DFT Study of N2O Adsorption onto the Surface of M-Decorated Graphene Oxide (M = Mg, Cu or Ag)
Abstract
1. Introduction
2. Model and Computational Methods
3. Results and Discussions
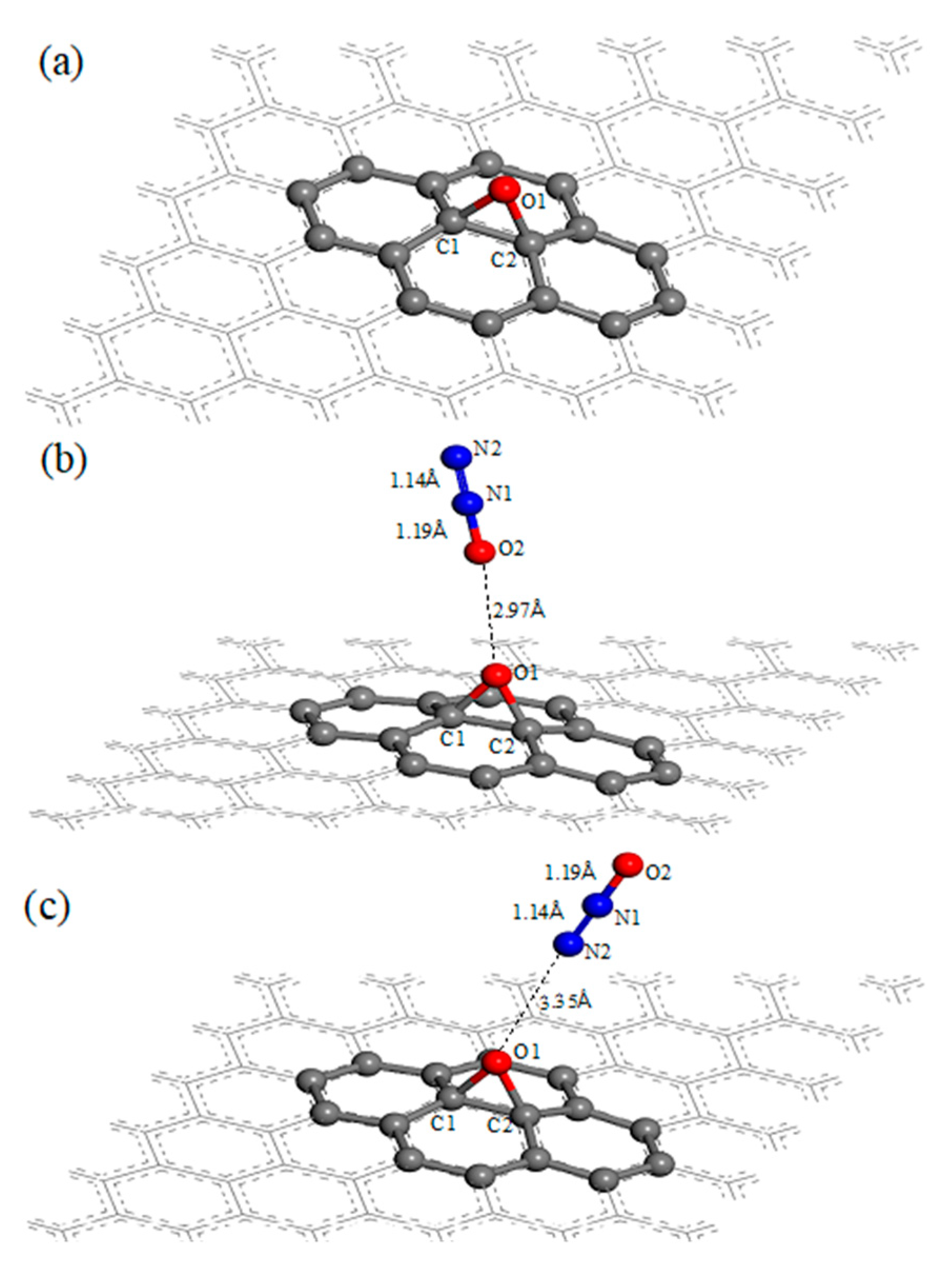
3.1. N2O Adsorbed on the Surface of GO
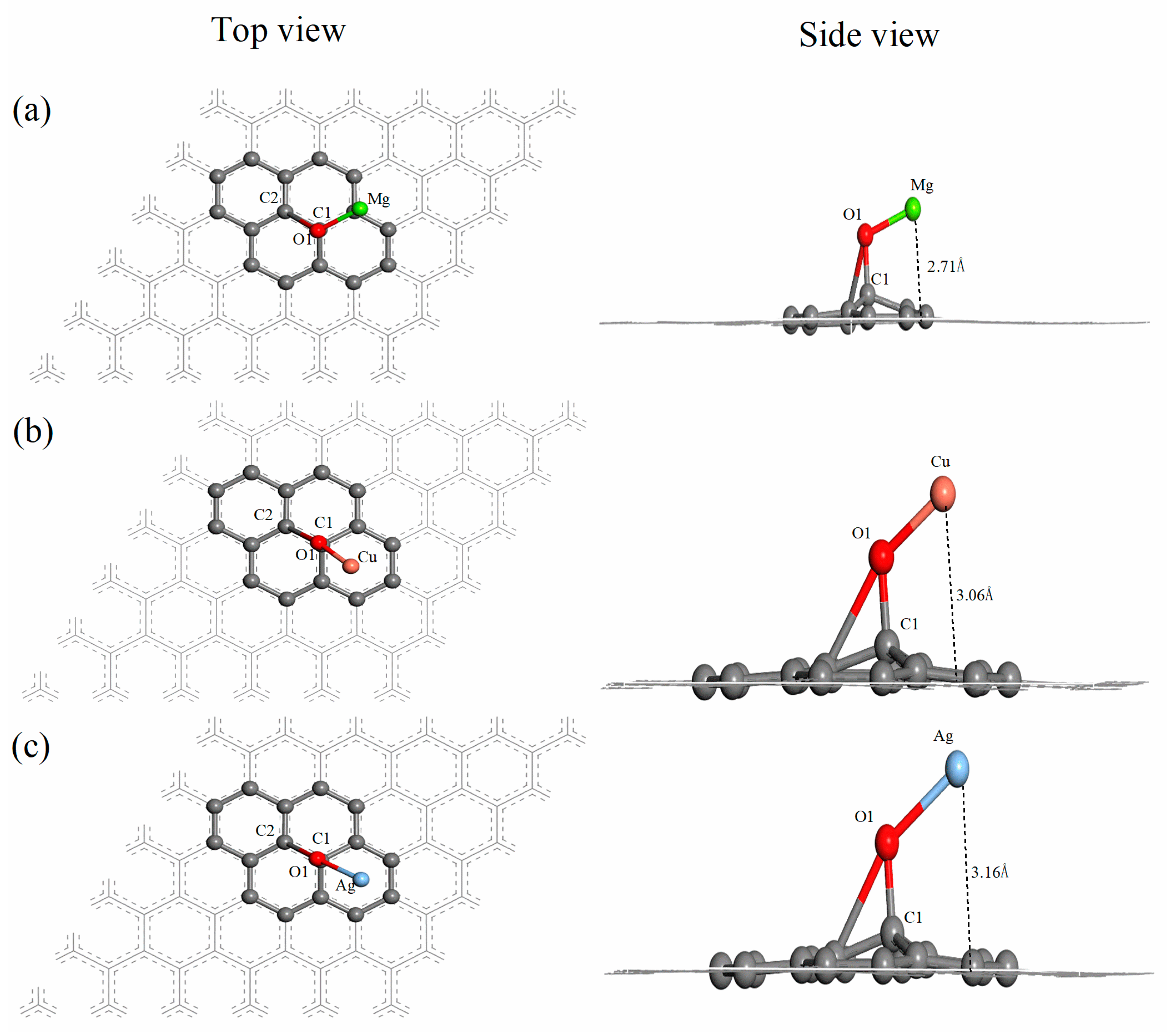
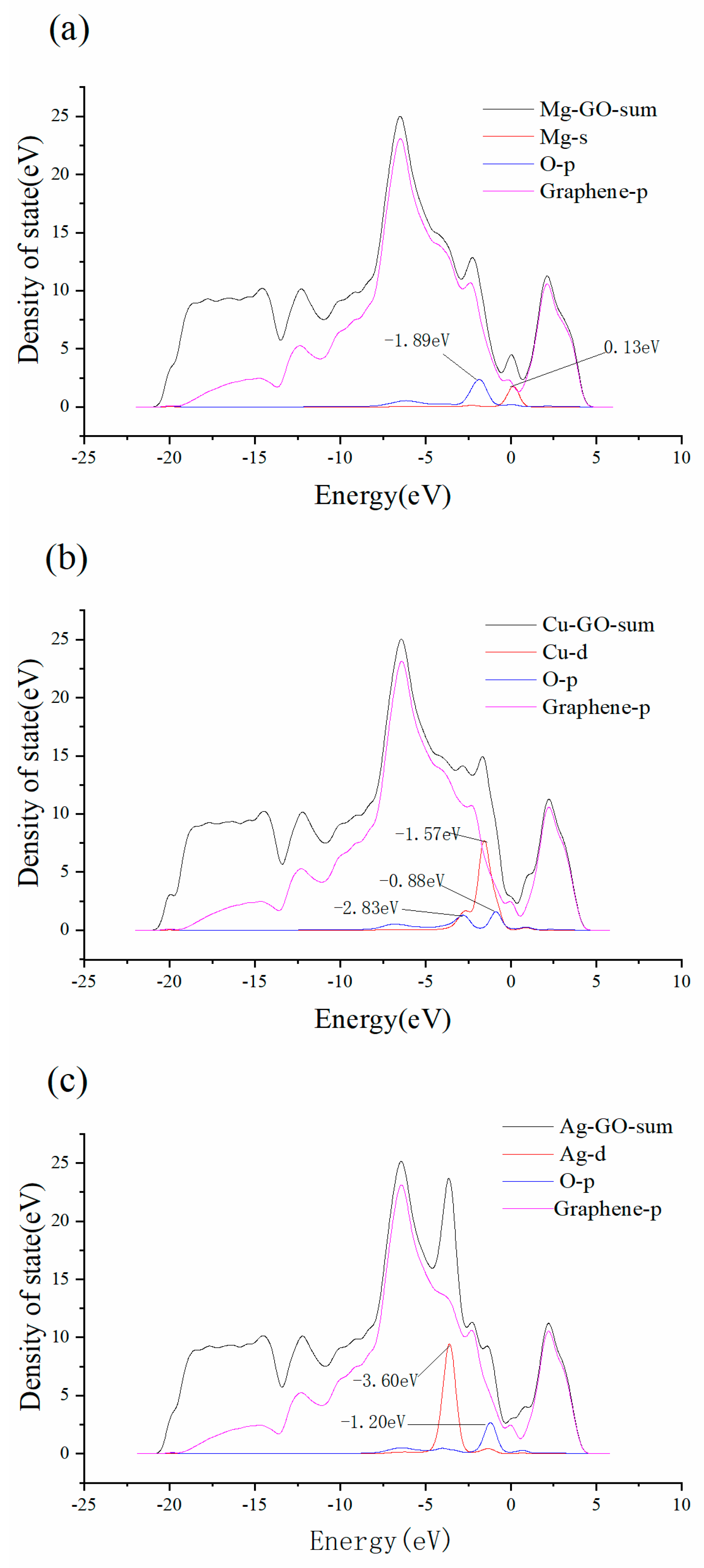
3.2. Mg-, Cu- and Ag-Decorated Graphene Oxide
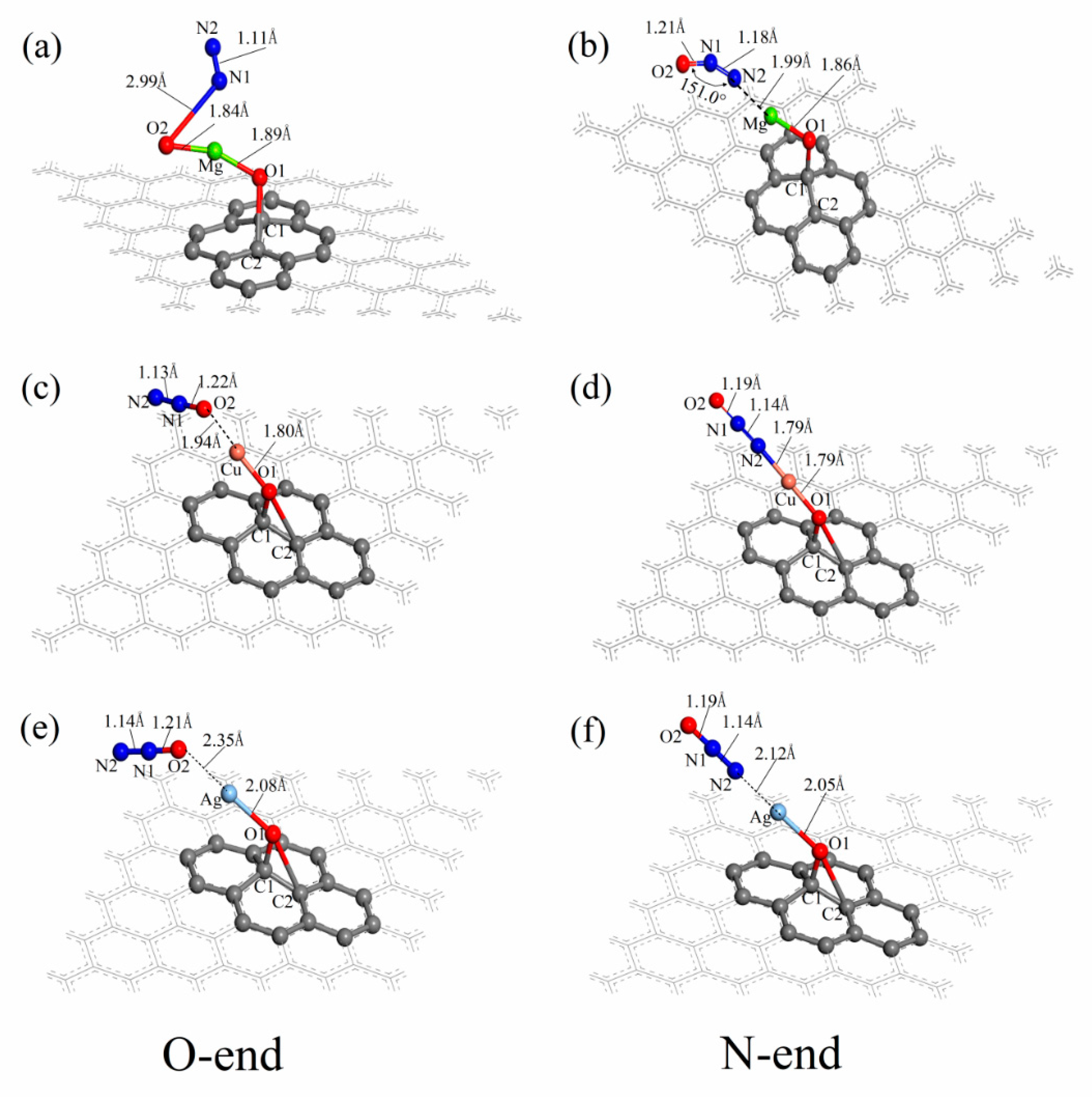
3.3. N2O Adsorbed on the Surface of M-GO (M = Mg, Cu or Ag)
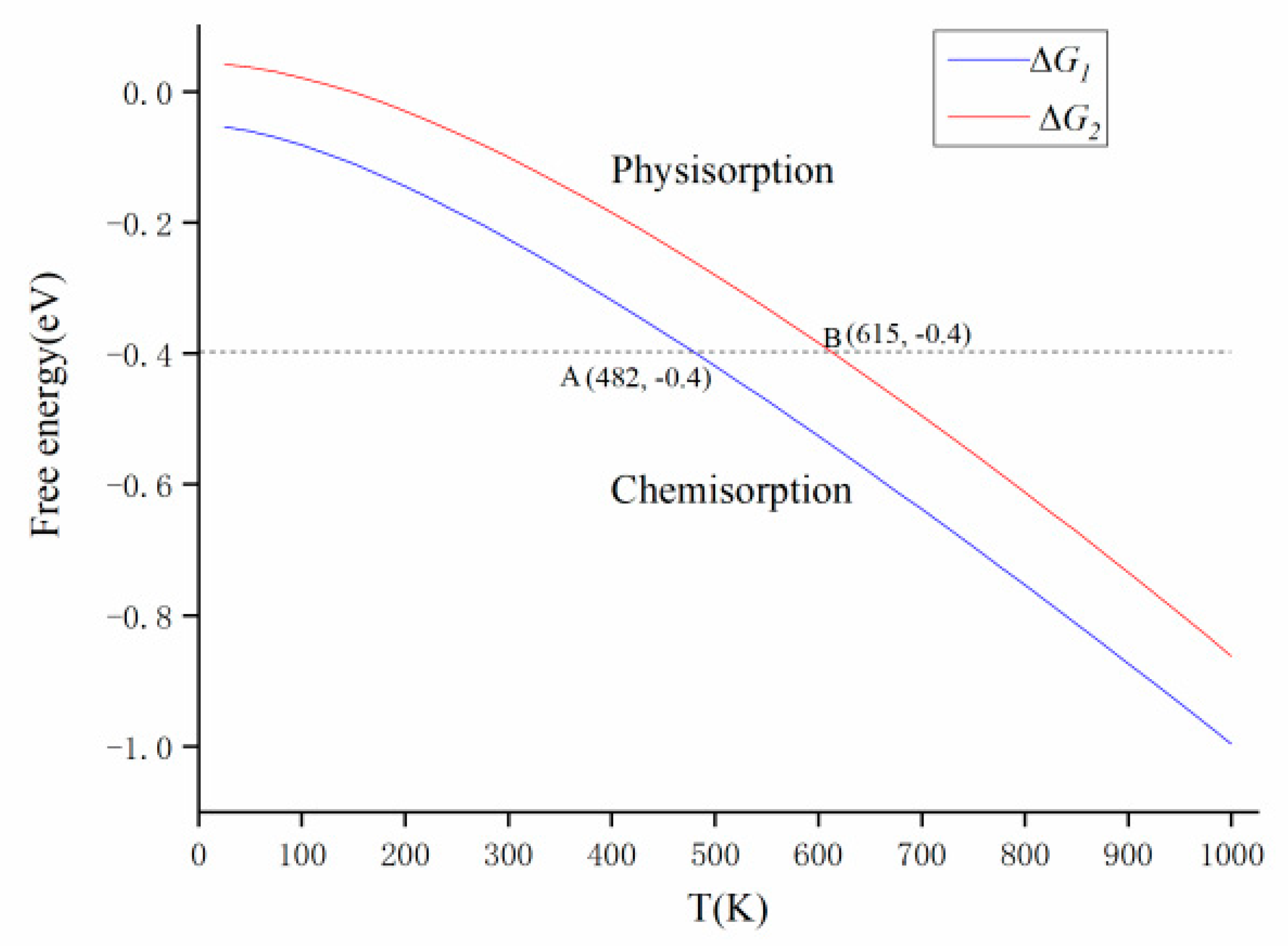
3.4. Gibbs Free Energy Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Ravishankara, J.S.; Daniel, J.S.; Portman, R.W. Nitrous Oxide (N2O): The Dominant Ozone-Depleting Substance Emitted in the 21st Century. Science 2009, 326, 123–125. [Google Scholar] [CrossRef] [PubMed]
- Duce, R.A.; LaRoche, J.; Altieri, K. Impacts of Atmospheric Anthropogenic Nitrogen on the Open Ocean. Science 2008, 320, 893–897. [Google Scholar] [CrossRef] [PubMed]
- Montzka, S.A.; Dlugokencky, E.J.; Butler, J.H. Non-CO2 greenhouse gases and climate change. Nature 2011, 476, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.X.; Wang, X.P.; Zhao, X.J.; Xu, Y.Z.; Gao, H.; Zhang, F.F. An investigation on N2O formation route over Pt/HY in H2-SCR. Chem. Eng. J. 2014, 252, 288–297. [Google Scholar] [CrossRef]
- Rockmann, T.; Kaiser, J.; Brenninkmeijer, C.A.M. The isotopic fingerprint of the pre-industrial and the anthropogenic N2O source. Atmos. Chem. Phys. 2003, 3, 315–323. [Google Scholar] [CrossRef]
- Pérez-Ramírez, J. Prospects of N2O emission regulations in the European fertilizer industry. Appl. Catal. B Environ. 2007, 70, 31–35. [Google Scholar] [CrossRef]
- Ivanova, Y.A.; Sutormina, E.F.; Isupova, I.A. Catalytic Activity of the Oxide Catalysts Based on Ni0.75Co2.25O4 Decomposition Modified with Cesium Cations in a Reaction of N2O Decomposition. Kinet. Catal. 2017, 58, 793–799. [Google Scholar] [CrossRef]
- Wang, A.; Wang, Y.; Walter, E.D. Catalytic N2O decomposition and reduction by NH3 over Fe/Beta and Fe/SSZ-13 catalysts. J. Catal. 2018, 358, 199–210. [Google Scholar] [CrossRef]
- Klyushina, A.; Pacultová, K.; Karásková, K. Effect of preparation method on catalytic properties of Co-Mn-Al mixed oxides for N2O decomposition. J. Mol. Catal. A-Chem. 2016, 425, 237–247. [Google Scholar] [CrossRef]
- Zheng, X.; Zhang, R.; Bai, F. Catalytic Decomposition of N2O over Cu–Zn/ZnAl2O4 Catalysts. Catalysts 2017, 7, 166. [Google Scholar] [CrossRef]
- Carabineiro, S.A.C.; Papista, E.; Marnellos, G.E. Catalytic decomposition of N2O on inorganic oxides: Εffect of doping with Au nanoparticles. Mol. Catal. 2017, 436, 78–89. [Google Scholar] [CrossRef]
- Abu-Zied, B.M.; Bawaked, S.M.; Kosa, S.A. Effects of Nd-, Pr-, Tb- and Y-doping on the structural, textural, electrical and N2O decomposition activity of mesoporous NiO nanoparticles. Appl. Surf. Sci. 2017, 419, 399–408. [Google Scholar] [CrossRef]
- Yu, H.; Wang, X.; Wu, X. Promotion of Ag for Co3O4 catalyzing N2O decomposition under simulated real reaction conditions. Chem. Eng. J. 2017, 334, 800–806. [Google Scholar] [CrossRef]
- Wu, L.N.; Hu, X.Y.; Qin, W. Effect of CaO on the selectivity of N2O decomposition products: A combined experimental and DFT study. Surf. Sci. 2016, 651, 128–136. [Google Scholar] [CrossRef]
- Wang, L.; Song, W.; Deng, J. Facet-dependent photocatalytic decomposition of N2O on the anatase TiO2: A DFT study. Nanoscale 2018, 10, 6024–6038. [Google Scholar] [CrossRef]
- Fransisco, H.; Bertin, V.; Agacino, E. Dissociation of N2O Promoted by Rh6 Clusters: A ZORA/DFT/PBE Study. J. Mol. Catal. A-Chem. 2015, 406, 238–250. [Google Scholar] [CrossRef]
- Wongnongwa, Y.; Namuangruk, S.; Kungwan, N. Mechanistic study of CO oxidation by N2O over Ag7Au6 cluster investigated by DFT methods. Appl. Catal. A-Gen. 2017, 538, 99–106. [Google Scholar] [CrossRef]
- Esrafili, M.D. N2O reduction over a fullerene-like boron nitride nanocage: A DFT study. Phys. Lett. A 2017, 381, 2085–2091. [Google Scholar] [CrossRef]
- Ketrat, S.; Maihom, T.; Wannakao, S. Coordinatively Unsaturated Metal-Organic Frameworks M3(btc)2 (M = Cr, Fe, Co, Ni, Cu, and Zn) Catalyzing the Oxidation of CO by N2O: Insight from DFT Calculations. Inorg. Chem. 2017, 56, 14005–14012. [Google Scholar] [CrossRef]
- Chen, H.; Lu, Q.; Yi, C. Design of bimetallic Rh-M catalysts for N2O decomposition: From DFT calculation to experimental study. Mol. Catal. 2018, 446, 1–9. [Google Scholar] [CrossRef]
- Zhang, J.; Hu, H.; Xu, J.; Wu, G.M.; Zeng, Z.W. N2O decomposition over K/Na-promoted Mg/Zn–Ce–cobalt mixed oxides catalysts. J. Environ. Sci. 2014, 26, 1437–1443. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Tao, M.; Ma, Z.; Wu, G.; Zeng, Z. Catalytic decomposition of N2O over RhOx supported on metal phosphates. J. Ind. Eng. Chem. 2015, 28, 138–146. [Google Scholar] [CrossRef]
- Li, Y.; Wang, X. MgO Modifying Al2O3 to Load Cobalt Oxide for Catalytic N2O Decomposition. Catal. Lett. 2019, 149, 1856–1863. [Google Scholar] [CrossRef]
- Cortes-Arriagada, D.; Villegas-Escobar, N. A DFT analysis of the adsorption of nitrogen oxides on Fe-doped graphene, and the electric field induced desorption. Appl. Surf. Sci. 2017, 420, 446–455. [Google Scholar] [CrossRef]
- Lv, Y.; Zhuang, G.; Wang, J. Enhanced role of Al or Ga-doped graphene on the adsorption and dissociation of N2O under electric field, Physical chemistry chemical physics. Phys. Chem. Chem. Phys. 2011, 13, 12472–12477. [Google Scholar] [CrossRef] [PubMed]
- Rad, A.S. First principles study of Al-doped graphene as nanostructure adsorbent for NO2 and N2O: DFT calculations. Appl. Surf. Sci. 2015, 357, 1217–1224. [Google Scholar] [CrossRef]
- Gholizadeh, R.; Yu, Y. N2O + CO reaction over Si- and Se-doped graphenes: An ab initio DFT study. Appl. Surf. Sci. 2015, 357, 1187–1195. [Google Scholar] [CrossRef]
- Chen, Y.; Gao, B.; Zhao, J. Si-doped graphene: an ideal sensor for NO- or NO2-detection and metal-free catalyst for N2O-reduction. J. Mol. Model. 2012, 18, 2043–2054. [Google Scholar] [CrossRef]
- Zhao, J.; Chen, Y.; Fu, H. Si-embedded graphene: an efficient and metal-free catalyst for CO oxidation by N2O or O2. Theor. Chem. Acc. 2012, 131, 1242–1252. [Google Scholar] [CrossRef]
- Tong, Y.; Wang, Y.; Wang, Q. Theoretical investigation for the reaction of N2O with CO catalyzed by Pt-Graphene. Struct. Chem. 2017, 28, 1679–1685. [Google Scholar] [CrossRef]
- Gholizadeh, R.; Yu, Y.; Wang, Y. N2O Adsorption and Decomposition over ZnO (0001) Doped Graphene: Density Functional Theory Calculations. Appl. Surf. Sci. 2017, 420, 944–953. [Google Scholar] [CrossRef]
- Cortes-Arriagada, D. Global and local reactivity indexes applied to understand the chemistry of graphene oxide and doped graphene. J. Mol. Model. 2013, 19, 919–930. [Google Scholar] [CrossRef] [PubMed]
- Lv, Z.; Mo, H.; Chen, C. The effective adsorption and decomposition of N2O on Al-decorated graphene oxide under electric field. RSC Adv. 2015, 5, 18761–18766. [Google Scholar] [CrossRef]
- Esrafili, M.D.; Sharifi, F.; Nematollahi, P. Al- or Si-decorated graphene oxide: A favorable metal-free catalyst for the N2O reduction. Appl. Surf. Sci. 2016, 387, 454–460. [Google Scholar] [CrossRef]
- Delley, B. DMol3 DFT studies: from molecules and molecular environments to surfaces and solids. Comp. Mater. Sci. 2000, 17, 122–126. [Google Scholar] [CrossRef]
- Delley, B. From molecules to solids with the DMol3 approach. J. Chem. Phy. 2000, 13, 7756–7764. [Google Scholar] [CrossRef]
- Delley, B. An all-electron numerical method for solving the local density functional for polyatomic molecules. J. Chem. Phy. 1990, 92, 508–517. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Wang, Y. Generalized gradient approximation for the exchange-correlation hole of a many-electron system. Phys. Rev. B 1996, 54, 16533–16539. [Google Scholar] [CrossRef]
- Grimme, S. Semiempirical GGA-type density functional constructed with a long-range dispersion correction. J. Comput. Chem. 2006, 27, 1787–1799. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, Y.L.; Wang, B.Y.; Cheng, H.; Cheng, X.R.; Huang, Z.C. DFT study on Al-doped defective graphene towards adsorption of elemental mercury. Appl. Surf. Sci. 2018, 427, 547–553. [Google Scholar] [CrossRef]
- Li, F.; Zhao, J.; Chen, Z. Fe-Anchored Graphene Oxide: A Low-Cost and Easily Accessible Catalyst for Low-Temperature CO Oxidation. J. Phys. Chem. C 2011, 116, 2507–2514. [Google Scholar] [CrossRef]
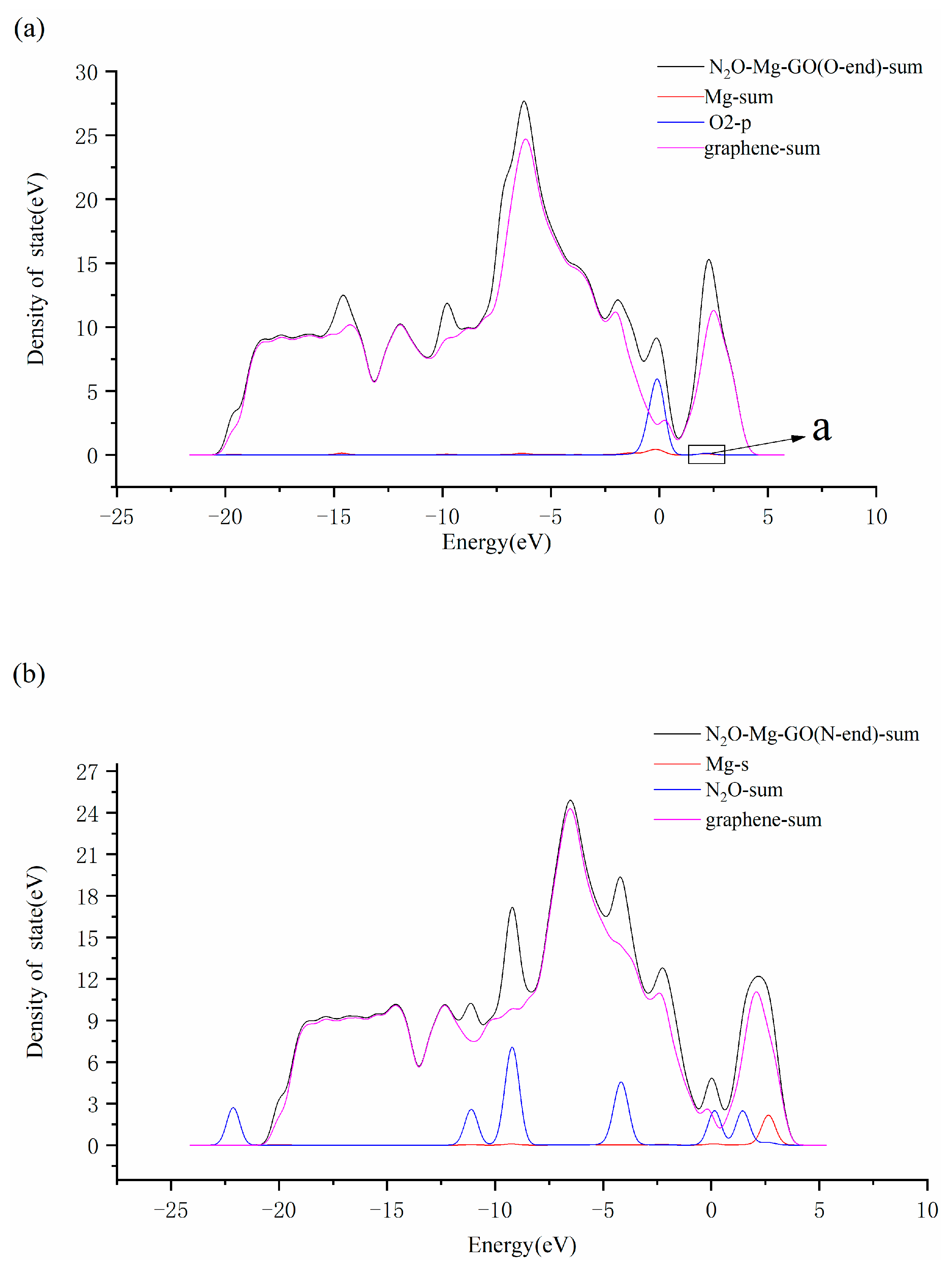
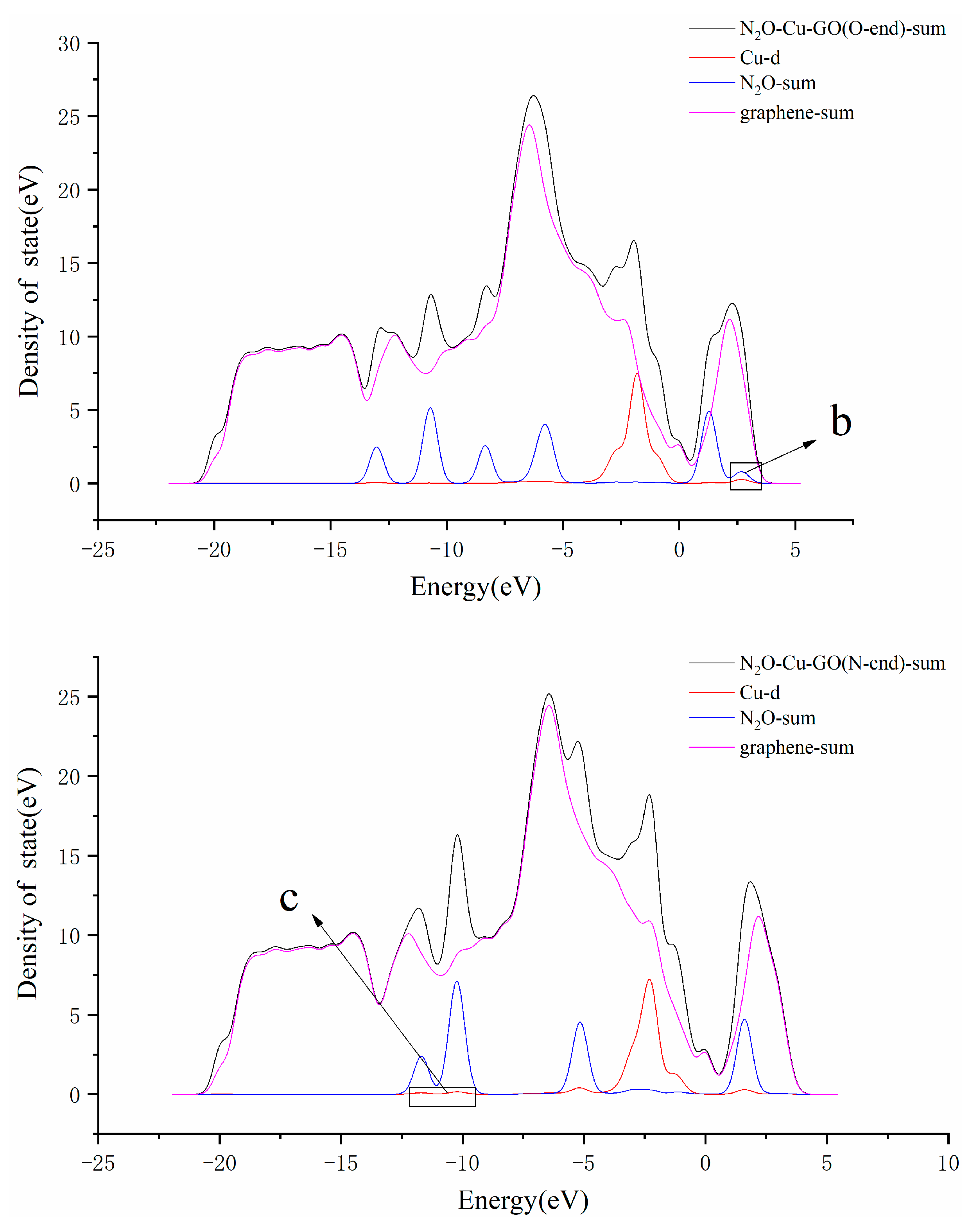
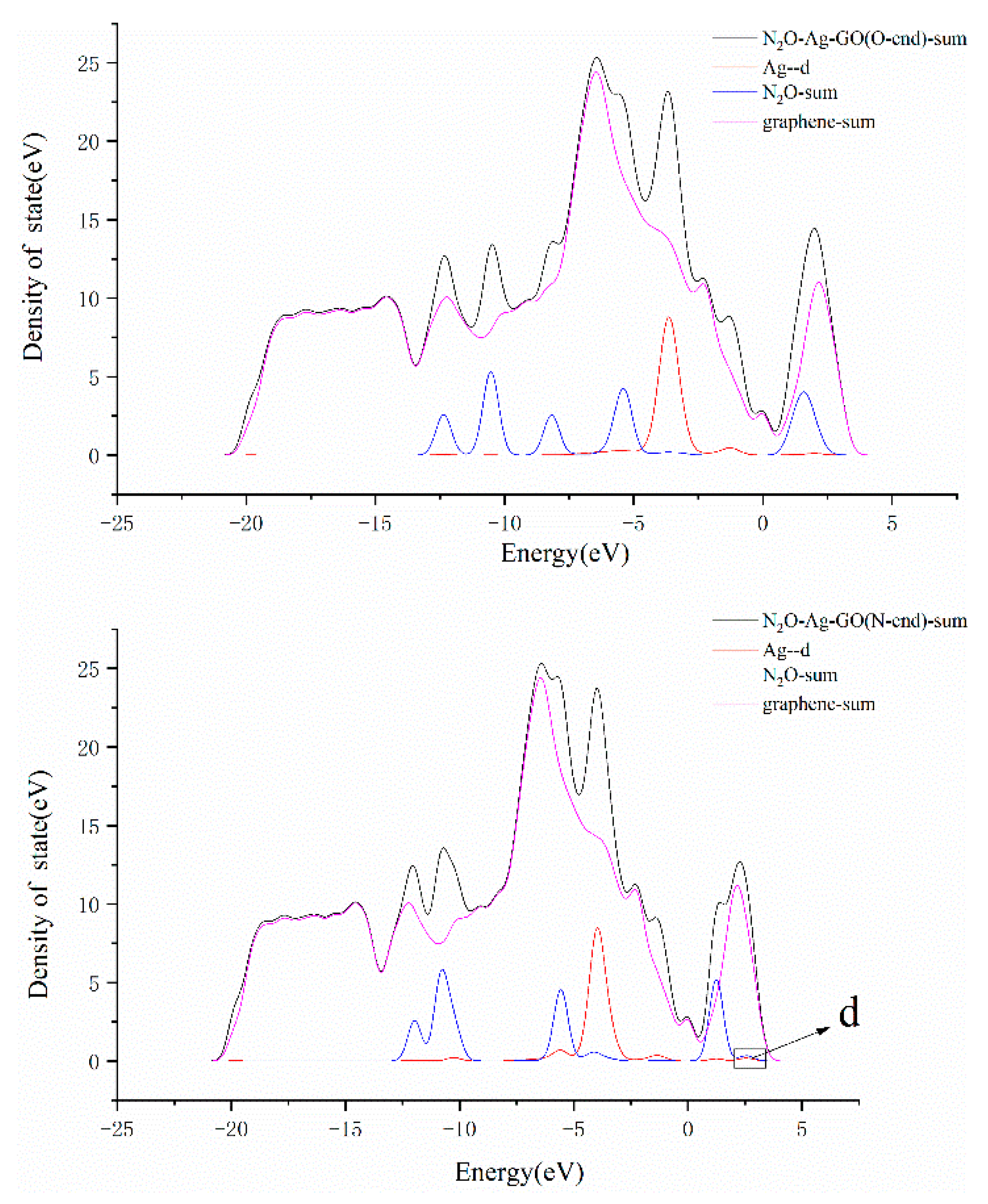
| System | Bond Length/Å | Bond Angle/° | ||||
|---|---|---|---|---|---|---|
| C1–O1 | C2–O1 | M–O1 | ∠C2–C1–O1 | ∠C1–C2–O1 | ∠C1–O1–M | |
| GO | 1.46 | 1.46 | - | 58.7 | 58.7 | - |
| Mg–GO | 1.47 | 2.35 | 1.87 | 105.0 | 37.0 | 106.7 |
| Cu–GO | 1.47 | 2.28 | 1.80 | 100.3 | 39.4 | 120.4 |
| Ag–GO | 1.45 | 2.26 | 2.10 | 100.1 | 39.1 | 159.4 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Cheng, X.-r.; Yang, Y.-m.; Jia, H.-z.; Bai, B.-q.; Zhao, L. DFT Study of N2O Adsorption onto the Surface of M-Decorated Graphene Oxide (M = Mg, Cu or Ag). Materials 2019, 12, 2611. https://doi.org/10.3390/ma12162611
Liu Z, Cheng X-r, Yang Y-m, Jia H-z, Bai B-q, Zhao L. DFT Study of N2O Adsorption onto the Surface of M-Decorated Graphene Oxide (M = Mg, Cu or Ag). Materials. 2019; 12(16):2611. https://doi.org/10.3390/ma12162611
Chicago/Turabian StyleLiu, Zhong, Xi-ren Cheng, Yi-min Yang, Hong-zhang Jia, Bao-quan Bai, and Li Zhao. 2019. "DFT Study of N2O Adsorption onto the Surface of M-Decorated Graphene Oxide (M = Mg, Cu or Ag)" Materials 12, no. 16: 2611. https://doi.org/10.3390/ma12162611
APA StyleLiu, Z., Cheng, X.-r., Yang, Y.-m., Jia, H.-z., Bai, B.-q., & Zhao, L. (2019). DFT Study of N2O Adsorption onto the Surface of M-Decorated Graphene Oxide (M = Mg, Cu or Ag). Materials, 12(16), 2611. https://doi.org/10.3390/ma12162611





