Operando Laboratory X-Ray Imaging of Silver-Based Gas Diffusion Electrodes during Oxygen Reduction Reaction in Highly Alkaline Media
Abstract
1. Introduction
2. Materials and Methods
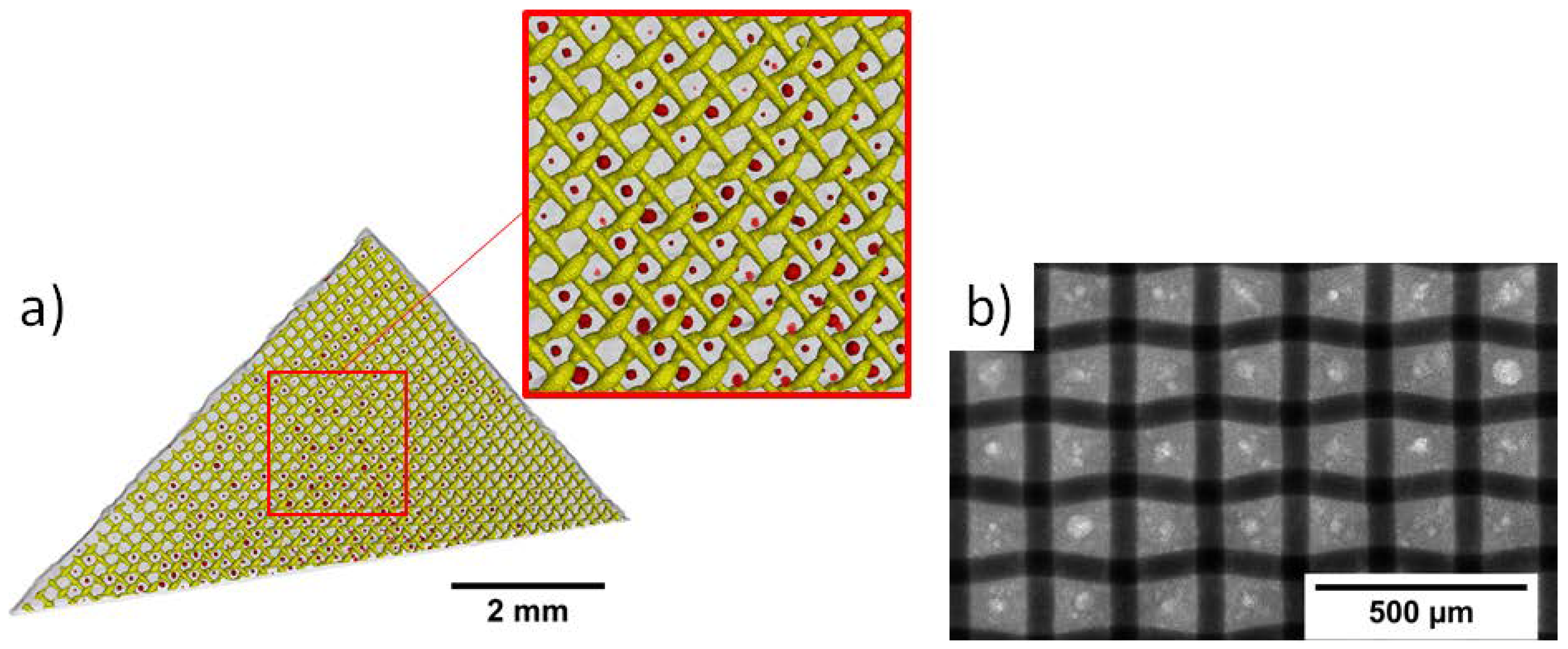
2.1. Preparation of Silver-Based Gas Diffusion Electrodes
2.2. Electrochemical Measurement
2.3. Focused Ion Beam
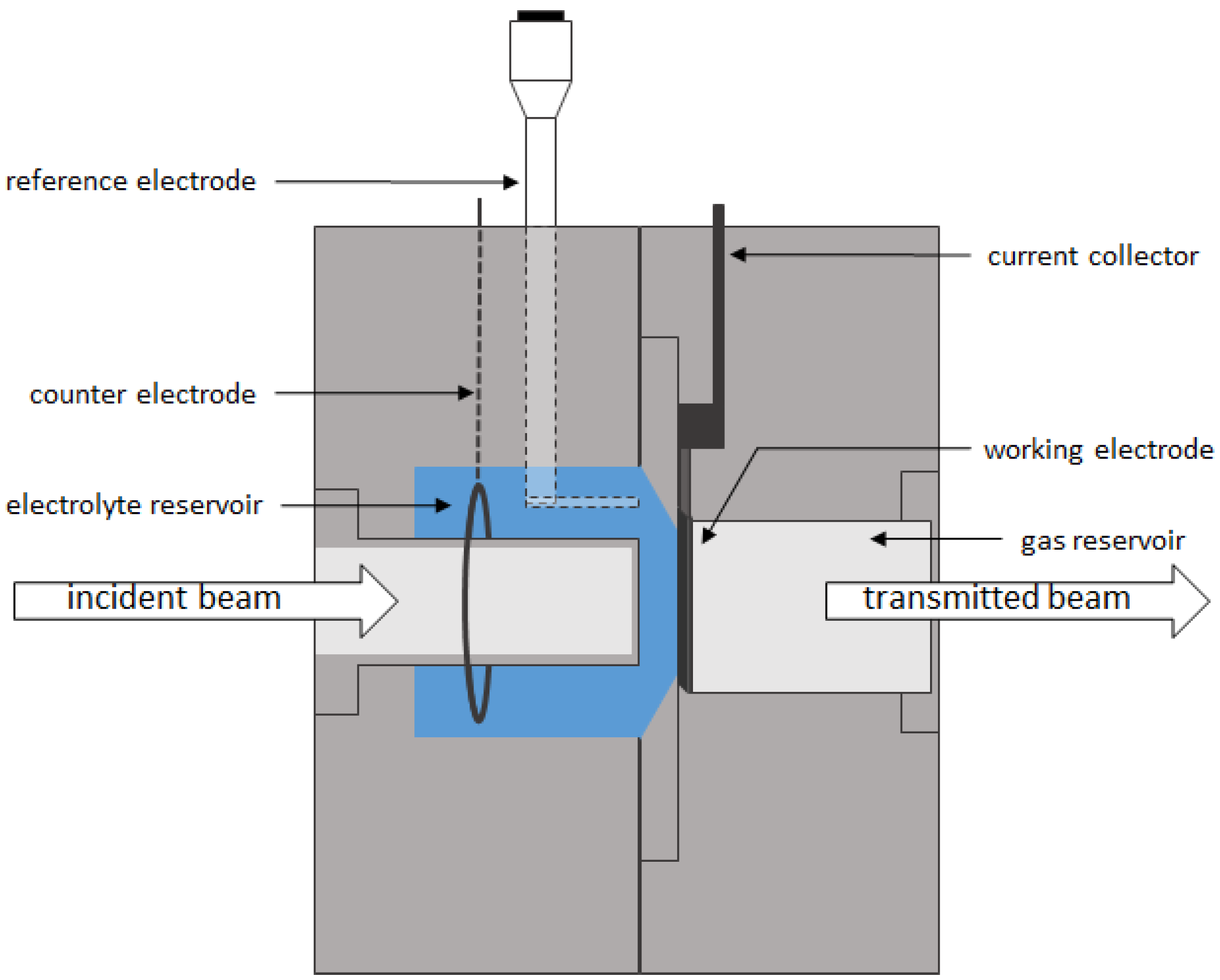
2.4. Laboratory X-Ray Measurements
2.5. Synchrotron
3. Results
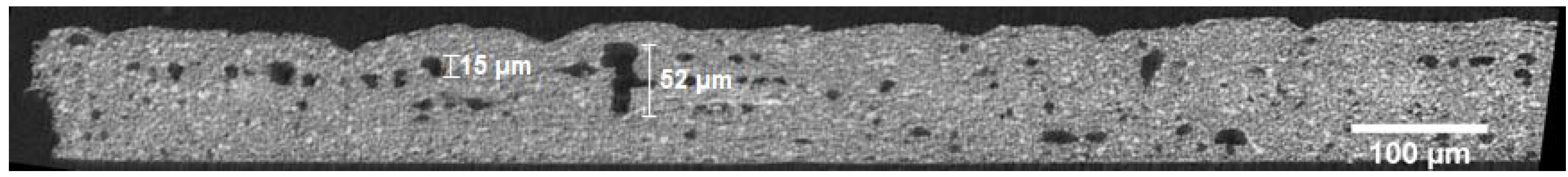
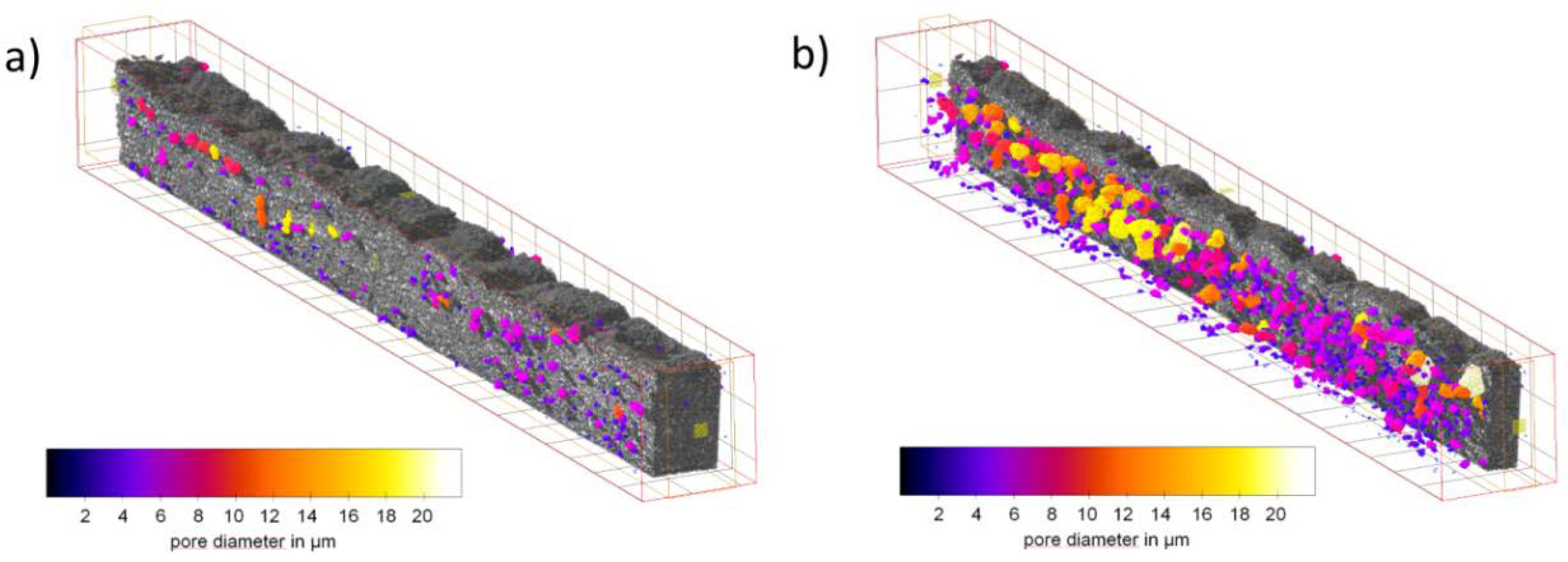
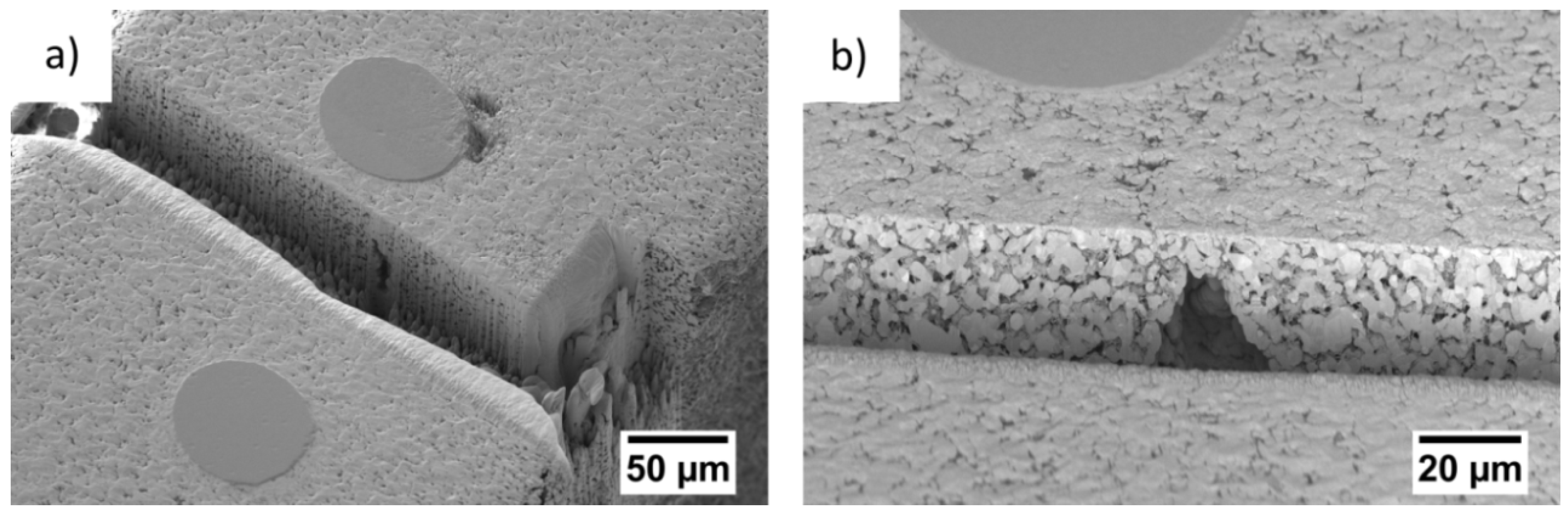
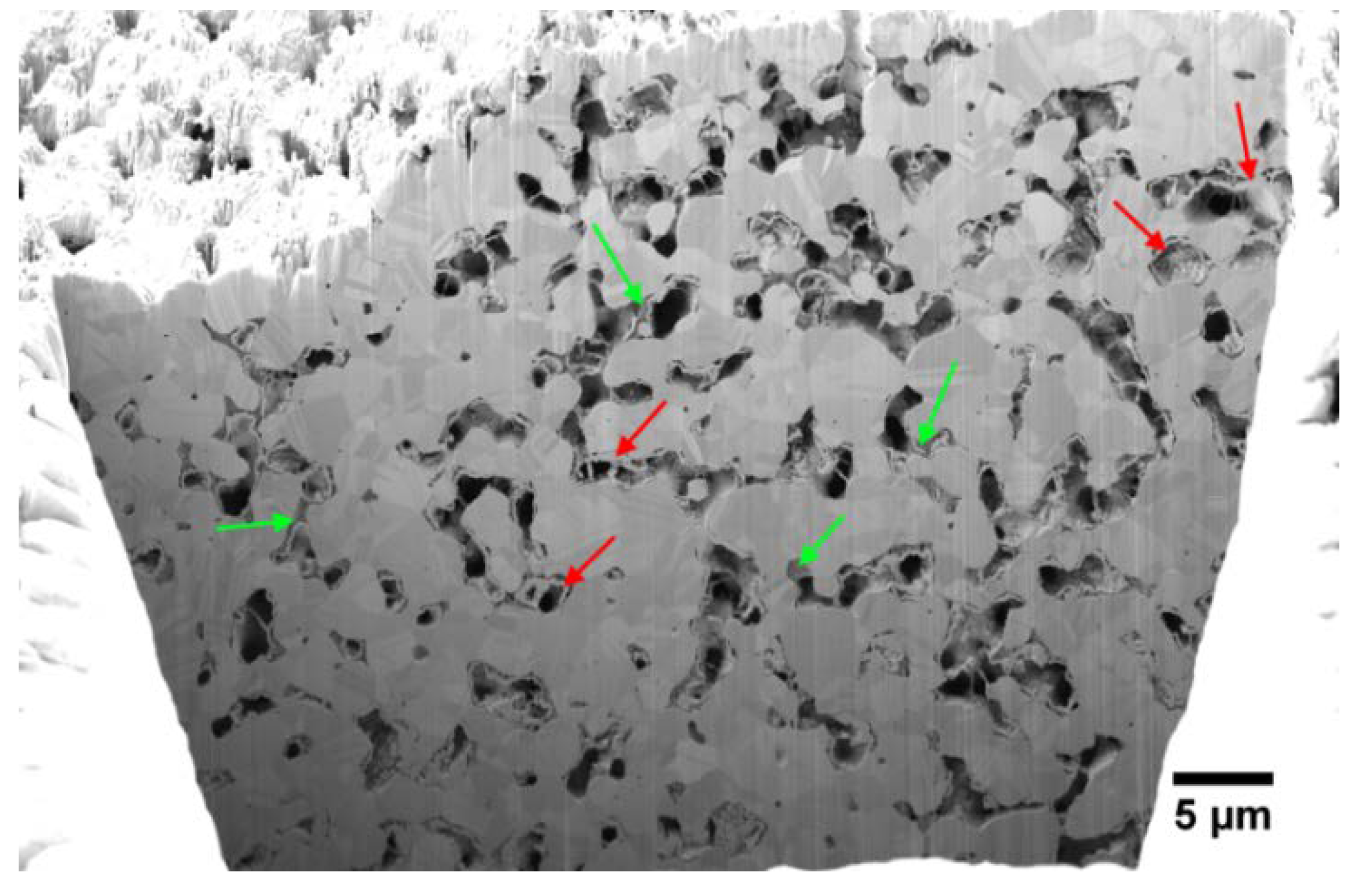
3.1. Ex Situ Analysis of the Microstructure
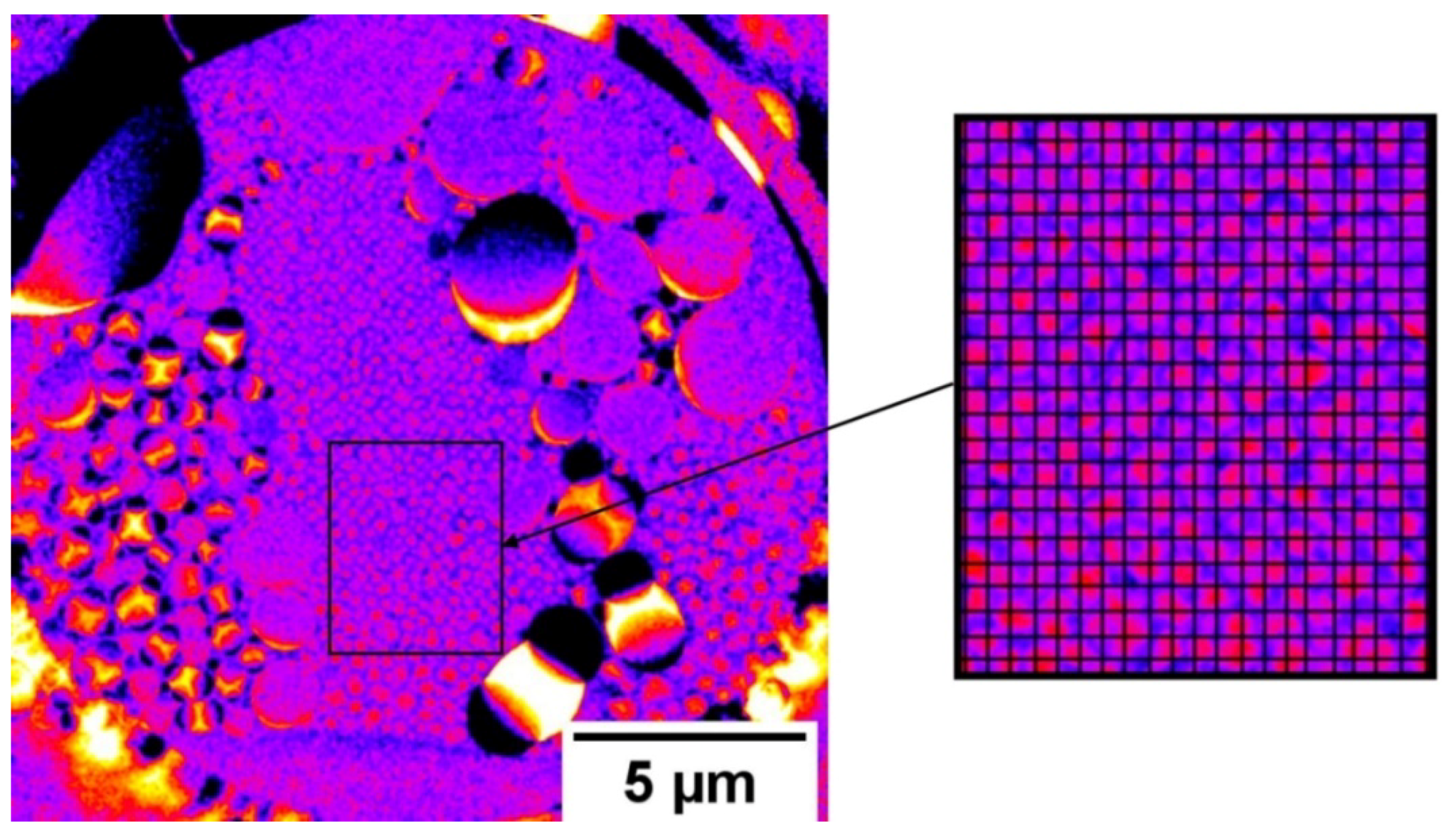
3.2. Operando Radiography of the Electrochemical Process
4. Discussion
4.1. Microstructure of the Gas Diffusion Electrodes
4.2. Electrolyte Distribution during Electrochemical Tests
4.3. Comparison of GDEs with Different Chemical Composition
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kintrup, J.; Millaruelo, M.; Trieu, V.; Bulan, A.; Mojica, E.S. Gas Diffusion Electrodes for Efficient Manufacturing of Chlorine and Other Chemicals. Electrochem. Soc. Interface 2017, 26, 73–76. [Google Scholar] [CrossRef]
- Moussallem, I.; Jörissen, J.; Kunz, U.; Pinnow, S.; Turek, T. Chlor-alkali electrolysis with oxygen depolarized cathodes: History, present status and future prospects. J. Appl. Electrochem. 2008, 38, 1177–1194. [Google Scholar] [CrossRef]
- Jörissen, J.; Turek, T.; Weber, R. Chlorherstellung mit Sauerstoffverzehrkathoden. Energieeinsparung bei der Elektrolyse. Chem. Unserer Zeit 2011, 45, 172–183. [Google Scholar] [CrossRef]
- Garcia-Herrero, I.; Margallo, M.; Onandía, R.; Aldaco, R.; Irabien, A. Environmental challenges of the chlor-alkali production: Seeking answers from a life cycle approach. Sci. Total Environ. 2017, 580, 147–157. [Google Scholar] [CrossRef]
- Jung, J.; Postels, S.; Bardow, A. Cleaner chlorine production using oxygen depolarized cathodes? A life cycle assessment. J. Clean. Prod. 2014, 80, 46–56. [Google Scholar] [CrossRef]
- Chatenet, M.; Genies-Bultel, L.; Aurousseau, M.; Durand, R.; Andolfatto, F. Oxygen reduction on silver catalysts in solutions containing various concentrations of sodium hydroxide—Comparison with platinum. J. Appl. Electrochem. 2002, 32, 1131–1140. [Google Scholar] [CrossRef]
- Moussallem, I.; Pinnow, S.; Wagner, N.; Turek, T. Development of high-performance silver-based gas-diffusion electrodes for chlor-alkali electrolysis with oxygen depolarized cathodes. Chem. Eng. Process. Intensif. 2012, 52, 125–131. [Google Scholar] [CrossRef]
- Franzen, D.; Ellendorff, B.; Paulisch, M.C.; Hilger, A.; Osenberg, M.; Manke, I.; Turek, T. Influence of binder content in silver-based gas diffusion electrodes on pore system and electrochemical performance. J. Appl. Electrochem. 2019, 49, 705–713. [Google Scholar] [CrossRef]
- Al Rwashdeh, S.S.; Manke, I.; Markötter, H.; Klages, M.; Göbel, M.; Haußmann, J.; Scholta, J.; Banhart, J. In Operando Quantification of Three-Dimensional Water Distribution in Nanoporous Carbon-Based Layers in Polymer Electrolyte Membrane Fuel Cells. ACS Nano 2017, 11, 5944–5949. [Google Scholar] [CrossRef]
- Boillat, P.; Frei, G.; Lehmann, E.H.; Scherer, G.G.; Wokaun, A. Neutron Imaging Resolution Improvements Optimized for Fuel Cell Applications. Electrochem. Solid State Lett. 2010, 13, B25–B27. [Google Scholar] [CrossRef]
- Fazeli, M.; Hinebaugh, J.; Fishman, Z.; Tötzke, C.; Lehnert, W.; Manke, I.; Bazylak, A. Pore network modeling to explore the effects of compression on multiphase transport in polymer electrolyte membrane fuel cell gas diffusion layers. J. Power Sources 2016, 335, 162–171. [Google Scholar] [CrossRef]
- Manke, I.; Hartnig, C.; Grünerbel, M.; Lehnert, W.; Kardjilov, N.; Haibel, A.; Hilger, A.; Banhart, J.; Riesemeier, H. Investigation of water evolution and transport in fuel cells with high resolution synchrotron X-ray radiography. Appl. Phys. Lett. 2007, 90, 4105. [Google Scholar] [CrossRef]
- Markötter, H.; Manke, I.; Krüger, P.; Arlt, T.; Haussmann, J.; Klages, M.; Riesemeier, H.; Hartnig, C.; Scholta, J.; Banhart, J. Investigation of 3D water transport paths in gas diffusion layers by combined in-situ synchrotron X-ray radiography and tomography. Electrochem. Commun. 2011, 13, 1001–1004. [Google Scholar] [CrossRef]
- Sasabe, T.; Deevanhxay, P.; Tsushima, S.; Hirai, S. Investigation on the effect of microstructure of proton exchange membrane fuel cell porous layers on liquid water behavior by soft X-ray radiography. J. Power Sources 2011, 196, 8197–8206. [Google Scholar] [CrossRef]
- Schröder, A.; Wippermann, K.; Lehnert, W.; Stolten, D.; Sanders, T.; Baumhöfer, T.; Kardjilov, N.; Hilger, A.; Banhart, J.; Manke, I. The influence of gas diffusion layer wettability on direct methanol fuel cell performance: A combined local current distribution and high resolution neutron radiography study. J. Power Sources 2010, 195, 4765–4771. [Google Scholar] [CrossRef]
- Gebhard, M.; Paulisch, M.; Hilger, A.; Franzen, D.; Ellendorff, B.; Turek, T.; Manke, I.; Roth, C. Design of an In-Operando Cell for X-ray and Neutron Imaging of Oxygen-Depolarized Cathodes in Chlor-Alkali Electrolysis. Materials 2019, 12, 1275. [Google Scholar] [CrossRef]
- Moussallem, I. Development of Gas Diffusion Electrodes for a New Energy Saving Chlor-Alkali Electrolysis Process. Ph.D. Thesis, Clausthal University of Technology, Clausthal-Zellerfeld, Germany, January 2011. [Google Scholar]
- Kardjilov, N.; Hilger, A.; Manke, I. CONRAD-2: Cold Neutron Tomography and Radiography at BER II (V7). J. Large Scale Res. Facil. 2016, 2, A98. [Google Scholar] [CrossRef]
- Kardjilov, N.; Hilger, A.; Manke, I.; Woracek, R.; Banhart, J. CONRAD-2: The new neutron imaging instrument at the Helmholtz-Zentrum Berlin. J. Appl. Crystallogr. 2016, 49, 195–202. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Legland, D.; Arganda-Carreras, I.; Andrey, P. MorphoLibJ: Integrated library and plugins for mathematical morphology with ImageJ. Bioinformatics 2016, 32, 3532–3534. [Google Scholar] [CrossRef]
- Kunz, P.; Paulisch, M.; Osenberg, M.; Bischof, B.; Manke, I.; Nieken, U. Prediction of Electrolyte Distribution in Technical Gas Diffusion Electrodes: From Imaging to SPH Simulations. Transp. Porous Med. 2019, in press. [Google Scholar]
- Haußmann, J.; Markötter, H.; Alink, R.; Bauder, A.; Dittmann, K.; Manke, I.; Scholta, J. Synchrotron radiography and tomography of water transport in perforated gas diffusion media. J. Power Sources 2013, 239, 611–622. [Google Scholar] [CrossRef]
- Alink, R.; Haußmann, J.; Markötter, M.; Schwager, M.; Manke, I.; Gerteisen, D. The Influence of Porous Transport Layer Modifications on the Water Management in PEM Fuel Cells. J. Power Sources 2013, 233, 358–368. [Google Scholar] [CrossRef]
- Jeanty, P.; Scherer, C.; Magori, E.; Wiesner-Fleischer, K.; Hinrichsen, O.; Fleischer, M. Upscaling and continuous operation of electrochemical CO2 to CO conversion in aqueous solutions on silver gas diffusion electrodes. J. CO2 Util. 2018, 24, 454–462. [Google Scholar] [CrossRef]
- Burchardt, T. An evaluation of electrocatalytic activity and stability for air electrodes. J. Power Sources 2004, 135, 192–197. [Google Scholar] [CrossRef]
- Bidault, F.; Brett, D.; Middleton, P.H.; Brandon, N.P. Review of gas diffusion cathodes for alkaline fuel cells. J. Power Sources 2009, 187, 39–48. [Google Scholar] [CrossRef]

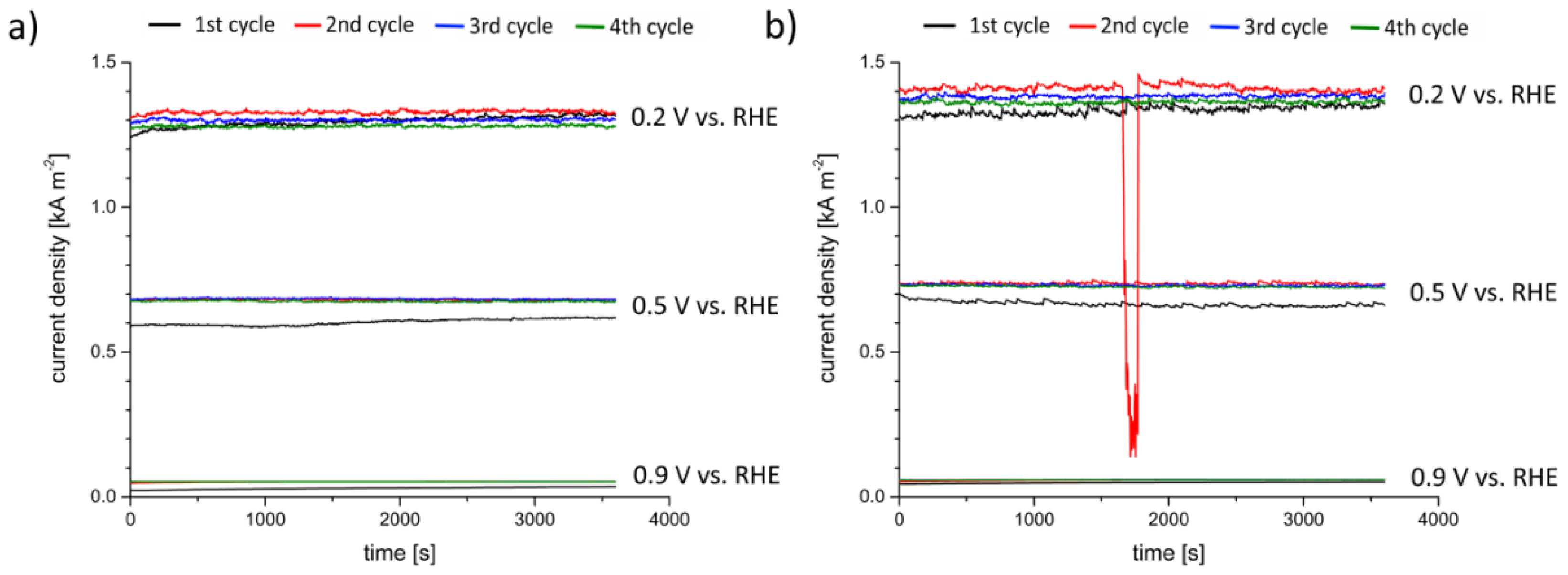

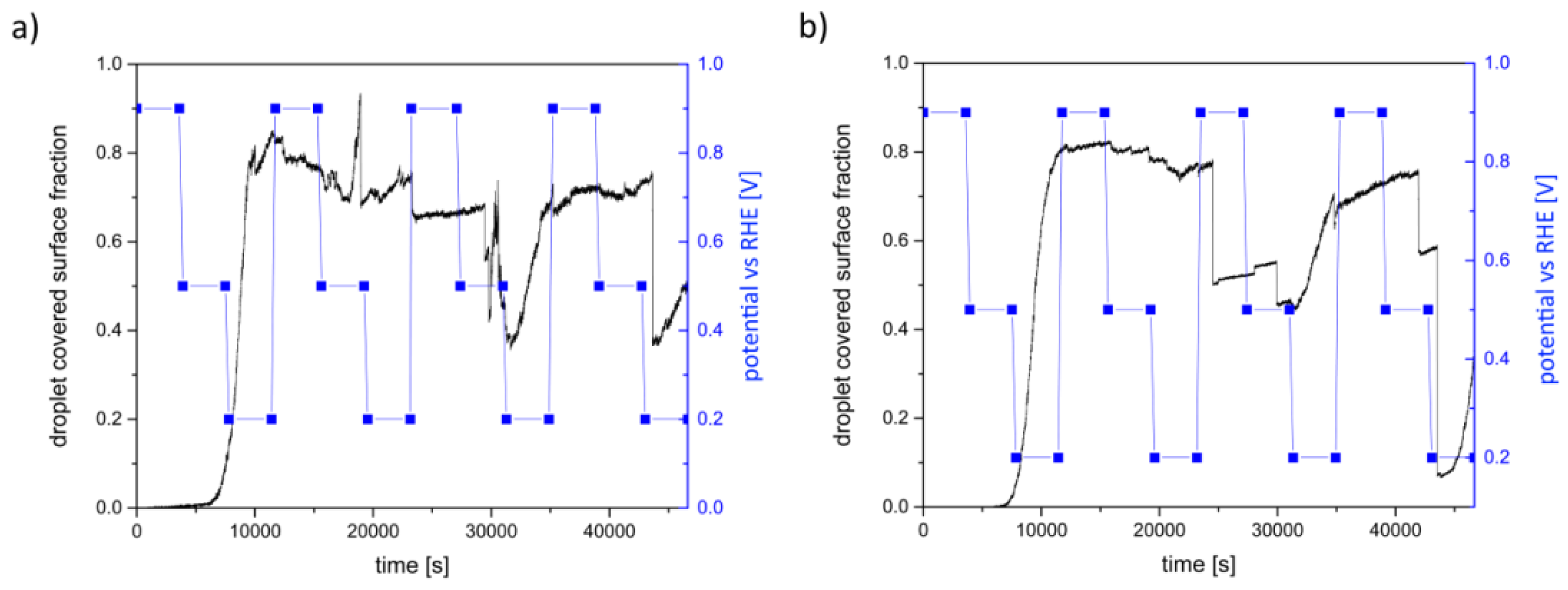

| Potential [V] | Cycle | Saturation [µm·s−1] | Imaging |
|---|---|---|---|
| 0.9 vs. RHE | first | decrease | no changes of the electrolyte distribution |
| 0.5 vs. RHE | |||
| 0.2 vs. RHE | increase of 0.094 | small droplets form | |
| 0.9 vs. RHE | second | almost constant | no changes of droplets |
| 0.5 vs. RHE | |||
| 0.2 vs. RHE | increase of 0.063 | small droplets agglomerate | |
| 0.9 vs. RHE | third | increase of 0.033 | no changes of droplets |
| 0.5 vs. RHE | sudden decrease | drop down of large droplets | |
| 0.2 vs. RHE | increase of 0.080 | new droplets form | |
| 0.9 vs. RHE | fourth | sharp peak | peak caused by a large droplet dropping into the ROI; apart from this saturation constant |
| 0.5 vs. RHE | increase of 0.027 | droplets agglomerate; in the end of this cycle, droplets dropped down | |
| 0.2 vs. RHE | increase of 0.062 | no changes |
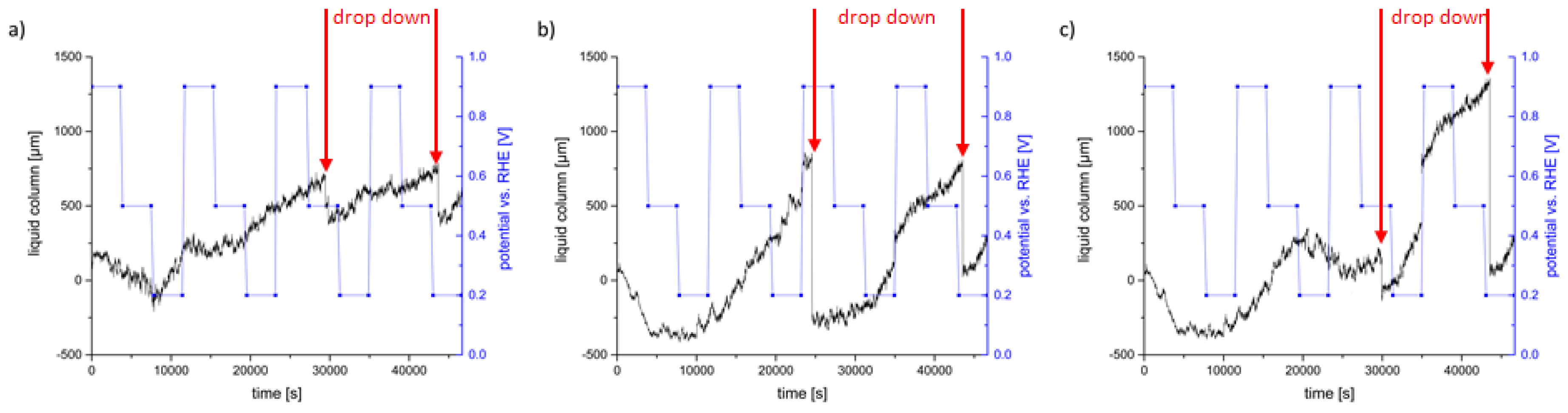
| Potential [V] | Cycle | Saturation [µm·s−1] | Imaging |
|---|---|---|---|
| 0.9 vs. RHE | first | decrease | no changes of the electrolyte distribution |
| 0.5 vs. RHE | |||
| 0.2 vs. RHE | sudden increase | small droplets form | |
| 0.9 vs. RHE | second | increase of 0.094 | no changes of droplets |
| 0.5 vs. RHE | increase of 0.099 | no changes of droplets | |
| 0.2 vs. RHE | increase of 0.145 | droplets agglomerate | |
| 0.9 vs. RHE | third | almost constant | droplets coagulate and drop down, afterwards no changes of the electrolyte distribution |
| 0.5 vs. RHE | increase of 0.022 | no changes of the electrolyte distribution | |
| 0.2 vs. RHE | increase of 0.107 | new droplets form | |
| 0.9 vs. RHE | fourth | saturation increases | caused by a droplet, which dropped into ROI |
| 0.5 vs. RHE | increase of 0.056 | drop down in the end of this cycle | |
| 0.2 vs. RHE | increase of 0.121 | no changes of the electrolyte distribution |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paulisch, M.C.; Gebhard, M.; Franzen, D.; Hilger, A.; Osenberg, M.; Kardjilov, N.; Ellendorff, B.; Turek, T.; Roth, C.; Manke, I. Operando Laboratory X-Ray Imaging of Silver-Based Gas Diffusion Electrodes during Oxygen Reduction Reaction in Highly Alkaline Media. Materials 2019, 12, 2686. https://doi.org/10.3390/ma12172686
Paulisch MC, Gebhard M, Franzen D, Hilger A, Osenberg M, Kardjilov N, Ellendorff B, Turek T, Roth C, Manke I. Operando Laboratory X-Ray Imaging of Silver-Based Gas Diffusion Electrodes during Oxygen Reduction Reaction in Highly Alkaline Media. Materials. 2019; 12(17):2686. https://doi.org/10.3390/ma12172686
Chicago/Turabian StylePaulisch, Melanie Cornelia, Marcus Gebhard, David Franzen, André Hilger, Markus Osenberg, Nikolay Kardjilov, Barbara Ellendorff, Thomas Turek, Christina Roth, and Ingo Manke. 2019. "Operando Laboratory X-Ray Imaging of Silver-Based Gas Diffusion Electrodes during Oxygen Reduction Reaction in Highly Alkaline Media" Materials 12, no. 17: 2686. https://doi.org/10.3390/ma12172686
APA StylePaulisch, M. C., Gebhard, M., Franzen, D., Hilger, A., Osenberg, M., Kardjilov, N., Ellendorff, B., Turek, T., Roth, C., & Manke, I. (2019). Operando Laboratory X-Ray Imaging of Silver-Based Gas Diffusion Electrodes during Oxygen Reduction Reaction in Highly Alkaline Media. Materials, 12(17), 2686. https://doi.org/10.3390/ma12172686






