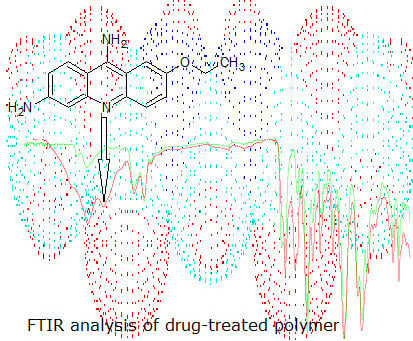
Application of FTIR Method for the Assessment of Immobilization of Active Substances in the Matrix of Biomedical Materials
Abstract
1. Introduction
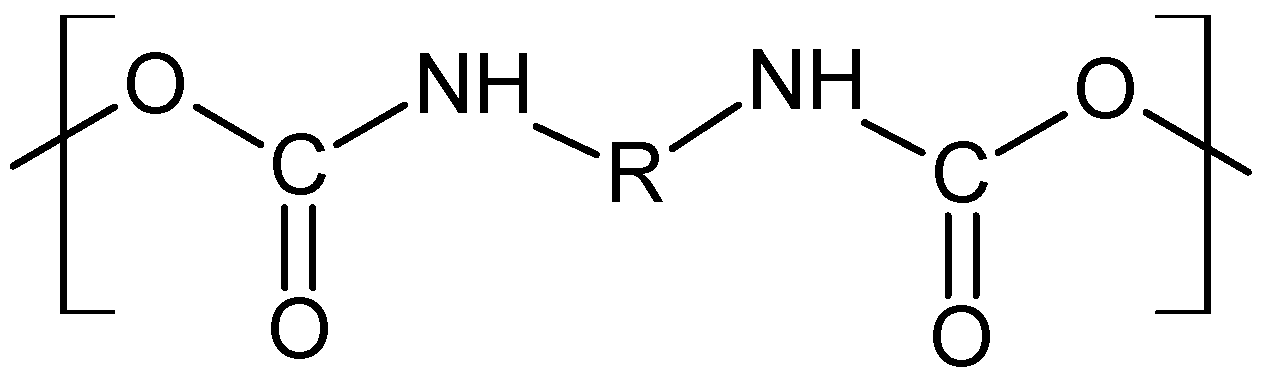
2. Materials and Methods
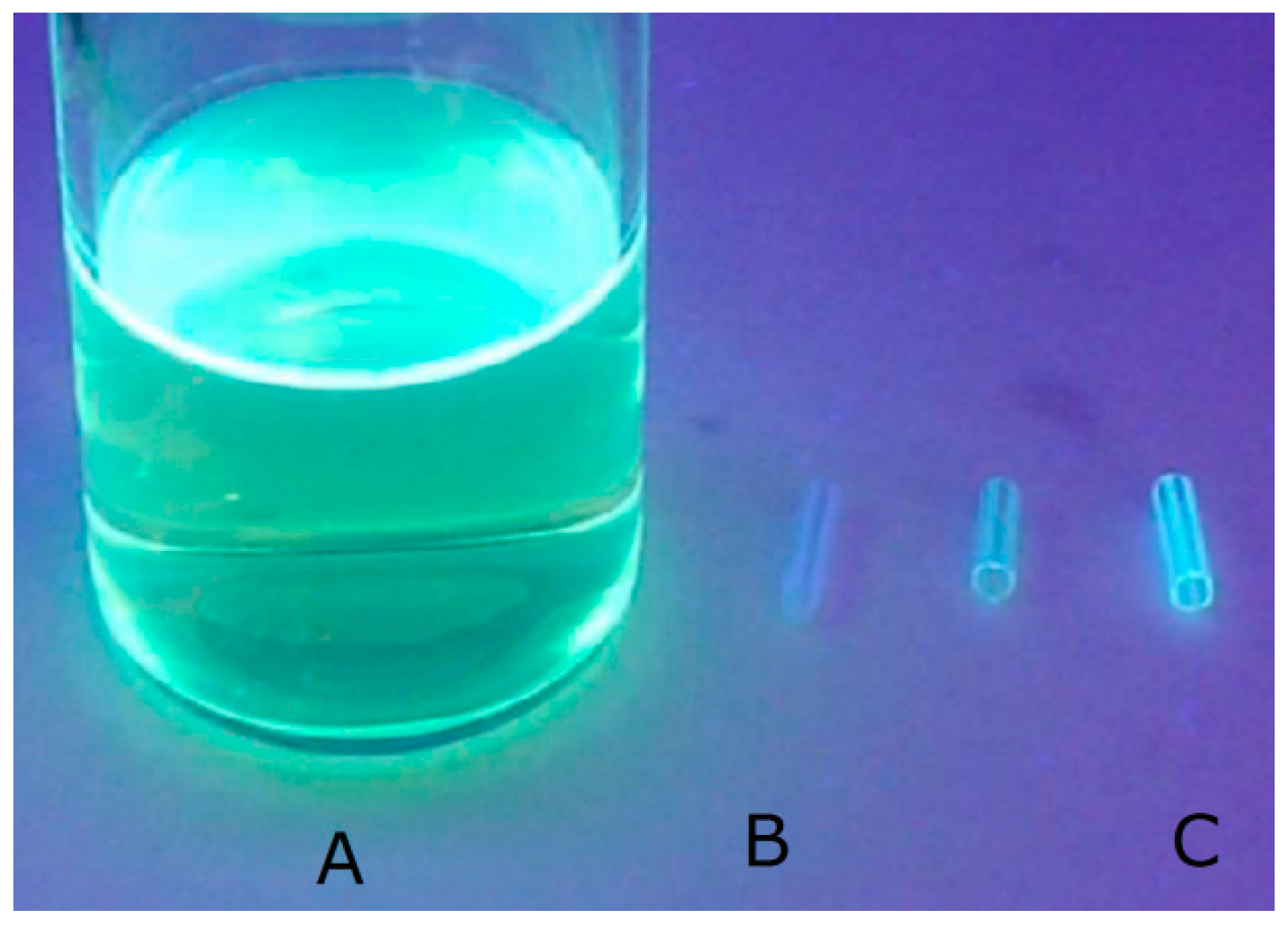
2.1. Preparation of P-2-CA-Treated Catheter Samples
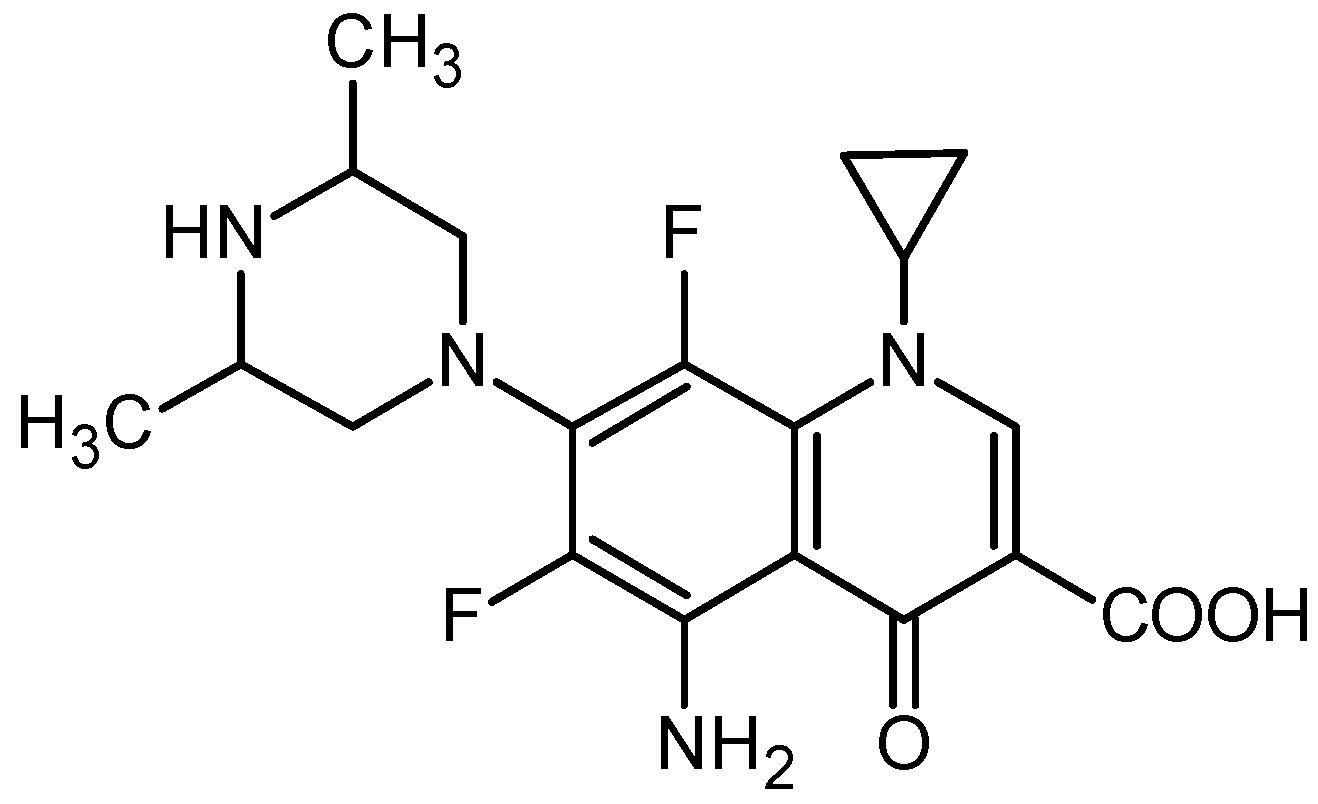
2.2. Preparation of Sparfloxacin-Treated Catheter Samples
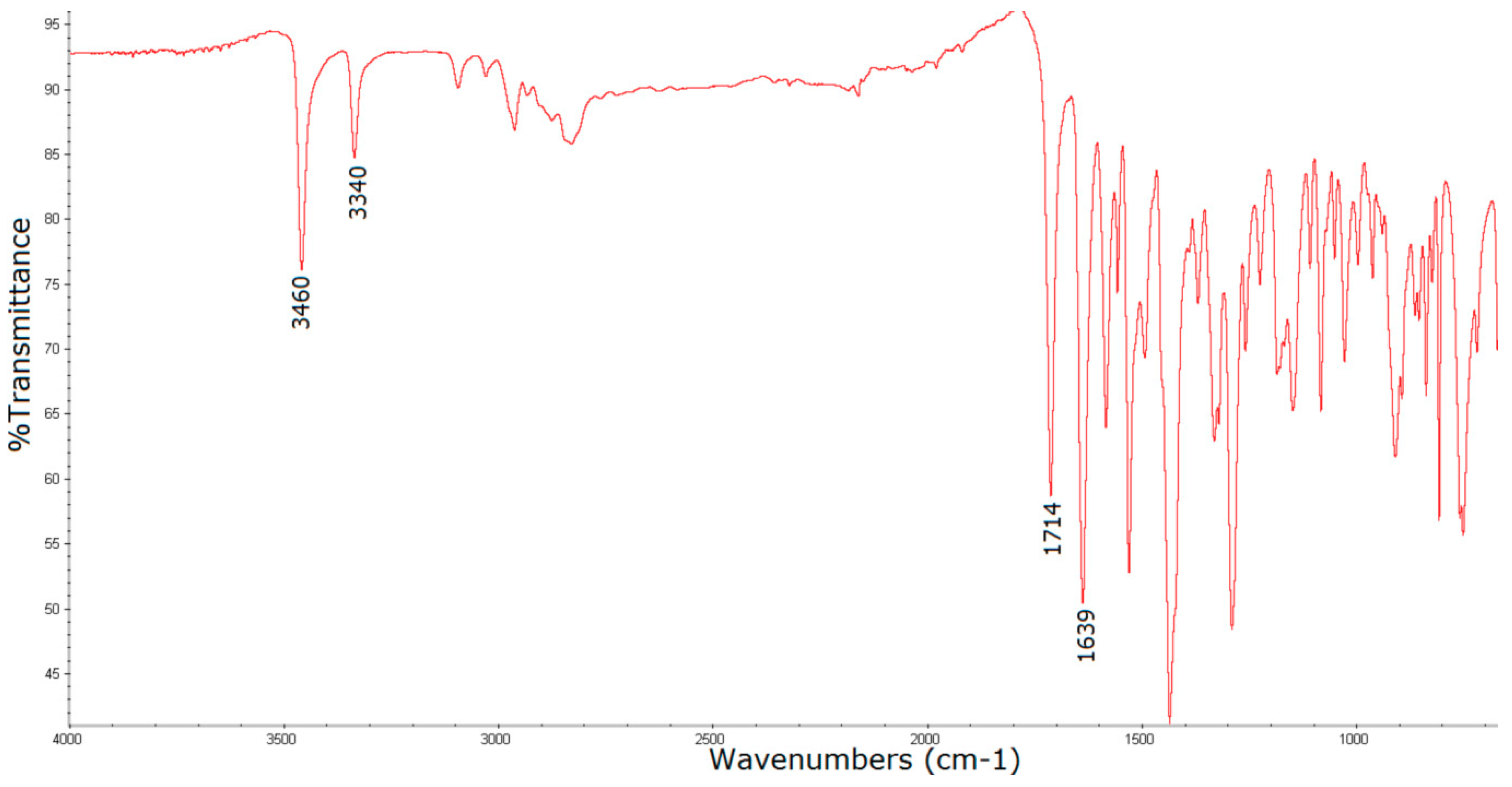
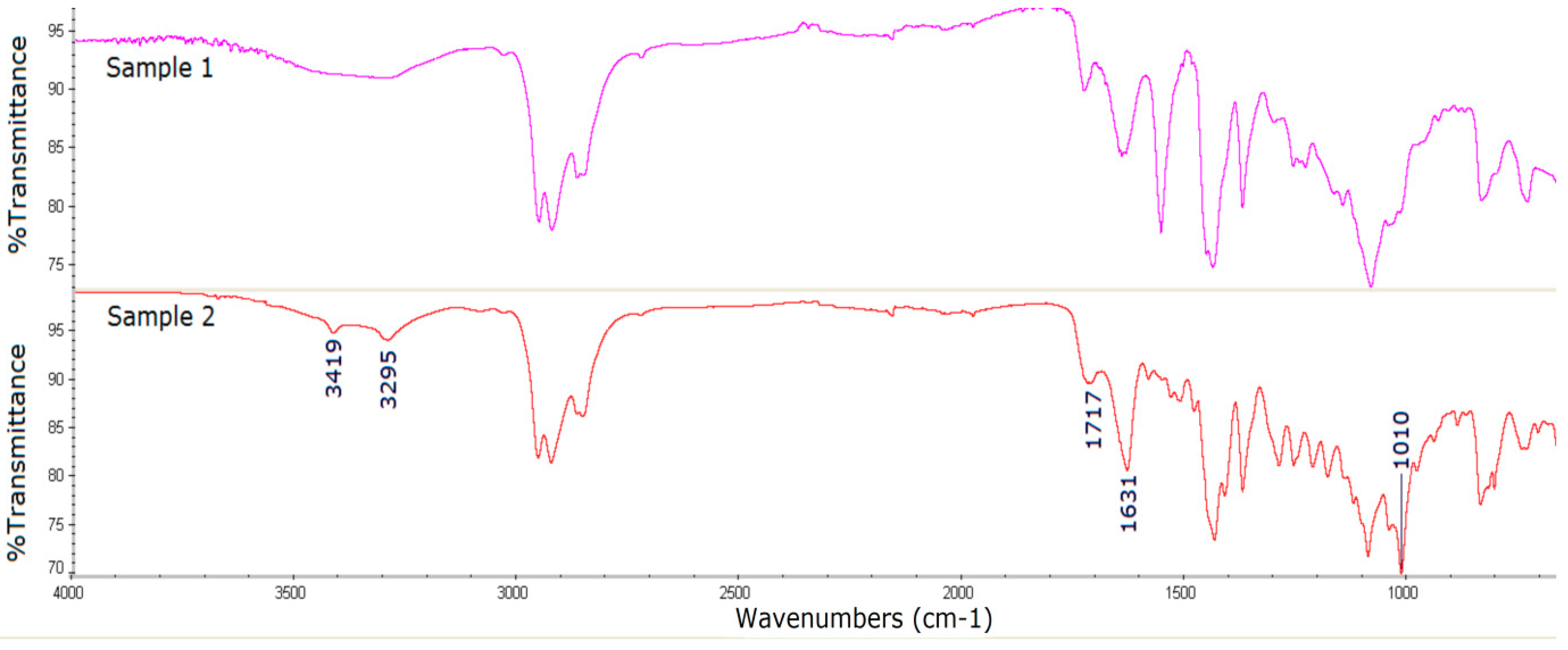
2.3. Preparation of Sethacridine-Treated Catheter Samples
2.4. Fourier Transform Infrared Spectroscopy (FTIR) Characterization
3. Results and Discussion
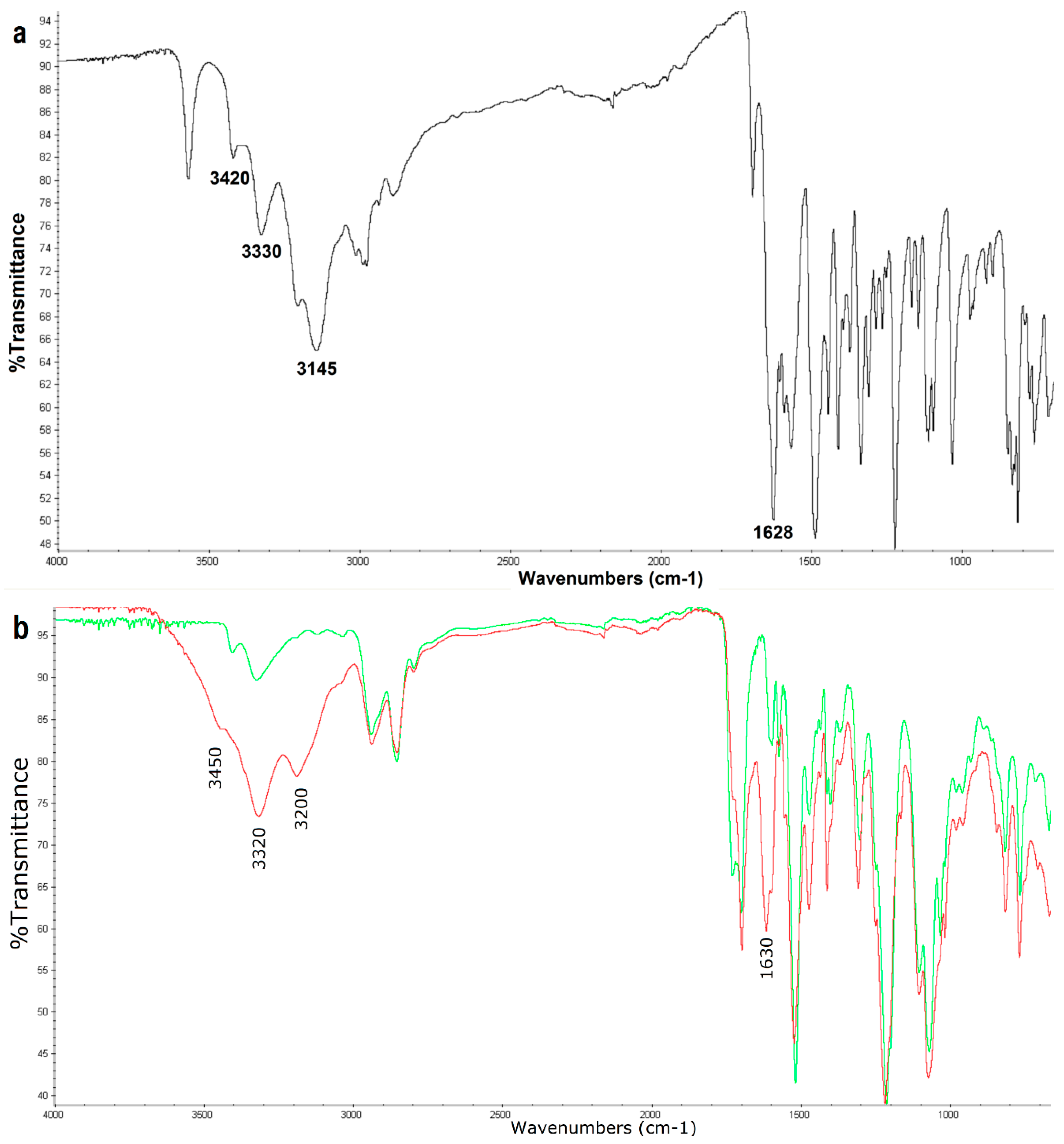
3.1. Surface Characterization of the Porphyrin-Coated Catheter
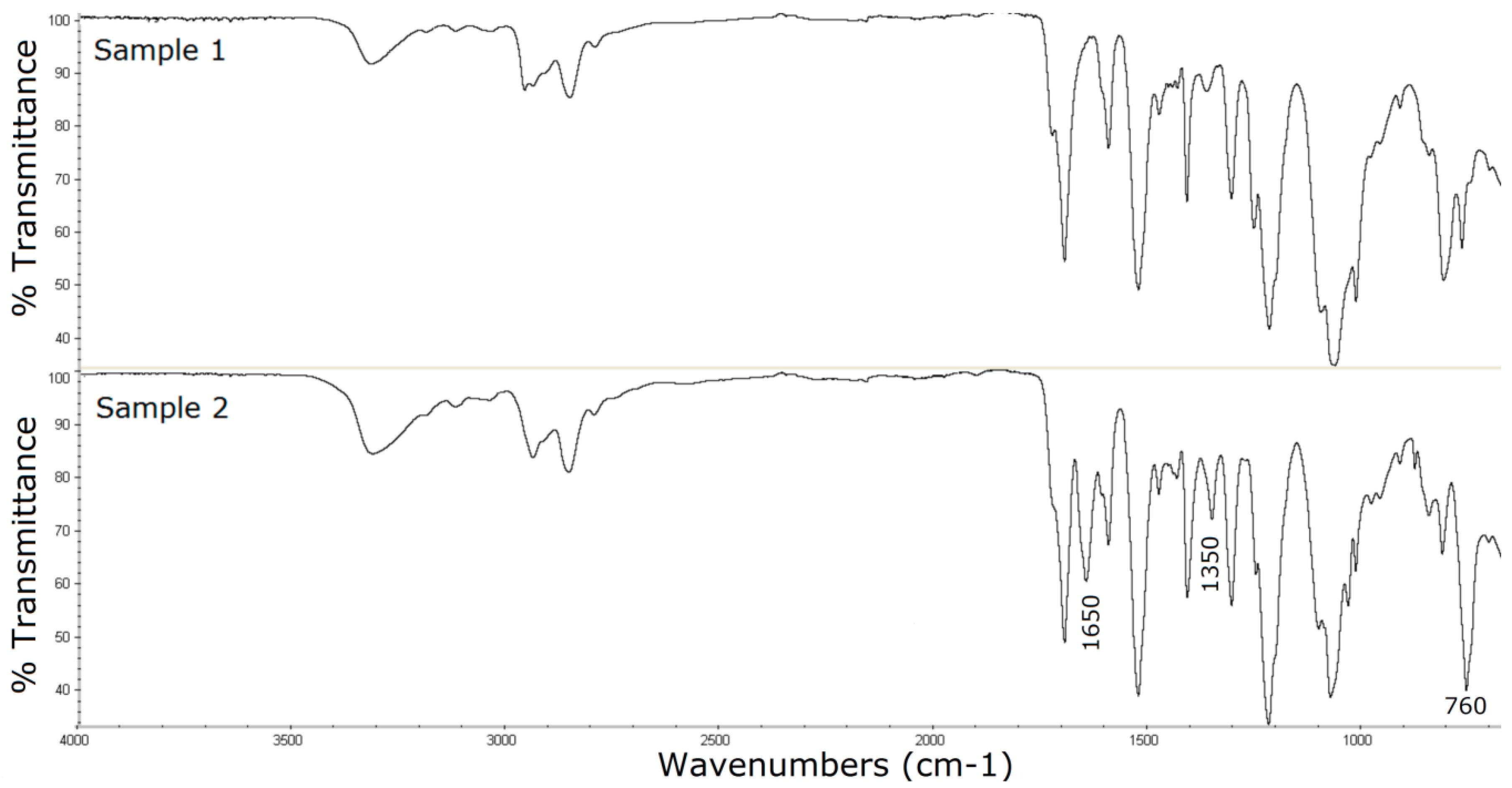
3.2. Surface Characterization of the Catheter Coated with a Chemotherapeutic
3.3. Surface Characterization of the Catheter Coated with an Antiseptic
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dole, M.N.; Patel, P.A.; Sawant, S.D.; Shedpure, P.S. Advance Applications of Fourier Transform Infrared Spectroscopy. Int. J. Pharm. Sci. Rev. Res. 2011, 7, 159–166. [Google Scholar]
- Haas, J.; Mizaikoff, B. Advances in Mid-Infrared Spectroscopy for Chemical Analysis. Annu. Rev. Anal. Chem. 2016, 9, 45–68. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Jing, W.; Wen, Z. Identification of Unknown Mixtures of Materials from Biopharmaceutical Manufacturing Processes by Microscopic-FTIR and Library Searching. Am. Pharm. Rev. 2011, 14, 60. [Google Scholar]
- Stefanowics, Z.; Stefanowics, J.; Mulas, K. Determination of Tropicamide And Its Major Impurity in Raw Material By the HPLC-DAD Analysis and Identification of This Impurity Using the Offline HPLC-FT-IR Coupling. J. Pharm. Biomed. Anal. 2009, 49, 214–220. [Google Scholar] [CrossRef]
- Basiuk, V.A. Quantum chemical calculations of infrared spectra for the identification of unknown compounds by GC/FTIR/MS in exobiological simulation experiments. Adv. Space Res. 2001, 27, 255–260. [Google Scholar] [CrossRef]
- Kumar Pandey, A.; Rapolu, R.; Raju, Ch.K.; Sasalamari, G.; Kumar, G.S.; Awasthi, A.; Navalgund, S.G.; Surendranath, K.V. The novel acid degradation products of losartan: Isolation and characterization using Q-TOF, 2D-NMR and FTIR. J. Pharm. Biomed. Anal. 2016, 120, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Stefan, M.; Vlaicu, I.D.; Nistor, L.C.; Ghica, D.; Nistor, S.V. Origin and chemical composition of the amorphous material from the intergrain pores of self-assembled cubic ZnS:Mn nanocrystals. Appl. Surf. Sci. 2017, 426, 342–350. [Google Scholar] [CrossRef]
- Hakim, S.H.; Shanks, B.H. Synthesis and characterization of hierarchically structured aluminosilicates. J. Mater. Chem. 2011, 21, 7364–7375. [Google Scholar] [CrossRef]
- Spiridon, I.; Teaca, C.-A.; Bodirlau, R. Structural changes evidenced by FTIR spectroscopy in cellulosic materials after pre-treatment with ionic liquid and enzymatic hydrolysis. Bioresources 2011, 6, 400–413. [Google Scholar]
- Roychoudhury, P.; Harvey, L.M.; McNeil, B. The potential of mid infrared spectroscopy (MIRS) for real time bioprocess monitoring. Anal. Chim. Acta. 2006, 571, 159–166. [Google Scholar] [CrossRef]
- Scholz, T.; Lopes, V.V.; Calado, C.R.C. High-throughput analysis of the plasmid bioproduction process in Escherichia coli by FTIR spectroscopy. Biotechnol. Bioeng. 2012, 109, 2279–2285. [Google Scholar] [CrossRef] [PubMed]
- Villar, A.; Gorritxategi, E.; Aranzabe, E.; Fernandez, S.; Otaduy, D.; Fernandez, L.A. Low-cost visible–near infrared sensor for on-line monitoring of fat and fatty acids content during the manufacturing process of the milk. Food Chem. 2012, 135, 2756–2760. [Google Scholar] [CrossRef] [PubMed]
- Wartewig, S.; Neubert, R.H.H. Pharmaceutical applications of Mid-IR and Raman spectroscopy. Adv. Drug Deliv. Rev. 2005, 57, 1144–1170. [Google Scholar] [CrossRef] [PubMed]
- Prati, S.; Joseph, E.; Sciutto, G.; Mazzeo, R. New Advances in the Application of FTIR Microscopy and Spectroscopy for the Characterization of Artistic Materials. Acc. Chem. Res. 2010, 43, 792–801. [Google Scholar] [CrossRef]
- Bi, X.; Yang, X.; Bostrom, M.P.G.; Pleshko Camacho, N. Fourier transform infrared imaging spectroscopy investigations in the pathogenesis and repair of cartilage. Biochim. Biophys. Acta 2006, 1758, 934–941. [Google Scholar] [CrossRef]
- Nabers, A.; Ollesch, J.; Schartner, J.; Kotting, C.; Genius, J.; Haußmann, U.; Klafki, H.; Wiltfang, J.; Gerwert, K. An infrared sensor analysing label-free the secondary structure of the Abeta peptide in presence of complex fluids. J. Biophotonics 2016, 9, 224–234. [Google Scholar] [CrossRef]
- Barrios, V.A.E.; Mendez, J.R.R.; Aguilar, N.V.P.; Espinosa, G.A.; Rodríguez, J.L.D. FTIR—An Essential Characterization Technique for Polymeric Materials. In Materials Science, Engineering and Technology; Theophanides, T., Ed.; IntechOpen: London, UK, 2012; Available online: https://www.intechopen.com/books/infrared-spectroscopy-materials-science-engineering-and-technology/ftir-an-essential-characterization-technique-for-polymeric-materials (accessed on 10 August 2019).
- Bhargava, R.; Wang, S.Q.; Koening, J.L. FTIR Microspectroscopy of polymeric systems. Adv. Polym. Sci. 2003, 163, 137–191. [Google Scholar]
- Al-Ali, A.A.S.; Kassab-Bashi, T.Y. Fourier Transform Infra Red (FTIR) Spectroscopy of New Copolymers of Acrylic Resin Denture Base Materials. IJERSTE 2015, 4, 172–180. [Google Scholar]
- Sardon, H.; Engler, A.C.; Chan, J.M.W.; Coady, D.J.; O’Brien, J.M.; Mecerreyes, D.; Yang, Y.Y.; Hedrick, J.L. Homogeneous isocyanate- and catalyst-free synthesisof polyurethanes in aqueous media. Green Chem. 2013, 15, 1121–1126. [Google Scholar] [CrossRef]
- Schartner, J.; Hoeck, N.; Guldenhaupt, J.; Mavarani, L.; Nabers, A.; Gerwert, K.; Kotting, C. Chemical Functionalization of Germanium with Dextran Brushes for Immobilization of Proteins Revealed by Attenuated Total Reflection Fourier Transform Infrared Difference Spectroscopy. Anal. Chem. 2015, 87, 7467–7475. [Google Scholar] [CrossRef]
- Wilkie, C.A. TGA/FTIR: An extremely useful technique for studying polymer degradation. Polym. Degrad. Stabil. 1999, 66, 301–306. [Google Scholar] [CrossRef]
- Duemichen, E.; Braun, U.; Senz, R.; Fabian, G.; Sturm, H. Assessment of a new method for the analysis of decomposition gases of polymers by a combining thermogravimetric solid-phase extraction and thermal desorption gas chromatography mass spectrometry. J. Chromatogr. A 2014, 1354, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-Y.; Li, M.-S.; Ku, C.-L.; Hsieh, H.-C.; Li, K.-C. Chemical characterization of failures and process materials for microelectronics assembly. Microelectron. Int. 2009, 26, 41–48. [Google Scholar] [CrossRef]
- Zarante, P.H.B.; Sodre, J.R. Comparison of aldehyde emissions simulation with FTIR measurements in the exhaust of a spark ignition engine fueled by ethanol. Int. J. Heat Mass Transf. 2018, 54, 2079–2087. [Google Scholar] [CrossRef]
- Fulk, S.M.; Rochelle, G.T. Quantification of gas and aerosol-phase piperazine emissions by FTIR under variable bench-scale absorber conditions. Energy Procedia 2014, 63, 871–883. [Google Scholar] [CrossRef]
- Navarra, G.; Cannas, M.; D’Amico, M.; Giacomazza, D.; Militello, V.; Vaccaro, L.; Leone, M. Thermal oxidative process in extra-virgin olive oils studied by FTIR, rheology and time-resolved luminescence. Food Chem. 2011, 126, 1226–1231. [Google Scholar] [CrossRef]
- Poiana, M.-A.; Alexa, E.; Melania-Florina Munteanu, M.-F.; Gligor, R.; Moigradean, D.; Mateescu, C. Use of ATR-FTIR spectroscopy to detect the changes in extra virgin olive oil by adulteration with soybean oil and high temperature heat treatment. Open Chem. 2015, 13, 689–698. [Google Scholar] [CrossRef]
- Malek, M.A.; Nakazawa, T.; Kang, H.-W.; Tsuji, K.; Ro, C.-U. Multi-Modal Compositional Analysis of Layered Paint Chips of Automobiles by the Combined Application of ATR-FTIR Imaging, Raman Microspectrometry, and SEM/EDX. Molecules 2019, 24, 1381–1397. [Google Scholar] [CrossRef] [PubMed]
- Amenabar, I.; Poly, S.; Goikoetxea, M.; Nuansing, W.; Lasch, P.; Hillenbrand, R. Hyperspectral infrared nanoimaging of organic samples based on Fourier transform infrared nanospectroscopy. Nat. Commun. 2017, 8, 14402. [Google Scholar] [CrossRef] [PubMed]
- Asadinezhad, A.; Novak, I.; Lehocky, M.; Sedlarık, V.; Vesel, A.; Junkar, I.; Saha, P.; Chodak, I. A Physicochemical Approach to Render Antibacterial Surfaces on Plasma-Treated Medical-Grade PVC: Irgasan Coating. Plasma Process. Polym. 2010, 7, 504–514. [Google Scholar] [CrossRef]
- He, C.; Wang, M.; Cai, X.; Huang, X.; Li, L.; Zhu, H.; Shen, J.; Yuan, J. Chemically induced graft copolymerization of 2-hydroxyethyl methacrylate onto polyurethane surface for improving blood compatibility. Appl. Surf. Sci. 2011, 258, 755–760. [Google Scholar] [CrossRef]
- Otto, D.P.; Vosloo, H.C.M.; Liebenberg, W.; de Villiers, M.M. Development of microporous drug-releasing films cast from artificial nanosized latexes of poly (styrene-co-methyl methacrylate) or poly(styrene-co-ethyl methacrylate). Eur. J. Pharm. Biopharm. 2008, 69, 1121–1134. [Google Scholar] [CrossRef] [PubMed]
- Abenojar, J.; Martınez, M.A.; Encinas, N.; Velasco, F. Modification of glass surfaces adhesion properties by atmospheric pressure plasma torch. Int. J. Adhes. Adhes. 2013, 44, 1–8. [Google Scholar] [CrossRef]
- Aksoy, A.E.; Hasirci, V.; Hasirci, N. Surface Modification of Polyurethanes with Covalent Immobilization of Heparin. Macromol. Symp. 2008, 269, 145–153. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, T.; Jiang, X.; Gu, N. The Surface Modification of Medical Polyurethane to Improve the Hydrophilicity and Lubricity: The Effect of Pretreatment. J. Appl. Polym. Sci. 2010, 116, 1284–1290. [Google Scholar] [CrossRef]
- You, D.; Liang, H.; Mai, W.; Zeng, R.; Tu, M.; Zhao, J.; Zha, Z. Microwave-assisted functionalization of polyurethane surface for improving blood compatibility. J. Ind. Eng. Chem. 2013, 19, 1587–1592. [Google Scholar] [CrossRef]
- Ignat, L.; Ignat, M.; Ciobanu, C.; Doroftei, F.; Popa, V.I. Effects of flax lignin addition on enzymatic oxidation of poly (ethylene adipate) urethanes. Ind. Crops Prod. 2011, 34, 1017–1028. [Google Scholar] [CrossRef]
- Rytwo, G.; Zakai, R.; Wicklein, B. The Use of ATR-FTIR Spectroscopy for Quantification of Adsorbed Compounds. J. Spectrosc. 2015, 727595. [Google Scholar] [CrossRef]
- Morhardt, C.; Ketterer, B.; Heißler, S.; Franzreb, M. Direct quantification of immobilized enzymes by means of FTIR ATR spectroscopy—A process analytics tool for biotransformations applying non-porous magnetic enzyme carriers. J. Mol. Catal. B-Enzym. 2014, 107, 55–63. [Google Scholar] [CrossRef]
- Quintelas, C.; Ferreira, E.C.; Lopes, J.A.; Sousa, C. An Overview of the Evolution of Infrared Spectroscopy Applied to Bacterial Typing. Biotechnol. J. 2018, 13, 1700449. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Perez-Guaita, D.; Andrew, D.W.; Richards, J.S.; McNaughton, D.; Heraud, P.; Wood, B.R. Simultaneous ATR-FTIR Based Determination of Malaria Parasitemia, Glucose and Urea in Whole Blood Dried onto a Glass Slide. Anal. Chem. 2017, 89, 5238–5245. [Google Scholar] [CrossRef] [PubMed]
- Paraskevaidia, M.; Moraisb, K.; Limab, K.; Snowden, J.; Saxon, J.; Richardson, A.; Jones, M.; Mann, D.; Allsop, D.; Martin-Hirsch, P.; et al. Differential diagnosis of Alzheimer’s disease using spectrochemical analysis of blood. Proc. Natl. Acad. Sci. USA 2017, 114, E7929–E7938. [Google Scholar] [CrossRef] [PubMed]
- Georgakilas, V.; Otyepka, M.; Bourlinos, A.; Chandra, V.; Kim, N.; Kemp, K.; Hobza, P.; Zboril, R.; Kim, K. Functionalization of Graphene: Covalent and Non-Covalent Approaches, Derivatives and Applications. Chem. Rev. 2012, 112, 6156–6214. [Google Scholar] [CrossRef] [PubMed]
- Garand, E.; Kamrath, M.; Jordan, P.; Wolk, A.; Leavitt, C.; McCoy, A.; Miller, S.; Johnson, M. Determination of non-covalent docking by IR spectroscopy of cold gas-phase complexes. Science 2012, 335, 694–698. [Google Scholar] [CrossRef] [PubMed]
- Kowalczuk, D.; Ginalska, G.; Golus, J. Characterization of the developed antimicrobial urological catheters. Int. J. Pharm. 2010, 402, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Kowalczuk, D.; Ginalska, G.; Przekora, A. The cytotoxicity assessment of the novel latex urinary catheter with prolonged antimicrobial activity. J. Biomed. Mater. Res. Part A 2011, 98A, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Ginalska, G.; Kowalczuk, D. The Method of Obtaining an Antimicrobial Matrix by Immobilization of a Chemotherapeutic Agent on a Bioactive Surface. Medical University of Lublin, Poland. Patent PL 208173 B1, 31 March 2011. [Google Scholar]
- Kowalczuk, D.; Ginalska, G. The Method for Preparing the Antimicrobial Biomaterial Via Immobilization of the Antibacterial Substance on its Surface. Medical University of Lublin, Poland. Patent PL 214742 B1, 30 September 2013. [Google Scholar]
- Kharkwal, G.B.; Sharma, S.K.; Huang, Y.-Y.; Dai, T.; Hamblin, M.R. Photodynamic Therapy for Infections: Clinical Applications. Lasers Surg Med. 2011, 43, 755–767. [Google Scholar] [CrossRef]
- Rodrigues, G.B.; Dias-Baruffi, M.; Holman, N.; Wainwright, M.; Braga, G.U. In vitro photodynamic inactivation of Candida species and mouse fibroblasts with phenothiazinium photosensitisers and red light. Photodiagnosis Photodyn. Ther. 2013, 10, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Hu, M.; Ma, D.; Lei, J.; Xu, J. Photodynamic inactivation of antibiotic-resistant bacteria and biofilms by hematoporphyrin monomethyl ether. Lasers Med. Sci. 2016, 31, 297–304. [Google Scholar] [CrossRef]
- Khatoon, Z.; McTiernan, C.D.; Suuronen, E.J.; Mah, T.-F.; Alarcon, E.I. Bacterial biofilm formation on implantable devices and approaches to its treatment and prevention. Heliyon 2018, 4, e01067. [Google Scholar] [CrossRef]
- Martínez-Carpio, P.A.; Alcolea-López, J.M.; Vélez, M. Efficacy of photodynamic therapy in the short and medium term in the treatment of actinic keratosis, basal cell carcinoma, acne vulgaris and photoaging: Results from four clinical trials. Laser Ther. 2012, 21, 199–208. [Google Scholar] [PubMed]
- Xu, Z.; Gao, Y.; Meng, S.; Yang, B.; Pang, L.; Wang, C.; Liu, T. Mechanism and In Vivo Evaluation: Photodynamic Antibacterial Chemotherapy of Lysine-Porphyrin Conjugate. Front Microbiol. 2016, 7, 242. [Google Scholar] [CrossRef] [PubMed]
- Lindsey, J.S. Synthesis of meso-Substituted Porphyrins. In The Porphyrin Handbook.; Kadish, K.M., Smith, K.M., Guilard, R., Eds.; Academic Press: San Diego, CA, USA, 2000; Volume 1, pp. 45–118. [Google Scholar]
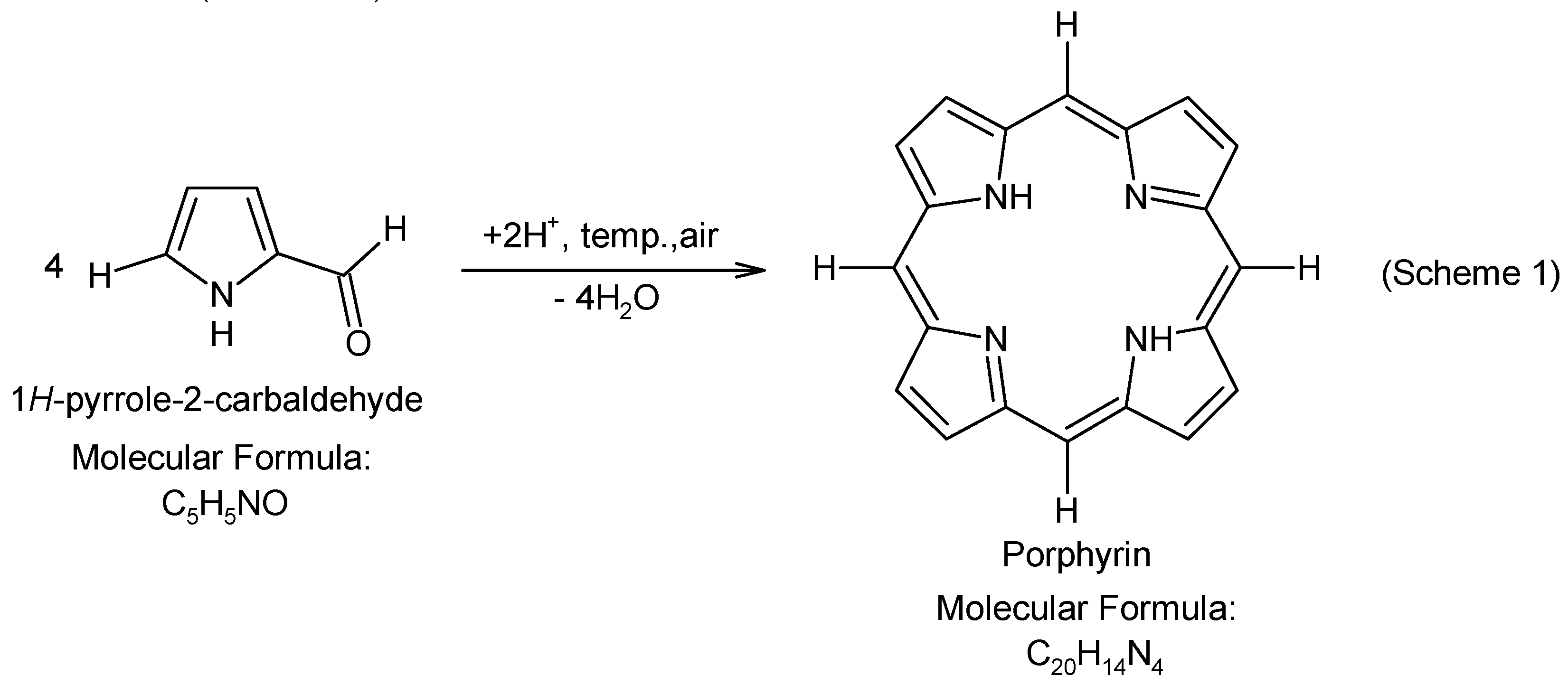
- Gomes, A.T.P.C. Acid Catalysis in the Way to Porphyrins: Reaction of Pyrrole/Aldehydes in the Synthesis of meso-Substituted Porphyrins. Rev. Virtual Quim. 2013, 5, 312–317. [Google Scholar] [CrossRef]
- Mohammadi, A.; Barikani, M.; Barmar, M. Synthesis and investigation of thermal and mechanical properties of in situ prepared biocompatible Fe3O4/polyurethane elastomer nanocomposites. Polym. Bull. 2015, 72, 219–234. [Google Scholar] [CrossRef]
- Asefnejad, A.; Khorasani, M.T.; Behnamghader, A.; Farsadzadeh, B.; Bonakdar, S. Manufacturing of biodegradable polyurethane scaffolds based on polycaprolactone using a phase separation method: Physical properties and in vitro assay. Int. J. Nanomed. 2011, 6, 2375–2384. [Google Scholar] [CrossRef] [PubMed]
- Datta, J.; Kosiorek, P.; Włoch, M. Synthesis, structure and properties of poly(ether urethane)s synthesized using a tri-functional oxypropylated glycerol as a polyol. J. Therm. Anal. Calorim. 2017, 128, 155–167. [Google Scholar] [CrossRef][Green Version]
- Sun, Z.-C.; She, Y.-B.; Zhou, Y.; Song, X.-F.; Li, K. Synthesis, Characterization and Spectral Properties of Substituted Tetraphenylporphyrin Iron Chloride Complexes. Molecules 2011, 16, 2960–2970. [Google Scholar] [CrossRef] [PubMed]
- Tenke, P.; Kovacs, B.; Jackel, M.; Nagy, E. The role of biofilm infection in urology. World J. Urol. 2006, 24, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Poh, B.T.; Lee, K.S. FTIR study of thermal oxidation of ENR. Eur. Polym. J. 1994, 30, 17–23. [Google Scholar] [CrossRef]
- Marjanovic, B.; Juranic, I.; Ciric-Marjanovic, G.; Mojovic, M.; Pašti, I.; Janoševic, A.; Trchova, M.; Holler, P.; Horsky, J. Chemical oxidative polymerization of ethacridine. React. Funct. Polym. 2012, 72, 25–35. [Google Scholar] [CrossRef]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kowalczuk, D.; Pitucha, M. Application of FTIR Method for the Assessment of Immobilization of Active Substances in the Matrix of Biomedical Materials. Materials 2019, 12, 2972. https://doi.org/10.3390/ma12182972
Kowalczuk D, Pitucha M. Application of FTIR Method for the Assessment of Immobilization of Active Substances in the Matrix of Biomedical Materials. Materials. 2019; 12(18):2972. https://doi.org/10.3390/ma12182972
Chicago/Turabian StyleKowalczuk, Dorota, and Monika Pitucha. 2019. "Application of FTIR Method for the Assessment of Immobilization of Active Substances in the Matrix of Biomedical Materials" Materials 12, no. 18: 2972. https://doi.org/10.3390/ma12182972
APA StyleKowalczuk, D., & Pitucha, M. (2019). Application of FTIR Method for the Assessment of Immobilization of Active Substances in the Matrix of Biomedical Materials. Materials, 12(18), 2972. https://doi.org/10.3390/ma12182972