Influence of the Ni/Co Mass Ratio on the Microstructure and Properties of Quaternary Cu-Ni-Co-Si Alloys
Abstract
:1. Introduction
2. Experimental
3. Results and Discussion
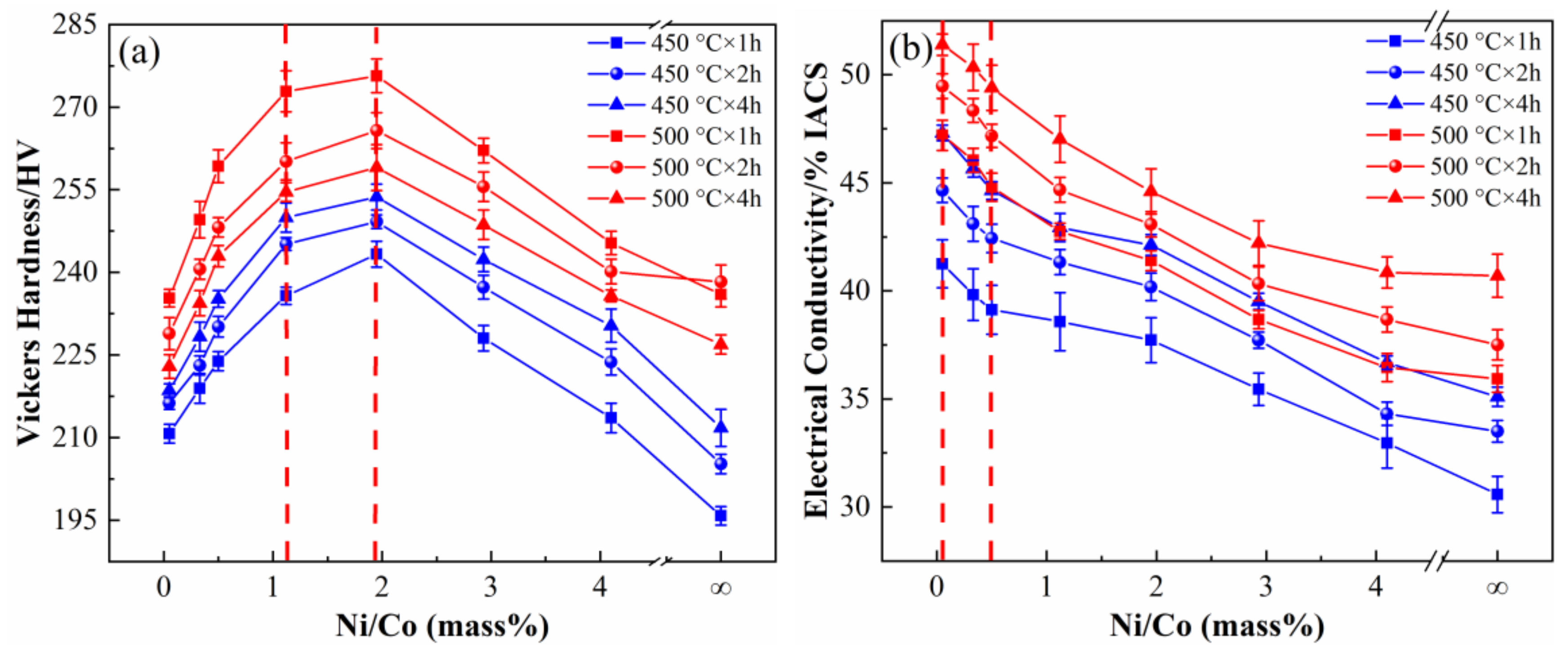
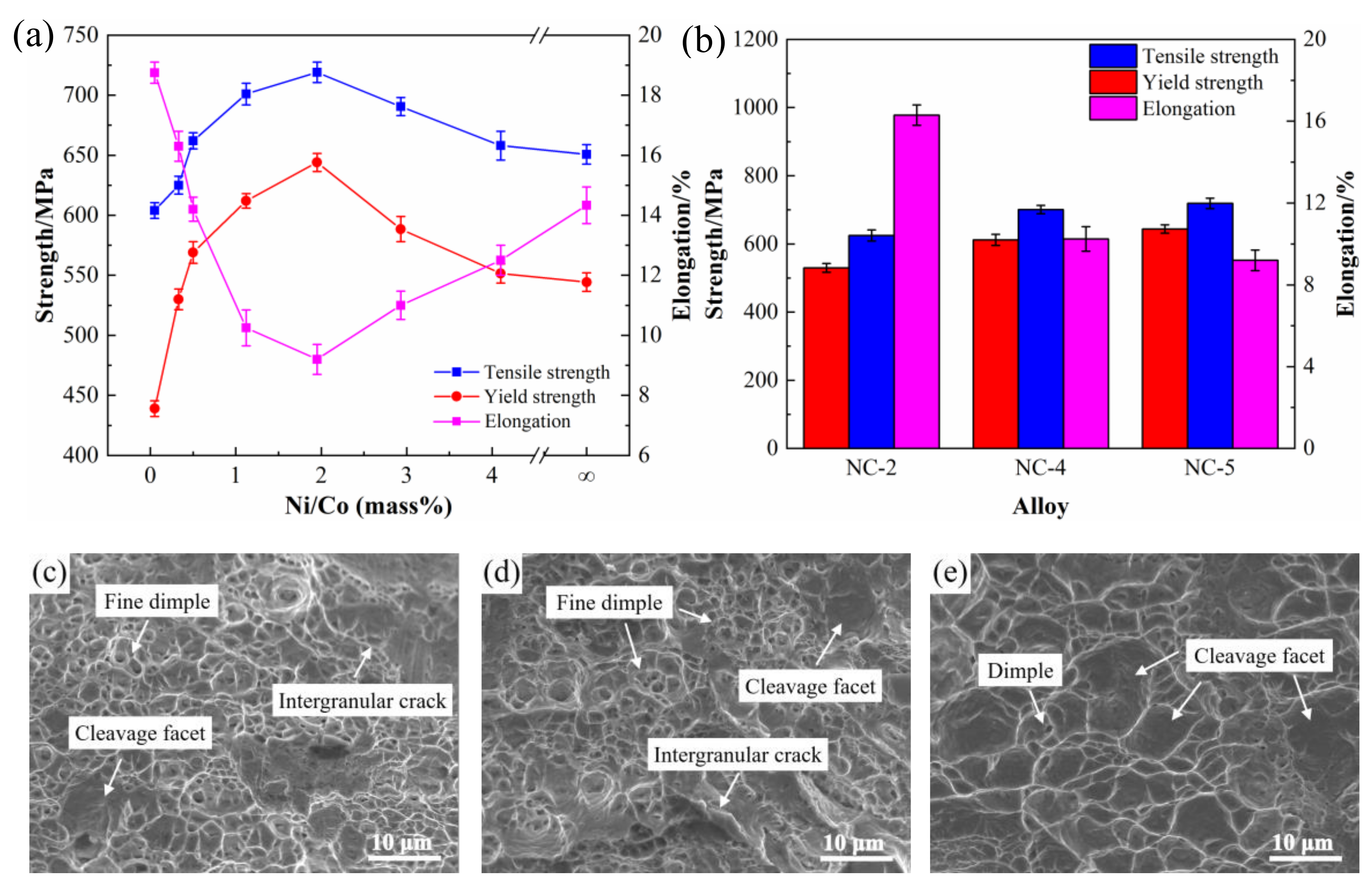
3.1. Physical and Mechanical Properties
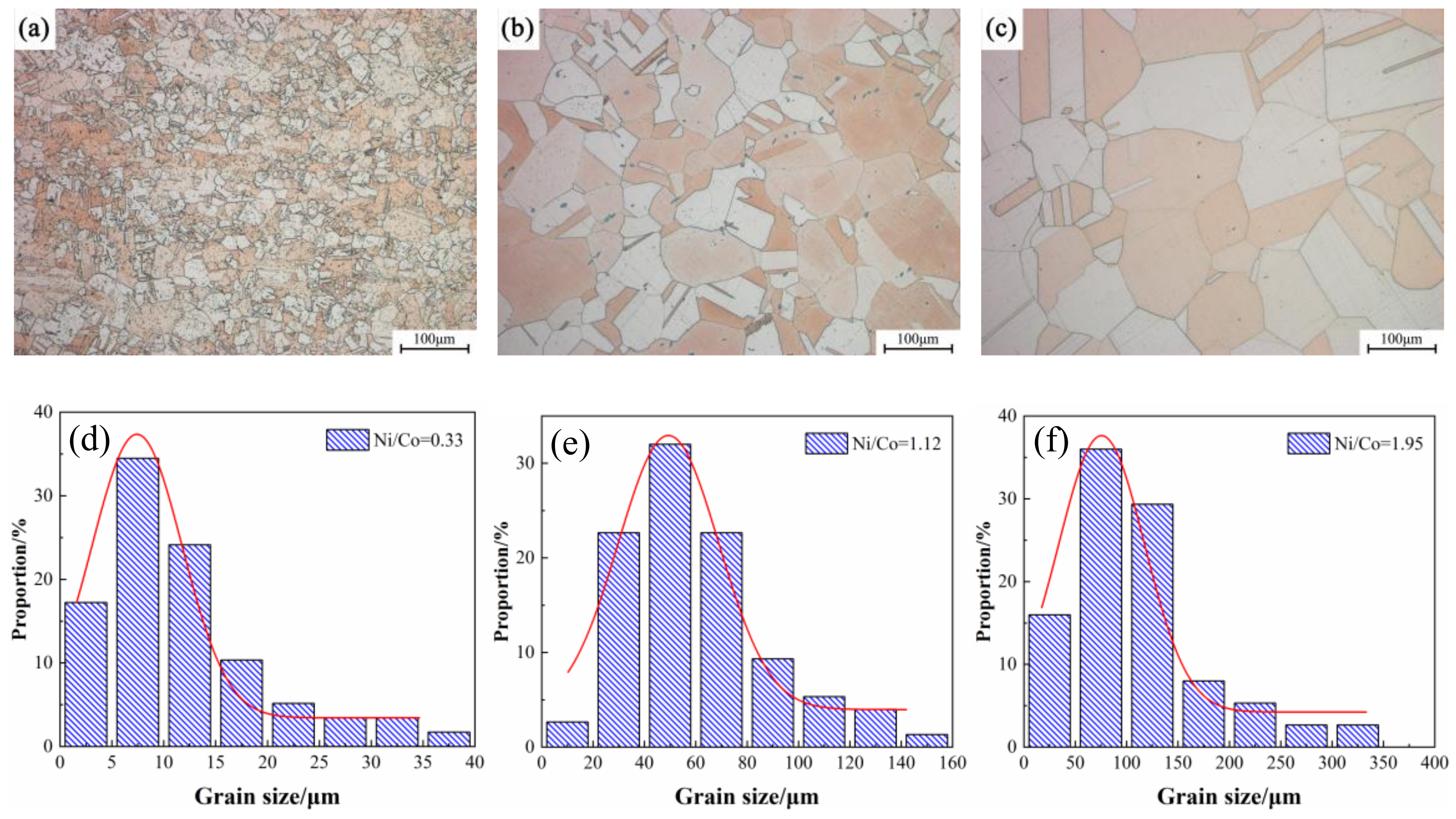
3.2. Microstructure Observation
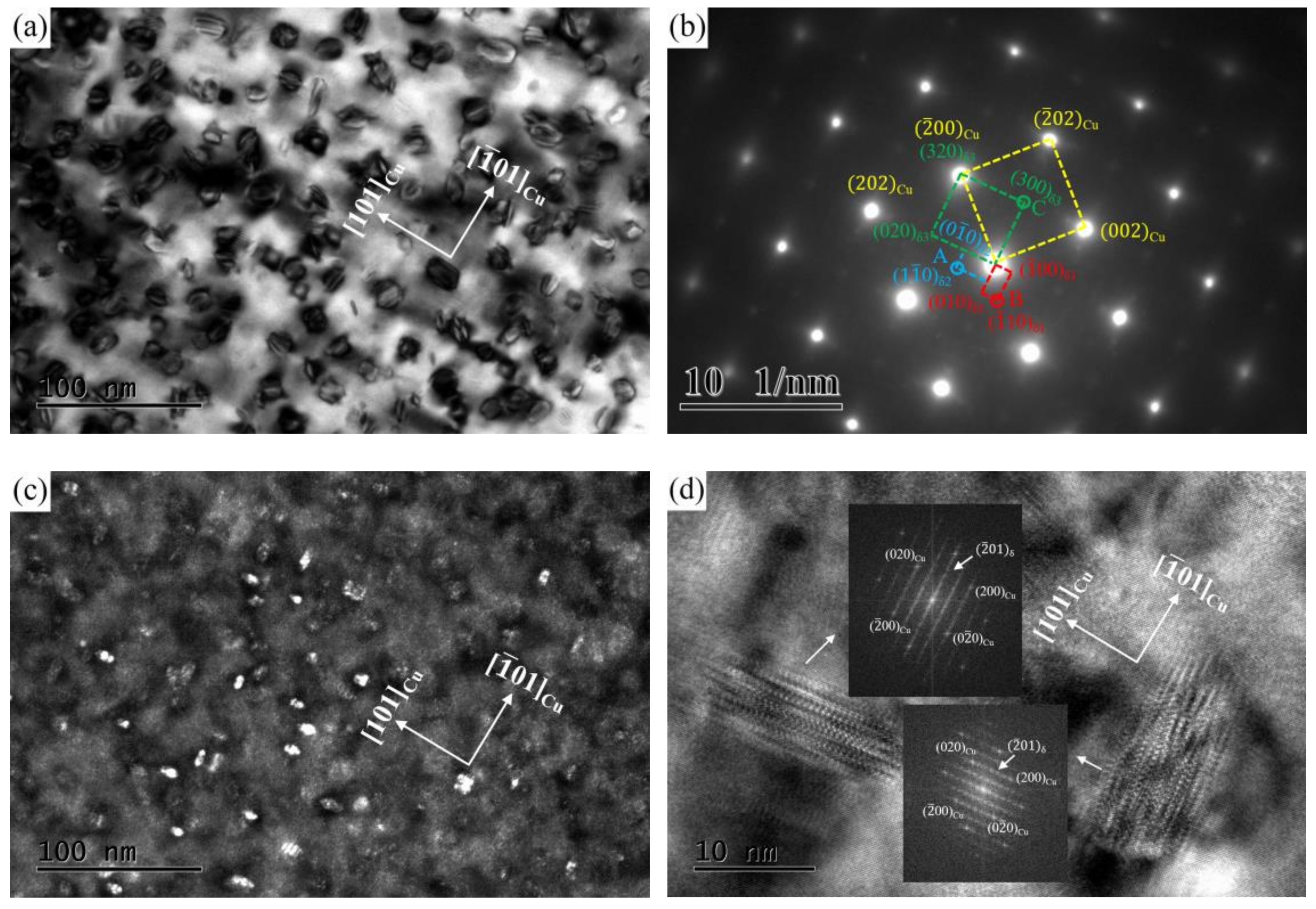
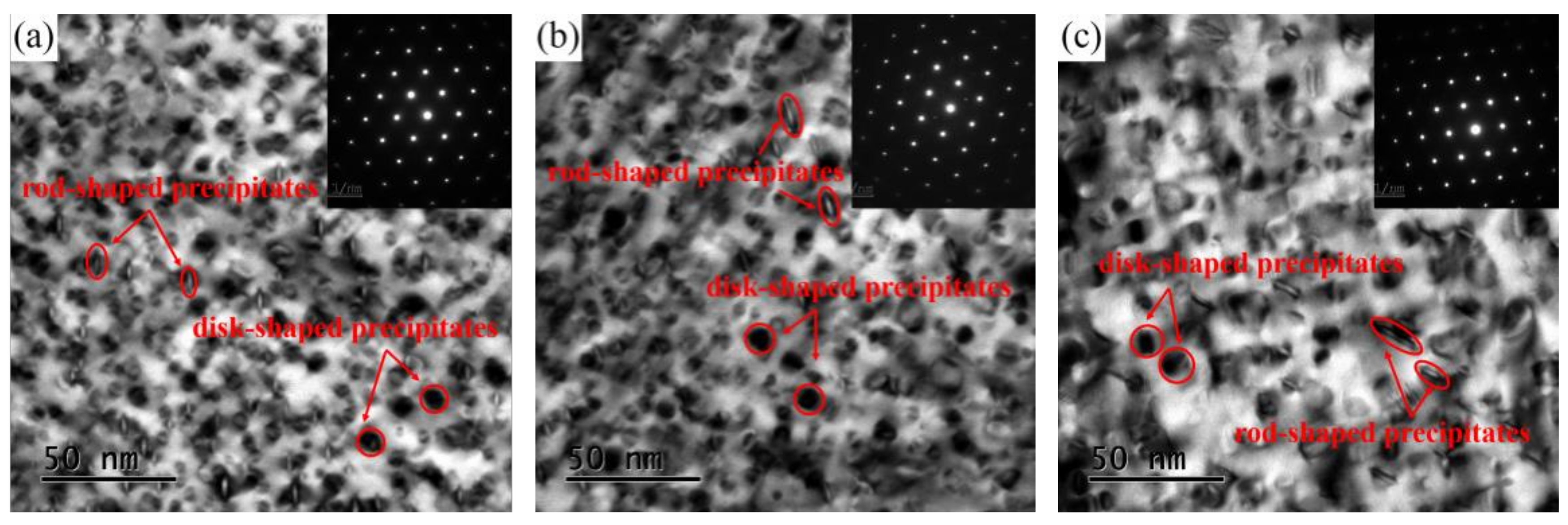
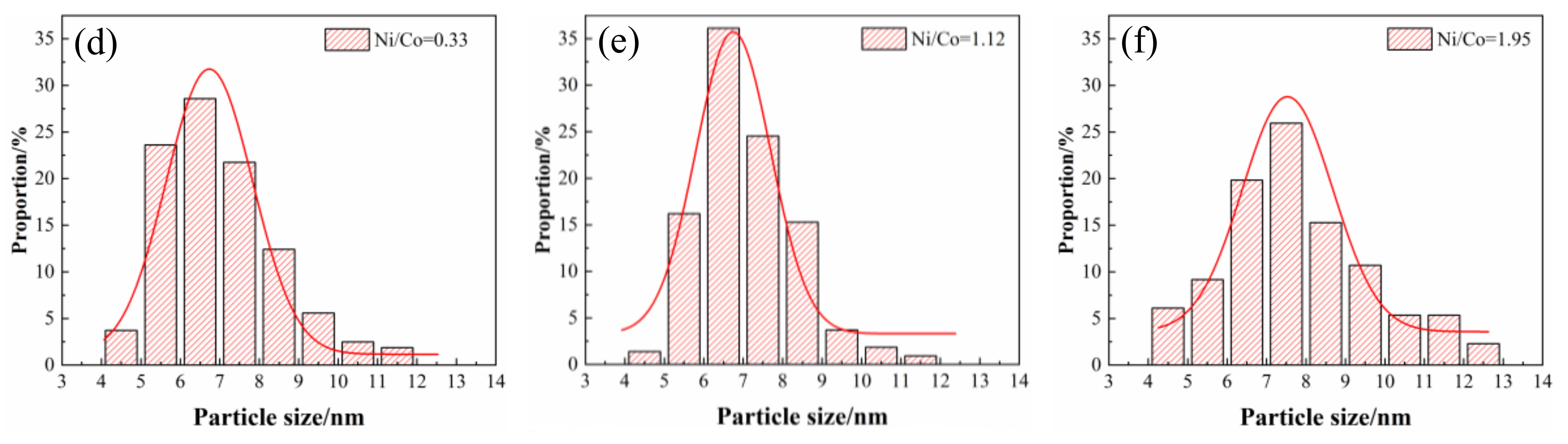
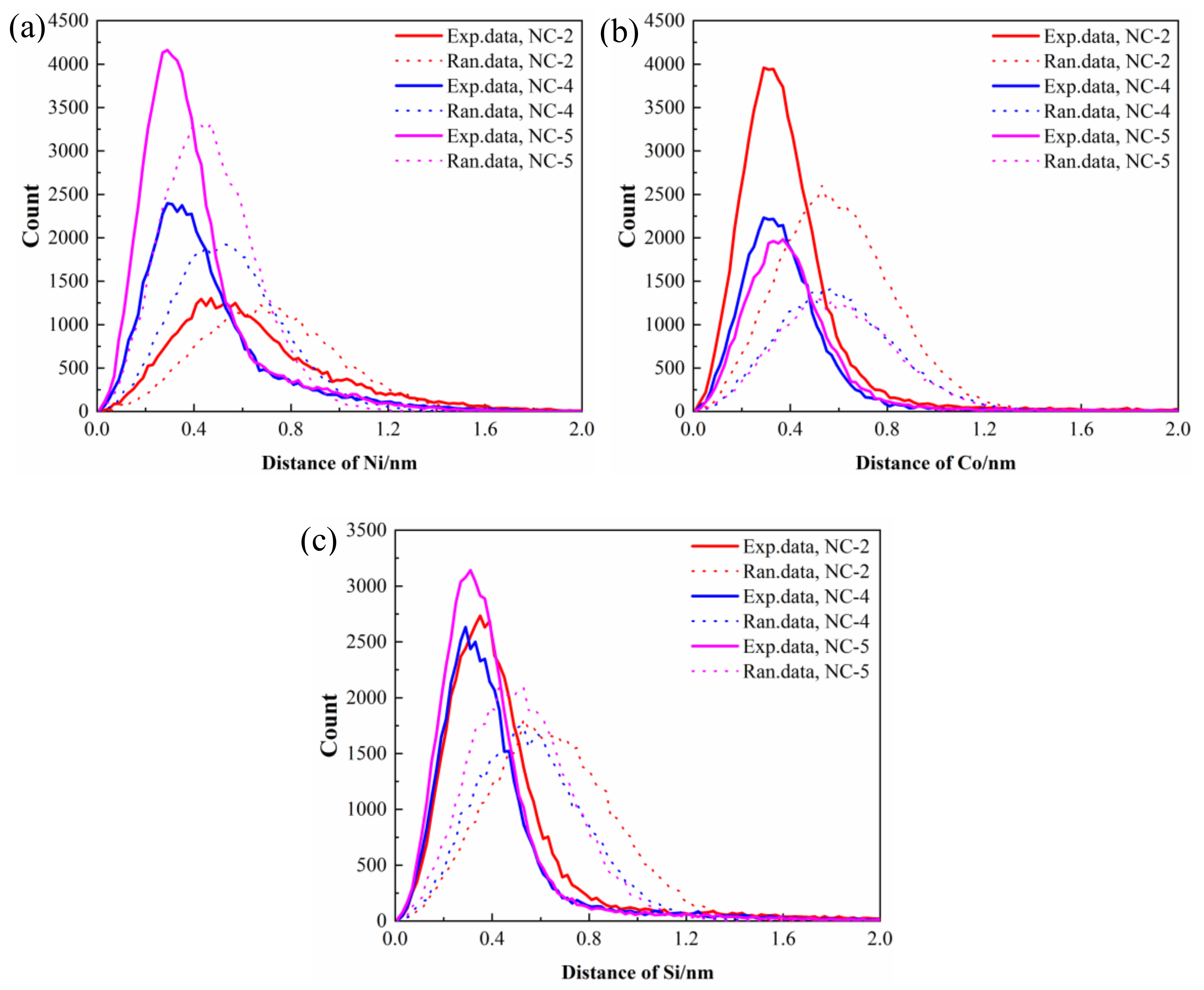
3.3. TEM Observation
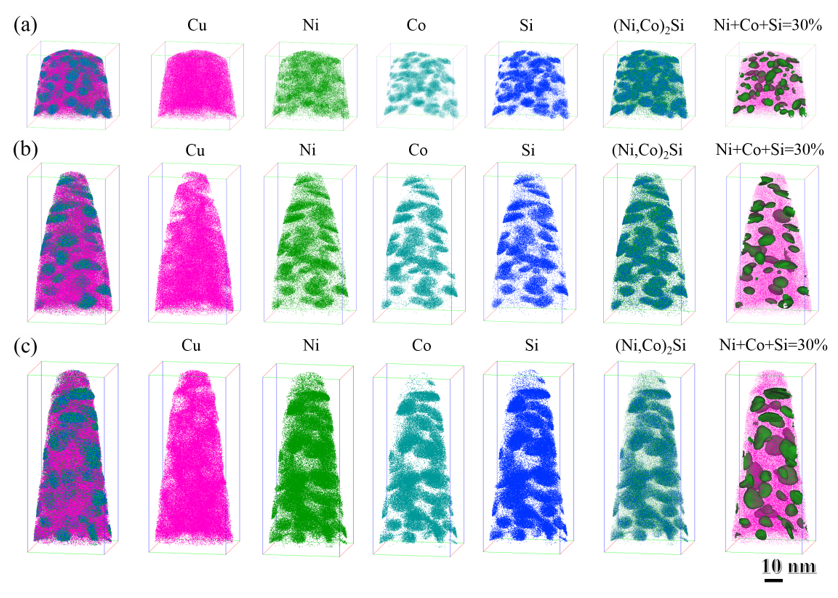
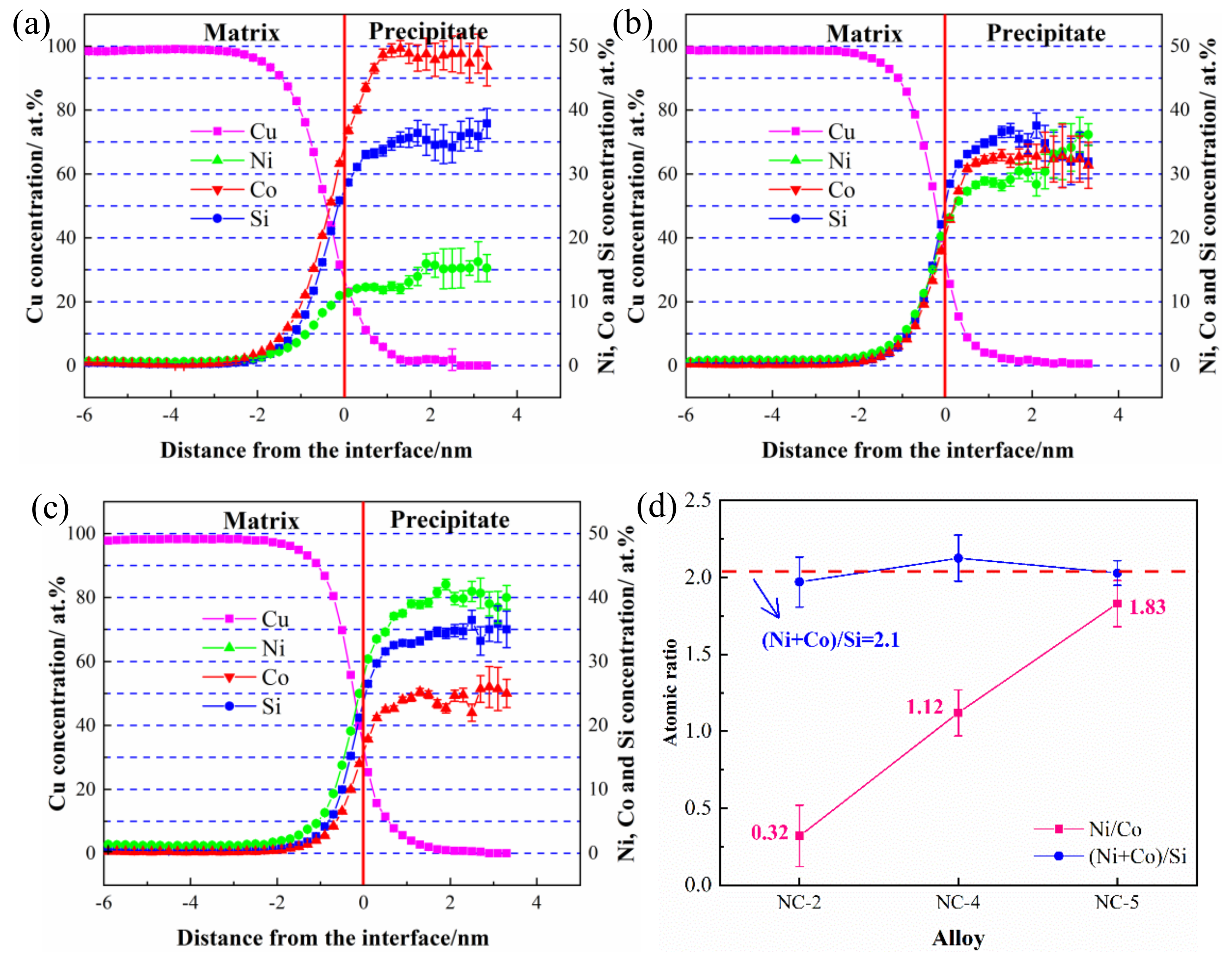
3.4. 3DAP Analysis
3.5. Microstructure—Properties Relationship
3.5.1. Mechanical Properties
3.5.2. Conductive Property
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Li, D.M.; Wang, Q.; Jiang, B.B.; Li, X.N.; Zhou, W.L.; Dong, C. Minor-alloyed Cu-Ni-Si alloys with high hardness and electric conductivity designed by a cluster formula approach. Prog. Nat. Sci. Mater. 2017, 27, 467–473. [Google Scholar] [CrossRef]
- Wang, H.-S.; Chen, H.-G.; Gu, J.-W.; Hsu, C.-E.; Wu, C.-Y. Improvement in strength and thermal conductivity of powder metallurgy produced Cu-Ni-Si-Cr alloy by adjusting Ni/Si weight ratio and hot forging. J. Alloys Compd. 2015, 633, 59–64. [Google Scholar] [CrossRef]
- Li, J.; Huang, G.; Mi, X.; Peng, L.; Xie, H.; Kang, Y. Effect of Ni/Si Mass Ratio and Thermomechanical Treatment on the Microstructure and Properties of Cu-Ni-Si Alloys. Materials 2019, 12, 2076. [Google Scholar] [CrossRef] [PubMed]
- Lei, Q.; Li, S.Y.; Zhu, J.L.; Xiao, Z.; Zhang, F.F.; Li, Z. Microstructural evolution, phase transition, and physics properties of a high strength Cu-Ni-Si-Al alloy. Mater. Charact. 2019, 147, 315–323. [Google Scholar] [CrossRef]
- Lei, Q.; Li, Z.; Xiao, T.; Pang, Y.; Xiang, Z.Q.; Qiu, W.T.; Xiao, Z. A new ultrahigh strength Cu-Ni-Si alloys. Intermetallics 2013, 42, 77–84. [Google Scholar] [CrossRef]
- Lee, E.; Han, S.; Euh, K.; Lim, S.; Kim, S. Effect of Ti addition on tensile properties of Cu-Ni-Si alloys. Met. Mater. Int. 2011, 17, 569–576. [Google Scholar] [CrossRef]
- Wang, H.S.; Chen, H.G.; Gu, J.W.; Hsu, C.E.; Wu, C.Y. Effects of heat treatment processes on the microstructures and properties of powder metallurgy produced Cu-Ni-Si-Cr alloy. Mater. Sci. Eng. A 2014, 619, 221–227. [Google Scholar] [CrossRef]
- Wang, W.; Kang, H.J.; Chen, Z.N.; Chen, Z.J.; Zou, C.L.; Li, R.G. Effects of Cr and Zr additions on microstructure and properties of Cu-Ni-Si alloys. Mater. Sci. Eng. A 2016, 673, 378–390. [Google Scholar] [CrossRef]
- Han, S.Z.; Gu, J.H.; Lee, J.H.; Que, Z.P.; Shin, J.H.; Lim, S.H.; Kim, S.S. Effect of V addition on hardness and electrical conductivity in Cu-Ni-Si alloys. Met. Mater. Int. 2013, 19, 637–641. [Google Scholar] [CrossRef]
- Li, J.; Huang, G.J.; Mi, X.J.; Peng, L.J.; Xie, H.F.; Kang, Y.L. Effect of Co Addition on Microstructure and Properties of Cu-Ni-Si Alloy. Adv. Mater. Process. 2018, 33, 353–360. [Google Scholar]
- Xiao, X.P.; Yi, Z.Y.; Chen, T.T.; Liu, R.Q.; Wang, H. Suppressing spinodal decomposition by adding Co into Cu-Ni-Si alloy. J. Alloys Compd. 2016, 660, 178–183. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhang, Y.; Tian, B.H.; Jia, Y.L.; Liu, Y.; Song, K.X.; Volinsky, A.A. Co effects on Cu-Ni-Si alloys microstructure and physical properties. J. Alloys Compd. 2019, 797, 1327–1337. [Google Scholar] [CrossRef]
- Huang, J.Z.; Xiao, Z.; Dai, J.; Li, Z.; Jiang, H.Y.; Wang, W.; Zhang, X.X. Microstructure and properties of a novel Cu-Ni-Co-Si-Mg alloy with super-high strength and conductivity. Mater. Sci. Eng. A 2019, 744, 754–763. [Google Scholar] [CrossRef]
- Li, J.; Huang, G.J.; Mi, X.J.; Peng, L.J.; Xie, H.F.; Kang, Y.L. Relationship between the microstructure and properties of a peak aged Cu-Ni-Co-Si alloy. Mater. Sci. Technol. 2019, 35, 606–614. [Google Scholar] [CrossRef]
- Lockyer, S.A.; Noble, F.W. Precipitate structure in a Cu-Ni-Si alloy. J. Mater. Sci. 1994, 29, 218–226. [Google Scholar] [CrossRef]
- Fujiwara, H.; Sato, T.; Kamio, A. Effect of alloy composition on precipitation behavior in Cu-Ni-Si alloys. J. Jpn. Inst. Met. 1998, 62, 301–309. [Google Scholar] [CrossRef]
- Liu, J.; Wang, X.H.; Chen, J.; Liu, J.T. The effect of cold rolling on age hardening of Cu-3Ti-3Ni-0.5Si alloy. J. Alloys Compd. 2019, 797, 370–379. [Google Scholar] [CrossRef]
- Yang, C.; Zhang, P.; Shao, D.; Wang, R.H.; Cao, L.F.; Zhang, J.Y.; Liu, G. The influence of Sc solute partitioning on the microalloying effect and mechanical properties of Al-Cu alloys with minor Sc addition. Acta Mater. 2016, 119, 68–79. [Google Scholar] [CrossRef]
- Fujiwara, H. Designing high-strength copper alloys based on the crystallographic structure of precipitates. Furukawa Rev. 2004, 26, 39–43. [Google Scholar]
- Ryu, H.J.; Baik, H.K.; Hong, S.H. Effect of thermomechanical treatments on microstructure and properties of Cu-base lead frame alloy. J. Mater. Sci. 2000, 35, 3641–3646. [Google Scholar] [CrossRef]
- Jia, Y.L.; Wang, M.P.; Chen, C.; Dong, Q.Y.; Wang, S. Orientation and diffraction patterns of δ-Ni2Si precipitates in Cu-Ni-Si alloy. J. Alloys Compd. 2013, 557, 147–151. [Google Scholar] [CrossRef]
- Jeong, Y.B.; Jo, H.R.; Kim, J.T.; Hong, S.H.; Kim, K.B. A study on the micro-evolution of mechanical property and microstructures in (Cu-30Fe)-2X alloys with the addition of minor alloying elements. J. Alloys Compd. 2019, 786, 341–345. [Google Scholar] [CrossRef]
- Wen, H.M.; Topping, T.D.; Isheim, D.; Seidman, D.N.; Lavernia, E.J. Strengthening mechanisms in a high-strength bulk nanostructured Cu-Zn-Al alloy processed via cryomilling and spark plasma sintering. Acta Mater. 2013, 61, 2769–2782. [Google Scholar] [CrossRef]
- Jumaev, E.; Hong, S.H.; Kim, J.T.; Park, H.J.; Kim, Y.S. Chemical evolution-induced strengthening on AlCoCrNi dual-phase high-entropy alloy with high specific strength. J. Alloys Compd. 2019, 777, 828–834. [Google Scholar] [CrossRef]
- Chen, J.; Liu, Y.J.; Lei, F.Y.; Sheng, G.; Kang, Z.T. Mobilities, diffusivities and kinetic characteristics for fcc Cu-Co-Ni alloys. Calphad 2014, 47, 123–128. [Google Scholar] [CrossRef]
- Ying, P.Y.; Liu, Z.Y.; Bai, S.; Liu, M.; Lin, L.H.; Xia, P.; Xia, L.Y. Effects of pre-strain on Cu-Mg co-clustering and mechanical behavior in a naturally aged Al-Cu-Mg alloy. Mater. Sci. Eng. A 2017, 704, 18–24. [Google Scholar] [CrossRef]
- Wu, Y.; Li, Y.; Lu, J.Y.; Tan, S.; Jiang, F.; Sun, J. Correlations between microstructures and properties of Cu-Ni-Si-Cr alloy. Mater. Sci. Eng. A 2018, 731, 403–412. [Google Scholar] [CrossRef]
- Semboshi, S.; Sato, S.; Iwase, A.; Takasugi, T. Discontinuous precipitates in age-hardening Cu-Ni-Si alloys. Mater. Charact. 2016, 115, 39–45. [Google Scholar] [CrossRef]
- Xie, G.L.; Wang, Q.S.; Xie, X.J.; Xiong, B.Q.; Peng, L.J. The precipitation behavior and strengthening of a Cu-2.0 wt% Be alloy. Mater. Sci. Eng. A 2012, 558, 326–330. [Google Scholar]
- Yang, B.; Wu, M.Z.; Li, X.; Zhang, J.W.; Wang, H.Q. Effects of cold working and corrosion on fatigue properties and fracture behaviors of precipitate strengthened Cu-Ni-Si alloy. Int. J. Fatigue 2018, 116, 118–127. [Google Scholar] [CrossRef]
- Hall, E.O. The Deformation and ageing of mild Steel: III discussion of results. Proc. Phys. Soc. Sect. B 1951, 64, 747–753. [Google Scholar] [CrossRef]
- Petch, N.J. The cleavage strength of polycrystals. J. Iron Steel Inst. 1953, 174, 25–28. [Google Scholar]
- Hansen, N. Hall-Petch relation and boundary strengthening. Scr. Mater. 2004, 51, 801–806. [Google Scholar] [CrossRef]
- Wu, Y.; Li, Y.; Lu, J.Y.; Tan, S.; Jiang, F.; Sun, J. Effects of pre-deformation on precipitation behaviors and properties in Cu-Ni-Si-Cr alloy. Mater. Sci. Eng. A 2019, 742, 501–507. [Google Scholar] [CrossRef]
- Luca, A.D.; Dunand, D.C.; Seidman, D.N. Microstructure and mechanical properties of a precipitation-strengthened Al-Zr-Sc-Er-Si alloy with a very small Sc content. Acta Mater. 2018, 144, 80–91. [Google Scholar] [CrossRef]
- Varschavsky, A.; Donoso, E. A microcalorimetric study of fatigue crack propagation in precipitation-hardened Cu-Co-Si alloys. Mater. Lett. 1992, 15, 207–211. [Google Scholar] [CrossRef]
- Zhang, L.; Du, Y.; Xu, Y.O.H.; Lu, X.G.; Liu, Y.; Kong, Y.; Wang, J. Atomic mobilities, diffusivities and simulation of diffusion growth in the Co-Si system. Acta Mater. 2008, 56, 3940–3950. [Google Scholar] [CrossRef]



| Alloy | Cu | Ni | Co | Si | Ni/Co | (Ni + Co)/Si | Ni + Co + Si |
|---|---|---|---|---|---|---|---|
| NC-1 | Bal. | 0.17 | 3.36 | 0.84 | 0.05 | 4.2 | 4.3 |
| NC-2 | Bal. | 0.85 | 2.67 | 0.82 | 0.32 | 4.3 | 4.3 |
| NC-3 | Bal. | 1.16 | 2.30 | 0.82 | 0.50 | 4.2 | 4.3 |
| NC-4 | Bal. | 1.84 | 1.64 | 0.82 | 1.12 | 4.2 | 4.3 |
| NC-5 | Bal. | 2.31 | 1.18 | 0.82 | 1.95 | 4.3 | 4.3 |
| NC-6 | Bal. | 2.60 | 0.89 | 0.82 | 2.92 | 4.3 | 4.3 |
| NC-7 | Bal. | 2.80 | 0.68 | 0.82 | 4.12 | 4.3 | 4.3 |
| NC-8 | Bal. | 3.48 | - | 0.82 | ∞ | 4.2 | 4.3 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Huang, G.; Mi, X.; Peng, L.; Xie, H.; Kang, Y. Influence of the Ni/Co Mass Ratio on the Microstructure and Properties of Quaternary Cu-Ni-Co-Si Alloys. Materials 2019, 12, 2855. https://doi.org/10.3390/ma12182855
Li J, Huang G, Mi X, Peng L, Xie H, Kang Y. Influence of the Ni/Co Mass Ratio on the Microstructure and Properties of Quaternary Cu-Ni-Co-Si Alloys. Materials. 2019; 12(18):2855. https://doi.org/10.3390/ma12182855
Chicago/Turabian StyleLi, Jiang, Guojie Huang, Xujun Mi, Lijun Peng, Haofeng Xie, and Yonglin Kang. 2019. "Influence of the Ni/Co Mass Ratio on the Microstructure and Properties of Quaternary Cu-Ni-Co-Si Alloys" Materials 12, no. 18: 2855. https://doi.org/10.3390/ma12182855
APA StyleLi, J., Huang, G., Mi, X., Peng, L., Xie, H., & Kang, Y. (2019). Influence of the Ni/Co Mass Ratio on the Microstructure and Properties of Quaternary Cu-Ni-Co-Si Alloys. Materials, 12(18), 2855. https://doi.org/10.3390/ma12182855





