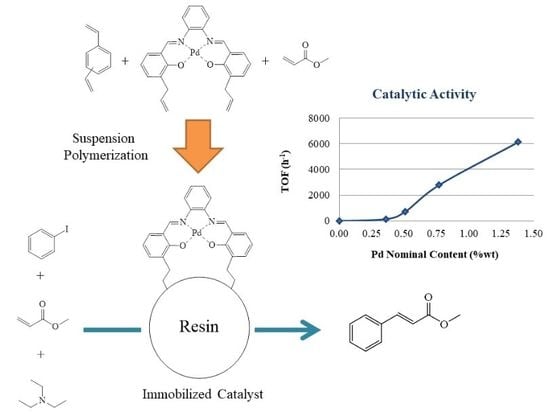
Mesoporous Palladium N,N’-Bis(3-Allylsalicylidene)o-Phenylenediamine-Methyl Acrylate Resins as Heterogeneous Catalysts for the Heck Coupling Reaction
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Synthesis of the Schiff Base Ligands and Pd(II) Complexes
2.3. Synthesis of the PdAS(x)-MA, (x = 1, 2, 5 or 10 wt.%) Resins
2.4. Characterization
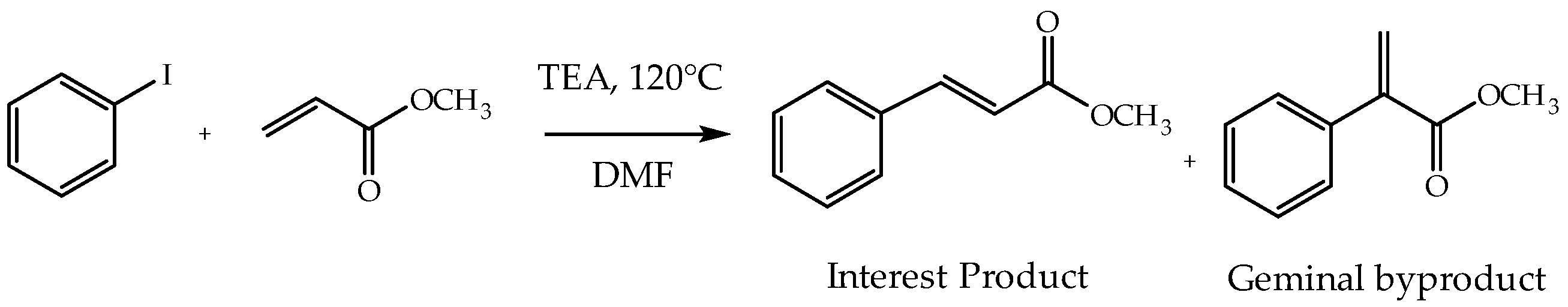
2.5. Catalytic Activity
3. Results and Discussion
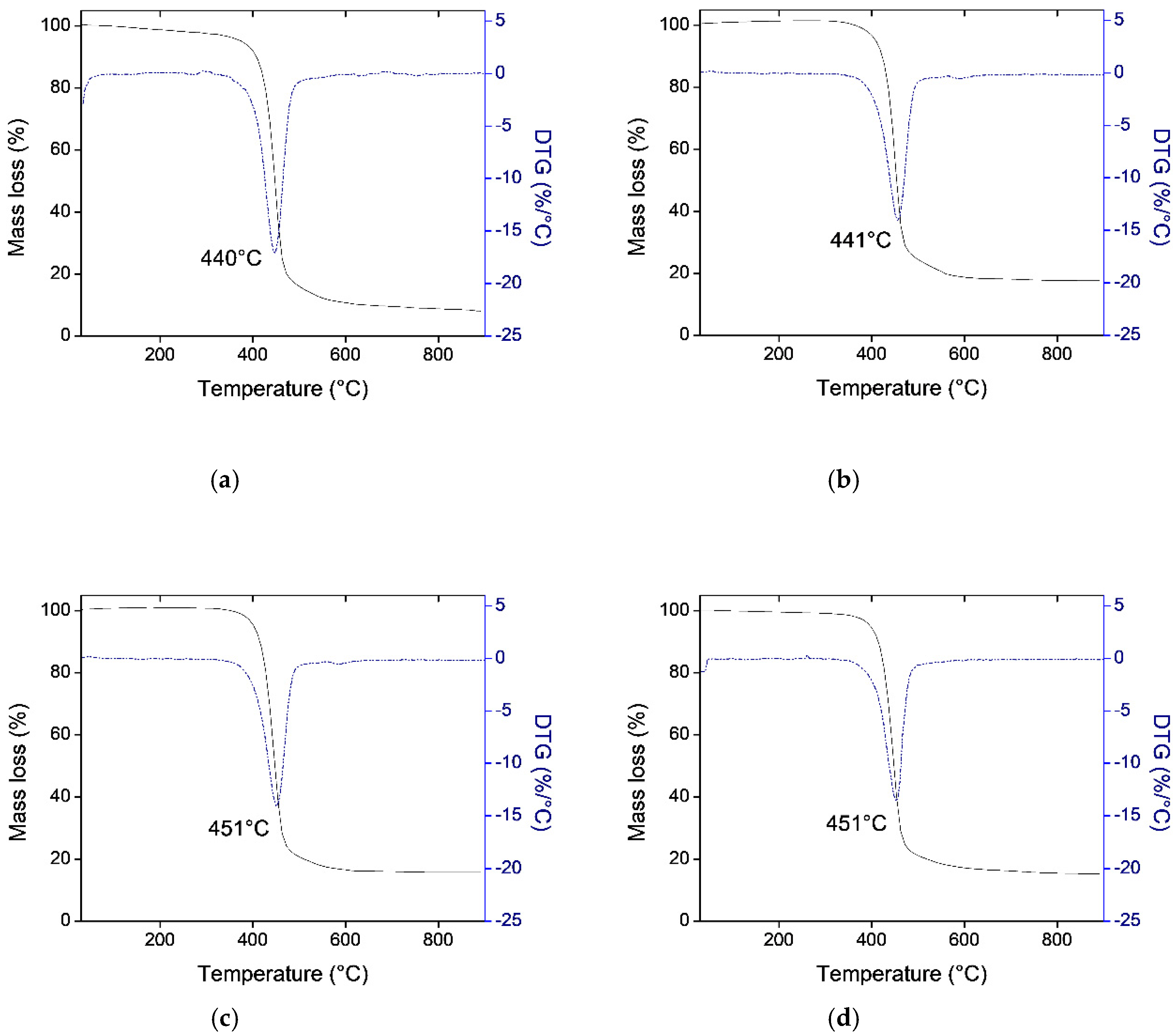
3.1. Thermogravimetric Analysis (TGA)
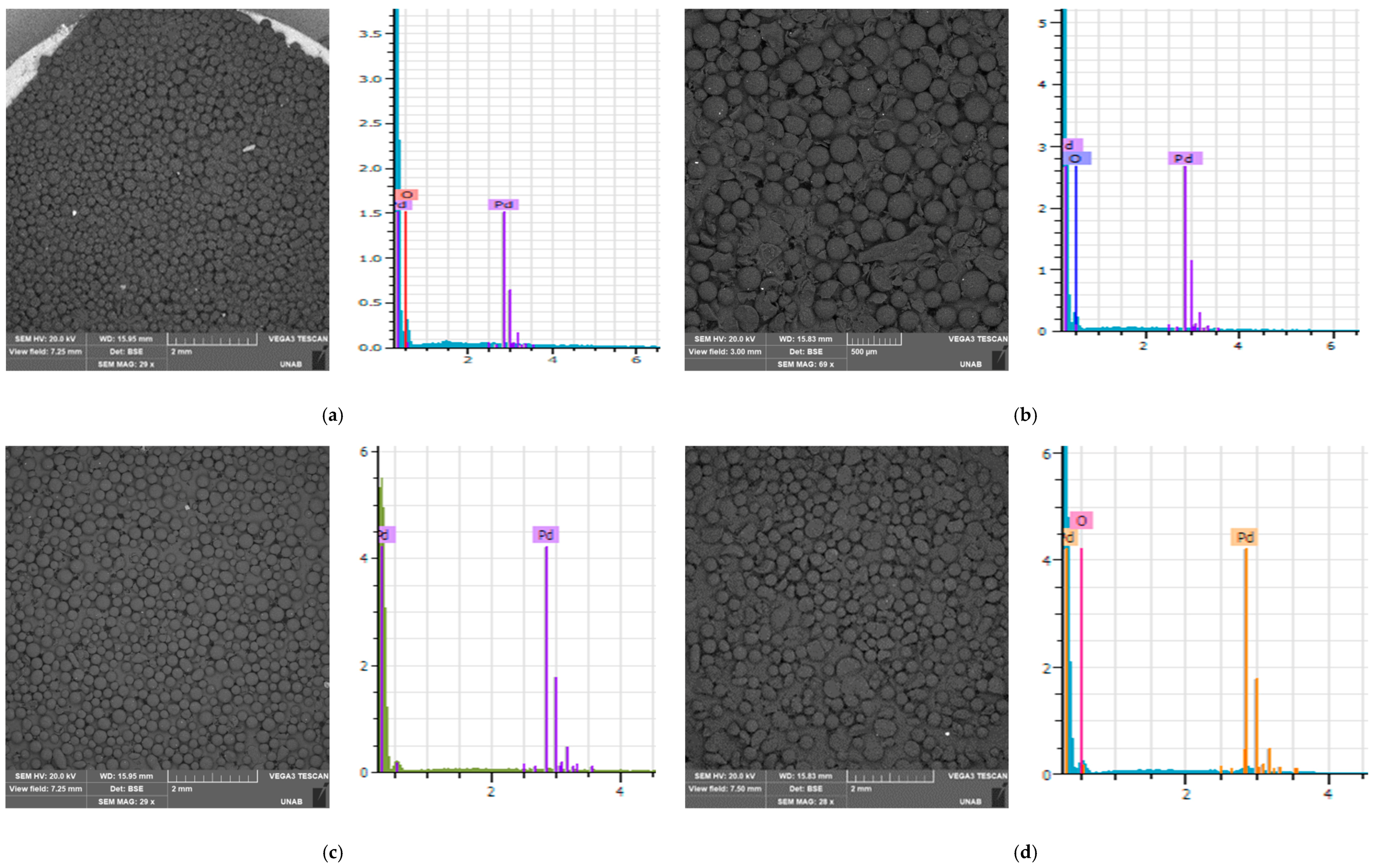
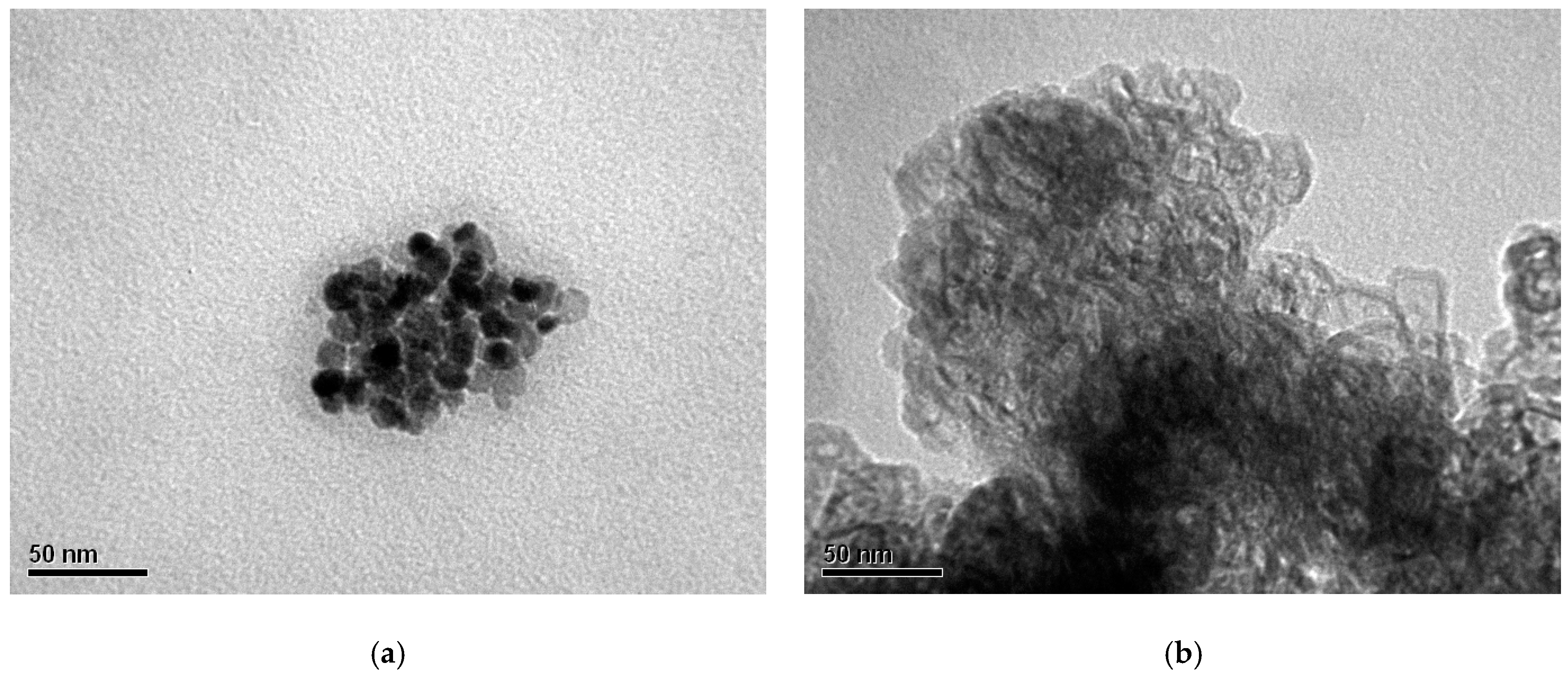
3.2. Scanning Electron Microscopy (SEM) Coupled with Energy-Dispersive X-ray Spectroscopy (EDS)
3.3. Nitrogen Adsorption-Desorption Isotherms at −196 °C
3.4. CP/MAS 13C NMR Spectroscopy
3.5. DRS UV–Vis Measurements
3.6. ICP-OES
3.7. X-ray Photoelectron Spectroscopy (XPS)
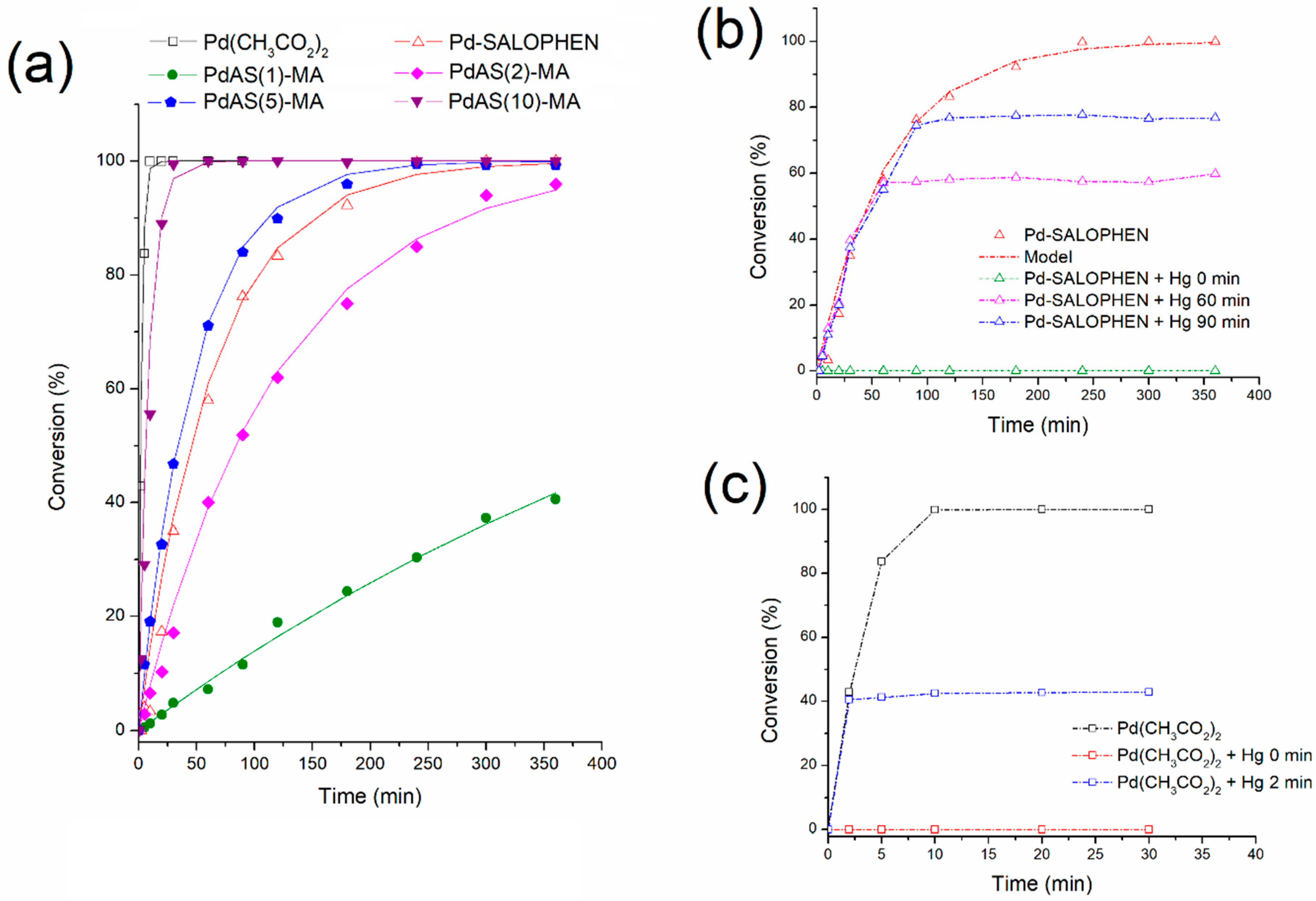
3.8. Catalytic Activity
3.8.1. Effect of PdAS Loading
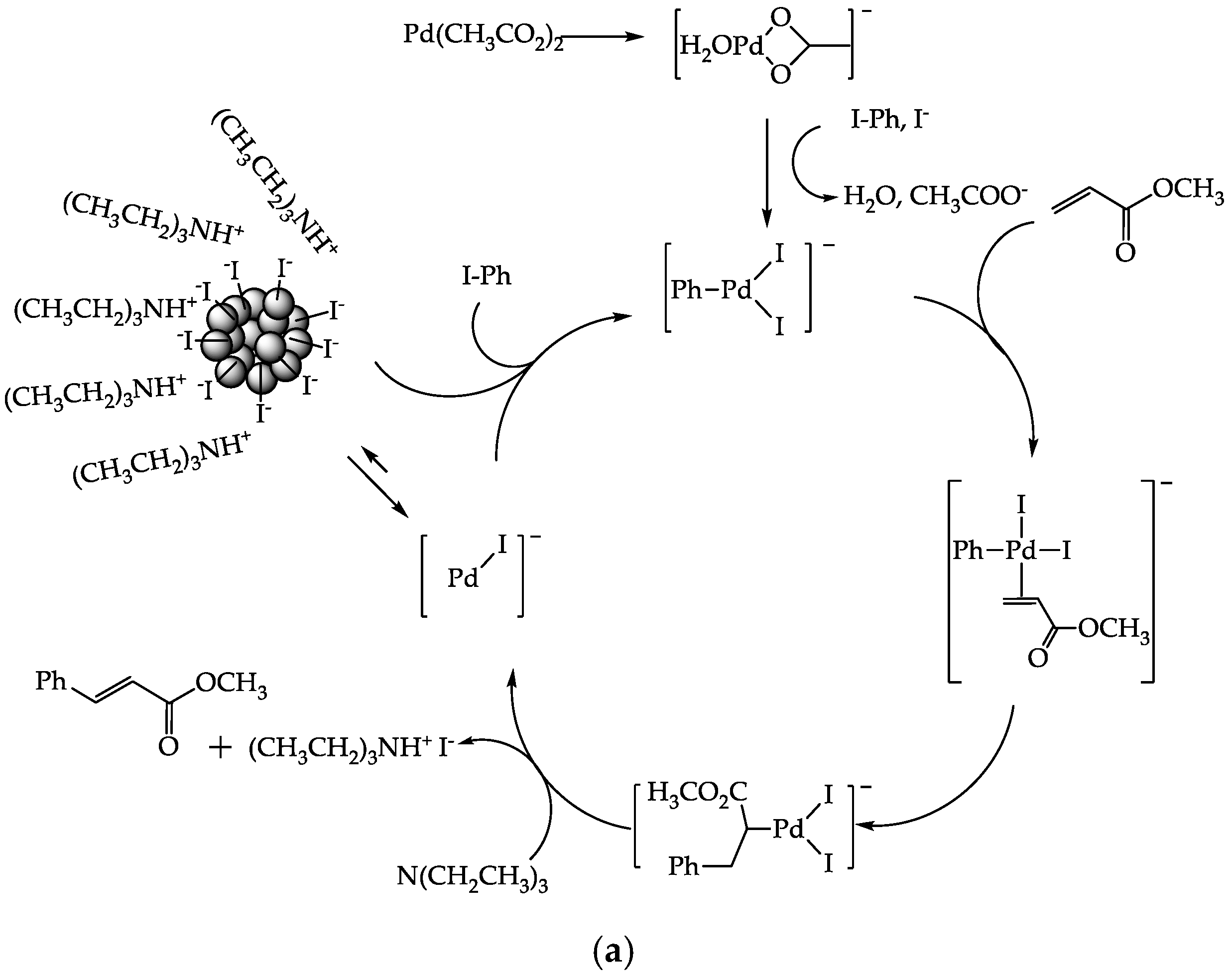

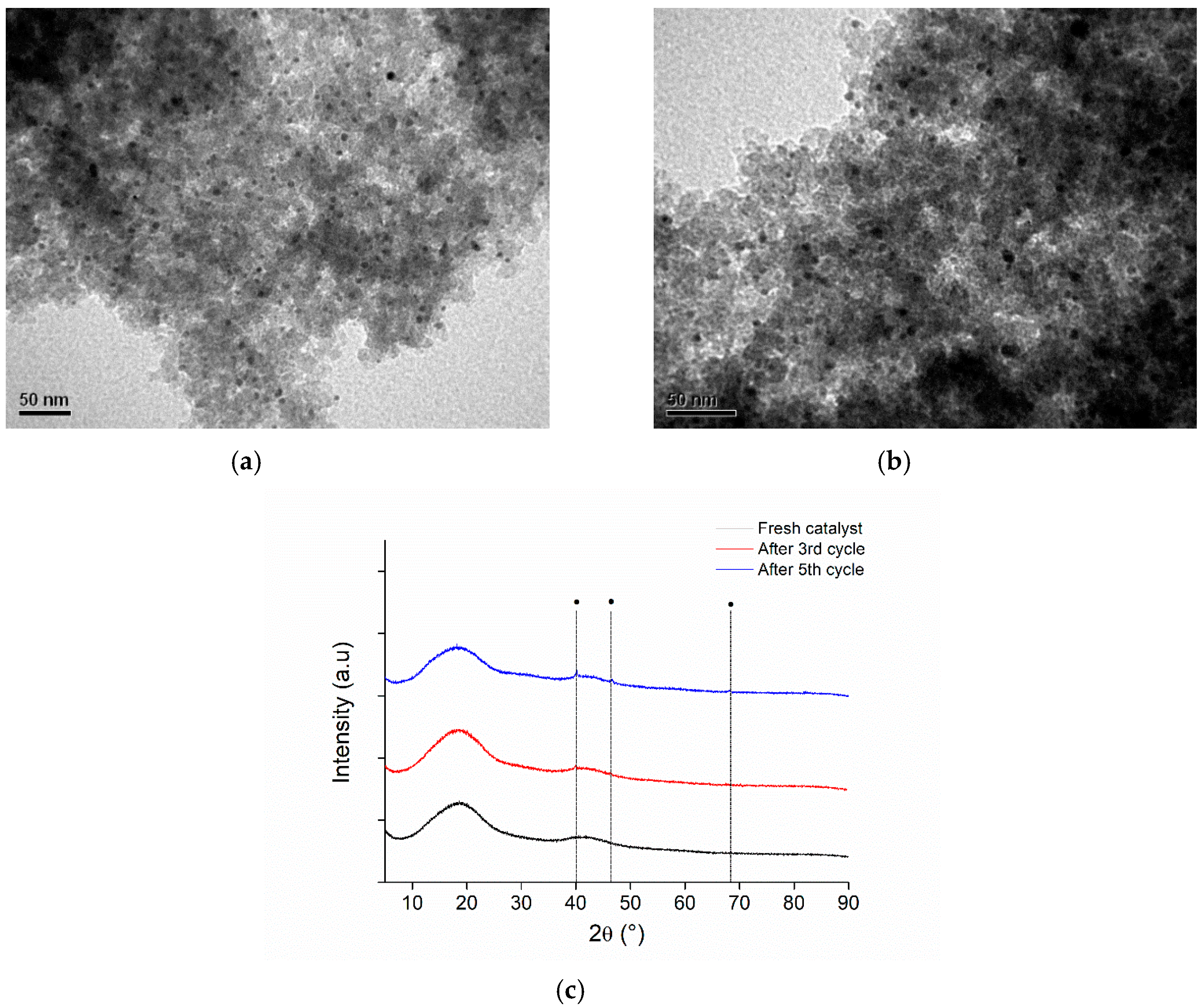
3.8.2. Reusability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Magano, J.; Dunetz, J.R. Large-Scale Applications of Transition Metal-Catalyzed Couplings for the Synthesis of Pharmaceuticals. Chem. Rev. 2011, 111, 2177–2250. [Google Scholar] [CrossRef] [PubMed]
- Blaser, H.-U.; Indolese, A.; Schnyder, A. Applied homogeneous catalysis by organometallic complexes. Curr. Sci. 2000, 78, 1336–1344. [Google Scholar]
- Zapf, A.; Beller, M. Fine Chemical Synthesis with Homogeneous Palladium Catalysts: Examples, Status and Trends. Top. Catal. 2002, 19, 101–109. [Google Scholar] [CrossRef]
- Jagtap, S. Heck Reaction—State of the Art. Catalysts 2017, 7, 267. [Google Scholar] [CrossRef]
- Beletskaya, I.P.; Cheprakov, A.V. The Heck Reaction as a Sharpening Stone of Palladium Catalysis. Chem. Rev. 2000, 100, 3009–3066. [Google Scholar] [CrossRef] [PubMed]
- Negishi, E.-I. A genealogy of Pd-catalyzed cross-coupling. J. Organomet. Chem. 2002, 653, 34–40. [Google Scholar] [CrossRef]
- Heck, R.F. Palladium-catalyzed reactions of organic halides with olefins. Acc. Chem. Res. 1979, 12, 146–151. [Google Scholar] [CrossRef]
- Yao, Q.; Kinney, E.P.; Yang, Z. Ligand-Free Heck Reaction: Pd(OAc)2 as an Active Catalyst Revisited. J. Org. Chem. 2003, 68, 7528–7531. [Google Scholar] [CrossRef]
- Johansson Seechurn, C.C.C.; Kitching, M.O.; Colacot, T.J.; Snieckus, V. Palladium-Catalyzed Cross-Coupling: A Historical Contextual Perspective to the 2010 Nobel Prize. Angew. Chem. Int. Ed. 2012, 51, 5062–5085. [Google Scholar] [CrossRef]
- Cole-Hamilton, D.J.; Tooze, R.P. Homogeneous Catalysis—Advantages and Problems. In Catalyst Separation, Recovery and Recycling: Chemistry and Process Design; Cole-Hamilton, D.J., Tooze, R.P., Eds.; Springer: Dordrecht, The Netherlands, 2006; pp. 1–8. [Google Scholar]
- Anastas, P.T.; Kirchhoff, M.M.; Williamson, T.C. Catalysis as a foundational pillar of green chemistry. Appl. Catal. A 2001, 221, 3–13. [Google Scholar] [CrossRef]
- Heveling, J. Heterogeneous Catalytic Chemistry by Example of Industrial Applications. J. Chem. Educ. 2012, 89, 1530–1536. [Google Scholar] [CrossRef]
- Hinner, M.J.; Grosche, M.; Herdtweck, E.; Thiel, W.R. A Merrifield Resin Functionalized with Molybdenum Peroxo Complexes: Synthesis and Catalytic Properties. Z. Anorg. Allg. Chem. 2003, 629, 2251–2257. [Google Scholar] [CrossRef]
- Iyer, S.; Kulkarni, G.M.; Ramesh, C. Mizoroki–Heck reaction, catalysis by nitrogen ligand Pd complexes and activation of aryl bromides. Tetrahedron 2004, 60, 2163–2172. [Google Scholar] [CrossRef]
- Shaikh, T.M.; Hong, F.-E. Palladium(II)-catalyzed Heck reaction of aryl halides and arylboronic acids with olefins under mild conditions. Beilstein J. Org. Chem. 2013, 9, 1578–1588. [Google Scholar] [CrossRef] [PubMed]
- Zamboulis, A.; Moitra, N.; Moreau, J.J.E.; Cattoen, X.; Man, M.W.C. Hybrid materials: Versatile matrices for supporting homogeneous catalysts. Mater. Chem. 2010, 20, 9322–9338. [Google Scholar] [CrossRef]
- Cozzi, F. Catalyst Immobilization Strategy: Some General Considerations and a Comparison of the Main Features of Different Supports. In Recoverable and Recyclable Catalysts; Wiley-VCH Verlag GmbH: Weinheim, Germany, 2009; pp. 427–461. [Google Scholar]
- Zhao, X.S.; Bao, X.Y.; Guo, W.; Lee, F.Y. Immobilizing catalysts on porous materials. Mater. Today 2006, 9, 32–39. [Google Scholar] [CrossRef]
- Dhakshinamoorthy, A.; Asiri, A.M.; Garcia, H. Metal-organic frameworks catalyzed C-C and C-heteroatom coupling reactions. Chem. Soc. Rev. 2015, 44, 1922–1947. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Toy, P.H. Organic Polymer Supports for Synthesis and for Reagent and Catalyst Immobilization. Chem. Rev. 2009, 109, 815–838. [Google Scholar] [CrossRef] [PubMed]
- Dong, K.; Sun, Q.; Meng, X.; Xiao, F.-S. Strategies for the design of porous polymers as efficient heterogeneous catalysts: From co-polymerization to self-polymerization. Catal. Sci. Technol. 2017, 7, 1028–1039. [Google Scholar] [CrossRef]
- Taylor, R.A.; Santora, B.P.; Gagné, M.R. A Polymer-Supported Rhodium Catalyst that Functions in Polar Protic Solvents. Org. Lett. 2000, 2, 1781–1783. [Google Scholar] [CrossRef]
- Leadbeater, N.E.; Marco, M. Preparation of Polymer-Supported Ligands and Metal Complexes for Use in Catalysis. Chem. Rev. 2002, 102, 3217–3274. [Google Scholar] [CrossRef] [PubMed]
- Boruah, J.J.; Das, S.P.; Ankireddy, S.R.; Gogoi, S.R.; Islam, N.S. Merrifield resin supported peroxomolybdenum(vi) compounds: Recoverable heterogeneous catalysts for the efficient, selective and mild oxidation of organic sulfides with H2O2. Green Chem. 2013, 15, 2944–2959. [Google Scholar] [CrossRef]
- Dzhardimalieva, G.I.; Uflyand, I.E. Metal Chelate Monomers as Precursors of Polymeric Materials. J. Inorg. Organomet. Polym. Mater. 2016, 26, 1112–1173. [Google Scholar] [CrossRef]
- Dzhardimalieva, G.I.; Uflyand, I.E. Review: Recent advances in the chemistry of metal chelate monomers. Coord. Chem. 2017, 70, 1468–1527. [Google Scholar] [CrossRef]
- Zhang, Y.; Riduan, S.N. Functional porous organic polymers for heterogeneous catalysis. Chem. Soc. Rev. 2012, 41, 2083–2094. [Google Scholar] [CrossRef] [PubMed]
- Pulko, I.; Wall, J.; Krajnc, P.; Cameron, N.R. Ultra-High Surface Area Functional Porous Polymers by Emulsion Templating and Hypercrosslinking: Efficient Nucleophilic Catalyst Supports. Chem. Eur. J. 2010, 16, 2350–2354. [Google Scholar] [CrossRef]
- Duan, C.; Zou, W.; Du, Z.; Li, H.; Zhang, C. Fabrication of micro-mesopores in macroporous poly (formaldehyde-melamine) monoliths via reaction-induced phase separation in high internal phase emulsion template. Polymer 2019, 167, 78–84. [Google Scholar] [CrossRef]
- Ben, T.; Ren, H.; Ma, S.; Cao, D.; Lan, J.; Jing, X.; Wang, W.; Xu, J.; Deng, F.; Simmons, J.M.; et al. Targeted Synthesis of a Porous Aromatic Framework with High Stability and Exceptionally High Surface Area. Angew. Chem. Int. Ed. 2009, 48, 9457–9460. [Google Scholar] [CrossRef]
- Bi, J.; Dong, Y.; Meng, D.; Zhu, D.; Li, T. The study and application of three highly porous hyper-crosslinked catalysts possessing similar catalytic centers. Polymer 2019, 164, 183–190. [Google Scholar] [CrossRef]
- Wei, J.; Zhao, X.; Yan, J. Pore structure of water-wettable hydrophobic resins based on divinylbenzene and methyl acrylate. J. Appl. Polym. Sci. 2004, 92, 2681–2688. [Google Scholar] [CrossRef]
- Mohamed, M.H.; Wilson, L.D. Porous Copolymer Resins: Tuning Pore Structure and Surface Area with Non Reactive Porogens. Nanomaterials 2012, 2, 163. [Google Scholar] [CrossRef] [PubMed]
- Ghosh Dastidar, D.; Saha, S.; Chowdhury, M. Porous microspheres: Synthesis, characterisation and applications in pharmaceutical & medical fields. Int. J. Pharm. 2018, 548, 34–48. [Google Scholar] [PubMed]
- Shi, S.; Russell, T.P. Nanoparticle Assembly at Liquid–Liquid Interfaces: From the Nanoscale to Mesoscale. Adv. Mater. 2018, 30, 1800714. [Google Scholar] [CrossRef] [PubMed]
- Alacid, E.; Alonso, D.A.; Botella, L.; Nájera, C.; Pacheco, M.C. Oxime palladacycles revisited: Stone-stable complexes nonetheless very active catalysts. Chem. Rec. 2006, 6, 117–132. [Google Scholar] [CrossRef] [PubMed]
- Alonso, D.A.; Nájera, C.; Pacheco, M.C. Oxime Palladacycles: Stable and Efficient Catalysts for Carbon−Carbon Coupling Reactions. Org. Lett. 2000, 2, 1823–1826. [Google Scholar] [CrossRef] [PubMed]
- Khodja, W.; Leclair, A.; Rull-Barrull, J.; Zammattio, F.; Kutonova, K.V.; Trusova, M.E.; Felpin, F.-X.; Rodriguez-Zubiri, M. The promoting effect of pyridine ligands in the Pd-catalysed Heck–Matsuda reaction. New J. Chem. 2016, 40, 8855–8862. [Google Scholar] [CrossRef]
- Shahnaz, N.; Banik, B.; Das, P. A highly efficient Schiff-base derived palladium catalyst for the Suzuki–Miyaura reactions of aryl chlorides. Tetrahedron Lett. 2013, 54, 2886–2889. [Google Scholar] [CrossRef]
- Das, P.; Linert, W. Schiff base-derived homogeneous and heterogeneous palladium catalysts for the Suzuki–Miyaura reaction. Coord. Chem. Rev. 2016, 311, 1–23. [Google Scholar] [CrossRef]
- Andrade, A.P.S.; Arantes, L.M.; Kadooca, J.Y.; Carvalho, R.L.; de Fátima, Â.; Sabino, A.A. Palladium Complexes with Tetradentate Schiff Bases or their Corresponding Amines: Synthesis and Application in Heck Reactions. ChemistrySelect 2016, 1, 886–890. [Google Scholar] [CrossRef]
- Borhade, S.R.; Waghmode, S.B. Phosphine-free Pd–salen complexes as efficient and inexpensive catalysts for Heck and Suzuki reactions under aerobic conditions. Tetrahedron Lett. 2008, 49, 3423–3429. [Google Scholar] [CrossRef]
- Yuan, L.; Xu, Y.; Hu, X.; Yang, G.; Wu, Y. A water-soluble palladium-salen catalyst modified by pyridinium salt showing higher reactivity and recoverability for Heck coupling reaction. J. Mol. Catal. A Chem. 2015, 396, 55–60. [Google Scholar] [CrossRef]
- Ghabdian, M.; Nasseri, M.A.; Allahresani, A.; Motavallizadehkakhky, A. Pd salen complex@CPGO as a convenient, effective heterogeneous catalyst for Suzuki–Miyaura and Heck–Mizoroki cross-coupling reactions. Res. Chem. Intermed. 2018. [Google Scholar] [CrossRef]
- Jin, X.; Zhang, K.; Sun, J.; Wang, J.; Dong, Z.; Li, R. Magnetite nanoparticles immobilized Salen Pd (II) as a green catalyst for Suzuki reaction. Catal. Comm. 2012, 26, 199–203. [Google Scholar] [CrossRef]
- Liu, P.; Feng, X.-J.; He, R. Salen and half-salen palladium(II) complexes: Synthesis, characteriztion and catalytic activity toward Suzuki–Miyaura reaction. Tetrahedron 2010, 66, 631–636. [Google Scholar] [CrossRef]
- Movassagh, B.; Parvis, F.S.; Navidi, M. Pd(II) salen complex covalently anchored to multi-walled carbon nanotubes as a heterogeneous and reusable precatalyst for Mizoroki–Heck and Hiyama cross-coupling reactions. Appl. Organomet. Chem. 2015, 29, 40–44. [Google Scholar] [CrossRef]
- Nikoorazm, M.; Ghorbani-Choghamarani, A.; Jabbari, A. A facile preparation of palladium Schiff base complex supported into MCM-41 mesoporous and its catalytic application in Suzuki and Heck reactions. J. Porous Mater. 2016, 23, 967–975. [Google Scholar] [CrossRef]
- Che Soh, S.K.; Shamsuddin, M. Tetradentate N2O2 Chelated Palladium(II) Complexes: Synthesis, Characterization, and Catalytic Activity towards Mizoroki-Heck Reaction of Aryl Bromides. J. Chem. 2013, 2013, 8. [Google Scholar] [CrossRef]
- Tobiasz, A.; Walas, S.; Trzewik, B.; Grzybek, P.; Zaitz, M.M.; Gawin, M.; Mrowiec, H. Cu(II)-imprinted styrene–divinylbenzene beads as a new sorbent for flow injection-flame atomic absorption determination of copper. Microchem. J. 2009, 93, 87–92. [Google Scholar] [CrossRef]
- Li, Y.; Fan, Y.; Ma, J. Thermal, physical and chemical stability of porous polystyrene-type beads with different degrees of crosslinking. Polym. Degrad. Stab. 2001, 73, 163–167. [Google Scholar] [CrossRef]
- Hao, D.-X.; Gong, F.-L.; Wei, W.; Hu, G.-H.; Ma, G.-H.; Su, Z.-G. Porogen effects in synthesis of uniform micrometer-sized poly(divinylbenzene) microspheres with high surface areas. J. Colloid Interface Sci. 2008, 323, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Campos, C.H.; Belmar, J.B.; Jeria, S.E.; Urbano, B.F.; Torres, C.C.; Alderete, J.B. Rhodium(I) diphenylphosphine complexes supported on porous organic polymers as efficient and recyclable catalysts for alkene hydrogenation. RSC Adv. 2017, 7, 3398–3407. [Google Scholar] [CrossRef]
- Liu, Q.; Li, Y.; Shen, S.; Xiao, Q.; Chen, L.; Liao, B.; Ou, B.; Ding, Y. Preparation and characterization of crosslinked polymer beads with tunable pore morphology. J. Appl. Polym. Sci. 2011, 121, 654–659. [Google Scholar] [CrossRef]
- Law, R.V.; Sherrington, D.C.; Snape, C.E. Quantitative Solid State 13C NMR Studies of Highly Cross-Linked Poly(divinylbenzene) Resins. Macromolecules 1997, 30, 2868–2875. [Google Scholar] [CrossRef]
- Zhu, X.X.; Banana, K.; Liu, H.Y.; Krause, M.; Yang, M. Cross-Linked Porous Polymer Resins with Reverse Micellar Imprints: Factors Affecting the Porosity of the Polymers. Macromolecules 1999, 32, 277–281. [Google Scholar] [CrossRef]
- Choudhary, A.; Kumari, S.; Ray, S. Tuning of Catalytic Property Controlled by the Molecular Dimension of Palladium–Schiff Base Complexes Encapsulated in Zeolite Y. ACS Omega 2017, 2, 6636–6645. [Google Scholar] [CrossRef]
- Fonseca, J.; Tedim, J.; Biernacki, K.; Magalhães, A.L.; Gurman, S.J.; Freire, C.; Hillman, A.R. Structural and electrochemical characterisation of [Pd(salen)]-type conducting polymer films. Electrochim. Acta 2010, 55, 7726–7736. [Google Scholar] [CrossRef]
- Sobhani, S.; Falatooni, Z.M.; Asadi, S.; Honarmand, M. Palladium-Schiff Base Complex Immobilized Covalently on Magnetic Nanoparticles as an Efficient and Recyclable Catalyst for Heck and Suzuki Cross-Coupling Reactions. Catal. Lett. 2016, 146, 255–268. [Google Scholar] [CrossRef]
- Sobhani, S.; Ghasemzadeh, M.S.; Honarmand, M.; Zarifi, F. Acetamidine–palladium complex immobilized on γ-Fe2O3 nanoparticles: A novel magnetically separable catalyst for Heck and Suzuki coupling reactions. RSC Adv. 2014, 4, 44166–44174. [Google Scholar] [CrossRef]
- Alamgholiloo, H.; Rostamnia, S.; Hassankhani, A.; Khalafy, J.; Baradarani, M.M.; Mahmoudi, G.; Liu, X. Stepwise post-modification immobilization of palladium Schiff-base complex on to the OMS-Cu (BDC) metal–organic framework for Mizoroki-Heck cross-coupling reaction. Appl. Organomet. Chem. 2018, 32, e4539. [Google Scholar] [CrossRef]
- Ohtaka, A.; Yamaguchi, T.; Teratani, T.; Shimomura, O.; Nomura, R. Linear polystyrene-stabilized PdO nanoparticle-catalyzed Mizoroki-Heck reactions in water. Molecules 2011, 16, 9067–9076. [Google Scholar] [CrossRef] [PubMed]
- Fernández, E.; Rivero-Crespo, M.A.; Domínguez, I.; Rubio-Marqués, P.; Oliver-Meseguer, J.; Liu, L.; Cabrero-Antonino, M.; Gavara, R.; Hernández-Garrido, J.C.; Boronat, M.; et al. Base-Controlled Heck, Suzuki, and Sonogashira Reactions Catalyzed by Ligand-Free Platinum or Palladium Single Atom and Sub-Nanometer Clusters. J. Am. Chem. Soc. 2019, 141, 1928–1940. [Google Scholar] [CrossRef] [PubMed]
- Rajender Reddy, K.; Kumar, N.S.; Surendra Reddy, P.; Sreedhar, B.; Lakshmi Kantam, M. Cellulose supported palladium(0) catalyst for Heck and Sonogashira coupling reactions. J. Mol. Catal. A Chem. 2006, 252, 12–16. [Google Scholar] [CrossRef]
- Kiviaho, J.; Hanaoka, T.; Kubota, Y.; Sugi, Y. Heterogeneous palladium catalysts for the Heck reaction. J. Mol. Catal. A Chem. 1995, 101, 25–31. [Google Scholar] [CrossRef]
- de Vries, J.G. A unifying mechanism for all high-temperature Heck reactions. The role of palladium colloids and anionic species. Dalton Trans. 2006, 21, 421–429. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.; Maddipoti, K.; Das, B.; Ray, S. Palladium–Schiff Base Complexes Encapsulated in Zeolite-Y Host: Functionality Controlled by the Structure of a Guest Complex. Inorg. Chem. 2019, 58, 1527–1540. [Google Scholar] [CrossRef] [PubMed]
- Layek, S.; Anuradha; Agrahari, B.; Pathak, D.D. Synthesis and characterization of a new Pd(II)-Schiff base complex [Pd(APD)2]: An efficient and recyclable catalyst for Heck-Mizoroki and Suzuki-Miyaura reactions. J. Organomet. Chem. 2017, 846, 105–112. [Google Scholar] [CrossRef]
- Fortea-Pérez, F.R.; Julve, M.; Dikarev, E.V.; Filatov, A.S.; Stiriba, S.-E. Synthesis and structural characterization of well-defined bis(oxamato)palladate(II) precatalysts for Suzuki and Heck reactions. Inorg. Chim. Acta 2018, 471, 788–796. [Google Scholar] [CrossRef]
- Gholivand, K.; Salami, R.; Rastegar, S.F.; Roe, S.M. Dithiophosphorus-Palladium Complexes as a Catalyst in the Heck Reaction via Pd(II)/Pd(IV) Catalytic Cycle: A Combined Experimental and Computational Study. ChemistrySelect 2018, 3, 7822–7829. [Google Scholar] [CrossRef]
- Rao, K.U.; Lakshmidevi, J.; Appa, R.M.; Prasad, S.S.; Narasimhulu, M.; Vijitha, R.; Rao, K.S.V.K.; Venkateswarlu, K. Palladium(II)-Porphyrin Complexes as Efficient and Eco-Friendly Catalysts for Mizoroki-Heck Coupling. ChemistrySelect 2017, 2, 7394–7398. [Google Scholar] [CrossRef]
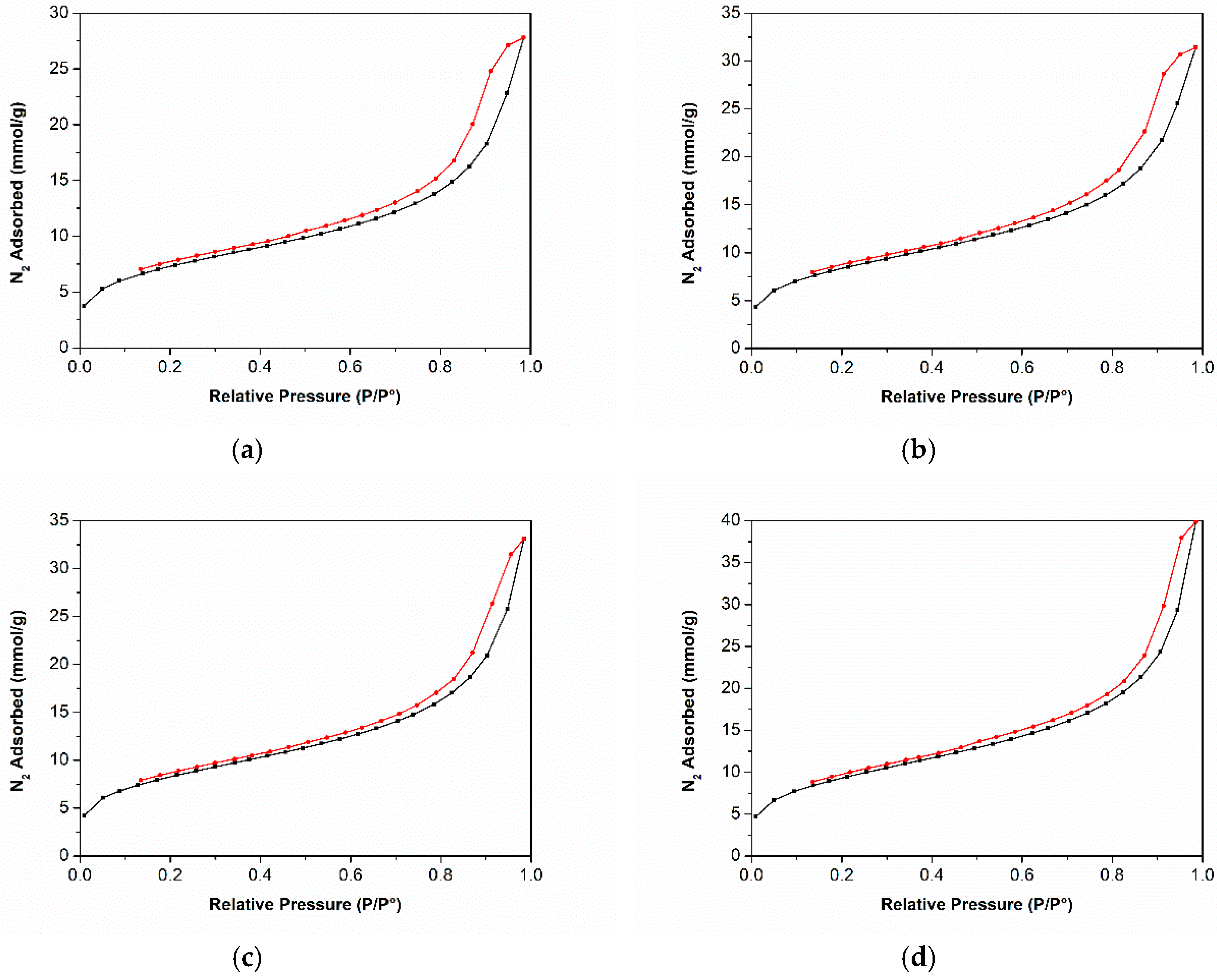
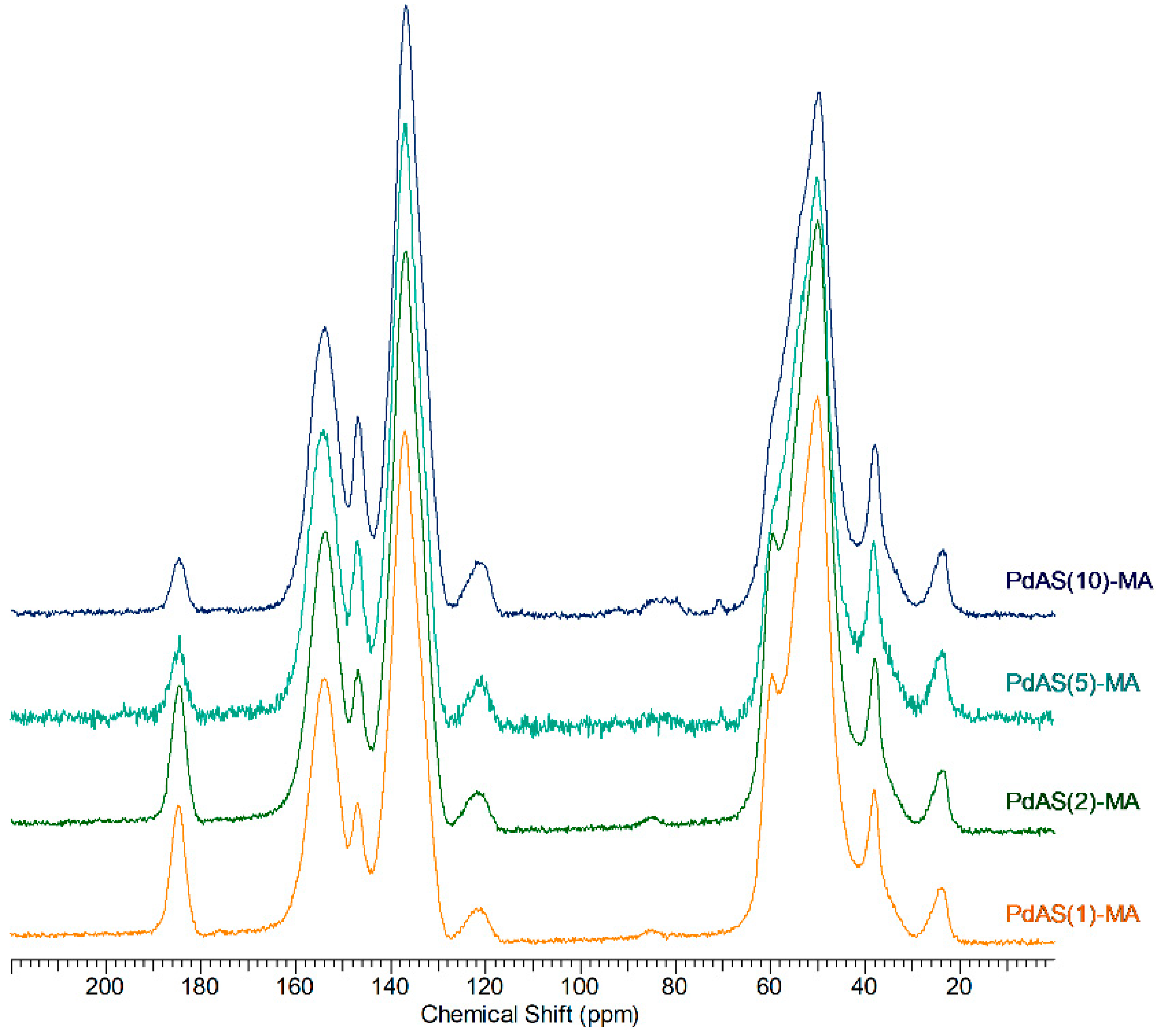
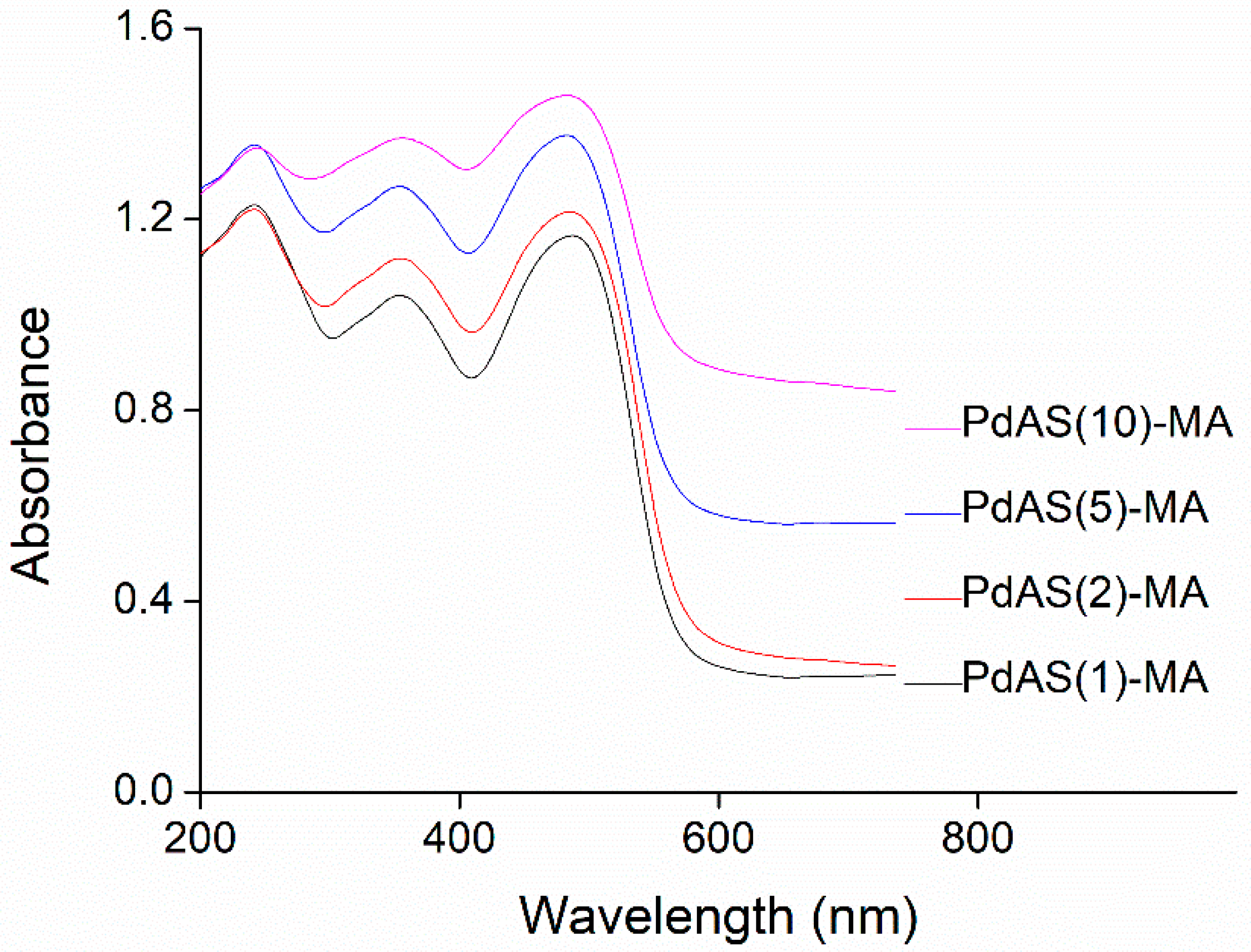
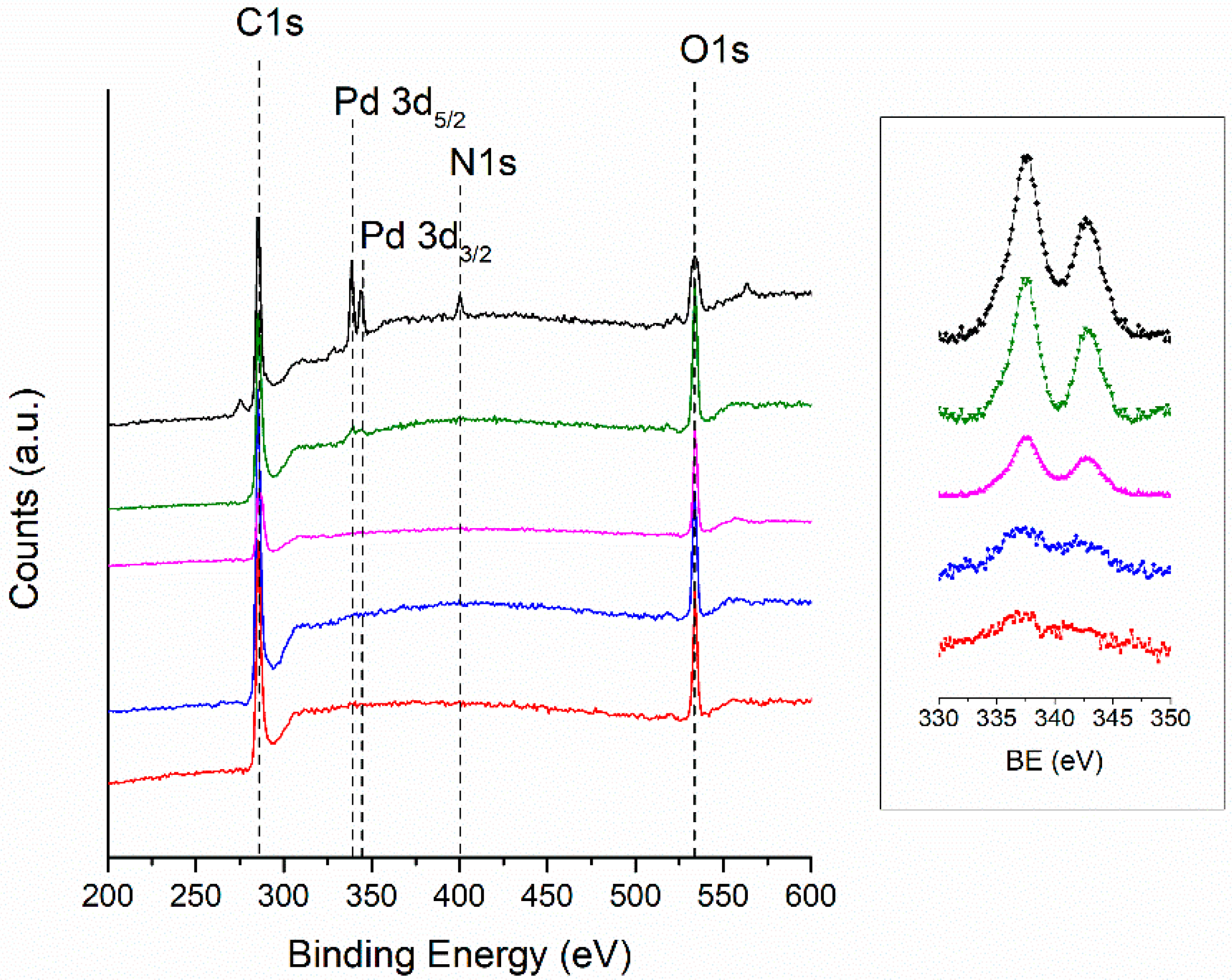


| Label | wt.% | Grain Diameter 1 µm | SBET m2g−1 | Pore Size nm | |||
|---|---|---|---|---|---|---|---|
| DVB | MA | AIBN | PdAS | ||||
| PdAS(1)-MA | 60 | 38 | 1 | 1 | 184 ± 20 | 586 | 17.0 |
| PdAS(2)-MA | 60 | 37 | 1 | 2 | 196 ± 28 | 665 | 19.7 |
| PdAS(5)-MA | 60 | 34 | 1 | 5 | 227 ± 32 | 671 | 17.3 |
| PdAS(10)-MA | 60 | 29 | 1 | 10 | 272 ± 41 | 754 | 22.3 |
| Label | Pd wt.% | Pd exp/Pd nom | Immobilized PdAS mmol gcat−1 | Binding Energy eV | |
|---|---|---|---|---|---|
| Pd 3d5/2 | N 1s | ||||
| PdAS | - | - | - | 338.4 | 400.0 |
| PdAS(1)-MA | 0.36 | 1.3 | 0.034 | 338.0 | 400.4 |
| PdAS(2)-MA | 0.51 | 0.8 | 0.050 | 338.5 | 400.0 |
| PdAS(5)-MA | 0.77 | 0.6 | 0.074 | 338.5 | 400.0 |
| PdAS(10)-MA | 1.38 | 0.5 | 0.132 | 338.3 | 400.4 |
| Catalyst | Conversion % | Selectivity MCIN 1 % | t5% conversion 2 min | ro ×104 mol·L−1min−1 | TOF h−1 |
|---|---|---|---|---|---|
| Pd(CH3CO2)2 | 100 | 100 | 0.2 | 1280 | 29554 |
| PdAS | 100 | 100 | 6.4 | 40 | 929 |
| PdAS(1)-MA | 58 | 100 | 43.7 | 2 | 137 |
| PdAS(2)-MA | 99 | 100 | 8.3 | 9 | 720 |
| PdAS(5)-MA | 99 | 100 | 2.2 | 67 | 2782 |
| PdAS(10)-MA | 100 | 100 | 1.0 | 265 | 6122 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mella, C.; Torres, C.C.; Pecchi, G.; Campos, C.H. Mesoporous Palladium N,N’-Bis(3-Allylsalicylidene)o-Phenylenediamine-Methyl Acrylate Resins as Heterogeneous Catalysts for the Heck Coupling Reaction. Materials 2019, 12, 2612. https://doi.org/10.3390/ma12162612
Mella C, Torres CC, Pecchi G, Campos CH. Mesoporous Palladium N,N’-Bis(3-Allylsalicylidene)o-Phenylenediamine-Methyl Acrylate Resins as Heterogeneous Catalysts for the Heck Coupling Reaction. Materials. 2019; 12(16):2612. https://doi.org/10.3390/ma12162612
Chicago/Turabian StyleMella, Claudio, Cecilia C. Torres, Gina Pecchi, and Cristian H. Campos. 2019. "Mesoporous Palladium N,N’-Bis(3-Allylsalicylidene)o-Phenylenediamine-Methyl Acrylate Resins as Heterogeneous Catalysts for the Heck Coupling Reaction" Materials 12, no. 16: 2612. https://doi.org/10.3390/ma12162612
APA StyleMella, C., Torres, C. C., Pecchi, G., & Campos, C. H. (2019). Mesoporous Palladium N,N’-Bis(3-Allylsalicylidene)o-Phenylenediamine-Methyl Acrylate Resins as Heterogeneous Catalysts for the Heck Coupling Reaction. Materials, 12(16), 2612. https://doi.org/10.3390/ma12162612