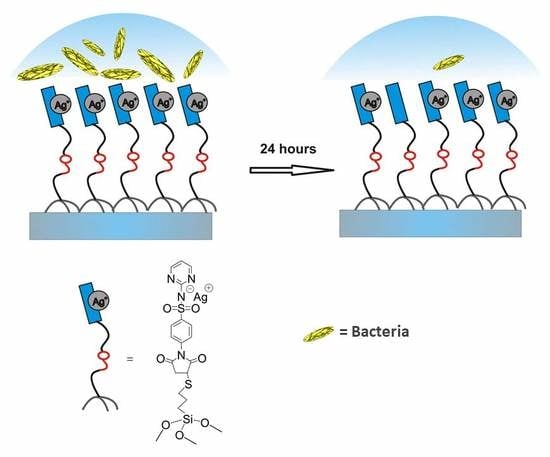
High Bactericidal Self-Assembled Nano-Monolayer of Silver Sulfadiazine on Hydroxylated Material Surfaces
Abstract
1. Introduction
2. Results
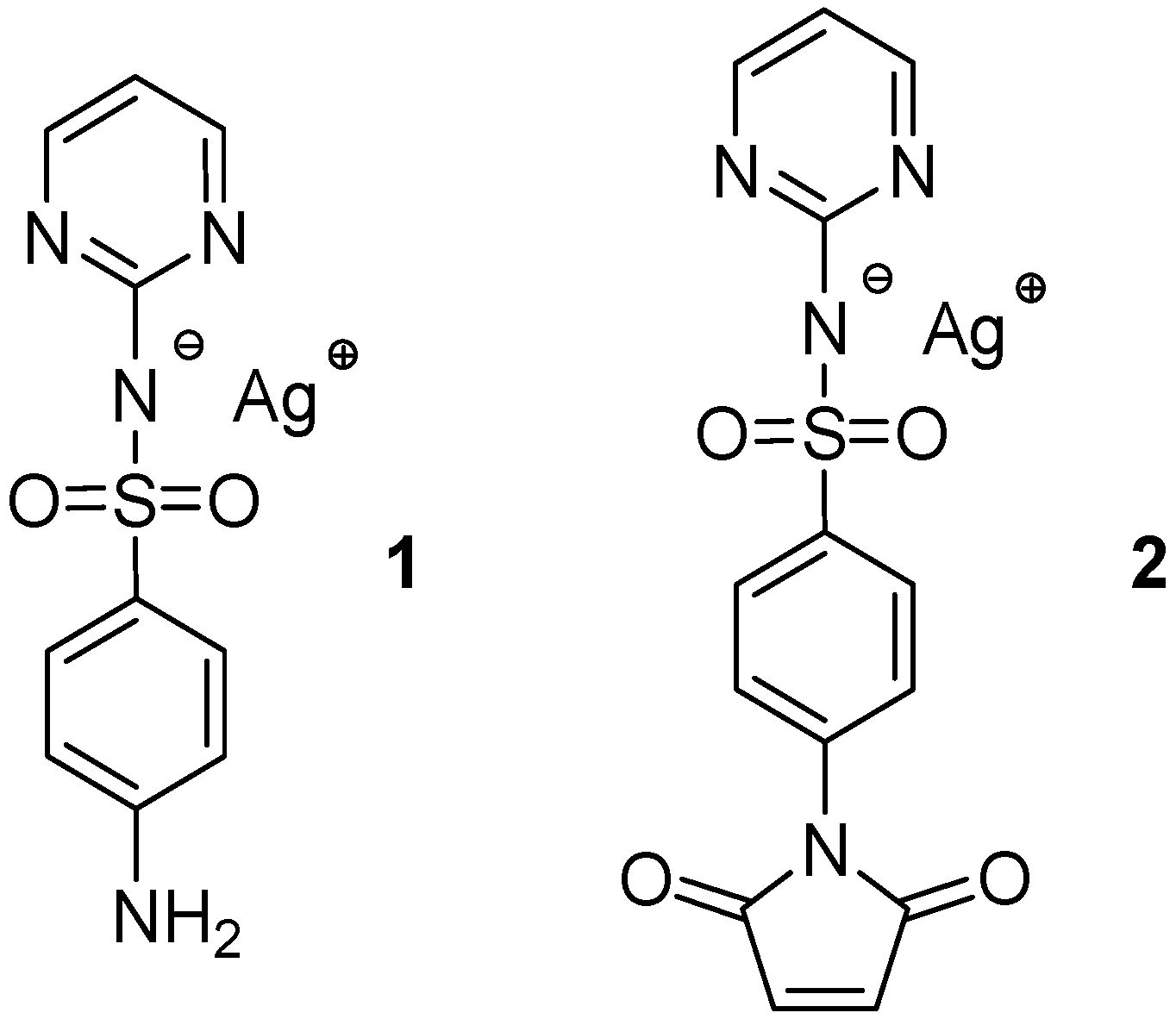
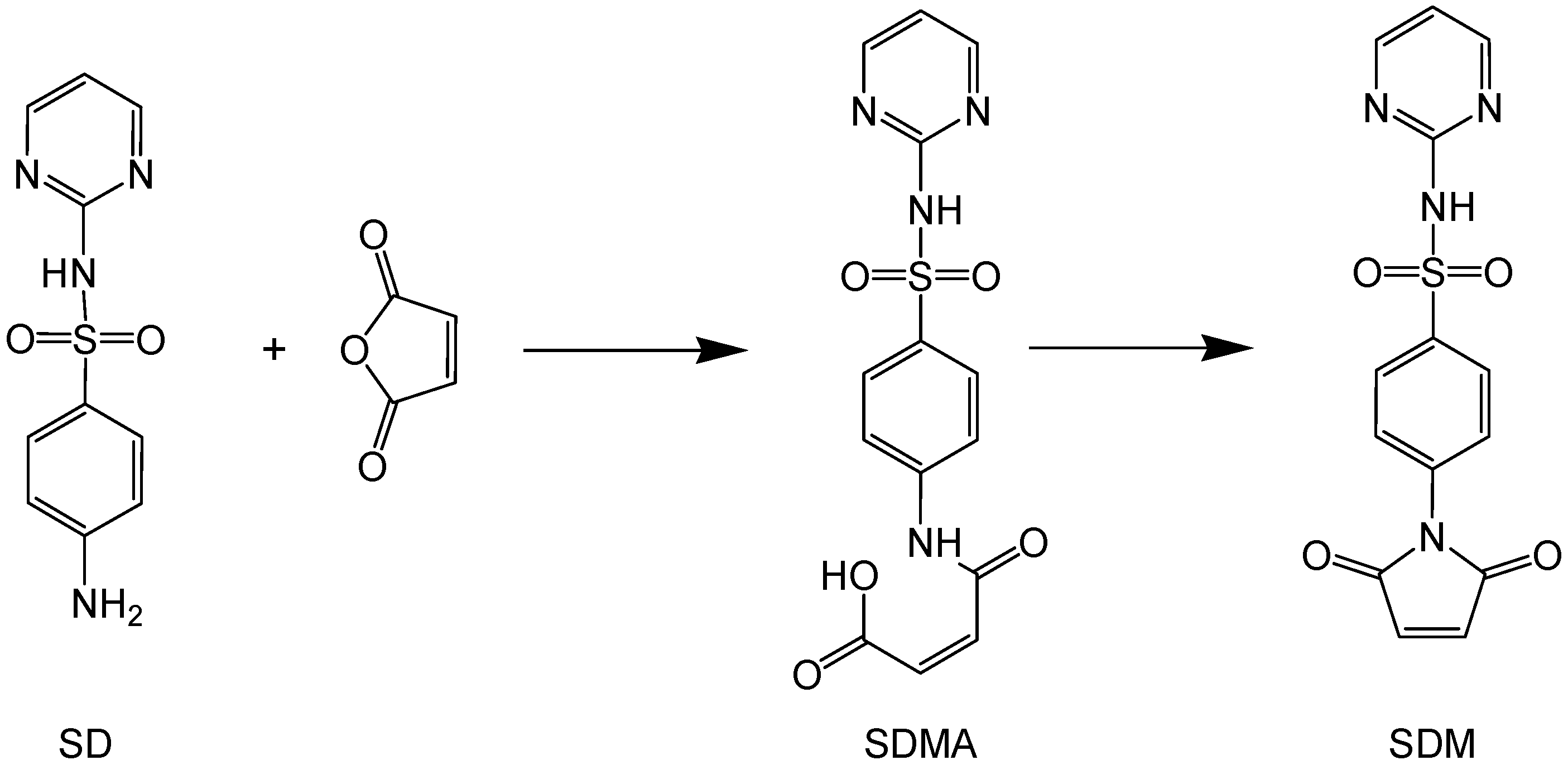
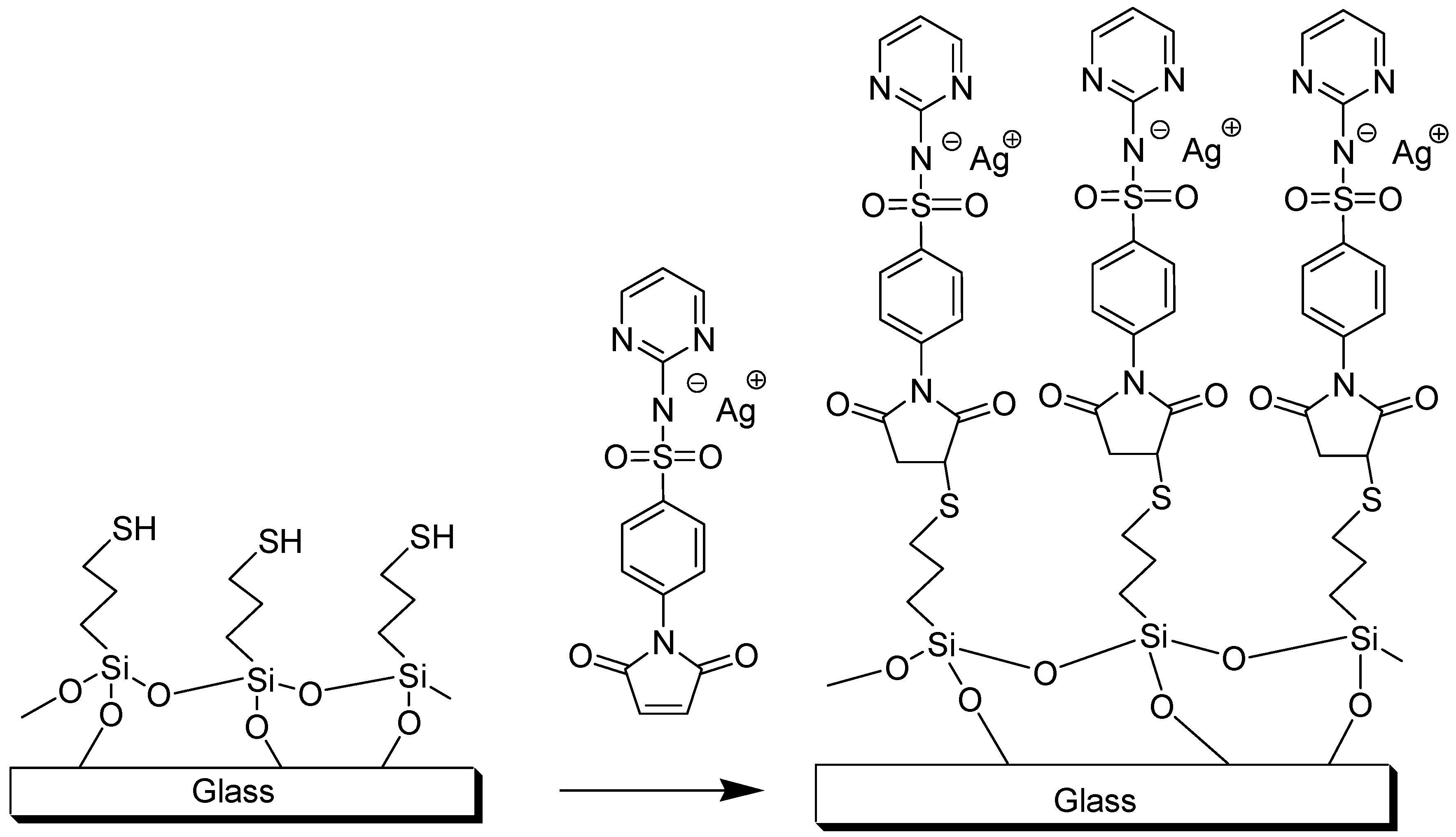
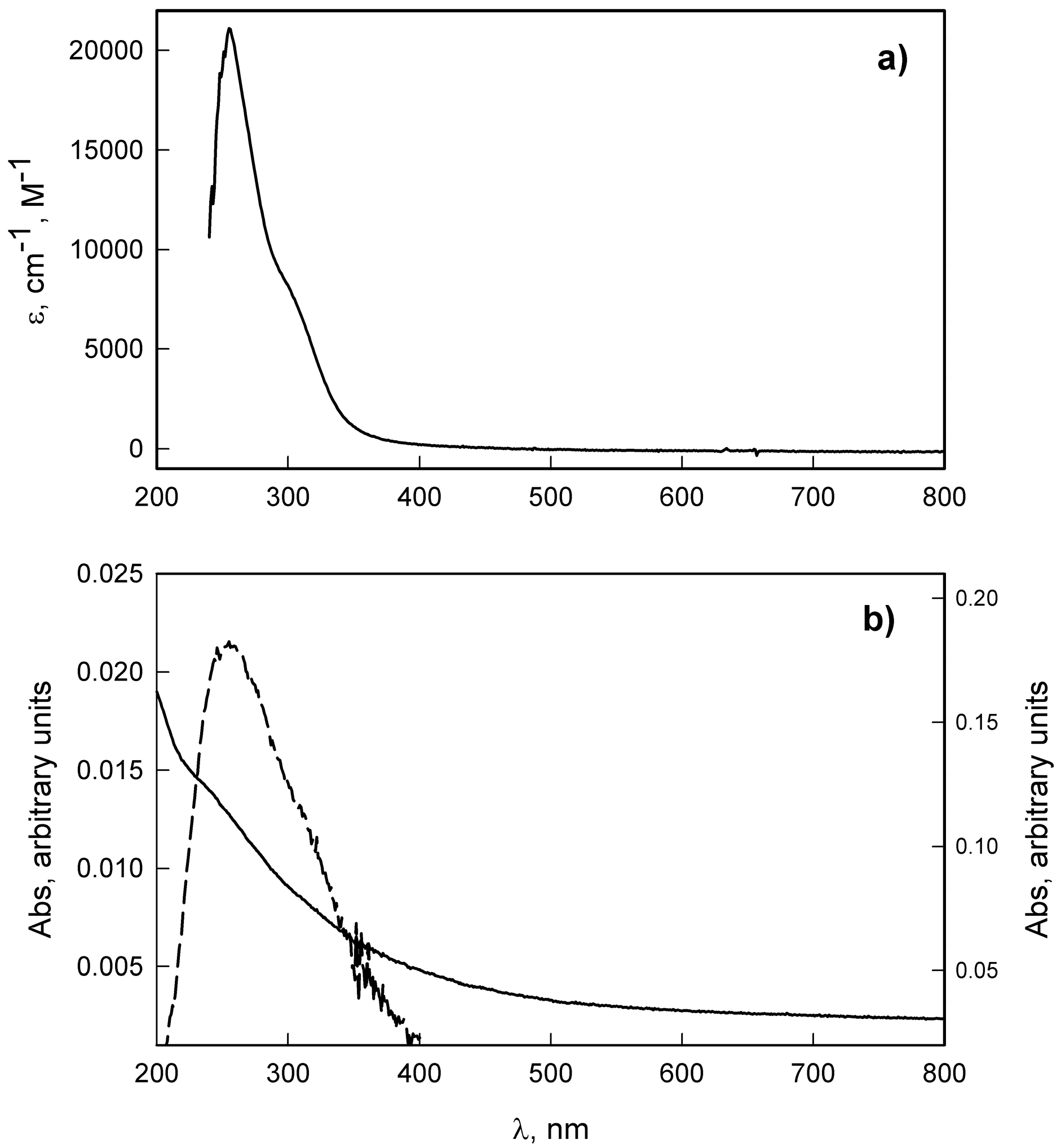
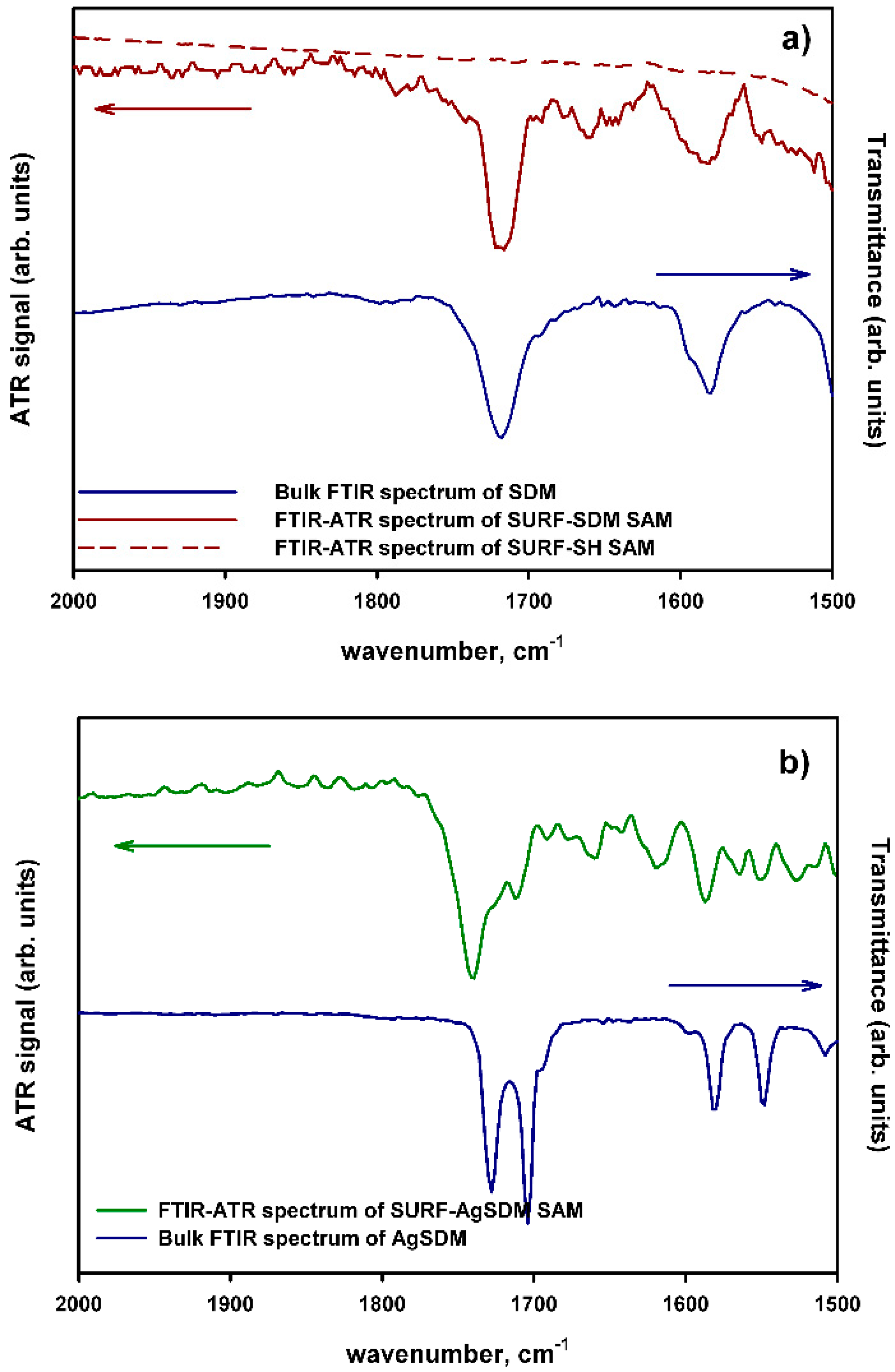
2.1. Synthesis and Characterization
2.2. Antibacterial Tests
3. Discussion
4. Materials and Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Arciola, C.R.; Campoccia, D.; Montanaro, L. Implant infections: Adhesion, biofilm formation and immune evasion. Nat. Rev. Microbiol. 2018, 16, 397. [Google Scholar] [CrossRef] [PubMed]
- Costerton, J.W.; Montanaro, L.; Arciola, C.R. Biofilm in implant infections: Its production and regulation. Int. J. Artif. Organs 2005, 28, 1062–1068. [Google Scholar] [CrossRef] [PubMed]
- Campoccia, D.; Montanaro, L.; Arciola, C.R. A review of the biomaterials technologies for infection-resistant surfaces. Biomaterials 2013, 34, 8533–8554. [Google Scholar] [CrossRef]
- Ulman, A. Formation and Structure of Self-Assembled Monolayers. Chem. Rev. 1996, 96, 1533–1554. [Google Scholar] [CrossRef]
- Tegoulia, V.A.; Cooper, S.L. Staphylococcus aureus adhesion to self-assembled monolayers: Effect of surface chemistry and fibrinogen presence. Colloids Surf. B Biointerfaces 2002, 24, 217–228. [Google Scholar] [CrossRef]
- Emerson, R.J.I.V.; Bergstrom, T.S.; Liu, Y.; Soto, E.R.; Brown, C.A.; McGimpsey, W.G.; Camesano, T.A. Microscale correlation between surface chemistry, texture, and the adhesive strength of Staphylococcus epidermidis. Langmuir 2006, 22, 11311–11321. [Google Scholar] [CrossRef]
- Ploux, L.; Beckendorff, S.; Nardin, M.; Neunlist, S. Quantitative and morphological analysis of biofilm formation on self-assembled monolayers. Colloids Surf. B Biointerfaces 2007, 57, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Simchi, A.; Tamjid, E.; Pishbin, F.; Boccaccini, A.R. Recent progress in inorganic and composite coatings with bactericidal capability for orthopaedic applications. Nanomed. NBM 2011, 7, 22–39. [Google Scholar] [CrossRef] [PubMed]
- Gottenbos, B.; van der Mei, H.C.; Klatter, F.; Nieuwenhuis, P.; Busscher, H.J. In vitro and in vivo antimicrobial activity of covalently coupled quaternary ammonium silane coatings on silicone rubber. Biomaterials 2002, 23, 1417–1423. [Google Scholar] [CrossRef]
- Jose, B.; Antoci, V.J.; Zeiger, A.R.; Wickstrom, E.; Hickok, N. Vancomycin covalently bonded to titanium beads kills Staphylococcus aureus. Chem. Biol. 2005, 12, 1041–1048. [Google Scholar] [CrossRef]
- Torres, N.; Oh, S.; Appleford, M.; Dean, D.D.; Jorgensen, J.H.; Ong, J.L.; Agrawal, C.M.; Mani, G. Stability of antibacterial self-assembled monolayers on hydroxyapatite. Acta Biomaterialia 2010, 6, 3242–3255. [Google Scholar] [CrossRef] [PubMed]
- Amalric, J.; Mutin Ph Guerrero, G.; Ponche, A.; Sotto, A.; Lavigne, J.-P. Phosphonate monolayers functionalized by silver thiolate species as antibacterial nanocoatings on titanium and stainless steel. J. Mater. Chem. 2009, 19, 141–149. [Google Scholar] [CrossRef]
- Humblot, V.; Yala, J.-F.; Thebault, P.; Boukerma, K.; Héquet, A.; Berjeaud, J.-M.; Pradier, C.-M. The antibacterial activity of Magainin I immobilized onto mixed thiols Self-Assembled Monolayers. Biomaterials 2009, 30, 3503–3512. [Google Scholar] [CrossRef] [PubMed]
- Bouloussa, O.; Rondelez, F.; Semetey, V. A new, simple approach to confer permanent antimicrobial properties to hydroxylated surfaces by surface functionalization. Chem. Commun. 2008, 8, 951–953. [Google Scholar] [CrossRef] [PubMed]
- Schoefield, W.C.E.; Badyal, J.P.S. A substrate-independent approach for bactericidal surfaces. ACS Appl. Mater. Interfaces 2009, 1, 2763–2767. [Google Scholar] [CrossRef]
- Dhende, P.V.; Samanta, S.; Jones, D.M.; Hardin, I.R.; Locklin, J. One-step photochemical synthesis of permanent, nonleaching, ultrathin antimicrobial coatings for textiles and plastics. ACS Appl. Mater. Interfaces 2011, 3, 2830–2837. [Google Scholar] [CrossRef] [PubMed]
- Tiraferri, A.; Vecitis, C.D.; Elimelech, M. Covalent Binding of Single-Walled Carbon Nanotubes to Polyamide Membranes for Antimicrobial Surface Properties. ACS Appl. Mater. Interfaces 2011, 3, 2869–2877. [Google Scholar] [CrossRef]
- Netzer, L.; Savig, J. A new approach to construction of artificial monolayer assemblies. J. Am. Chem. Soc. 1983, 105, 674–676. [Google Scholar] [CrossRef]
- Lee, H.; Kepley, L.J.; Hong, H.G.; Mallouk, T.E. Adsorption of ordered zirconium phosphonate multilayer films on silicon and gold surfaces. J. Am. Chem. Soc. 1988, 110, 618–620. [Google Scholar] [CrossRef]
- Yang, H.C.; Aoki, K.; Hong, H.G.; Sackett, D.D.; Arendt, M.F.; Yau, S.L.; Bell, C.M.; Mallouk, T.E. Growth and characterization of metal(ii) alkanebisphosphonate multilayer thin-films on gold surfaces. J. Am. Chem. Soc. 1993, 115, 11855–11862. [Google Scholar] [CrossRef]
- Aroca, R.F.; Goulet, P.J.G.; dos Santos, D.S.; Alvarez-Puebla, R.A.; Oliveira, O.N. Silver nanowire layer-by-layer films as substrates for surface-enhanced Raman scattering. Anal. Chem. 2005, 77, 378–382. [Google Scholar] [CrossRef] [PubMed]
- Peczonka, N.P.W.; Goulet, P.J.G.; Aroca, R.F. Chemically selective sensing through layer-by-layer incorporation of biorecognition into thin film substrates for surface-enhanced resonance Raman scattering. J. Am. Chem. Soc. 2006, 128, 12626–12627. [Google Scholar] [CrossRef] [PubMed]
- Dacarro, G.; Cucca, L.; Grisoli, P.; Pallavicini, P.; Patrini, M.; Taglietti, A. Monolayers of polyethilenimine on flat glass: A versatile platform for cations coordination and nanoparticles grafting in the preparation of antibacterial surfaces. Dalton Trans. 2012, 41, 2456–2463. [Google Scholar] [CrossRef] [PubMed]
- Biesuz, R.; Emma, G.; Milanese, C.; Dacarro, G.; Taglietti, A.; Nurchi, V.M.; Alberti, G. Novel DFO-SAM on mesoporous silica for iron sensing. Part I. Synthesis optimization and characterization of the material. Analyst 2014, 139, 3932–3939. [Google Scholar] [CrossRef] [PubMed]
- Taglietti, A.; Arciola, C.R.; D’Agostino, A.; Dacarro, G.; Montanaro, L.; Campoccia, D.; Cucca, L.; Vercellino, M.; Poggi, A.; Pallavicini, P.; et al. Antibiofilm activity of a monolayer of silver nanoparticles anchored to an amino-silanized glass surface. Biomaterials 2014, 35, 1779–1788. [Google Scholar] [CrossRef] [PubMed]
- Pallavicini, P.; Dacarro, G.; Diaz-Fernandez, Y.A.; Taglietti, A. Coordination chemistry of surface-grafted ligands for antibacterial materials. Coord. Chem. Rev. 2014, 275, 37–53. [Google Scholar] [CrossRef]
- Taglietti, A.; Grisoli, P.; Dacarro, G.; Gattesco, A.; Mangano, C.; Pallavicini, P. Grafted monolayers of the neutral Cu (ii) complex of a dioxo-2, 3, 2 ligand: Surfaces with decreased antibacterial action. New J. Chem. 2018, 42, 7595–7598. [Google Scholar] [CrossRef]
- Pallavicini, P.; Arciola, C.R.; Bertoglio, F.; Curtosi, S.; Dacarro, G.; D’Agostino, A.; Ferrari, F.; Merli, D.; Milanese, C.; Rossi, S.; et al. Silver nanoparticles synthesized and coated with pectin: An ideal compromise for anti-bacterial and anti-biofilm action combined with wound-healing properties. J. Colloid Interface Sci. 2017, 498, 271–281. [Google Scholar] [CrossRef]
- Pallavicini, P.; Bassi, B.; Chirico, G.; Collini, M.; Dacarro, G.; Fratini, E.; Grisoli, P.; Patrini, M.; Sironi, L.; Taglietti, A.; et al. Modular approach for bimodal antibacterial surfaces combining photo-switchable activity and sustained biocidal release. Sci. Rep. 2017, 7, 5259. [Google Scholar] [CrossRef]
- Pissinis, D.E.; Benítez, G.A.; Schilardi, P.L. Two-step biocompatible surface functionalization for two-pathway antimicrobial action against Gram-positive bacteria. Colloids Surf. B Biointerfaces 2018, 164, 262–271. [Google Scholar] [CrossRef]
- Yuan, Z.; Liu, P.; Hao, Y.; Ding, Y.; Cai, K. Construction of Ag-incorporated coating on Ti substrates for inhibited bacterial growth and enhanced osteoblast response. Colloids Surf. B Biointerfaces 2018, 171, 597–605. [Google Scholar] [CrossRef] [PubMed]
- Basera, P.; Lavania, M.; Agnihotri, A.; Lal, B. Analytical Investigation of Cymbopogon citratus and Exploiting the Potential of Developed Silver Nanoparticle Against the Dominating Species of Pathogenic Bacteria. Front. Microbiol. 2019, 10, 282. [Google Scholar] [CrossRef] [PubMed]
- Pal, I.; Bhattacharyya, D.; Kar, R.K.; Zarena, D.; Bhunia, A.; Atreya, H.S. A Peptide-Nanoparticle System with Improved Efficacy against Multidrug Resistant Bacteria. Sci. Rep. 2019, 9, 4485. [Google Scholar] [CrossRef] [PubMed]
- Pradeep, T. Noble metal nanoparticles for water purification: A critical review. Thin Solid Film 2009, 517, 6441–6478. [Google Scholar] [CrossRef]
- Pal, S.; Tak, Y.K.; Song, J.M. Does the antibacterial activity of silver nanoparticles depend on the shape of the nanoparticle? A study of the gram-negative bacterium Escherichia coli. Appl. Environ. Microbiol. 2007, 73, 1712–1720. [Google Scholar] [CrossRef]
- Shahverdi, A.R.; Fakhimi, A.; Shahverdi, H.R.; Minaian, S. Synthesis and effect of silver nanoparticles on the antibacterial activity of different antibiotics against Staphylococcus aureus and Escherichia coli. Nanomedicine 2007, 3, 168–171. [Google Scholar] [CrossRef] [PubMed]
- Amato, E.; Diaz-Fernandez, Y.A.; Taglietti, A.; Pallavicini, P.; Pasotti, L.; Cucca, L.; Milanese, C.; Grisoli, P.; Dacarro, C.; Fernandez-Hechavarria, J.M.; et al. Antibacterial activity of glutathione-coated silver nanoparticles against gram positive and gram negative bacteria. Langmuir 2011, 27, 9165–9173. [Google Scholar] [CrossRef]
- Huda, S.; Smoukov, S.K.; Nakanishi, H.; Kowalczyk, B.; Bishop, K.; Grybowski, B.A. Antibacterial Nanoparticle Monolayers Prepared on Chemically Inert Surfaces by Cooperative Electrostatic Adsorption (CELA). ACS Appl. Mater. Interfaces 2010, 2, 1206–1210. [Google Scholar] [CrossRef]
- Samberg, M.E.; Orndorff, P.E.; Monteiro-Riviere, N.A. Antibacterial efficacy of silver nanoparticles of different sizes, surface conditions and synthesis methods. Nanotoxycology 2011, 5, 244–253. [Google Scholar] [CrossRef]
- Klasen, H.J. A historical review of the use of silver in the treatment of burns. II. Renewed interest for silver. Burns 2000, 26, 131–138. [Google Scholar] [CrossRef]
- Xu, H.; Shi, X.; Ma, H.; Lv, Y.; Zhang, L.; Mao, Z. The preparation and antibacterial effects of dopa-cotton/AgNPs. Appl. Surf. Sci. 2011, 15, 6799–6803. [Google Scholar] [CrossRef]
- Chen, W.; Liu, Y.; Courtney, H.S.; Bettenga, M.; Agrawal, C.M.; Bumgradner, J.D.; Ong, J.L. In vitro anti-bacterial and biological properties of magnetron co-sputtered silver-containing hydroxyapatite coating. Biomaterials 2006, 27, 5512–5517. [Google Scholar] [CrossRef] [PubMed]
- Salesh, S.; Taha, M.O.; Haddadin, R.N.; Marzooqa, D.; Hodali, H. Preparation of Silver-and Zinc-Doped Mullite-Based Ceramics Showing Anti-Bacterial Biofilm Properties. Molecules 2011, 16, 2862–2870. [Google Scholar] [CrossRef]
- Pallavicini, P.; Taglietti, A.; Dacarro, G.; Diaz Fernandez, Y.A.; Galli, M.; Grisoli, P.; Patrini, M.; Santucci De Magistris, G.; Zanoni, R. Self-assembled monolayers of silver nanoparticles firmly grafted on glass surfaces: Low Ag+ release for an efficient antibacterial activity. J. Colloid Interf. Sci. 2010, 350, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Fox, C.L.; Modak, S.M. Mechanism of silver sulfadiazine action on burn wound infections. Antimicrob. Agents Chemother. 1974, 5, 582–588. [Google Scholar] [CrossRef] [PubMed]
- Mastrolorenzo, A.; Scozzafava, A.; Supuran, C.T. Antifungal activity of silver and zinc complexes of sulfadrug derivatives incorporating arylsulfonylureido moieties. Eur. J. Pharm. Sci. 2000, 11, 99–107. [Google Scholar] [CrossRef]
- Pallavicini, P.; Dacarro, G.; Taglietti, A. Self-assembled monolayers of silver nanoparticles: From intrinsic to switchable inorganic antibacterial surfaces. Eur. J. Inorg. Chem. 2018, 45, 4846–4855. [Google Scholar] [CrossRef]
- Nímia, H.H.; Carvalho, V.F.; Isaac, C.; Souza, F.Á.; Gemperli, R.; Paggiaro, A.O. Comparative study of Silver Sulfadiazine with other materials for healing and infection prevention in burns: A systematic review and meta-analysis. Burns 2019, 45, 282–292. [Google Scholar] [CrossRef]
- Atiyeh, B.S.; Costagliola, M.; Hayek, S.N.; Dibo, S.A. Effect of silver on burn wound infection control and healing: Review of the literature. Burns 2007, 33, 139–148. [Google Scholar] [CrossRef]
- Pallavicini, P.; Dacarro, G.; Galli, M.; Patrini, M. Spectroscopic evaluation of surface functionalization efficiency in the preparation of mercaptopropyltrimethoxysilane self-assembled monolayers on glass. J. Colloid Interface Sci. 2009, 332, 432–438. [Google Scholar] [CrossRef]
- Pallavicini, P.; Dacarro, G.; Cucca, L.; Denat, F.; Grisoli, P.; Patrini, M.; Sok, N.; Taglietti, A. A monolayer of a Cu2+-tetraazamacrocyclic complex on glass as the adhesive layer for silver nanoparticles grafting, in the preparation of surface-active antibacterial materials. New J. Chem. 2011, 35, 1198–1201. [Google Scholar] [CrossRef]
- Krasnoslobodtsev, A.; Smirnov, S. Surface assisted intermolecular interactions in self-assembled coumarin submonolayers. Langmuir 2001, 17, 7593–7599. [Google Scholar] [CrossRef]
- Krasnoslobodtsev, A.V.; Smirnov, S.N. Effect of water on silanization of silica by trimethoxysilanes. Langmuir 2002, 18, 3181–3184. [Google Scholar] [CrossRef]
- Russel, A.D.; Hugo, W.B.; Ayliffe, G.A.J. Principles and Practice of Disinfection, Preservation & Sterilization; Fraise, A.P., Lambert, P.A., Maillard, J.-Y., Eds.; Blackwell Publishing: Oxford, UK, 2004. [Google Scholar]
- Arciola, C.R.; Campoccia, D.; Speziale, P.; Montanaro, L.; Costerton, J.W. Biofilm formation in Staphylococcus implant infections. A review of molecular mechanisms and implications for biofilm-resistant materials. Biomaterials 2012, 33, 5967–5982. [Google Scholar] [CrossRef] [PubMed]
- Arciola, C.R.; Campoccia, D.; Ehrlich, G.D.; Montanaro, L. Biofilm-based implant infections in orthopaedics. Adv. Exp. Med. Biol. 2015, 830, 29–46. [Google Scholar]
- Li, W.T.; Chang, H.W.; Yang, W.C.; Lo, C.; Wang, L.Y.; Pang, V.F.; Chen, M.H.; Jeng, C.R. Immunotoxicity of SilverNanoparticles (AgNPs) on the Leukocytes of Common Bottlenose Dolphins (Tursiops truncatus). Sci. Rep. 2018, 8, 5593. [Google Scholar] [CrossRef]
- Davies, R.L.; Etris, S.F. The development and functions of silver in water purification and disease control. Catal. Today 1997, 36, 107–114. [Google Scholar] [CrossRef]
| S. aureus | E. coli | |||
|---|---|---|---|---|
| 5 h | 24 h | 5 h | 24 h | |
| SURF-SH | −0.1 | −0.1 | −0.2 | −0.3 |
| SURF-SDM | 0.4 | 0.2 | 0.3 | 0.1 |
| SURF-Ag-SDM | 0.8 | 6.1 | 1.0 | 4.0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taglietti, A.; Dacarro, G.; Barbieri, D.; Cucca, L.; Grisoli, P.; Patrini, M.; Arciola, C.R.; Pallavicini, P. High Bactericidal Self-Assembled Nano-Monolayer of Silver Sulfadiazine on Hydroxylated Material Surfaces. Materials 2019, 12, 2761. https://doi.org/10.3390/ma12172761
Taglietti A, Dacarro G, Barbieri D, Cucca L, Grisoli P, Patrini M, Arciola CR, Pallavicini P. High Bactericidal Self-Assembled Nano-Monolayer of Silver Sulfadiazine on Hydroxylated Material Surfaces. Materials. 2019; 12(17):2761. https://doi.org/10.3390/ma12172761
Chicago/Turabian StyleTaglietti, Angelo, Giacomo Dacarro, Daniele Barbieri, Lucia Cucca, Pietro Grisoli, Maddalena Patrini, Carla Renata Arciola, and Piersandro Pallavicini. 2019. "High Bactericidal Self-Assembled Nano-Monolayer of Silver Sulfadiazine on Hydroxylated Material Surfaces" Materials 12, no. 17: 2761. https://doi.org/10.3390/ma12172761
APA StyleTaglietti, A., Dacarro, G., Barbieri, D., Cucca, L., Grisoli, P., Patrini, M., Arciola, C. R., & Pallavicini, P. (2019). High Bactericidal Self-Assembled Nano-Monolayer of Silver Sulfadiazine on Hydroxylated Material Surfaces. Materials, 12(17), 2761. https://doi.org/10.3390/ma12172761