3D Printing of Bioceramic Scaffolds—Barriers to the Clinical Translation: From Promise to Reality, and Future Perspectives
Abstract
1. Introduction
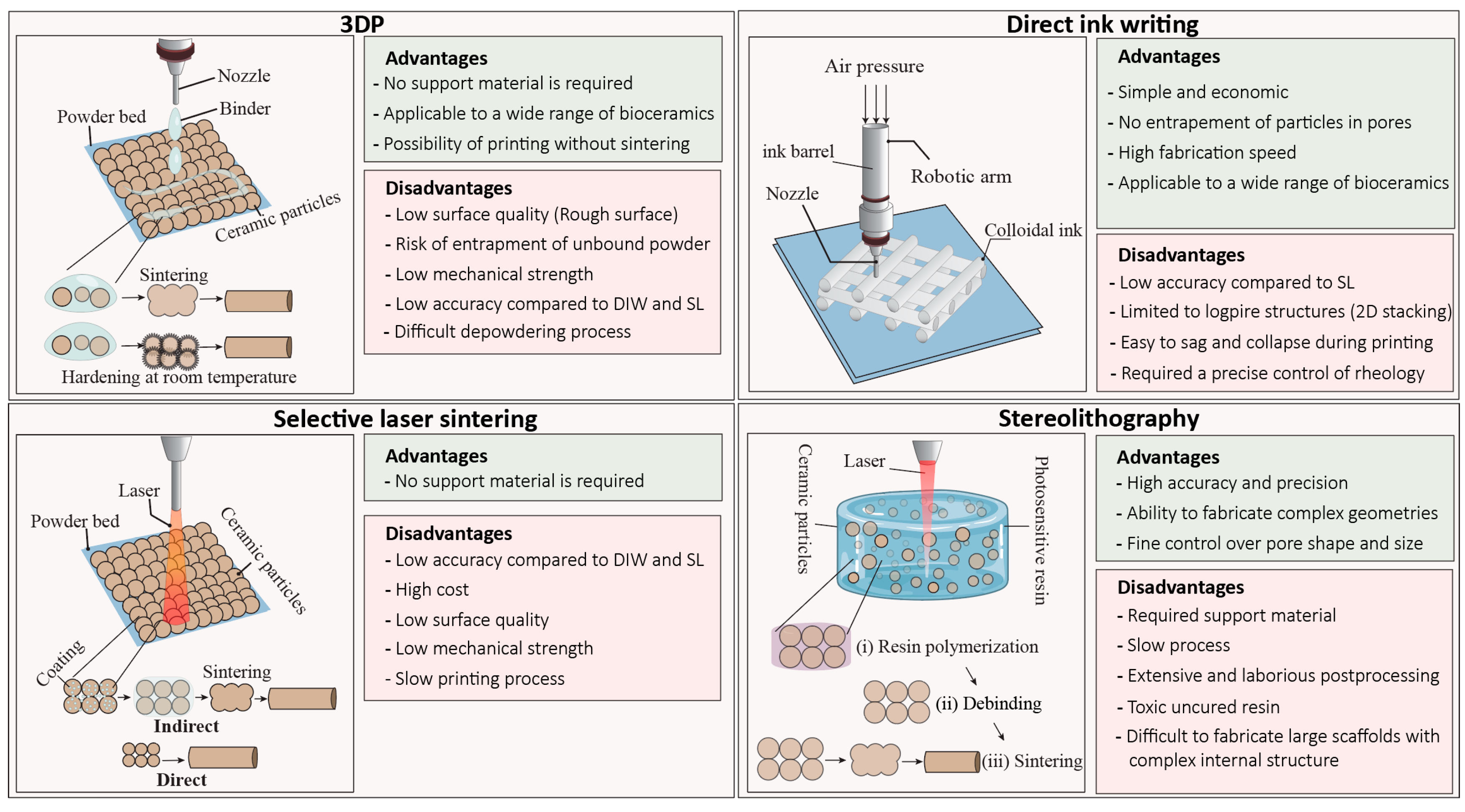
2. Powder-Based Techniques
2.1. Three-Dimensional Printing (3DP)
2.2. Selective Laser Sintering (SLS)
3. Slurry-Based Technology
3.1. Stereolithography (SL)
3.2. Direct Ink Writing (DIW)
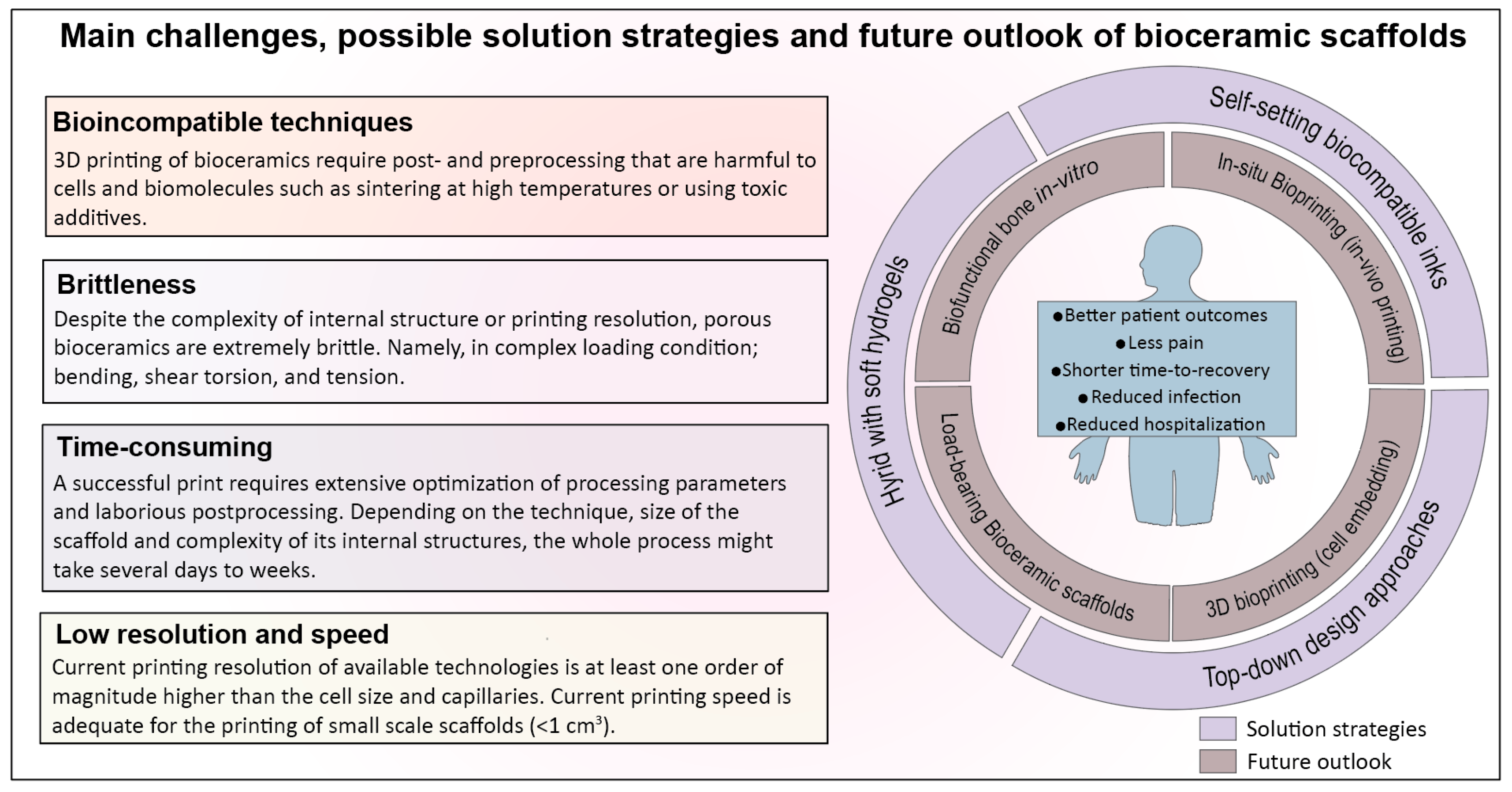
4. Barriers to Clinical Translation and Current Challenges
4.1. 3D Printing Techniques for Bioceramics Are Time-Consuming and Sensitive to Post/Preprocessing Steps
4.2. Bioceramic Scaffolds Are Brittle, Regardless of the Printing Technique or Complexity of Internal Structure
4.3. Prefabricated Bioceramic Scaffolds often Fail to Match with the Anatomical Shape and Size of the Defect
4.4. Bioceramics and Their Printing Techniques Are Incompatible in Integration with Cells and Biomolecule during Printing: 3D Bioprinting versus 3D Printing
4.5. Printing Resolution, Vascularization Strategies, and Practical Challenges in Adoption of Additive Manufacturing in Clinical Practice
5. Future Perspective
Funding
Acknowledgments
Conflicts of Interest
References
- Dumic-Cule, I.; Pecina, M.; Jelic, M.; Jankolija, M.; Popek, I.; Grgurevic, L.; Vukicevic, S. Biological aspects of segmental bone defects management. Int. Orthop. 2015, 39, 1005–1011. [Google Scholar] [CrossRef] [PubMed]
- Pobloth, A.M.; Checa, S.; Razi, H.; Petersen, A.; Weaver, J.C.; Chmidt-Bleek, K.; Windolf, M.; Tatai, A.A.; Roth, C.P.; Schaser, K.D.; et al. Mechanobiologically optimized 3D titanium-mesh scaffolds enhance bone regeneration in critical segmental defects in sheep. Sci. Transl. Med. 2018, 10, 1–16. [Google Scholar] [CrossRef]
- Petite, H.; Viateau, V.; Bensaïd, W.; Meunier, A.; De Pollak, C.; Bourguignon, M.; Oudina, K.; Sedel, L.; Guillemin, G. Tissue-engineered bone regeneration. Nat. Biotechnol. 2000, 18, 959–963. [Google Scholar] [CrossRef]
- Pilia, M.; Guda, T.; Appleford, M. Development of composite scaffolds for load-bearing segmental bone defects. Biomed Res. Int. 2013, 2013, 458253. [Google Scholar] [CrossRef]
- Schlundt, C.; Bucher, C.H.; Tsitsilonis, S.; Schell, H.; Duda, G.N.; Schmidt-Bleek, K. Clinical and Research Approaches to Treat Non-union Fracture. Curr. Osteoporos. Rep. 2018, 16, 155–168. [Google Scholar] [CrossRef]
- Berner, A.; Reichert, J.C.; Müller, M.B.; Zellner, J.; Pfeifer, C.; Dienstknecht, T.; Nerlich, M.; Sommerville, S. Treatment of long bone defects and non-unions: From research to clinical practice. Cell Tissue Res. 2012, 3, 501–519. [Google Scholar] [CrossRef] [PubMed]
- Myeroff, C.; Michael, A. Autogenous Bone Graft: Donor Sites and Techniques. J. Bone Jt. Surg. 2011, 93, 2227–2236. [Google Scholar] [CrossRef] [PubMed]
- Calori, G.M.; Colombo, M.; Mazza, E.L.; Mazzola, S.; Malagoli, E.; Mineo, G.V. Incidence of donor site morbidity following harvesting from iliac crest or RIA graft. Injury 2014, 45, S116–S120. [Google Scholar] [CrossRef] [PubMed]
- Klar, R.M. The Induction of Bone Formation: The Translation Enigma. Front. Bioeng. Biotechnol. 2018, 6, 1–13. [Google Scholar] [CrossRef]
- Grado De, G.F.; Keller, L.; Idoux-gillet, Y.; Wagner, Q.; Musset, A.; Benkirane-jessel, N.; Bornert, F.; Offner, D. Bone substitutes: A review of their characteristics, clinical use, and perspectives for large bone defects management. J. Tissue Eng. 2018, 9, 1–18. [Google Scholar]
- Dimitriou, R.; Jones, E.; McGonagle, D.; Giannoudis, P.V. Bone regeneration: Current concepts and future directions. BMC Med. 2011, 9, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Calori, G.M.; Mazza, E.; Colombo, M.; Ripamonti, C. The use of bone-graft substitutes in large bone defects: Any specific needs? Injury 2011, 42, S56–S63. [Google Scholar] [CrossRef] [PubMed]
- Hollister, S.J. Scaffold design and manufacturing: From concept to clinic. Adv. Mater. 2009, 21, 3330–3342. [Google Scholar] [CrossRef] [PubMed]
- De Witte, T.-M.; Fratila-Apachitei, L.E.; Zadpoor, A.A.; Peppas, N.A. Bone tissue engineering via growth factor delivery: From scaffolds to complex matrices. Regen. Biomater. 2018, 5, 197–211. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Fan, Y.; Dunne, N.; Li, X. Effect of microporosity on scaffolds for bone tissue engineering. Regen. Biomater. 2018, 5, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Rose, J.C.; De Laporte, L. Hierarchical Design of Tissue Regenerative Constructs. Adv. Healthc. Mater. 2018, 7, 1–31. [Google Scholar] [CrossRef]
- Entezari, A.; Roohani, I.; Li, G.; Dunstan, C.R.; Rognon, P.; Li, Q. Architectural Design of 3D Printed Scaffolds Controls the Volume and Functionality of Newly Formed Bone. Adv. Healthc. Mater. 2019, 8, 1801353. [Google Scholar] [CrossRef]
- Zadpoor, A.A. Bone tissue regeneration: The role of scaffold geometry. Biomater. Sci. 2015, 3, 231–245. [Google Scholar] [CrossRef]
- Wildemann, B.; Kadowromacker, A.; Haas, N.; Schmidmaier, G. Quantification of various growth factors in different demineralized bone matrix preparations. J. Biomed. Mater. Res.-Part A 2007, 2, 437–442. [Google Scholar] [CrossRef]
- Guillemin, G.; Patat, J.-L.; Fournie, J.; Chetail, M. The use of coral as a bone graft substitute. J. Biomed. Mater. Res. 1987, 21, 557–567. [Google Scholar] [CrossRef]
- Walsh, W.R.; Morberg, P.; Yu, Y.; Yang, J.; Haggard, W.; Sheath, P.; Svehla, M.; Bruce, W.J. Response of a Calcium Sulfate Bone Graft Substitute in a Confined Cancellous Defect. Clin. Orthop. Relat. Res. 2003, 406, 228–236. [Google Scholar] [CrossRef]
- Miguez-Pacheco, V.; Hench, L.L.; Boccaccini, A.R. Acta Biomaterialia Bioactive glasses beyond bone and teeth: Emerging applications in contact with soft tissues. ACTA Biomater. 2015, 13, 1–15. [Google Scholar] [CrossRef]
- Diker, N.; Gulsever, S.; Koroglu, T.; Yilmaz Akcay, E.; Oguz, Y. Effects of hyaluronic acid and hydroxyapatite/beta-tricalcium phosphate in combination on bone regeneration of a critical-size defect in an experimental model. J. Craniofac. Surg. 2018, 4, 1087–1093. [Google Scholar] [CrossRef] [PubMed]
- Darus, F.; Isa, R.M.; Mamat, N.; Jaafar, M. Techniques for fabrication and construction of three-dimensional bioceramic scaffolds: Effect on pores size, porosity and compressive strength. Ceram. Int. 2018, 44, 18400–18407. [Google Scholar] [CrossRef]
- Bártolo, P.J.; Chua, C.K.; Almeida, H.A.; Chou, S.M.; Lim, A.S.C. Biomanufacturing for tissue engineering: Present and future trends. Virtual Phys. Prototyp. 2009, 4, 203–216. [Google Scholar] [CrossRef]
- Peltola, S.M.; Melchels, F.P.W.; Grijpma, D.W.; Kellomäki, M. A review of rapid prototyping techniques for tissue engineering purposes. Ann. Med. 2008, 40, 268–280. [Google Scholar] [CrossRef]
- Bartolo, P.; Kruth, J.P.; Silva, J.; Levy, G.; Malshe, A.; Rajurkar, K.; Mitsuishi, M.; Ciurana, J.; Leu, M. Biomedical production of implants by additive electro-chemical and physical processes. CIRP Ann.-Manuf. Technol. 2012, 61, 635–655. [Google Scholar] [CrossRef]
- Yan, Q.; Dong, H.; Su, J.; Han, J.; Song, B.; Wei, Q.; Shi, Y. A Review of 3D Printing Technology for Medical Applications. Engineering 2018, 4, 729–742. [Google Scholar] [CrossRef]
- Trombetta, R.; Inzana, J.A.; Schwarz, E.M.; Kates, S.L.; Awad, H.A. 3D Printing of Calcium Phosphate Ceramics for Bone Tissue Engineering and Drug Delivery. Ann. Biomed. Eng. 2017, 45, 23–44. [Google Scholar] [CrossRef] [PubMed]
- Gmeiner, R.; Deisinger, U.; Schönherr, J.; Lechner, B.; Detsch, R.; Boccaccini, A.R.; Stampfl, J. Additive manufacturing of bioactive glasses and silicate bioceramics. J. Ceram. Sci. Technol. 2015, 6, 75–86. [Google Scholar]
- Bose, S.; Vahabzadeh, S.; Bandyopadhyay, A. Bone tissue engineering using 3D printing. Mater. Today 2013, 16, 496–504. [Google Scholar] [CrossRef]
- Sachs, E.; Cima, M.; Cornie, J. Three-Dimensional Printing: Rapid Tooling and Prototypes Directly from a CAD Model. CIRP Ann.-Manuf. Technol. 1990, 39, 201–204. [Google Scholar] [CrossRef]
- Diegel, O.; Withell, A.; de Beer, D.; Potgieter, J.; Noble, F. Low-cost 3D printing of controlled porosity ceramic parts. Int. J. Autom. Technol. 2012, 6, 618–626. [Google Scholar] [CrossRef]
- Gbureck, U.; Grolms, O.; Barralet, J.E.; Grover, L.M.; Thull, R. Mechanical activation and cement formation of β -tricalcium phosphate. Biomaterials 2003, 24, 4123–4131. [Google Scholar] [CrossRef]
- Warnke, P.H.; Seitz, H.; Warnke, F.; Becker, S.T.; Sivananthan, S.; Sherry, E.; Liu, Q.; Wiltfang, J.; Douglas, T. Ceramic scaffolds produced by computer-assisted 3D printing and sintering: Characterization and biocompatibility investigations. J. Biomed. Mater. Res.-Part B Appl. Biomater. 2010, 93, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Butscher, A.; Bohner, M.; Roth, C.; Ernstberger, A.; Heuberger, R.; Doebelin, N.; Rudolf Von Rohr, P.; Müller, R. Printability of calcium phosphate powders for three-dimensional printing of tissue engineering scaffolds. Acta Biomater. 2012, 8, 373–385. [Google Scholar] [CrossRef] [PubMed]
- Gbureck, U.; Hölzel, T.; Klammert, U.; Würzler, K.; Müller, F.A.; Barralet, J.E. Resorbable dicalcium phosphate bone substitutes prepared by 3D powder printing. Adv. Funct. Mater. 2007, 17, 3940–3945. [Google Scholar] [CrossRef]
- Tarafder, S.; Balla, V.K.; Davies, N.M.; Bandyopadhyay, A.; Bose, S. Microwave-sintered 3D printed tricalcium phosphate scaffolds for bone tissue engineering. J. Tissue Eng. Regen. Med. 2013, 7, 631–641. [Google Scholar] [CrossRef] [PubMed]
- Fierz, F.C.; Beckmann, F.; Huser, M.; Irsen, S.H.; Leukers, B.; Witte, F.; Degistirici, O.; Andronache, A.; Thie, M.; Müller, B. The morphology of anisotropic 3D-printed hydroxyapatite scaffolds. Biomaterials 2008, 29, 3799–3806. [Google Scholar] [CrossRef]
- Vaezi, M.; Seitz, H.; Yang, S. A review on 3D micro-additive manufacturing technologies. Int. J. Adv. Manuf. Technol. 2013, 67, 1721–1754. [Google Scholar] [CrossRef]
- Hwa, L.C.; Rajoo, S.; Noor, A.M.; Ahmad, N.; Uday, M.B. Recent advances in 3D printing of porous ceramics: A review. Curr. Opin. Solid State Mater. Sci. 2017, 21, 323–347. [Google Scholar] [CrossRef]
- Bose, S.; Tarafder, S. Calcium phosphate ceramic systems in growth factor and drug delivery for bone tissue engineering: A review. Acta Biomater. 2012, 8, 1401–1421. [Google Scholar] [CrossRef] [PubMed]
- Uhland, S.A.; Holman, R.K.; Morissette, S.; Cima, M.J.; Sachs, E.M. Strength of Green Ceramics with Low Binder Content. J. Am. Ceram. Soc. 2001, 84, 2809–2818. [Google Scholar] [CrossRef]
- Amirkhani, S.; Bagheri, R.; Zehtab Yazdi, A. Effect of pore geometry and loading direction on deformation mechanism of rapid prototyped scaffolds. Acta Mater. 2012, 60, 2778–2789. [Google Scholar] [CrossRef]
- Zhou, Z.; Buchanan, F.; Mitchell, C.; Dunne, N. Printability of calcium phosphate: Calcium sulfate powders for the application of tissue engineered bone scaffolds using the 3D printing technique. Mater. Sci. Eng. C 2014, 38, 1–10. [Google Scholar] [CrossRef]
- Strobel, L.; Rath, S.; Maier, A.; Beier, J.; Arkudas, A.; Greil, P.; Horch, R.; Kneser, U. Induction of bone formation in biphasic calcium phosphate scaffolds by bone morphogenetic protein-2 and primary osteoblasts. J. Tissue Eng. Regen. Med. 2014, 8, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Castilho, M.; Dias, M.; Vorndran, E.; Gbureck, U.; Fernandes, P.; Pires, I.; Gouveia, B.; Armes, H.; Pires, E.; Rodrigues, J. Application of a 3D printed customized implant for canine cruciate ligament treatment by tibial tuberosity advancement. Biofabrication 2014, 6, 025005. [Google Scholar] [CrossRef]
- Castilho, M.; Moseke, C.; Ewald, A.; Gbureck, U.; Groll, J.; Pires, I.; Teßmar, J.; Vorndran, E. Direct 3D powder printing of biphasic calcium phosphate scaffolds for substitution of complex bone defects. Biofabrication 2014, 6, 015006. [Google Scholar] [CrossRef] [PubMed]
- Fielding, G.A.; Bandyopadhyay, A.; Bose, S. Effects of silica and zinc oxide doping on mechanical and biological properties of 3D printed tricalcium phosphate tissue engineering scaffolds. Dent. Mater. 2012, 28, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Seitz, H.; Rieder, W.; Irsen, S.; Leukers, B.; Tille, C. Three-dimensional printing of porous ceramic scaffolds for bone tissue engineering. J. Biomed. Mater. Res.-Part B Appl. Biomater. 2005, 74, 782–788. [Google Scholar] [CrossRef]
- Mandal, S.; Meininger, S.; Gbureck, U.; Basu, B. 3D powder printed tetracalcium phosphate scaffold with phytic acid binder: Fabrication, microstructure and in situ X-Ray tomography analysis of compressive failure. J. Mater. Sci. Mater. Med. 2018, 29, 29. [Google Scholar] [CrossRef] [PubMed]
- Vorndran, E.; Klarner, M.; Klammert, U.; Grover, L.M.; Patel, S.; Barralet, J.E.; Gbureck, U. 3D powder printing of β-tricalcium phosphate ceramics using different strategies. Adv. Eng. Mater. 2008, 10, B67–B71. [Google Scholar] [CrossRef]
- Zocca, A.; Elsayed, H.; Bernardo, E.; Gomes, C.M.; Lopez-Heredia, M.A.; Knabe, C.; Colombo, P.; Günster, J. 3D-printed silicate porous bioceramics using a non-sacrificial preceramic polymer binder. Biofabrication 2015, 7, 025008. [Google Scholar] [CrossRef] [PubMed]
- Tesavibul, P.; Felzmann, R.; Gruber, S.; Liska, R.; Thompson, I.; Boccaccini, A.R.; Stampfl, J. Processing of 45S5 Bioglass® by lithography-based additive manufacturing. Mater. Lett. 2012, 74, 81–84. [Google Scholar] [CrossRef]
- Schmidleithner, C.; Malferarri, S.; Palgrave, R.; Bomze, D.; Schwentenwein, M.; Kalaskar, D.M. Application of high resolution DLP stereolithography for fabrication of tricalcium phosphate scaffolds for bone regeneration. Biomed. Mater. 2019, 14, 45018. [Google Scholar] [CrossRef] [PubMed]
- Thavornyutikarn, B.; Tesavibul, P.; Sitthiseripratip, K.; Chatarapanich, N.; Feltis, B.; Wright, P.F.A.; Turney, T.W. Porous 45S5 Bioglass®-based scaffolds using stereolithography: Effect of partial pre-sintering on structural and mechanical properties of scaffolds. Mater. Sci. Eng. C 2017, 75, 1281–1288. [Google Scholar] [CrossRef]
- Elsayed, H.; Schmidt, J.; Bernardo, E.; Colombo, P. Comparative Analysis of Wollastonite-Diopside Glass-Ceramic Structures Fabricated via Stereo-Lithography. Adv. Eng. Mater. 2019, 21, 1801160. [Google Scholar] [CrossRef]
- Putlyaev, V.I.; Evdokimov, P.V.; Safronova, T.V.; Klimashina, E.S.; Orlov, N.K. Fabrication of osteoconductive Ca3–xM2x(PO4)2 (M = Na, K) calcium phosphate bioceramics by stereolithographic 3D printing. Inorg. Mater. 2017, 53, 529–535. [Google Scholar] [CrossRef]
- Bian, W.; Li, D.; Lian, Q.; Zhang, W.; Zhu, L.; Li, X.; Jin, Z. Design and fabrication of a novel porous implant with pre-set channels based on ceramic stereolithography for vascular implantation. Biofabrication 2011, 3, 034103. [Google Scholar] [CrossRef]
- Yang, C.; Wang, X.; Ma, B.; Zhu, H.; Huan, Z.; Ma, N.; Wu, C.; Chang, J. 3D-Printed Bioactive Ca3SiO5 Bone Cement Scaffolds with Nano Surface Structure for Bone Regeneration. ACS Appl. Mater. Interfaces 2017, 9, 5757–5767. [Google Scholar] [CrossRef]
- Luo, Y.; Zhai, D.; Huan, Z.; Zhu, H.; Xia, L.; Chang, J.; Wu, C. Three-Dimensional Printing of Hollow-Struts-Packed Bioceramic Scaffolds for Bone Regeneration. ACS Appl. Mater. Interfaces 2015, 7, 24377–24383. [Google Scholar] [CrossRef]
- Zhang, Y.; Xia, L.; Zhai, D.; Shi, M.; Luo, Y.; Feng, C.; Fang, B.; Yin, J.; Chang, J.; Wu, C. Mesoporous bioactive glass nanolayer-functionalized 3D-printed scaffolds for accelerating osteogenesis and angiogenesis. Nanoscale 2015, 7, 19207–19221. [Google Scholar] [CrossRef] [PubMed]
- Pei, P.; Wei, D.; Zhu, M.; Du, X.; Zhu, Y. The effect of calcium sulfate incorporation on physiochemical and biological properties of 3D-printed mesoporous calcium silicate cement scaffolds. Microporous Mesoporous Mater. 2017, 241, 11–20. [Google Scholar] [CrossRef]
- Wang, X.; Li, T.; Ma, H.; Zhai, D.; Jiang, C.; Chang, J.; Wang, J.; Wu, C. A 3D-printed scaffold with MoS2 nanosheets for tumor therapy and tissue regeneration. NPG Asia Mater. 2017, 9, e376. [Google Scholar] [CrossRef]
- Liu, Y.; Li, T.; Ma, H.; Zhai, D.; Deng, C.; Wang, J.; Zhuo, S.; Chang, J.; Wu, C. 3D-printed scaffolds with bioactive elements-induced photothermal effect for bone tumor therapy. Acta Biomater. 2018, 73, 531–546. [Google Scholar] [CrossRef] [PubMed]
- Roohani-Esfahani, S.-I.; Newman, P.; Zreiqat, H. Design and Fabrication of 3D printed Scaffolds with a Mechanical Strength Comparable to Cortical Bone to Repair Large Bone Defects. Sci. Rep. 2016, 6, 19468. [Google Scholar] [CrossRef]
- Eqtesadi, S.; Motealleh, A.; Miranda, P.; Pajares, A.; Lemos, A.; Ferreira, J.M.F. Robocasting of 45S5 bioactive glass scaffolds for bone tissue engineering. J. Eur. Ceram. Soc. 2014, 34, 107–118. [Google Scholar] [CrossRef]
- Elsayed, H.; Colombo, P.; Bernardo, E. Direct ink writing of wollastonite-diopside glass-ceramic scaffolds from a silicone resin and engineered fillers. J. Eur. Ceram. Soc. 2017, 37, 4187–4195. [Google Scholar] [CrossRef]
- Kolan, K.C.R.; Leu, M.C.; Hilmas, G.E.; Velez, M. Effect of material, process parameters, and simulated body fluids on mechanical properties of 13-93 bioactive glass porous constructs made by selective laser sintering. J. Mech. Behav. Biomed. Mater. 2012, 13, 14–24. [Google Scholar] [CrossRef]
- Kolan, K.C.R.; Leu, M.C.; Hilmas, G.E.; Brown, R.F.; Velez, M. Fabrication of 13-93 bioactive glass scaffolds for bone tissue engineering using indirect selective laser sintering. Biofabrication 2011, 3, 025004. [Google Scholar] [CrossRef] [PubMed]
- Shuai, C.; Duan, S.; Wu, P.; Gao, D.; Feng, P.; Gao, C.; Peng, S. Development of bioceramic bone scaffolds by introducing triple liquid phases. J. Mater. Res. 2016, 31, 3498–3505. [Google Scholar] [CrossRef]
- Liu, J.; Gao, C.; Feng, P.; Peng, S.; Shuai, C. Selective laser sintering of β-TCP/nano-58S composite scaffolds with improved mechanical properties. Mater. Des. 2015, 84, 395–401. [Google Scholar] [CrossRef]
- Gao, C.; Yang, B.; Hu, H.; Liu, J.; Shuai, C.; Peng, S. Enhanced sintering ability of biphasic calcium phosphate by polymers used for bone scaffold fabrication. Mater. Sci. Eng. C 2013, 33, 3802–3810. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Hu, H.; Li, P.; Shuai, C.; Peng, S. Fabrication and characterization of porous 45S5 glass Scaffolds via direct selective laser sintering. Mater. Manuf. Process. 2013, 28, 610–615. [Google Scholar] [CrossRef]
- Gao, C.; Liu, T.; Shuai, C.; Peng, S. Enhancement mechanisms of graphene in nano-58S bioactive glass scaffold: Mechanical and biological performance. Sci. Rep. 2014, 4, 4712. [Google Scholar] [CrossRef]
- Wu, C.; Fan, W.; Zhou, Y.; Luo, Y.; Gelinsky, M.; Chang, J.; Xiao, Y. 3D-printing of highly uniform CaSiO3 ceramic scaffolds: Preparation, characterization and in vivo osteogenesis. J. Mater. Chem. 2012, 22, 12288–12295. [Google Scholar] [CrossRef]
- Cesaretti, G.; Dini, E.; De Kestelier, X.; Colla, V.; Pambaguian, L. Building components for an outpost on the Lunar soil by means of a novel 3D printing technology. Acta Astronaut. 2014, 93, 430–450. [Google Scholar] [CrossRef]
- Leukers, B.; Gülkan, H.; Irsen, S.H.; Milz, S.; Tille, C.; Seitz, H.; Schieker, M. Biocompatibility of ceramic scaffolds for bone replacement made by 3D printing. Materwiss. Werksttech. 2005, 36, 781–787. [Google Scholar] [CrossRef]
- Khalyfa, A.; Vogt, S.; Weisser, J.; Grimm, G.; Rechtenbach, A.; Meyer, W.; Schnabelrauch, M. Development of a new calcium phosphate powder-binder system for the 3D printing of patient specific implants. J. Mater. Sci. Mater. Med. 2007, 18, 909–916. [Google Scholar] [CrossRef]
- Farzadi, A.; Solati-Hashjin, M.; Asadi-Eydivand, M.; Osman, N.A.A. Effect of layer thickness and printing orientation on mechanical properties and dimensional accuracy of 3D printed porous samples for bone tissue engineering. PLoS ONE 2014, 9, e108252. [Google Scholar] [CrossRef]
- Suwanprateeb, J.; Sanngam, R.; Suvannapruk, W.; Panyathanmaporn, T. Mechanical and in vitro performance of apatite-wollastonite glass ceramic reinforced hydroxyapatite composite fabricated by 3D-printing. J. Mater. Sci. Mater. Med. 2009, 20, 1281–1289. [Google Scholar] [CrossRef] [PubMed]
- Klammert, U.; Vorndran, E.; Reuther, T.; Müller, F.A.; Zorn, K.; Gbureck, U. Low temperature fabrication of magnesium phosphate cement scaffolds by 3D powder printing. J. Mater. Sci. Mater. Med. 2010, 21, 2947–2953. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Mandal, S.; Barui, S.; Vasireddi, R.; Gbureck, U.; Gelinsky, M.; Basu, B. Low temperature additive manufacturing of three dimensional scaffolds for bone-tissue engineering applications: Processing related challenges and property assessment. Mater. Sci. Eng. R Rep. 2016, 103, 1–39. [Google Scholar] [CrossRef]
- Brunner, T.J.; Grass, R.N.; Bohner, M.; Stark, W.J. Effect of particle size, crystal phase and crystallinity on the reactivity of tricalcium phosphate cements for bone reconstruction. J. Mater. Chem. 2007, 17, 4072–4078. [Google Scholar] [CrossRef]
- Inzana, J.A.; Olvera, D.; Fuller, S.M.; Kelly, J.P.; Graeve, O.A.; Schwarz, E.M.; Kates, S.L.; Awad, H.A. 3D printing of composite calcium phosphate and collagen scaffolds for bone regeneration. Biomaterials 2014, 35, 4026–4034. [Google Scholar] [CrossRef]
- Klammert, U.; Gbureck, U.; Vorndran, E.; Rödiger, J.; Meyer-Marcotty, P.; Kübler, A.C. 3D powder printed calcium phosphate implants for reconstruction of cranial and maxillofacial defects. J. Cranio-Maxillofac. Surg. 2010, 38, 565–570. [Google Scholar] [CrossRef]
- Saijo, H.; Igawa, K.; Kanno, Y.; Mori, Y.; Kondo, K.; Shimizu, K.; Suzuki, S.; Chikazu, D.; Iino, M.; Anzai, M.; et al. Maxillofacial reconstruction using custom-made artificial bones fabricated by inkjet printing technology. J. Artif. Organs 2009, 12, 200–205. [Google Scholar] [CrossRef]
- Shivalkar, S.; Singh, S. Solid Freeform Techniques Application in Bone Tissue Engineering for Scaffold Fabrication. Tissue Eng. Regen. Med. 2017, 14, 187–200. [Google Scholar] [CrossRef] [PubMed]
- Wubneh, A.; Tsekoura, E.K.; Ayranci, C.; Uludağ, H. Current state of fabrication technologies and materials for bone tissue engineering. Acta Biomater. 2018, 80, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Shirazi, S.F.S.; Gharehkhani, S.; Mehrali, M.; Yarmand, H.; Metselaar, H.S.C.; Adib Kadri, N.; Osman, N.A.A. A review on powder-based additive manufacturing for tissue engineering: Selective laser sintering and inkjet 3D printing. Sci. Technol. Adv. Mater. 2015, 16, 1–20. [Google Scholar] [CrossRef]
- Shahzad, K.; Deckers, J.; Zhang, Z.; Kruth, J.-P.; Vleugels, J. Additive manufacturing of zirconia parts by indirect selective laser sintering. J. Eur. Ceram. Soc. 2014, 34, 81–89. [Google Scholar] [CrossRef]
- Drummer, D.; Rietzel, D.; Kühnlein, F. Development of a characterization approach for the sintering behavior of new thermoplastics for selective laser sintering. Proc. Phys. Procedia 2010, 5, 533–542. [Google Scholar] [CrossRef]
- Bakshi, K.R. A Review on Selective Laser Sintering: A Rapid Prototyping Technology. IOSR J. Mech. Civ. Eng. 2016, 4, 53–57. [Google Scholar] [CrossRef]
- Chia, H.N.; Wu, B.M. Recent advances in 3D printing of biomaterials. J. Biol. Eng. 2015, 9, 4. [Google Scholar] [CrossRef] [PubMed]
- Criales, L.E.; Arısoy, Y.M.; Özel, T. Sensitivity analysis of material and process parameters in finite element modeling of selective laser melting of Inconel 625. Int. J. Adv. Manuf. Technol. 2016, 86, 2653–2666. [Google Scholar] [CrossRef]
- Wang, J.; Yang, M.; Zhang, Y. A nonequilibrium thermal model for direct metal laser sintering. Numer. Heat Transf. Part A Appl. 2015, 67, 249–267. [Google Scholar] [CrossRef]
- Yves-Christian, H.; Jan, W.; Wilhelm, M.; Konrad, W.; Reinhart, P. Net shaped high performance oxide ceramic parts by Selective Laser Melting. Proc. Phys. Procedia 2010, 5, 587–594. [Google Scholar] [CrossRef]
- Miguel, C.; Estomba, C.; González Fernández, I.; Ángel, M.; Otero, I. 3D Printing for Biomedical Applications: Where Are We Now? Eur. Med. J. 2017, 2, 16–22. [Google Scholar]
- Lorrison, J.C.; Dalgarno, K.W.; Wood, D.J. Processing of an apatite-mullite glass-ceramic and an hydroxyapatite/ phosphate glass composite by selective laser sintering. J. Mater. Sci. Mater. Med. 2005, 16, 775–781. [Google Scholar] [CrossRef]
- XiaoHui, S.; Wei, L.; PingHui, S.; QingYong, S.; QingSong, W.; YuSheng, S.; Kai, L.; WenGuang, L. Selective laser sintering of aliphatic-polycarbonate/hydroxyapatite composite scaffolds for medical applications. Int. J. Adv. Manuf. Technol. 2015, 81, 15–25. [Google Scholar] [CrossRef]
- Deckers, J.P.; Shahzad, K.; Cardon, L.; Rombouts, M.; Vleugels, J.; Kruth, J.-P. Shaping ceramics through indirect selective laser sintering. Rapid Prototyp. J. 2016, 22, 544–558. [Google Scholar] [CrossRef]
- Chen, Z.; Li, Z.; Li, J.; Liu, C.; Lao, C.; Fu, Y.; Liu, C.; Li, Y.; Wang, P.; He, Y. 3D printing of ceramics: A review. J. Eur. Ceram. Soc. 2019, 39, 661–687. [Google Scholar] [CrossRef]
- Chartier, T.; Chaput, C.; Doreau, F.; Loiseau, M. Stereolithography of structural complex ceramic parts. J. Mater. Sci. 2002, 37, 3141–3147. [Google Scholar] [CrossRef]
- D’urso, P.S.; Earwaker, W.J.; Barker, T.M.; Redmond, M.J.; Thompson, R.G.; Effeney, D.J.; Tomlinson, F.H. Custom cranioplasty using stereolithography and acrylic. Br. J. Plast. Surg. 2000, 53, 200–204. [Google Scholar] [CrossRef]
- Sarment, D.P.; Sukovic, P.; Clinthorne, N. Accuracy of implant placement with a stereolithographic surgical guide. Int. J. Oral Maxillofac. Implant. 2003, 18, 571–577. [Google Scholar]
- Chu, T.-M.G.; Orton, D.G.; Hollister, S.J.; Feinberg, S.E.; Halloran, J.W. Mechanical and in vivo performance of hydroxyapatite implants with controlled architectures. Biomaterials 2002, 23, 1283–1293. [Google Scholar] [CrossRef]
- Kim, J.Y.; Lee, J.W.; Lee, S.-J.; Park, E.K.; Kim, S.-Y.; Cho, D.-W. Development of a bone scaffold using HA nanopowder and micro-stereolithography technology. Microelectron. Eng. 2007, 84, 1762–1765. [Google Scholar] [CrossRef]
- Hull, C.W. Apparatus for Production of Three-Dimensional Objects by Stereolithography. U.S. Patent 4,575,330, 11 March 1986. [Google Scholar]
- Brady, G.A.; Halloran, J.W. Stereolithography of ceramic suspensions. Rapid Prototyp. J. 1997, 3, 61–65. [Google Scholar] [CrossRef]
- Hinczewski, C.; Corbel, S.; Chartier, T. Ceramic suspensions suitable for stereolithography. J. Eur. Ceram. Soc. 1998, 18, 583–590. [Google Scholar] [CrossRef]
- Hinczewski, C.; Corbel, S.; Chartier, T. Stereolithography for the fabrication of ceramic three-dimensional parts. Rapid Prototyp. J. 1998, 4, 104–111. [Google Scholar] [CrossRef]
- Chen, Z.; Li, D.; Zhou, W. Process parameters appraisal of fabricating ceramic parts based on stereolithography using the Taguchi method. Proc. Inst. Mech. Eng. Part B J. Eng. Manuf. 2012, 226, 1249–1258. [Google Scholar] [CrossRef]
- Griffith, M.L.; Halloran, J.W. Ultraviolet Curable Ceramic Suspensions for Stereolithography of Ceramics; ASME: New York, NY, USA, 1994; Volume 68-2, pp. 529–534. [Google Scholar]
- De Hazan, Y.; Penner, D. SiC and SiOC ceramic articles produced by stereolithography of acrylate modified polycarbosilane systems. J. Eur. Ceram. Soc. 2017, 37, 5205–5212. [Google Scholar] [CrossRef]
- Mitteramskogler, G.; Gmeiner, R.; Felzmann, R.; Gruber, S.; Hofstetter, C.; Stampfl, J.; Ebert, J.; Wachter, W.; Laubersheimer, J. Light curing strategies for lithography-based additive manufacturing of customized ceramics. Addit. Manuf. 2014, 1, 110–118. [Google Scholar] [CrossRef]
- Gentry, S.P.; Halloran, J.W. Depth and width of cured lines in photopolymerizable ceramic suspensions. J. Eur. Ceram. Soc. 2013, 33, 1981–1988. [Google Scholar] [CrossRef]
- Xing, H.; Zou, B.; Li, S.; Fu, X. Study on surface quality, precision and mechanical properties of 3D printed ZrO2 ceramic components by laser scanning stereolithography. Ceram. Int. 2017, 43, 16340–16347. [Google Scholar] [CrossRef]
- Pfaffinger, M.; Mitteramskogler, G.; Gmeiner, R.; Stampfl, J. Thermal Debinding of Ceramic-Filled Photopolymers. Mater. Sci. Forum 2015, 825–826, 75–81. [Google Scholar] [CrossRef]
- Griffith, M.L.; Halloran, J.W. Freeform fabrication of ceramics via stereolithography. J. Am. Ceram. Soc. 1996, 79, 2601–2608. [Google Scholar] [CrossRef]
- Lewis, J.A. Direct Ink Writing of 3D Functional Materials. Adv. Funct. Mater. 2006, 16, 2193–2204. [Google Scholar] [CrossRef]
- Feilden, E.; Blanca, E.G.-T.; Giuliani, F.; Saiz, E.; Vandeperre, L. Robocasting of structural ceramic parts with hydrogel inks. J. Eur. Ceram. Soc. 2016, 36, 2525–2533. [Google Scholar] [CrossRef]
- Cesarano, J.; Segalman, R.; Calvert, P. Robocasting provides moldless fabrication from slurry deposition. Ceram. Ind. 1998, 148, 94–102. [Google Scholar]
- Miranda, P.; Saiz, E.; Gryn, K.; Tomsia, A.P. Sintering and robocasting of β-tricalcium phosphate scaffolds for orthopaedic applications. Acta Biomater. 2006, 2, 457–466. [Google Scholar] [CrossRef] [PubMed]
- Michna, S.; Wu, W.; Lewis, J.A. Concentrated hydroxyapatite inks for direct-write assembly of 3-D periodic scaffolds. Biomaterials 2005, 26, 5632–5639. [Google Scholar] [CrossRef] [PubMed]
- Lewis, J.A. Colloidal processing of ceramics. J. Am. Ceram. Soc. 2000, 83, 2341–2359. [Google Scholar] [CrossRef]
- Lewis, J.A. Direct-write assembly of ceramics from colloidal inks. Curr. Opin. Solid State Mater. Sci. 2002, 6, 245–250. [Google Scholar] [CrossRef]
- James, S. Reed Introduction to the Principles of Ceramic Processing; Wiley: New York, NY, USA, 1988. [Google Scholar]
- Li, Q.; Lewis, J.A. Nanoparticle Inks for Directed Assembly of Three-Dimensional Periodic Structures. Adv. Mater. 2003, 15, 1639–1643. [Google Scholar] [CrossRef]
- Dellinger, J.G. Development of Model Hydroxyapatite Bone Scaffolds with Multiscale Porosity for Potential Load Bearing Applications. Development of Model Hydroxyapatite Bone Scaffolds with Multiscale Porosity for Potential Load Bearing Applications; University of Illinois at Urbana-Champaign: Champaign, IL, USA, 2005. [Google Scholar]
- Miranda, P.; Pajares, A.; Saiz, E.; Tomsia, A.P.; Guiberteau, F. Mechanical properties of calcium phosphate scaffolds fabricated by robocasting. J. Biomed. Mater. Res. A 2008, 85, 218–227. [Google Scholar] [CrossRef]
- Cesarano, J., III; Dellinger, J.G.; Saavedra, M.P.; Gill, D.D.; Jamison, R.D.; Grosser, B.A.; Sinn-hanlon, J.M.; Goldwasser, M.S.; Iii, J.C.; Dellinger, J.G.; et al. Customization of load-bearing hydroxyapatite lattice scaffolds. Int. J. Appl. Ceram. Technol. 2005, 2, 212–220. [Google Scholar] [CrossRef]
- Vallet-Regí, M.; Ruiz-Hernández, E. Bioceramics: From bone regeneration to cancer nanomedicine. Adv. Mater. 2011, 23, 5177–5218. [Google Scholar] [CrossRef]
- Ahangar, P.; Cooke, M.E.; Weber, M.H.; Rosenzweig, D.H. Current biomedical applications of 3D printing and additive manufacturing. Appl. Sci. 2019, 9, 1713. [Google Scholar] [CrossRef]
- Jazayeri, H.E.; Rodriguez-Romero, M.; Razavi, M.; Tahriri, M.; Ganjawalla, K.; Rasoulianboroujeni, M.; Malekoshoaraie, M.H.; Khoshroo, K.; Tayebi, L. The cross-disciplinary emergence of 3D printed bioceramic scaffolds in orthopedic bioengineering. Ceram. Int. 2018, 44, 1–9. [Google Scholar] [CrossRef]
- Munch, E.; Launey, M.E.; Alsem, D.H.; Saiz, E.; Tomsia, A.P.; Ritchie, R.O. Tough, bio-inspired hybrid materials. Science 2008, 322, 1516–1520. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Bhushan, B. Hierarchical structure and mechanical properties of nacre: A review. RSC Adv. 2012, 2, 7617. [Google Scholar] [CrossRef]
- Di Bella, C.; Duchi, S.; O’Connell, C.D.; Blanchard, R.; Augustine, C.; Yue, Z.; Thompson, F.; Richards, C.; Beirne, S.; Onofrillo, C.; et al. In situ handheld three-dimensional bioprinting for cartilage regeneration. J. Tissue Eng. Regen. Med. 2018, 12, 611–621. [Google Scholar] [CrossRef] [PubMed]
- Martelli, N.; Serrano, C.; Van Den Brink, H.; Pineau, J.; Prognon, P.; Borget, I.; El Batti, S. Advantages and disadvantages of 3-dimensional printing in surgery: A systematic review. Surgery 2016, 159, 1485–1500. [Google Scholar] [CrossRef] [PubMed]
- Connell, C.D.O.; Bella, C.D.; Thompson, F.; Augustine, C.; Beirne, S. Development of the Biopen: A handheld device for surgical printing of adipose stem cells at a chondral wound site Development of the Biopen: A handheld device for surgical printing of adipose stem cells at a chondral wound site. Biofabrication 2016, 8, 015019. [Google Scholar] [CrossRef] [PubMed]
- Romanazzo, S.; Nemec, S.; Roohani, I. iPSC Bioprinting: Where are We at? Materials 2019, 1, 2453. [Google Scholar] [CrossRef] [PubMed]
- Daly, A.C.; Freeman, F.E.; Gonzalez-Fernandez, T.; Critchley, S.E.; Nulty, J.; Kelly, D.J. 3D Bioprinting for Cartilage and Osteochondral Tissue Engineering. Adv. Healthc. Mater. 2017, 6. [Google Scholar] [CrossRef]
- Singh, A.V.; Dad Ansari, M.H.; Wang, S.; Laux, P.; Luch, A.; Kumar, A.; Patil, R.; Nussberger, S. The adoption of three-dimensional additive manufacturing from biomedical material design to 3D organ printing. Appl. Sci. 2019, 9, 811. [Google Scholar] [CrossRef]
- Ma, H.; Feng, C.; Chang, J.; Wu, C. 3D-printed bioceramic scaffolds: From bone tissue engineering to tumor therapy. Acta Biomater. 2018, 79, 37–59. [Google Scholar] [CrossRef]
- Mercado-Pagán, Á.E.; Stahl, A.M.; Shanjani, Y.; Yang, Y. Vascularization in Bone Tissue Engineering Constructs. Ann. Biomed. Eng. 2015, 43, 718–729. [Google Scholar] [CrossRef]
- Ashammakhi, N.; Hasan, A.; Kaarela, O.; Byambaa, B.; Sheikhi, A.; Gaharwar, A.K.; Khademhosseini, A. Advancing Frontiers in Bone Bioprinting. Adv. Healthc. Mater. 2019, 1801048, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Vikram Singh, A.; Gharat, T.; Batuwangala, M.; Park, B.-W.; Endlein, T.; Sitti, M. Three-dimensional patterning in biomedicine: Importance and applications in neuropharmacology. J. Biomed. Mater. Res.-Part B Appl. Biomater. 2018, 106, 1369–1382. [Google Scholar] [CrossRef] [PubMed]
| Printing Technique | Composition | Densification Method | Strut Size (µm) | Pore Size (µm) | Porosity (%) | Finish Quality + | Reference | Pore Shape |
|---|---|---|---|---|---|---|---|---|
| 3DP | CaSO4/HA/β-TCP | Setting reaction | 1000 –2000 | 1000–2000 | 50 | E | Zhou et al. [45] | Cubic |
| 3DP | HA/β-TCP | 1200 °C | NA | 100–600 | 65.3 | E | Strobel et al. [46] | Spherical |
| 3DP | α-TCP | Setting reaction | 1500 | 844 | 59.2 | E | Castilho et al. [47] | Spherical to Cubic |
| 3DP | α-TCP/CaCO3 | Setting reaction | 1250 | 300 | 68 | E | Castilho et al. [48] | Cubic |
| 3DP | β-TCP, β-TCP/ZnO/SiO2 | 1250°C | 1000–2000 | 400–700 | 30–50 | E | Feidling et al. [49] | Cubic |
| 3DP | HA | 1250 °C | 330 | 450 | - | F | Seitz et al. [50] | cubic |
| 3DP | HA and β-TCP | 1250 °C | 900 | 500 | 40 | F | Warnke et al. [35] | Cubic |
| 3DP | Ca4(PO4)2O | Setting reaction | 200 | 750 | 40 | E | Mandal et al. [51] | Cubic |
| 3DP | α/β-TCP/Ca4(PO4)2O | Setting reaction and 1100 °C | 1000 | 500 | 56–61 | E | Vorndran et al. [52] | Cubic |
| 3DP | CaSiO3 precursors | 900 °C | ~1000 | ~2000 | 48–53 | F | Zocca et al. [53] | Cubic |
| SL | 45S5 Bioglass® | 1000 °C | 540–1000 | 1000 | 50 | B | Tesavibul et al. [54] | Cylindrical cellular |
| SL | β-TCP | 1200 °C | ~250 | 400 | 75 or 50 | A | Schmidleithner et al. [55] | Grid and Kagome structure |
| SL | 45S5 Bioglass® | 950 °C | 307 | 700–400 | ~60 | A | Thavornyutikarn et al. [56] | Diamond-like structures |
| SL | CaSiO3–CaMgSi2O6 | 1100 °C | ~500 | ~500 | 57–85 | B | Elsayed et al. [57] | Diamond, kelvin and cubic structures |
| SL | Ca3−xM2x(PO4)2 (M = Na, K) | 900–1400° C | 500 | 50–750 | 70–80 | B | Putlyaev et al. [58] | Kelvin structure |
| SL | β-TCP | 1150 °C | 1000 | 600–800 | 45 | A | Weiguo et al. [59] | Spherical and cylindrical |
| DIW | Ca3SiO5 | Setting reaction | 200 (minimum) | 200 (minimum) | 60–65 | C | Yang et al. [60] | Logpile* |
| DIW | Ca7Si2P2O16 | 1400 °C | 1000 | 200 (minimum) | Up to 86 | C | Uo et al. [61] | Logpile |
| DIW | Mesoporous bioactive glass/β-TCP | 1100 °C | 250 | 400 | 58 | C | Zhang et al. [62] | Logpile |
| DIW | Ca2O4Si/CaSO4 | Setting reaction | 450 | 350 | 67 | D | Pei et al. [63] | Logpile |
| DIW | Ca2MgSi2O7 | 1350 °C | 450 | 400 | 65 | D | Wang et al. [64] | Logpile |
| DIW | Cu, Fe, Mn, Co-doped bioactive glass | 1300°C | 500 | 250 | <50 | D | Liu et al. [65] | Logpile |
| DIW | Sr doped Ca2ZnSi2O7 /Al2O3 | 1250 °C | 540 | 450–1200 | 50–70 | D | Roohani et al. [66] | Logpile |
| DIW | 45S5 bioactive glass | 1050 °C | ~250 | 287–820 | 60 to 80 | C | Eqtesadi et al. [67] | Logpile |
| DIW | CaSiO3-CaMgSi2O6 | 1100 °C | 320 | 390 | 68–76 | D | Elsayed et al. [68] | Logpile |
| SLS | 13-93 bioactive glass | 700 °C | 1000 | 1100 | 50 | E | Kolan et al. [69] | Cubic |
| SLS | 13-93 bioactive glass | 695 °C | 1000 | 1000 | 50 | E | Kolan et al. [70] | Cubic |
| SLS | Ca2MgSi2O7 | 715–914 °C | 2000 | 1000 | <20 | E | Shuai et al. [71] | Cubic |
| SLS | β-TCP/58S bioactive glass | No post treatment | 1100 | 1500 | 56.04 | F | Liu et al. [72] | Cubic |
| SLS | HA/β-TCP | No post treatment | 800 | 1000 | 70.1 | F | Gao et al. [73] | Cubic |
| SLS | 45S5 bioglass | No post treatment | ~3000 | 2000 × 2000 × 5000 | ~15 | F | Liu et al. [74] | Channels |
| SLS | 58S Bioactive glass/graphene | No post treatment | ~1000 | 800 | ~50 | F | Gao et al. [75] | Cubic |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, K.; Sheikh, R.; Romanazzo, S.; Roohani, I. 3D Printing of Bioceramic Scaffolds—Barriers to the Clinical Translation: From Promise to Reality, and Future Perspectives. Materials 2019, 12, 2660. https://doi.org/10.3390/ma12172660
Lin K, Sheikh R, Romanazzo S, Roohani I. 3D Printing of Bioceramic Scaffolds—Barriers to the Clinical Translation: From Promise to Reality, and Future Perspectives. Materials. 2019; 12(17):2660. https://doi.org/10.3390/ma12172660
Chicago/Turabian StyleLin, Kang, Rakib Sheikh, Sara Romanazzo, and Iman Roohani. 2019. "3D Printing of Bioceramic Scaffolds—Barriers to the Clinical Translation: From Promise to Reality, and Future Perspectives" Materials 12, no. 17: 2660. https://doi.org/10.3390/ma12172660
APA StyleLin, K., Sheikh, R., Romanazzo, S., & Roohani, I. (2019). 3D Printing of Bioceramic Scaffolds—Barriers to the Clinical Translation: From Promise to Reality, and Future Perspectives. Materials, 12(17), 2660. https://doi.org/10.3390/ma12172660




