The Effects of Sintering Temperature and Addition of TiH2 on the Sintering Process of Cu
Abstract
1. Introduction
2. Materials and Methodology
3. Experimental Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Krishnan, S.; Haseeb, A.S.M.A.; Johan, M.R. Preparation and Low-Temperature Sintering of Cu Nanoparticles for High-Power Devices. IEEE Trans. Componen. Packaging Manuf. Technol. 2012, 2, 587–592. [Google Scholar] [CrossRef]
- Sundaram, R.; Yamada, T.; Hata, K.; Sekiguchi, A. Electrical performance of lightweight CNT-Cu composite wires impacted by surface and internal Cu spatial distribution. Sci. Rep. 2017, 7, 9267–9277. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.B.; Chen, J.H.; He, X.B.; Qu, X.H. Effect of matrix-alloying-element chromium on the microstructure and properties of graphite flakes/copper composites fabricated by hot pressing sintering. Carbon. 2018, 127, 412–423. [Google Scholar] [CrossRef]
- Han, L.T.; Liu, J.W.; Tang, H.G.; Ma, X.F.; Zhao, W. Investigation on the properties of nanostructured Cu alloy prepared by mechanical milling and reactive hot-pressing. J. Alloy Compd. 2018, 742, 284–289. [Google Scholar] [CrossRef]
- Yao, G.C.; Mei, Q.S.; Li, J.Y.; Li, C.L.; Ma, Y.; Chen, F.; Liu, M. Cu/C composites with a good combination of hardness and electrical conductivity fabricated from Cu and graphite by accumulative roll-bonding. Mater. Des. 2016, 110, 124–129. [Google Scholar] [CrossRef]
- Zhao, S.; Zheng, Z.; Huang, Z.X.; Dong, S.J.; Luo, P.; Zhang, Z.; Wang, Y.W. Cu matrix composites reinforced with aligned carbon nanotubes: mechanical, electrical and thermal properties. Mater. Sci. Eng. A 2016, 675, 82–91. [Google Scholar] [CrossRef]
- Shaik, M.A.; Golla, B.R. Development of highly wear resistant Cu-Al alloys processed via powder metallurgy. Tribol. Int. 2019, 136, 127–139. [Google Scholar] [CrossRef]
- Souli, I.; Gruber, G.C.; Terziyska, V.L.; Zechner, J.; Mitterer, C. Thermal stability of immiscible sputter-deposited Cu-Mo thin films. J. Alloy Compd. 2018, 783, 208–218. [Google Scholar] [CrossRef]
- Vincent, C.; Silvain, J.F.; Heintz, J.M.; Chandra, N. Effect of porosity on the thermal conductivity of copper processed by powder metallurgy. J. Phys. Chem. Solids. 2012, 73, 499–504. [Google Scholar] [CrossRef]
- Schubert, Th.; Trindade, B.; Weißgärber, T.; Kieback, B. Interfacial design of Cu-based composites prepared by powder metallurgy for heat sink applications. Mater. Sci. Eng. A. 2008, 475, 39–44. [Google Scholar] [CrossRef]
- Upadhyaya, A.; Upadhyaya, G.S. Sintering of copper-alumina composites through blending and mechanical alloying powder metallurgy routes. Mater. Des. 1995, 16, 41–45. [Google Scholar] [CrossRef]
- Deng, Z.H.; Yin, H.Q.; Zhang, C.; Zhang, G.F.; Li, W.Q.; Zhang, T.; Zhang, R.; Jiang, X.; Qu, X. Microstructure and mechanical properties of Cu–12Al–xNi alloy prepared using powder metallurgy. Mater. Sci. Eng. A. 2019, 759, 241–251. [Google Scholar] [CrossRef]
- Paderin, S.N.; Shil’nikov, E.V. Thermodynamic laws of the oxygen solubility in liquid metals (Ni, Co, Fe, Mn, Cr) and the formation of oxygen-containing solutions in the alloys based on them. Russian Metall. 2015, 11, 1005–1012. [Google Scholar] [CrossRef]
- Tang, R.Z.; Tian, R.Z. Binary Alloy Phase Diagrams and Crystal Structure of Intermediate Phase; Central South University Press: Changsha, China, 2009. [Google Scholar]
- Guo, R.Q.; Rohatgi, P.K.; Nath, D. Preparation of aluminium-fly ash particulate composite by powder metallurgy technique. J Mater. Sci. 1997, 32, 3971–3974. [Google Scholar] [CrossRef]
- Yu, P.; Qian, M.; Li, L.; Schaffer, G.B. On the infiltration mode during fabrication of aluminium composite. Acta Mater. 2010, 58, 3790–3797. [Google Scholar] [CrossRef]
- Homeny, J.; Buckley, M.M. Transmission electron microscopy study of an aluminum oxide fiber/aluminum-magnesium alloy metal matrix composite interface. Mater. Lett. 1991, 10, 421–424. [Google Scholar] [CrossRef]
- Lumley, R.N.; Sercombe, T.B.; Schaffer, G.B. Surface oxide and the role of magnesium during the sintering of aluminum. Metall. Mater. Trans. A. 1999, 30, 457–463. [Google Scholar] [CrossRef]
- Mcleod, A.D.; Gabryel, C.M. Kinetics of the growth of spinel, MgAl2O4, on alumina particulate in aluminum alloys containing magnesium. Met. Trans. A. 1992, 23, 1279–1283. [Google Scholar] [CrossRef]
- Mahmoud, M.M.; Link, G.; Thumm, M. The role of the native oxide shell on the microwave sintering of copper metal powder compacts. J. Alloy Compd. 2015, 627, 231–237. [Google Scholar] [CrossRef]
- Ramakrishnan, P.; Tendolkar, G.S. Influence of thin oxide films on the mechanical properties of sintered metal-powder compacts. Powder Metall. 1964, 7, 34–49. [Google Scholar] [CrossRef]
- Li, W.J.; Shao, W.Z.; Xie, N.; Zhang, L.; Li, Y.R.; Yang, M.S.; Chen, B.A.; Zhang, Q.; Wang, Q.; Zhen, L. Air arc erosion behavior of CuZr/Zn2SnO4 electrical contact materials. J. Alloy Compd. 2018, 743, 697–706. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, X.H.; Deng, T.Q.; Jiang, J. Develop an effective oxygen removal method for copper powder. Adv. Powder Technol. 2018, 29, 1904–1912. [Google Scholar] [CrossRef]
- Hao, H.L.; Mo, W.; Lv, Y.H.; Ye, S.L.; Gu, R.N.; Yu, P. The effect of trace amount of Ti and W on the powder metallurgy process of Cu. J. Alloy Compd. 2016, 660, 204–207. [Google Scholar] [CrossRef]
- Hao, H.L.; Ye, S.L.; Yu, K.P.; Chen, P.; Gu, R.N.; Yu, P. The role of alloying elements on the sintering of Cu. J. Alloy Compd. 2016, 684, 91–97. [Google Scholar] [CrossRef]
- Wang, C.M.; Pan, L.; Zhang, Y.; Xiao, S.; Chen, Y. Deoxidization mechanism of hydrogen in TiH2 dehydrogenation process. Int. J. Hydrogen Energy. 2016, 41, 14836–14841. [Google Scholar] [CrossRef]
- Leipunsky, I.O.; Zhigach, A.N.; Kuskov, M.L.; Berezkina, N.G.; Afanasenkova, E.S.; Kudrov, B.V.; Lopez, G.W.; Vorobjeva, G.A.; Naumkin, A.V. Synthesis of TiH2 nano powder via the guen-miller flow-levitation method and characterization. J. Alloys Comp. 2019, 778, 271–279. [Google Scholar] [CrossRef]
- Lide, D.R.; Lide, K.S. The Handbook of Chemistry and Physics; CRC press: Boca Raton, FL, USA, 2010. [Google Scholar]
- Rodriguez, J.A.; Kim, J.Y.; Hanson, J.C.; Pérez, M.; Frenkel, A.I. Reduction of CuO in H2: In Situ Time-Resolved XRD Studies. Catal. Letters. 2003, 85, 247–254. [Google Scholar] [CrossRef]
- Bhosle, V.; Baburaj, E.G.; Miranova, M.; Salama, K. Dehydrogenation of TiH2. Mater. Sci. Eng. A. 2003, 356, 190–199. [Google Scholar] [CrossRef]
- German, R. Sintering: from Empirical Observations to Scientific Principles; Butterworth-Heinemann: Oxford, UK, 2014. [Google Scholar]
- Azevedo, C.R.F.; Rodrigues, D.; Neto, F.B. Ti–Al–V powder metallurgy (PM) via the hydrogenation–dehydrogenation (HDH) process. J. Alloys Comp. 2003, 353, 217–227. [Google Scholar] [CrossRef]
- Jiménez, C.; Garcia-Moreno, F.; Rack, A.; Tucoulou, R.; Klaus, M.; Pfretzschner, B.; Rack, T.; Cloetens, P.; Banhart, J. Partial decomposition of TiH2 studied in situ by energy-dispersive diffraction and ex situ by diffraction microtomography of hard X-ray synchrotron radiation. Scr. Mater. 2012, 66, 757–760. [Google Scholar] [CrossRef]
- Matijasevic-Lux, B.; Banhart, J.; Fiechter, S.; Görke, O.; Wanderka, N. Modification of titanium hydride for improved aluminium foam manufacture. Acta Mater. 2006, 54, 1887–1900. [Google Scholar] [CrossRef]
- Liu, H.; He, P.; Feng, J.C.; Cao, J. Kinetic study on nonisothermal dehydrogenation of TiH2 powders. Int. J. Hydrogen Energy. 2009, 34, 3018–3025. [Google Scholar] [CrossRef]
- Oanh, N.T.H.; Viet, N.H.; Kim, J.S.; Junior, A.M.J. Characterization of In-Situ Cu–TiH2–C and Cu–Ti–C Nanocomposites Produced by Mechanical Milling and Spark Plasma Sintering. Metals. 2017, 7, 117. [Google Scholar] [CrossRef]
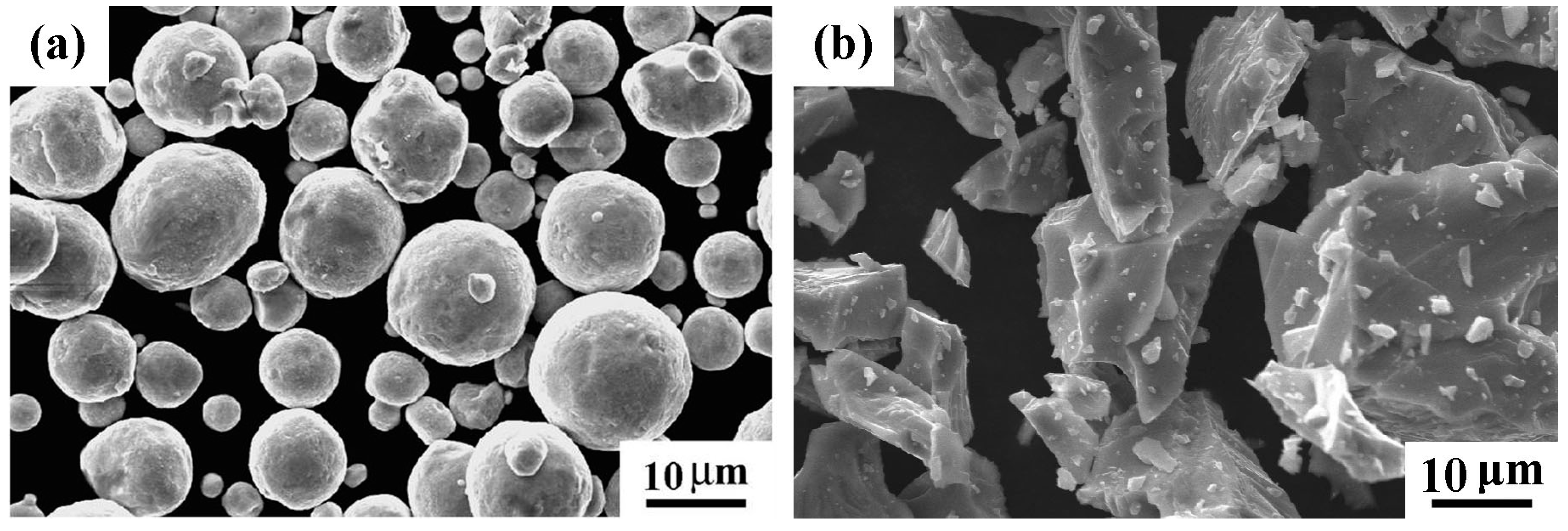
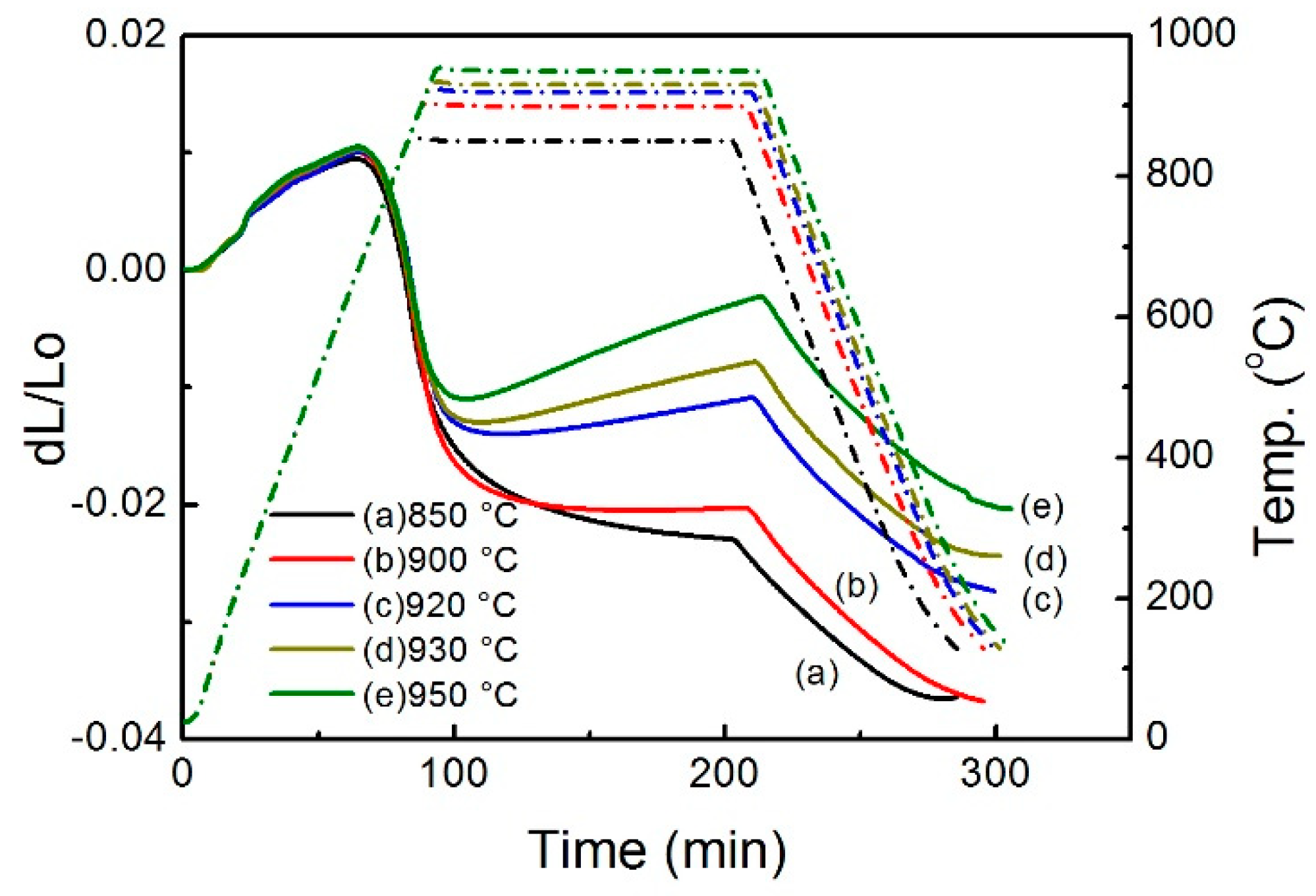
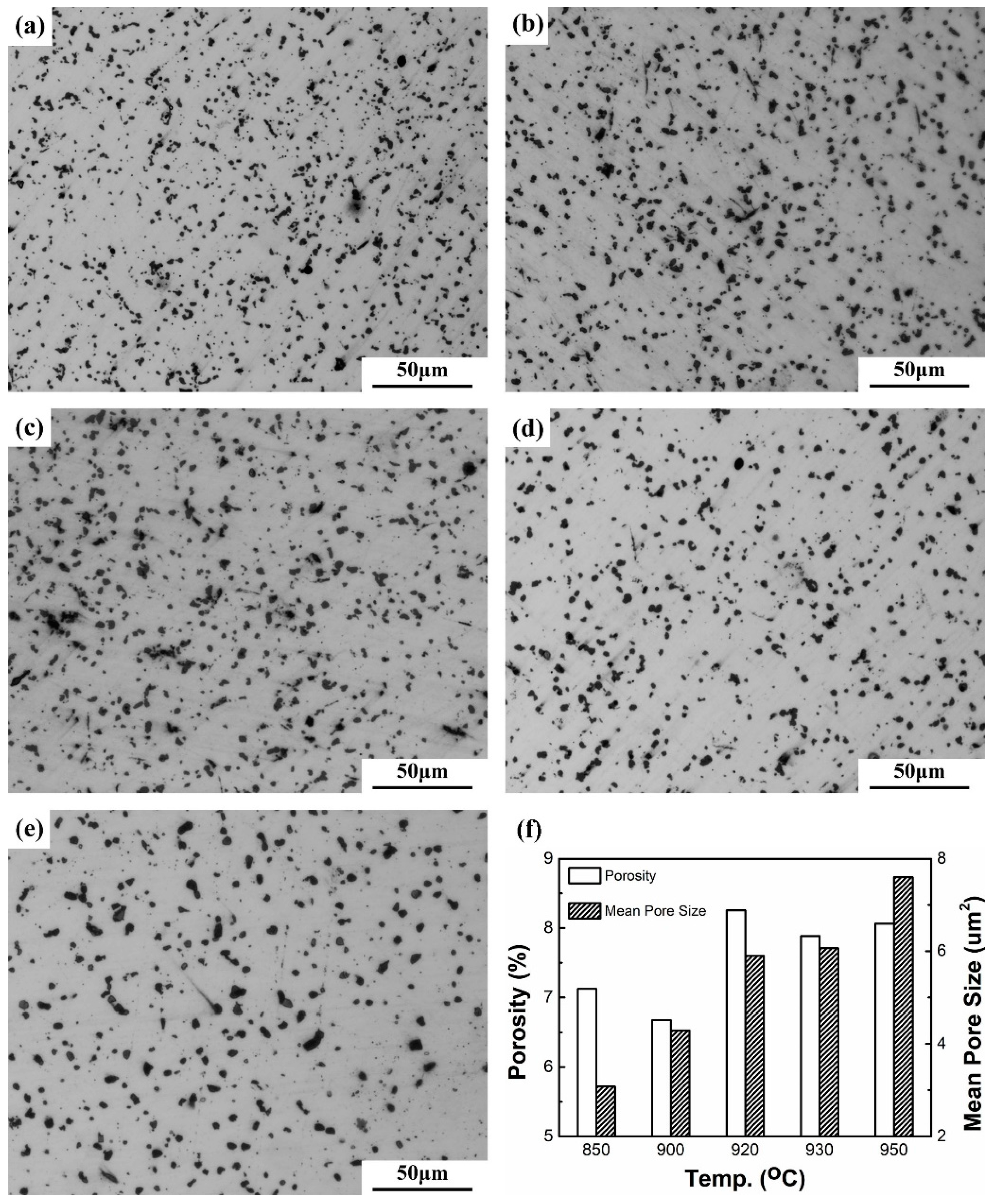
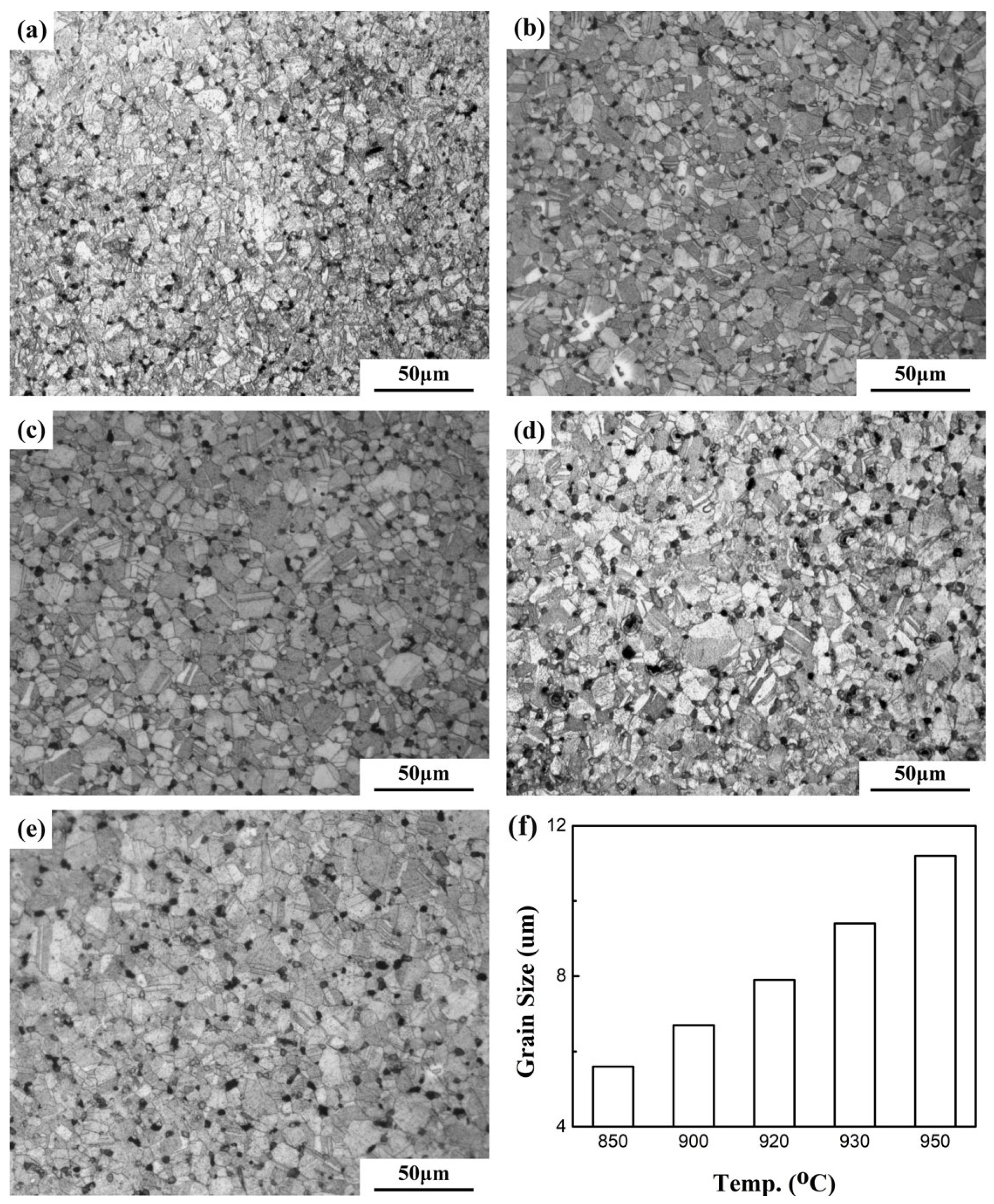

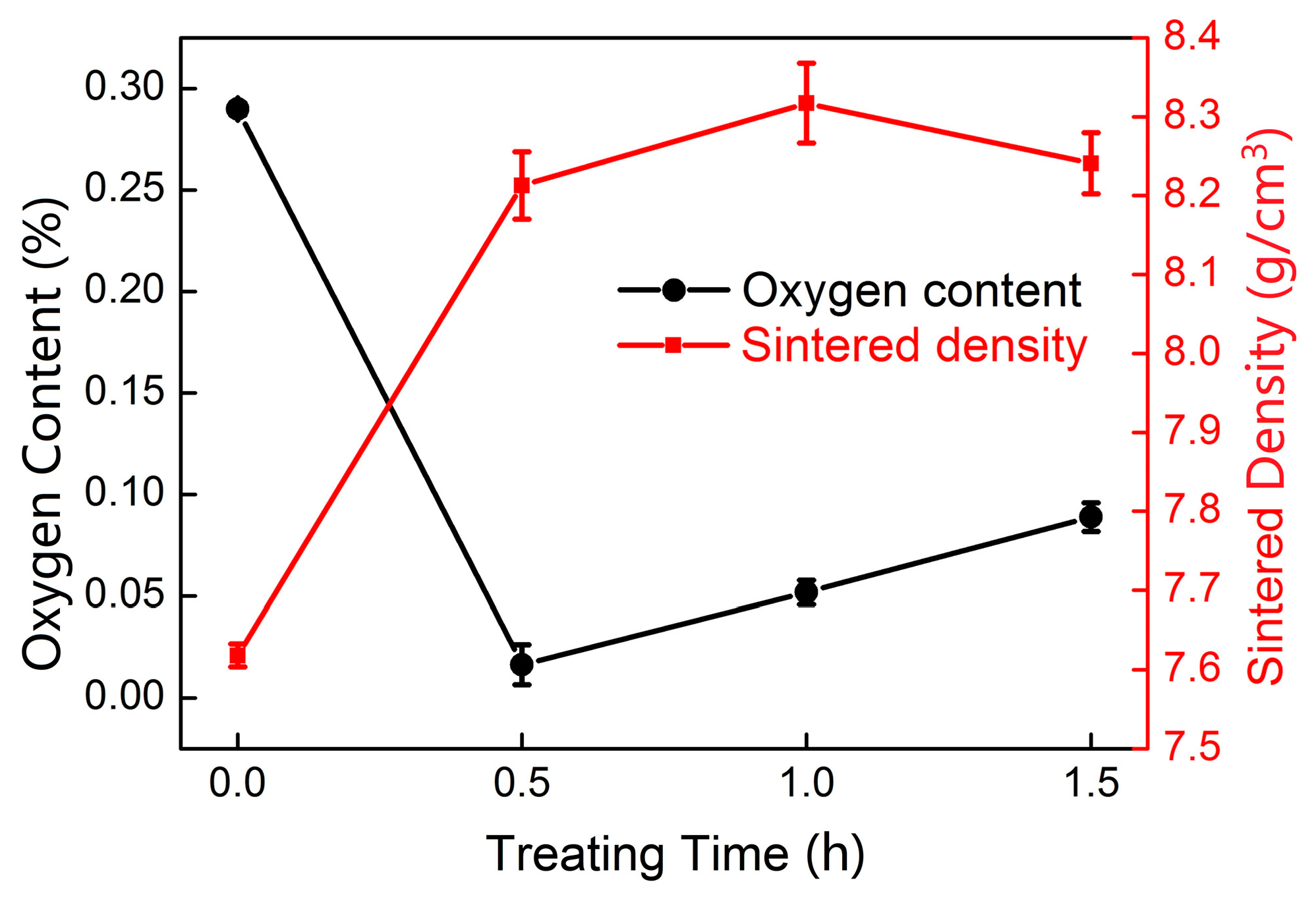
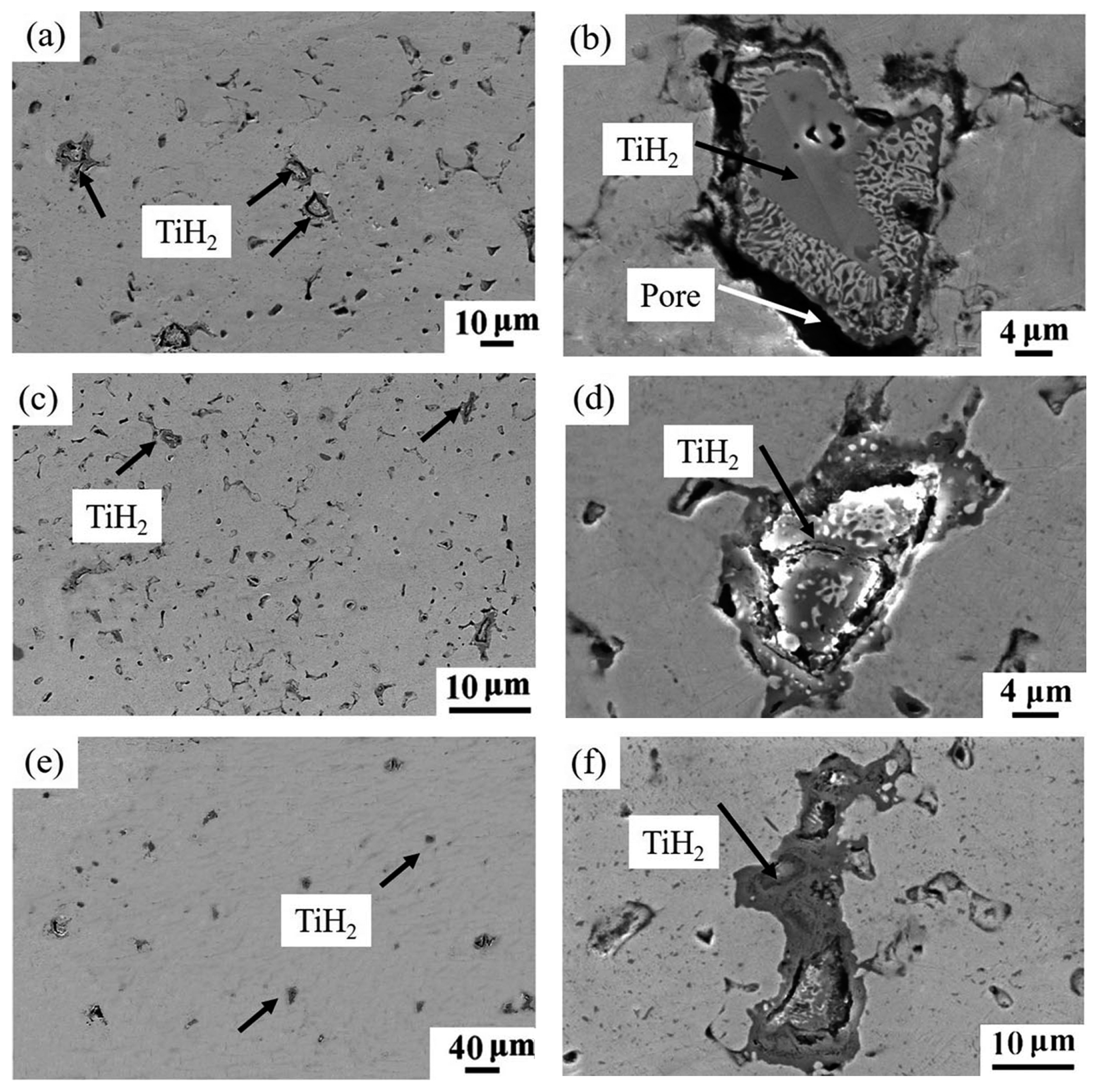
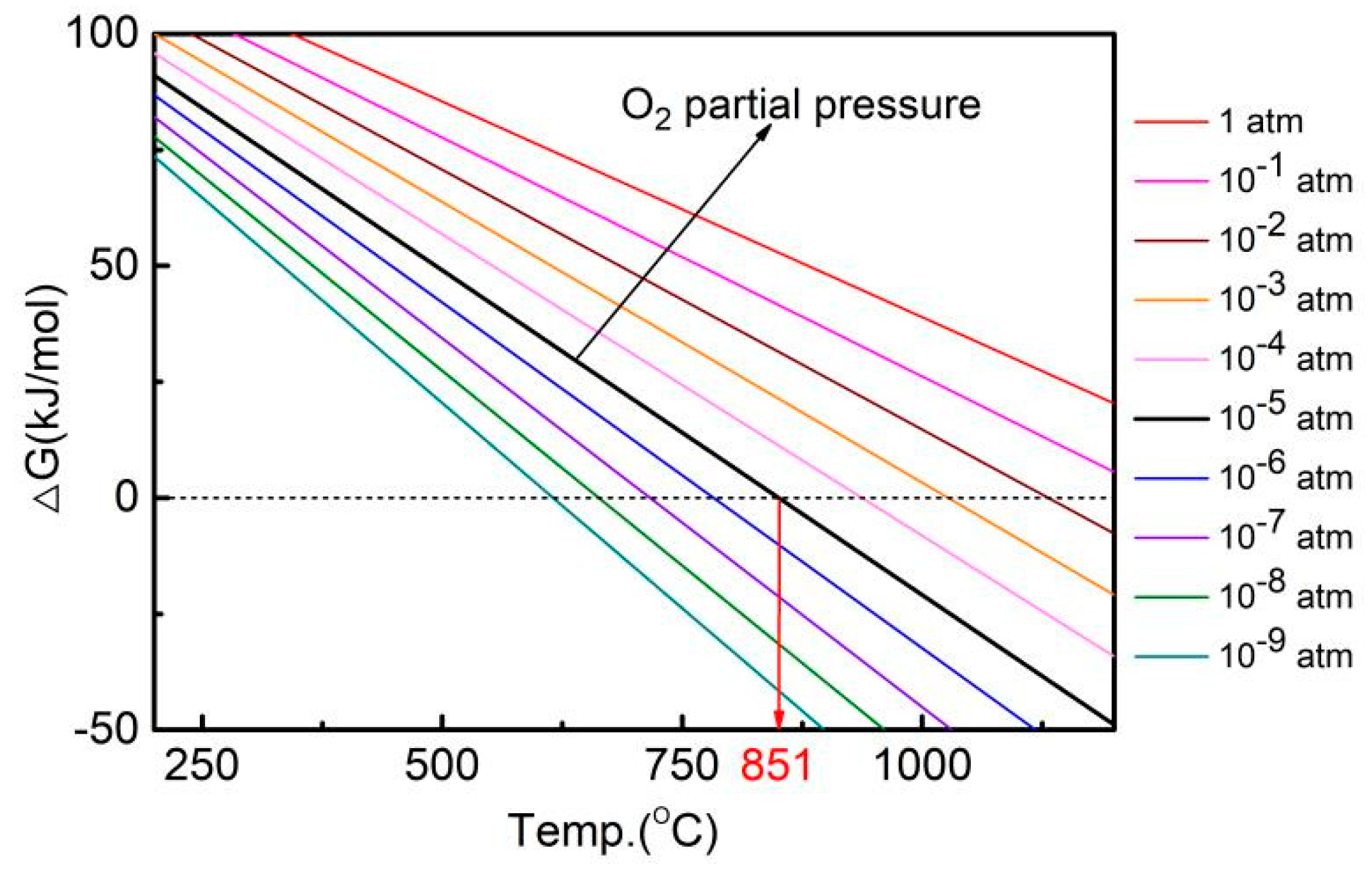
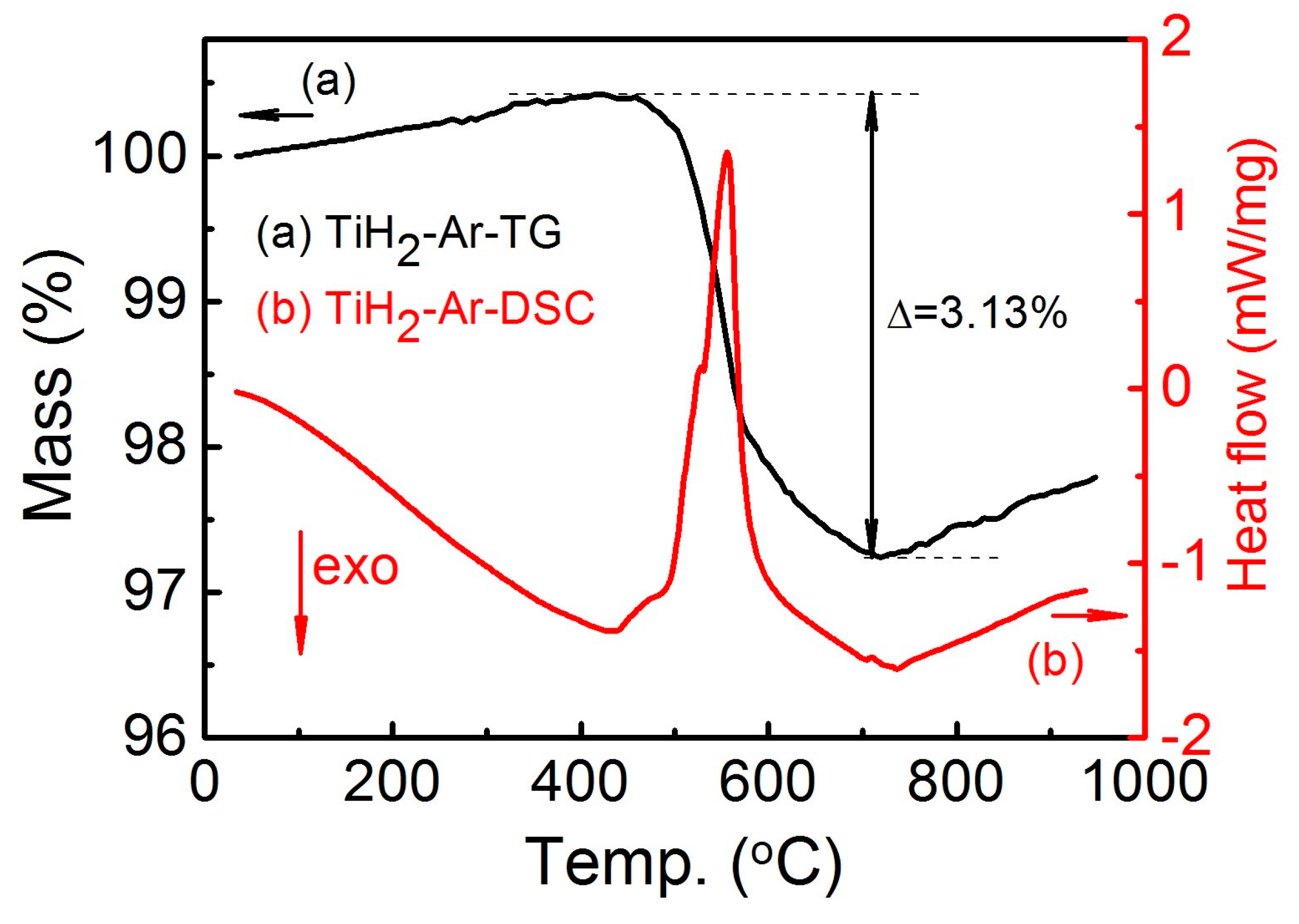
| Sintering Temperature (°C) | Sintered Density, g/cm3 | Relative Density, % | Porosity, % | Mean Pore Size, μm | Grain Size, μm | Oxygen Content, ppm |
|---|---|---|---|---|---|---|
| 850 | 8.33 | 92.9 | 7.12 | 3.1 | 5.6 | 1750 |
| 900 | 8.40 | 93.8 | 6.68 | 4.3 | 6.7 | 2020 |
| 920 | 8.01 | 89.7 | 8.25 | 5.9 | 7.9 | 2580 |
| 930 | 8.10 | 90.4 | 7.88 | 6.1 | 9.4 | 2240 |
| 950 | 8.00 | 89.3 | 8.06 | 7.6 | 11.2 | 2130 |
| Samples | Sintered Density, g/cm3 | Relative Density, % | Oxygen Content, ppm | Tp1, °C | Tp2, °C |
|---|---|---|---|---|---|
| Cu-TiH2-0 h | 7.62 | 85.0 | 2900 | 649 | 952 |
| Cu-TiH2-0.5 h | 8.21 | 91.8 | 160 | 650 | 762 |
| Cu-TiH2-1 h | 8.32 | 93.1 | 520 | 650 | 770 |
| Cu-TiH2-1.5 h | 8.24 | 90.9 | 890 | 650 | 775 |
| Pressure/atm | ∆H (kJ/mol) | ∆S (kJ/mol K) | ∆G = ∆H−T∆S (kJ/mol) |
|---|---|---|---|
| 1 | 157.3 | 0.093 | ∆G = 157.3 − 0.093 T |
| 10−1 | 0.103 | ∆G = 157.3 − 0. 103 T | |
| 10−2 | 0.112 | ∆G = 157.3 − 0.112 T | |
| 10−3 | 0.121 | ∆G = 157.3 − 0.121 T | |
| 10−4 | 0.130 | ∆G = 157.3 − 0.130 T | |
| 10−5 | 0.140 | ∆G = 157.3 − 0. 140 T | |
| 10−6 | 0.149 | ∆G = 157.3 − 0.149 T | |
| 10−7 | 0.159 | ∆G = 157.3 − 0.159 T | |
| 10−8 | 0.168 | ∆G = 157.3 − 0.168 T | |
| 10−9 | 0.177 | ∆G = 157.3 − 0.177 T |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hao, H.; Wang, Y.; Jafari Nodooshan, H.R.; Zhang, Y.; Ye, S.; Lv, Y.; Yu, P. The Effects of Sintering Temperature and Addition of TiH2 on the Sintering Process of Cu. Materials 2019, 12, 2594. https://doi.org/10.3390/ma12162594
Hao H, Wang Y, Jafari Nodooshan HR, Zhang Y, Ye S, Lv Y, Yu P. The Effects of Sintering Temperature and Addition of TiH2 on the Sintering Process of Cu. Materials. 2019; 12(16):2594. https://doi.org/10.3390/ma12162594
Chicago/Turabian StyleHao, Huali, Yanjing Wang, Hamid Reza Jafari Nodooshan, Yongyun Zhang, Shulong Ye, Yonghu Lv, and Peng Yu. 2019. "The Effects of Sintering Temperature and Addition of TiH2 on the Sintering Process of Cu" Materials 12, no. 16: 2594. https://doi.org/10.3390/ma12162594
APA StyleHao, H., Wang, Y., Jafari Nodooshan, H. R., Zhang, Y., Ye, S., Lv, Y., & Yu, P. (2019). The Effects of Sintering Temperature and Addition of TiH2 on the Sintering Process of Cu. Materials, 12(16), 2594. https://doi.org/10.3390/ma12162594




