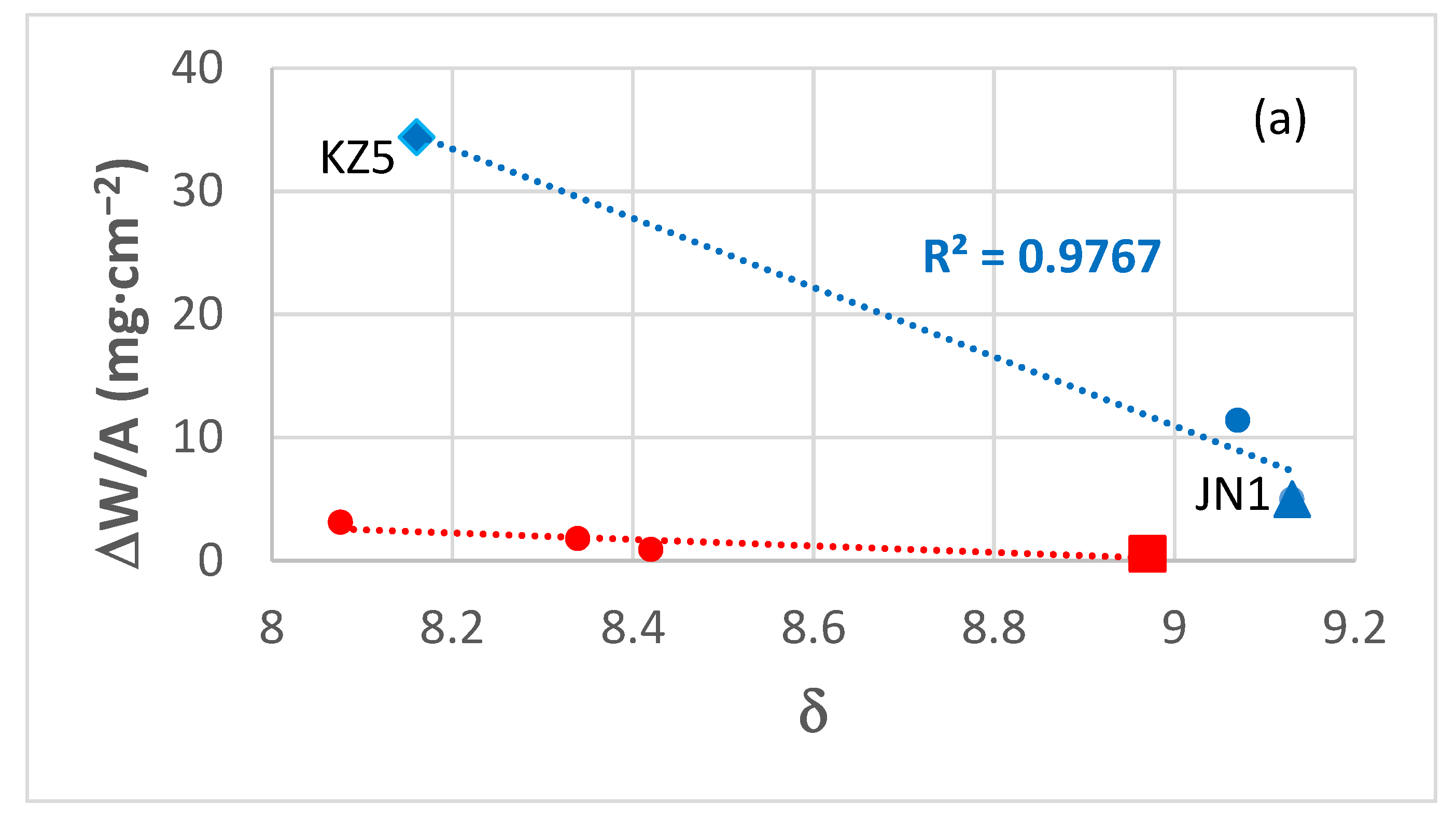
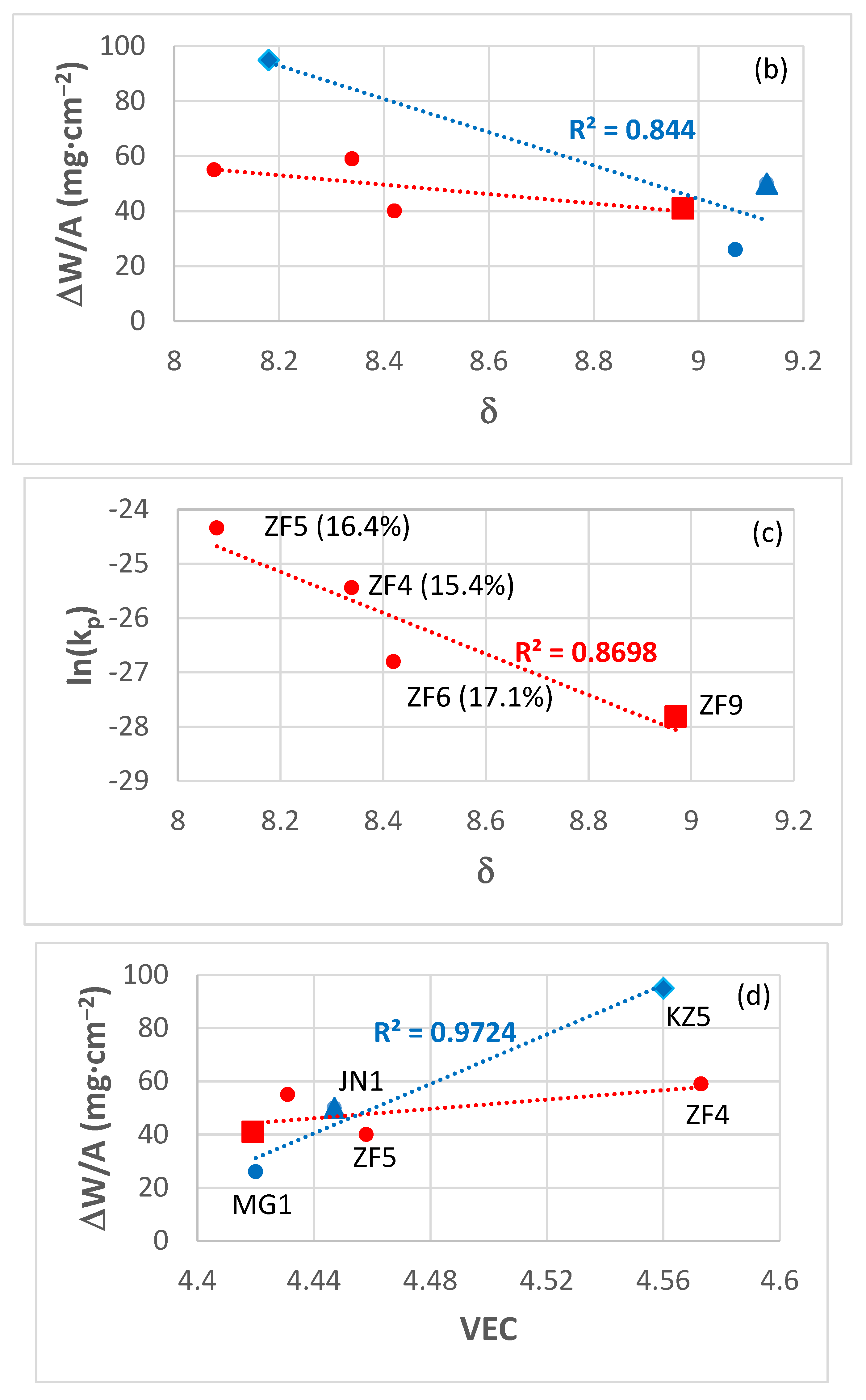
| Alloy | Phases | Ref. |
|---|---|---|
| ZF1 | Nbss, βNb5(Si,Ge)3, (Nbss + Nb5(Si,Ge)3)eutectic | [27] |
| ZF2 | Nbss, βNb5(Si,Ge)3, (Nbss + Nb5(Si,Ge)3)eutectic | [27] |
| ZF3 | Nbss, β(Nb,Ti)5(Si,Ge)3, (Ti,Nb)5(Si,Ge)3, (Nb,Ti)3(Si,Ge) | [28] |
| ZF4 | Nbss, βNb5(Si,Ge)3, (Nbss + Nb5(Si,Ge)3)eutectic, C14-NbCr2 | [29] |
| ZF5 | Nbss, βNb5(Si,Ge,Al)3, (Nbss + Nb5(Si,Ge,Al)3)eutectic | [30] |
| ZF6 | Nbss, βNb5(Si,Ge,Al)3, (Nbss + Nb5(Si,Ge,Al)3)eutectic, C14-NbCr2 | [31] |
| ZF9 | Nbss, βNb5(Si,Ge,Al)3, C14-NbCr2 | [31] |
| Alloy Code | Mass Change (mg·cm−2) | kl (g·cm−2·s−1) | kp (g2·cm−4·s−1) | Scale Thickness (μm) |
|---|---|---|---|---|
| ZF1 | 47.0 (0.67 h) | 1.9 × 10−5 (0.67 h) | — | |
| ZF2 | 33.2 (0.67 h) | 1.4 × 10−5 (0.67 h) | — | |
| ZF3 | 9.6 (10 h) | 2.7 × 10−7 (10 h) | — | |
| ZF4 | 1.79 (100 h) | — | 8.9 × 10−12 (100 h) | 50 |
| ZF5 | 3.11 (100 h) | — | 2.7 × 10−11 (100 h) | 13 |
| ZF6 | 0.91 (100 h) | — | 2.3 × 10−12 (100 h) | 8 |
| ZF9 | 0.55 (100 h) | — | 8.4 × 10−13 (100 h) | 1 |
| Area | Phase | Composition (at.%) | |||||
|---|---|---|---|---|---|---|---|
| Nb | Ti | Si | Ge | Cr | O | ||
| Diffusion Zone | Nbss | 51.5 | 18.2 | 1.6 | 1.4 | 5.7 | 21.6 |
| Nb5Si3 | 44.4 | 18.4 | 26.8 | 6.9 | 1.1 | 2.4 | |
| Bulk | Nbss | 69.2 | 21.6 | 1.6 | 1.8 | 5.8 | 0.0 |
| Nb5Si3 | 45.7 | 18.6 | 27.1 | 7.5 | 1.1 | 0.0 | |
| Area | Phase | Composition (at.%) | |||||
|---|---|---|---|---|---|---|---|
| Nb | Ti | Si | Ge | Al | O | ||
| Diffusion Zone | Nbss | 43.1 | 17.9 | 1.3 | 1.3 | 4.4 | 32.0 |
| Nb5Si3 | 44.1 | 17.7 | 25.6 | 6.9 | 3.1 | 2.6 | |
| Bulk | Nbss | 60.7 | 29.5 | 1.7 | 2.0 | 6.1 | 0.0 |
| Nb5Si3 | 42.6 | 22.3 | 23.9 | 7.2 | 4.0 | 0.0 | |
| Area | Phase | Nb | Ti | Si | Ge | Cr | Al | O |
|---|---|---|---|---|---|---|---|---|
| Bulk | Nbss | 53.4 | 27.3 | 1.5 | 1.4 | 8.7 | 7.7 | 0.0 |
| Nb5Si3 | 44.3 | 20.5 | 23.7 | 7.8 | 1.2 | 2.5 | 0.0 |
| Area | Phase | Nb | Ti | Si | Ge | Cr | Al | Hf | O |
|---|---|---|---|---|---|---|---|---|---|
| Bulk | Nbss | 44.4 | 29.1 | 1.5 | 1.1 | 13.4 | 7.5 | 3.0 | 0 |
| Nb5Si3 | 38.4 | 19.6 | 25.2 | 7.1 | 1.2 | 3.6 | 4.9 | 0 | |
| Hf-rich Nb5Si3 | 24.7 | 26.7 | 23.7 | 7.4 | 3.1 | 4.1 | 10.3 | 0 |
| Alloy Code | kp (g2·cm−4·s−1) | kl (g·cm−2·s−1) |
|---|---|---|
| ZF4 | 6.7 × 10−9 (≤35 h) | 1.3 × 10−7 (>35 h) |
| ZF5 | 6.4 × 10−9 (≤35 h) | 1.1 × 10−7 (>35 h) |
| ZF6 | 2.0 × 10−9 (≤20 h) | 9.7 × 10−8 (>20 h) |
| ZF9 | 1.8 × 10−9 (≤20 h) | 1.0 × 10−7 (>20 h) |
| Alloy Code | Thickness of Oxide Scale (µm) |
|---|---|
| ZF4 | 682 ± 20 |
| ZF5 | 686 ± 32 |
| ZF6 | 395 ± 12 |
| ZF9 | 465 ± 27 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Tsakiropoulos, P. The Effect of Ge Addition on the Oxidation of Nb-24Ti-18Si Silicide Based Alloys. Materials 2019, 12, 3120. https://doi.org/10.3390/ma12193120
Li Z, Tsakiropoulos P. The Effect of Ge Addition on the Oxidation of Nb-24Ti-18Si Silicide Based Alloys. Materials. 2019; 12(19):3120. https://doi.org/10.3390/ma12193120
Chicago/Turabian StyleLi, Zifu, and Panos Tsakiropoulos. 2019. "The Effect of Ge Addition on the Oxidation of Nb-24Ti-18Si Silicide Based Alloys" Materials 12, no. 19: 3120. https://doi.org/10.3390/ma12193120
APA StyleLi, Z., & Tsakiropoulos, P. (2019). The Effect of Ge Addition on the Oxidation of Nb-24Ti-18Si Silicide Based Alloys. Materials, 12(19), 3120. https://doi.org/10.3390/ma12193120






