The Role of Fluorine in F-La/TiO2 Photocatalysts on Photocatalytic Decomposition of Methanol-Water Solution
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Materials
2.2. Characterization of Materials
2.3. Photocatalytic Test
3. Results and Discussion
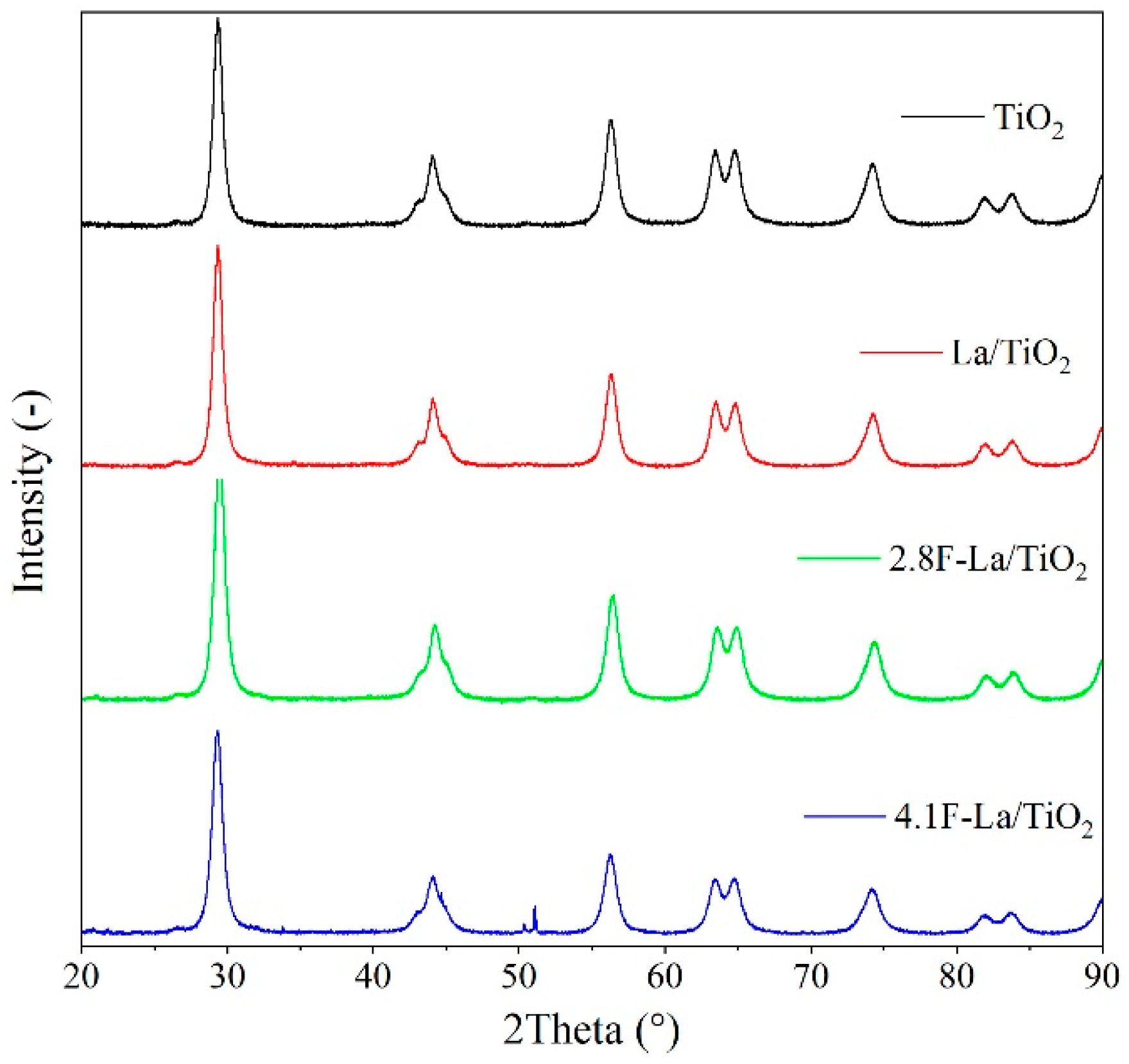
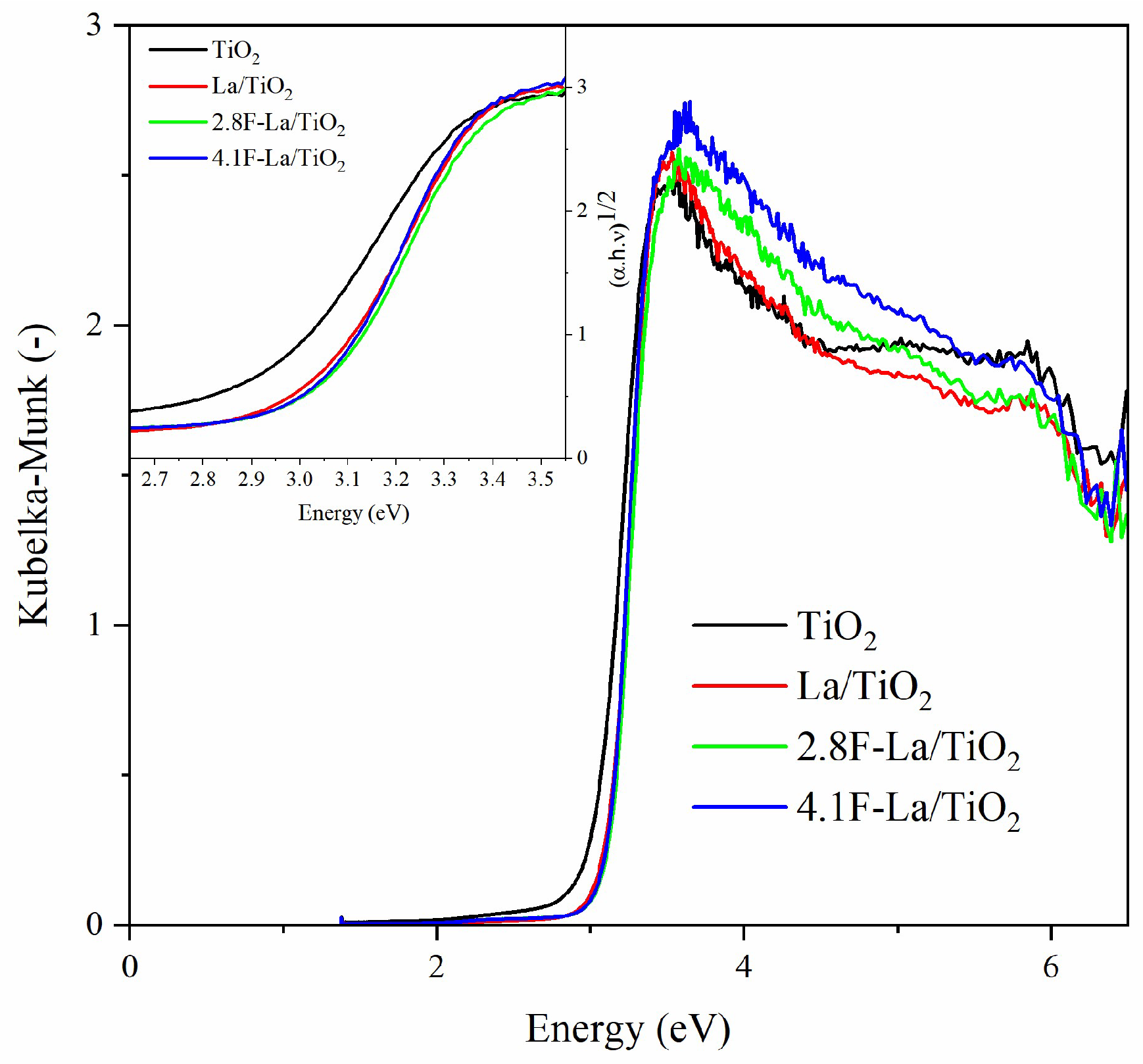
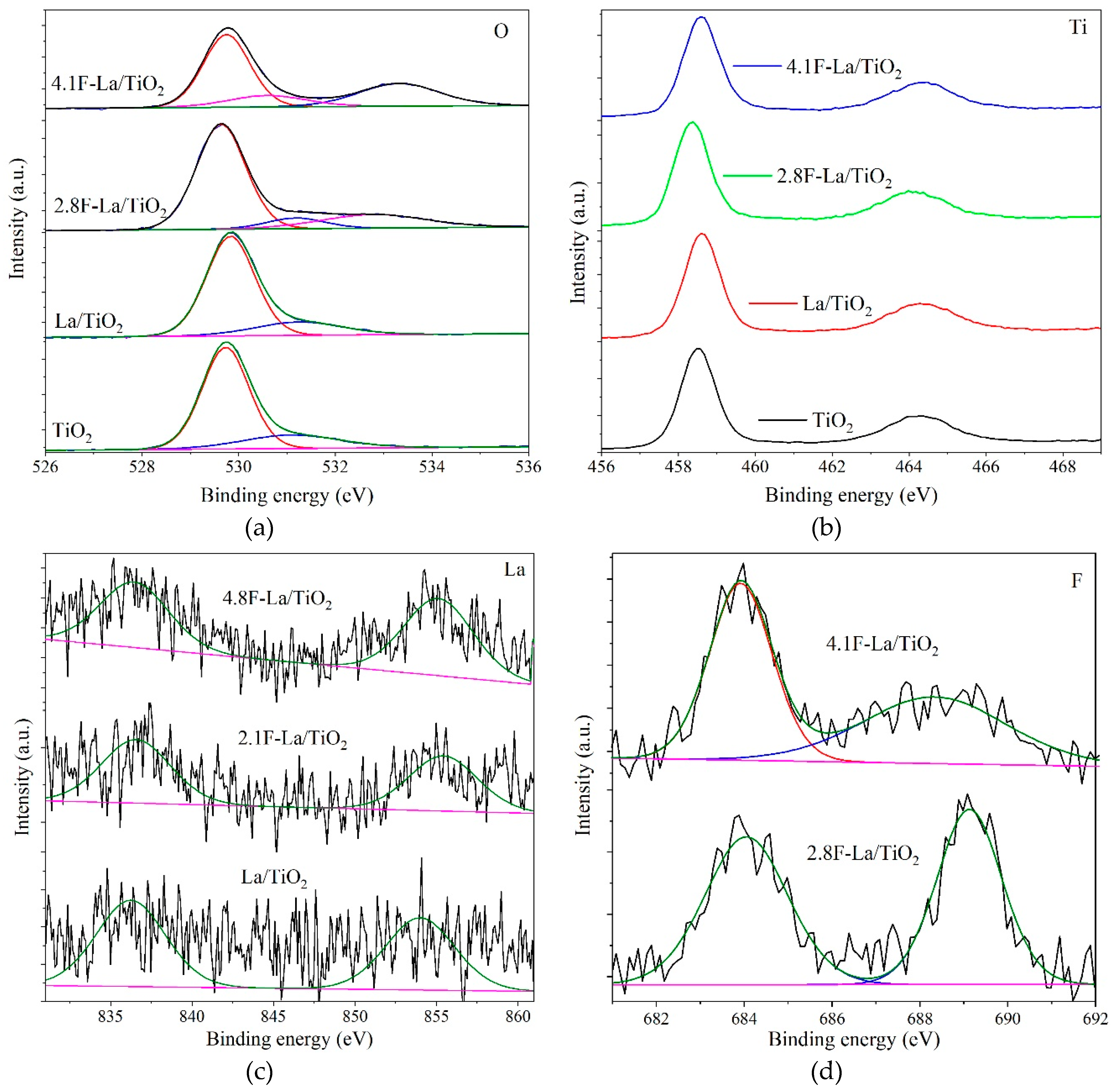
3.1. Structural and Optical Properties of La/and F-La/TiO2
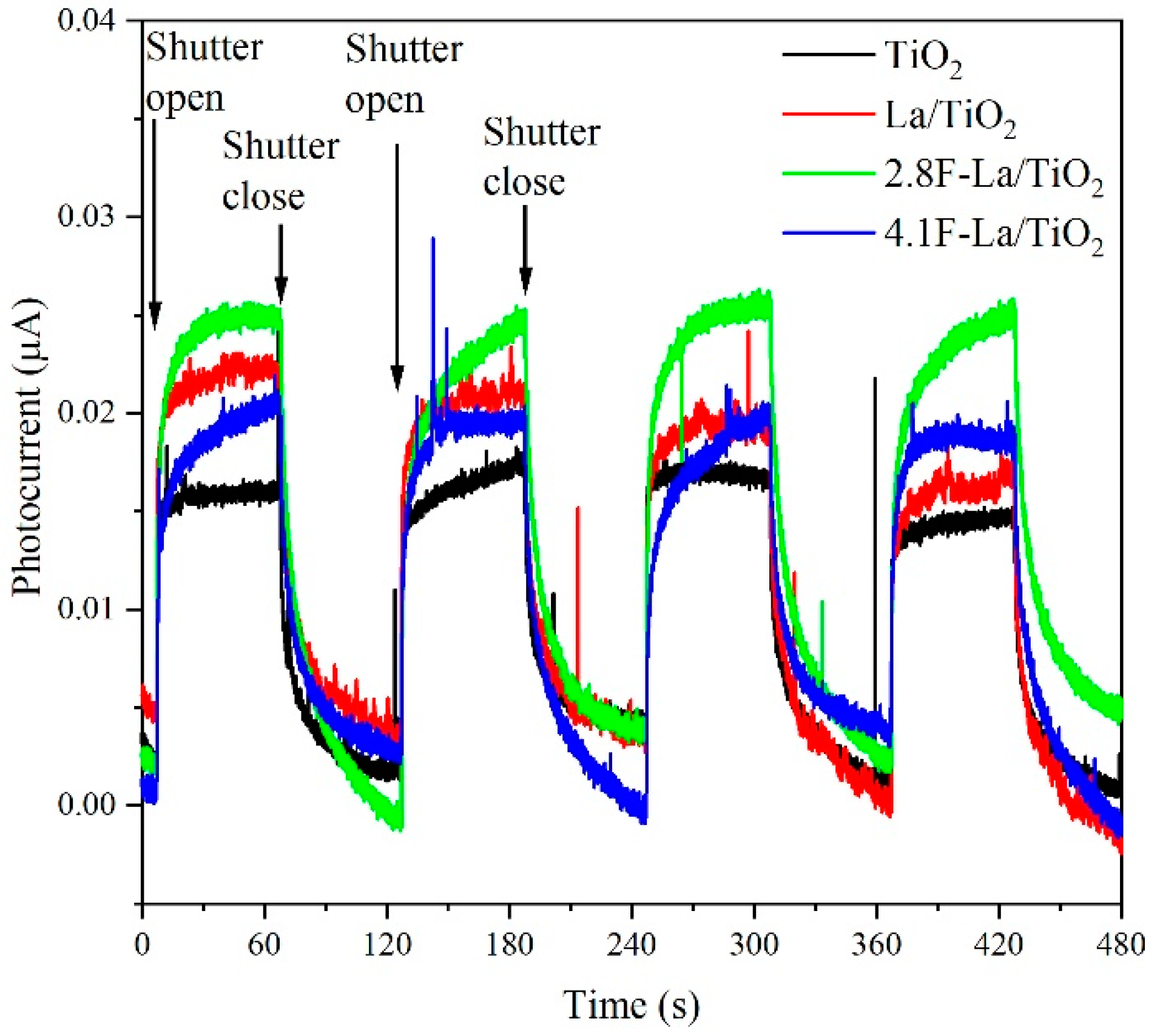
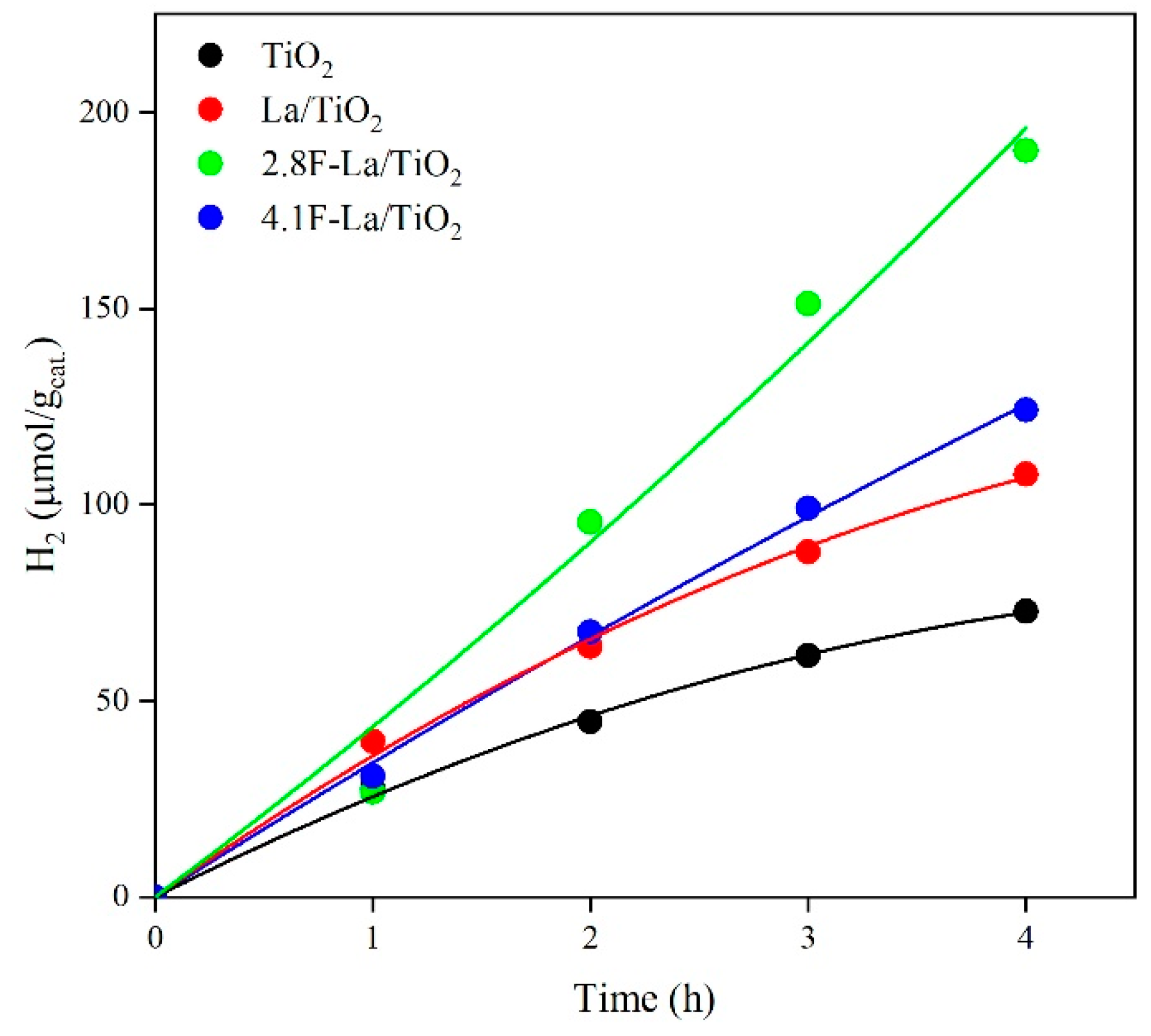
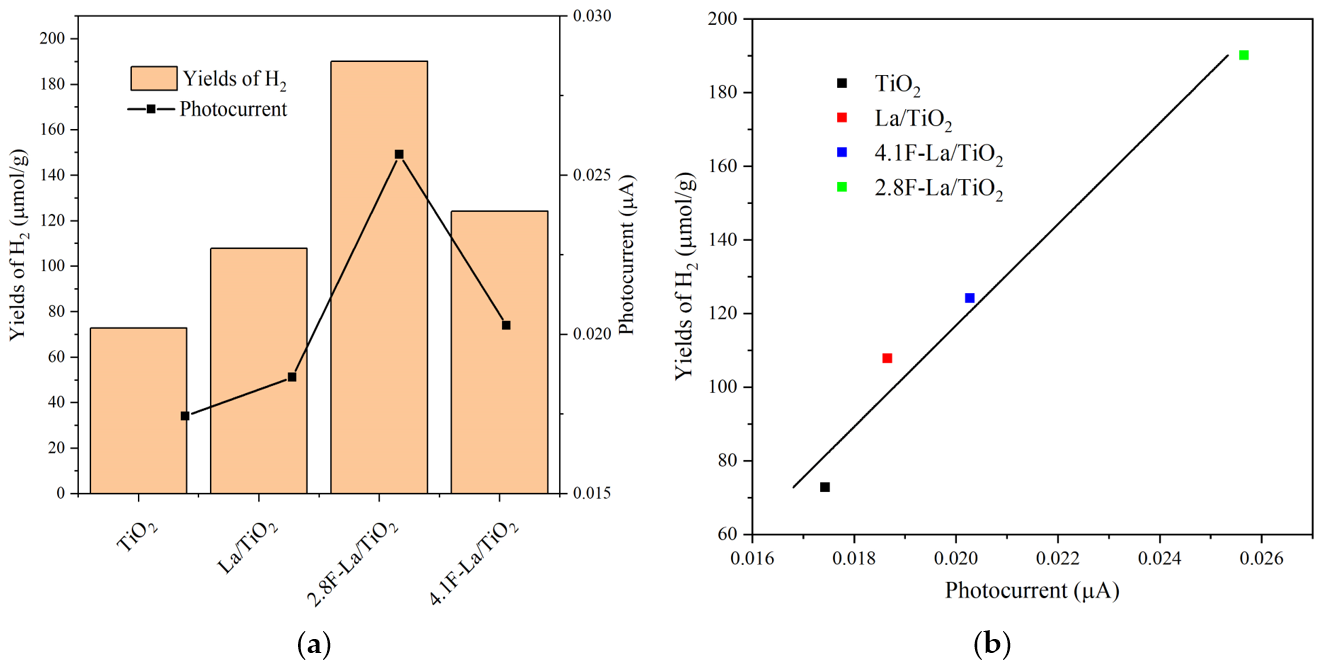
3.2. Photocatalytic Activity of La/and F-La/TiO2
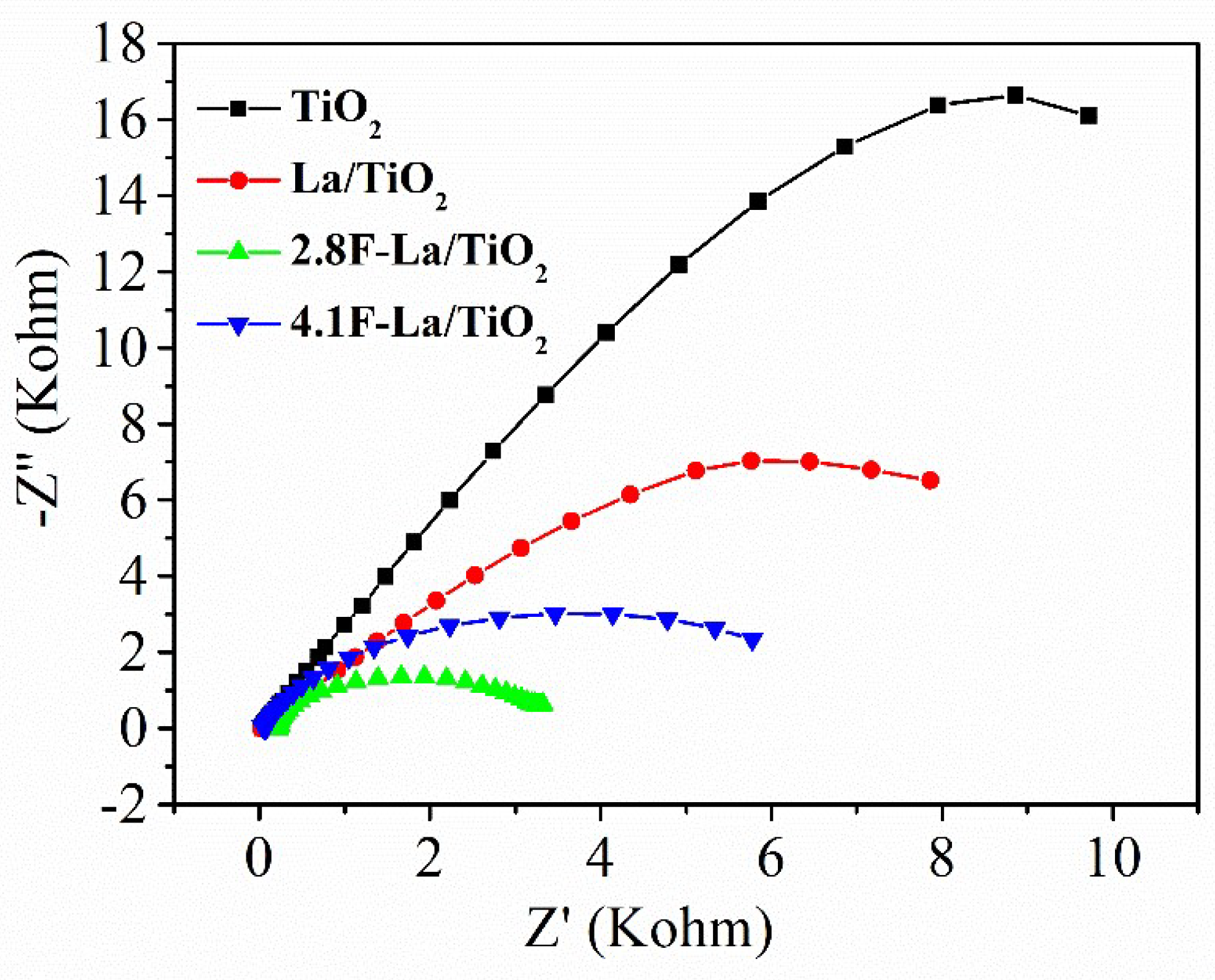
3.3. Properties of F-La/TiO2 Playing the Role in Photacatalytic Reaction
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Fajrina, N.; Tahir, M. A critical review in strategies to improve photocatalytic water splitting towards hydrogen production. Int. J. Hydrogen Energy 2019, 44, 540–577. [Google Scholar] [CrossRef]
- Kumaravel, V.; Mathew, S.; Bartlett, J.; Pillai, S.C. Photocatalytic hydrogen production using metal doped TiO2: A review of recent advances. Appl. Catal. B 2019, 244, 1021–1064. [Google Scholar] [CrossRef]
- Wang, Z.; Li, C.; Domen, K. Recent developments in heterogeneous photocatalysts for solar-driven overall water splitting. Chem. Soc. Rev. 2019, 48, 2109–2125. [Google Scholar] [CrossRef] [PubMed]
- Fang, W.; Xing, M.; Zhang, J. Modifications on reduced titanium dioxide photocatalysts: A review. J. Photochem. Photobiol. C 2017, 32, 21–39. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, X.; Jia, Y.; Chen, X.; Han, H.; Li, C. Titanium dioxide-based nanomaterials for photocatalytic fuel generations. Chem. Rev. 2014, 114, 9987–10043. [Google Scholar] [CrossRef] [PubMed]
- Low, J.; Cheng, B.; Yu, J. Surface modification and enhanced photocatalytic CO2 reduction performance of TiO2: A review. Appl. Surf. Sci. 2017, 392, 658–686. [Google Scholar] [CrossRef]
- Gomes, J.; Lincho, J.; Domingues, E.; Quinta-Ferreira, M.R.; Martins, C.R. N–TiO2 Photocatalysts: A Review of Their Characteristics and Capacity for Emerging Contaminants Removal. Water 2019, 11, 373. [Google Scholar] [CrossRef]
- Devaiah, D.; Jampaiah, D.; Saikia, P.; Reddy, B.M. Structure dependent catalytic activity of Ce0.8Tb0.2O2−δ and TiO2 supported Ce0.8Tb0.2O2−δ solid solutions for CO oxidation. J. Ind. Eng. Chem. 2014, 20, 444–453. [Google Scholar] [CrossRef]
- Smirniotis, P.G.; Boningari, T.; Damma, D.; Inturi, S.N.R. Single-step rapid aerosol synthesis of N-doped TiO2 for enhanced visible light photocatalytic activity. Catal. Commun. 2018, 113, 1–5. [Google Scholar] [CrossRef]
- Low, J.; Yu, J.; Jaroniec, M.; Wageh, S.; Al-Ghamdi, A.A. Heterojunction Photocatalysts. Adv. Mater. 2017, 29, 1601694. [Google Scholar] [CrossRef]
- Wei, L.; Yu, C.; Zhang, Q.; Liu, H.; Wang, Y. TiO2-based heterojunction photocatalysts for photocatalytic reduction of CO2 into solar fuels. J. Mater. Chem. A 2018, 6, 22411–22436. [Google Scholar] [CrossRef]
- Kubacka, A.; Fernández-García, M.; Colón, G. Advanced Nanoarchitectures for Solar Photocatalytic Applications. Chem. Rev. 2012, 112, 1555–1614. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Jeon, B.; Kim, Y.K. Dominant Influence of the Surface on the Photoactivity of Shape-Controlled Anatase TiO2 Nanocrystals. ACS Catal. 2015, 5, 3316–3322. [Google Scholar] [CrossRef]
- Pan, X.; Yang, M.-Q.; Fu, X.; Zhang, N.; Xu, Y.-J. Defective TiO2 with oxygen vacancies: Synthesis, properties and photocatalytic applications. Nanoscale 2013, 5, 3601–3614. [Google Scholar] [CrossRef] [PubMed]
- Bellardita, M.; Garlisi, C.; Venezia, A.M.; Palmisano, G.; Palmisano, L. Influence of fluorine on the synthesis of anatase TiO2 for photocatalytic partial oxidation: Are exposed facets the main actors? Catal. Sci. Technol. 2018, 8, 1606–1620. [Google Scholar] [CrossRef]
- Du, M.; Qiu, B.; Zhu, Q.; Xing, M.; Zhang, J. Fluorine doped TiO2/mesocellular foams with an efficient photocatalytic activity. Catal. Today 2019, 327, 340–346. [Google Scholar] [CrossRef]
- Pan, J.; Liu, G.; Lu, G.Q.; Cheng, H.-M. On the True Photoreactivity Order of {001}, {010}, and {101} Facets of Anatase TiO2 Crystals. Angew. Chem. Int. Ed. 2011, 50, 2133–2137. [Google Scholar] [CrossRef]
- Li, C.; Sun, Z.; Ma, R.; Xue, Y.; Zheng, S. Fluorine doped anatase TiO2 with exposed reactive (001) facets supported on porous diatomite for enhanced visible-light photocatalytic activity. Microporous Mesoporous Mater. 2017, 243, 281–290. [Google Scholar] [CrossRef]
- Kočí, K.; Troppová, I.; Edelmannová, M.; Starostka, J.; Matějová, L.; Lang, J.; Reli, M.; Drobná, H.; Rokicińska, A.; Kuśtrowski, P.; et al. Photocatalytic decomposition of methanol over La/TiO2 materials. Environ. Sci. Pollut. Res. Int. 2018, 25, 34818–34825. [Google Scholar] [CrossRef]
- Dubnová, L.; Zvolská, M.; Edelmannová, M.; Matějová, L.; Reli, M.; Drobná, H.; Kuśtrowski, P.; Kočí, K.; Čapek, L. Photocatalytic decomposition of methanol-water solution over N-La/TiO2 photocatalysts. Appl. Surf. Sci. 2019, 469, 879–886. [Google Scholar] [CrossRef]
- Wu, Y.; Zhou, Z.; Wang, W.; Huang, Y.; Shen, S. A novel and facile method to synthesize crystalline-disordered core–shell anatase (La, F)–TiO2. Mater. Lett. 2013, 98, 261–264. [Google Scholar] [CrossRef]
- Koci, K.; Troppova, I.; Reli, M.; Matejova, L.; Edelmannova, M.; Drobna, H.; Dubnova, L.; Rokicinska, A.; Kustrowski, P.; Capek, L. Nd/TiO2 Anatase-Brookite Photocatalysts for Photocatalytic Decomposition of Methanol. Front. Chem. 2018, 6, 44. [Google Scholar] [CrossRef] [PubMed]
- Kočí, K.; Reli, M.; Edelmannová, M.; Troppová, I.; Drobná, H.; Rokicińska, A.; Kuśtrowski, P.; Dvoranová, D.; Čapek, L. Photocatalytic hydrogen production from methanol over Nd/TiO2. J. Photochem. Photobiol. A 2018. [Google Scholar] [CrossRef]
- Reli, M.; Ambrozova, N.; Sihor, M.; Matejova, L.; Capek, L.; Obalova, L.; Matej, Z.; Kotarba, A.; Koci, K. Novel cerium doped titania catalysts for photocatalytic decomposition of ammonia. Appl. Catal. B 2015, 178, 108–116. [Google Scholar] [CrossRef]
- Wang, H.; Wu, D.; Liu, C.; Guan, J.; Li, J.; Huo, P.; Liu, X.; Wang, Q.; Yan, Y. Fabrication of Ag/In2O3/TiO2/HNTs hybrid-structured and plasma effect photocatalysts for enhanced charges transfer and photocatalytic activity. J. Ind. Eng. Chem. 2018, 67, 164–174. [Google Scholar] [CrossRef]
- Gao, Q.; Si, F.; Zhang, S.; Fang, Y.; Chen, X.; Yang, S. Hydrogenated F-doped TiO2 for photocatalytic hydrogen evolution and pollutant degradation. Int. J. Hydrogen Energy 2019, 44, 8011–8019. [Google Scholar] [CrossRef]
- Tang, J.; Quan, H.; Ye, J. Photocatalytic Properties and Photoinduced Hydrophilicity of Surface-Fluorinated TiO2. Chem. Mater. 2007, 19, 116–122. [Google Scholar] [CrossRef]
- Li, H.; Gao, Y.; Wu, X.; Lee, P.-H.; Shih, K. Fabrication of Heterostructured g-C3N4/Ag-TiO2 Hybrid Photocatalyst with Enhanced Performance in Photocatalytic Conversion of CO2 Under Simulated Sunlight Irradiation. Appl. Surf. Sci. 2017, 402, 198–207. [Google Scholar] [CrossRef]
- Huo, Y.; Zhu, J.; Li, J.; Li, G.; Li, H. An active La/TiO2 photocatalyst prepared by ultrasonication-assisted sol–gel method followed by treatment under supercritical conditions. J. Mol. Catal. A Chem. 2007, 278, 237–243. [Google Scholar] [CrossRef]
- Fu, W.; Ding, S.; Wang, Y.; Wu, L.; Zhang, D.; Pan, Z.; Wang, R.; Zhang, Z.; Qiu, S. F, Ca co-doped TiO2 nanocrystals with enhanced photocatalytic activity. Dalton Trans. 2014, 43, 16160–16163. [Google Scholar] [CrossRef]
- Czoska, A.M.; Livraghi, S.; Chiesa, M.; Giamello, E.; Agnoli, S.; Granozzi, G.; Finazzi, E.; Valentin, C.D.; Pacchioni, G. The Nature of Defects in Fluorine-Doped TiO2. J. Phys. Chem. C 2008, 112, 8951–8956. [Google Scholar] [CrossRef]
- Li, X.; Liu, C.; Wu, D.; Li, J.; Huo, P.; Wang, H. Improved charge transfer by size-dependent plasmonic Au on C3N4 for efficient photocatalytic oxidation of RhB and CO2 reduction. Chin. J. Catal. 2019, 40, 928–939. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, J.; Liu, C.; Huo, P.; Wang, H. Construction of 3D porous g-C3N4/AgBr/rGO composite for excellent visible light photocatalytic activity. Appl. Surf. Sci. 2018, 458, 586–596. [Google Scholar] [CrossRef]
- Liu, C.; Li, J.; Sun, L.; Zhou, Y.; Liu, C.; Wang, H.; Huo, P.; Ma, C.; Yan, Y. Visible-light driven photocatalyst of CdTe/CdS homologous heterojunction on N-rGO photocatalyst for efficient degradation of 2,4-dichlorophenol. J. Taiwan Inst. Chem. Eng. 2018, 93, 603–615. [Google Scholar] [CrossRef]
- Yu, W.; Liu, X.; Pan, L.; Li, J.; Liu, J.; Zhang, J.; Li, P.; Chen, C.; Sun, Z. Enhanced visible light photocatalytic degradation of methylene blue by F-doped TiO2. Appl. Surf. Sci. 2014, 319, 107–112. [Google Scholar] [CrossRef]
- Xu, J.; Ao, Y.; Fu, D.; Yuan, C. Low-temperature preparation of F-doped TiO2 film and its photocatalytic activity under solar light. Appl. Surf. Sci. 2008, 254, 3033–3038. [Google Scholar] [CrossRef]
- Yu, C.; Fan, Q.; Xie, Y.; Chen, J.; Shu, Q.; Jimmy, C.Y. Sonochemical Fabrication of Novel Square-Shaped F Doped TiO2 Nanocrystals with Enhanced Performance in Photocatalytic Degradation of Phenol. J. Hazard. Mater. 2012, 237–238, 38–45. [Google Scholar] [CrossRef]
- Yu, J.C.; Yu, J.; Ho, W.; Jiang, Z.; Zhang, L. Effects of F-Doping on the Photocatalytic Activity and Microstructures of Nanocrystalline TiO2 Powders. Chem. Mater. 2002, 14, 3808–3816. [Google Scholar] [CrossRef]
- Li, X.; Zhu, J.; Li, H. Influence of crystal facets and F-modification on the photocatalytic performance of anatase TiO2. Catal. Commun. 2012, 24, 20–24. [Google Scholar] [CrossRef]
- Li, X.; Zhang, H.; Zheng, X.; Yin, Z.; Wei, L. Visible light responsive N-F-codoped TiO2 photocatalysts for the degradation of 4-chlorophenol. J. Environ. Sci. 2011, 23, 1919–1924. [Google Scholar] [CrossRef]
- Bao, R.; Chen, C.; Xia, J.; Chen, H.; Li, H. Controlled synthesis and enhanced photoelectro-catalytic activity of a 3D TiO2 nanotube array/TiO2 nanoparticle heterojunction using a combined dielectrophoresis/sol–gel method. J. Mater. Chem. C 2019, 7, 4981–4987. [Google Scholar] [CrossRef]
- Negi, S.S. Enhanced light harvesting and charge separation over wormhole mesoporous TiO2−X nanocrystallites towards efficient hydrogen generation. Sustain. Energy Fuels 2019, 3, 1191–1200. [Google Scholar] [CrossRef]
- Wei, N.; Liu, Y.; Feng, M.; Li, Z.; Chen, S.; Zheng, Y.; Wang, D. Controllable TiO2 core-shell phase heterojunction for efficient photoelectrochemical water splitting under solar light. Appl. Catal. B 2019, 244, 519–528. [Google Scholar] [CrossRef]
- Zhou, J.K.; Lv, L.; Yu, J.; Li, H.L.; Guo, P.-Z.; Sun, H.; Zhao, X.S. Synthesis of Self-Organized Polycrystalline F-doped TiO2 Hollow Microspheres and Their Photocatalytic Activity under Visible Light. J. Phys. Chem. C 2008, 112, 5316–5321. [Google Scholar] [CrossRef]
| Photocatalyst | XRF | Textural Properties | DRS UV-vis |
|---|---|---|---|
| The Content of La (wt %) | SBET (m2.g−1) | Indirect Band Gap (eV) | |
| TiO2 | - | 80 | 2.85 |
| La/TiO2 | 0.13 | 73 | 2.95 |
| 2.8F-La/TiO2 | 0.20 | 84 | 3.01 |
| 4.1F-La/TiO2 | 0.22 | 79 | 3.00 |
| Photocatalyst | Anatase Crystallite Size nm | Lattice Parameters | |
|---|---|---|---|
| a nm | c nm | ||
| TiO2 | 14.4 | 0.3788 | 0.9510 |
| La/TiO2 | 14.7 | 0.3787 | 0.9504 |
| 2.8F-La/TiO2 | 13.3 | 0.3787 | 0.9506 |
| 4.1F-La/TiO2 | 12.9 | 0.3788 | 0.9505 |
| Photocatalyst | Ti (at %) | O (at %) | F (at %) | C (at %) | O/Ti |
|---|---|---|---|---|---|
| TiO2 | 31.27 | 60.44 | 0 | 8.30 | 1.93 |
| La/TiO2 | 30.84 | 59.58 | 0 | 9.58 | 1.93 |
| 2.8F-La/TiO2 | 26.26 | 49.46 | 2.83 | 21.45 | 1.88 |
| 4.1F-La/TiO2 | 30.63 | 58.31 | 4.14 | 6.93 | 1.90 |
| Photocatalyst | Content of F (at %) | Surface Fluorine (at %) | Lattice Fluorine (at %) | Ratio of Lattice and Surface Fluorine |
|---|---|---|---|---|
| 2.8F-La/TiO2 | 2.83 | 1.47 | 1.36 | 0.931 |
| 4.1F-La/TiO2 | 4.14 | 2.22 | 1.92 | 0.865 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Edelmannová, M.; Dubnová, L.; Reli, M.; Meinhardová, V.; Huo, P.; Lavrenčič Štangar, U.; Čapek, L.; Kočí, K. The Role of Fluorine in F-La/TiO2 Photocatalysts on Photocatalytic Decomposition of Methanol-Water Solution. Materials 2019, 12, 2867. https://doi.org/10.3390/ma12182867
Edelmannová M, Dubnová L, Reli M, Meinhardová V, Huo P, Lavrenčič Štangar U, Čapek L, Kočí K. The Role of Fluorine in F-La/TiO2 Photocatalysts on Photocatalytic Decomposition of Methanol-Water Solution. Materials. 2019; 12(18):2867. https://doi.org/10.3390/ma12182867
Chicago/Turabian StyleEdelmannová, Miroslava, Lada Dubnová, Martin Reli, Vendula Meinhardová, Pengwei Huo, Urška Lavrenčič Štangar, Libor Čapek, and Kamila Kočí. 2019. "The Role of Fluorine in F-La/TiO2 Photocatalysts on Photocatalytic Decomposition of Methanol-Water Solution" Materials 12, no. 18: 2867. https://doi.org/10.3390/ma12182867
APA StyleEdelmannová, M., Dubnová, L., Reli, M., Meinhardová, V., Huo, P., Lavrenčič Štangar, U., Čapek, L., & Kočí, K. (2019). The Role of Fluorine in F-La/TiO2 Photocatalysts on Photocatalytic Decomposition of Methanol-Water Solution. Materials, 12(18), 2867. https://doi.org/10.3390/ma12182867







