Viscosity and Structure of a CaO-SiO2-FeO-MgO System during a Modified Process from Nickel Slag by CaO
Abstract
:1. Introduction
2. Experimental Procedure
2.1. Experimental Materials
2.2. Experimental Method
2.2.1. Preparation of the Pre-Melted Slag Sample
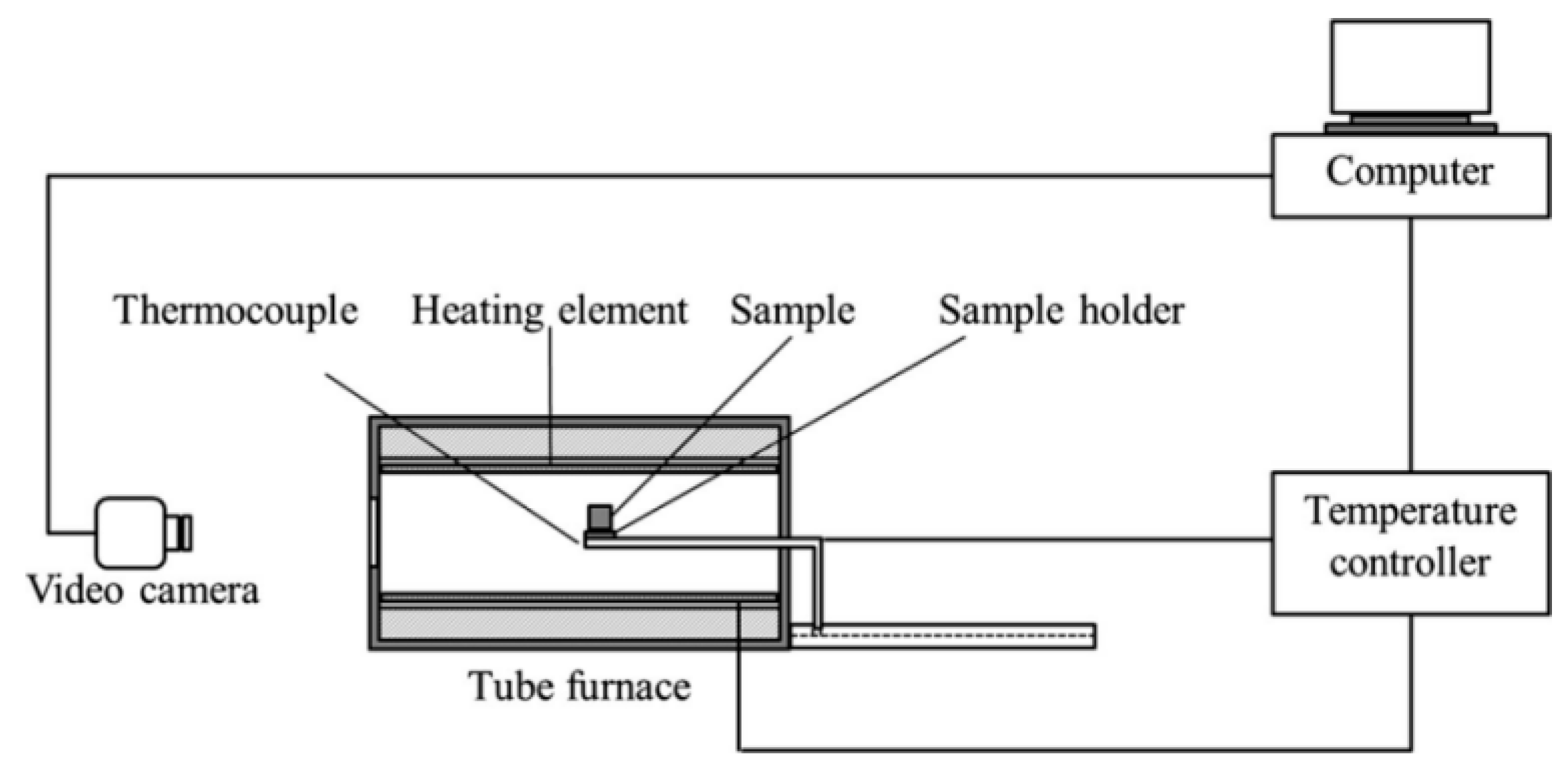
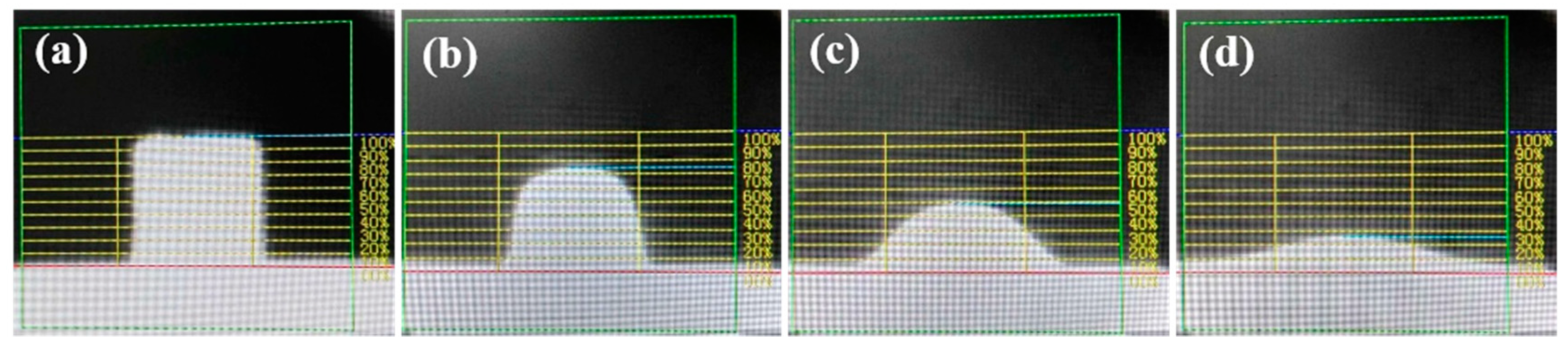
2.2.2. Determination of the Characteristic Temperature
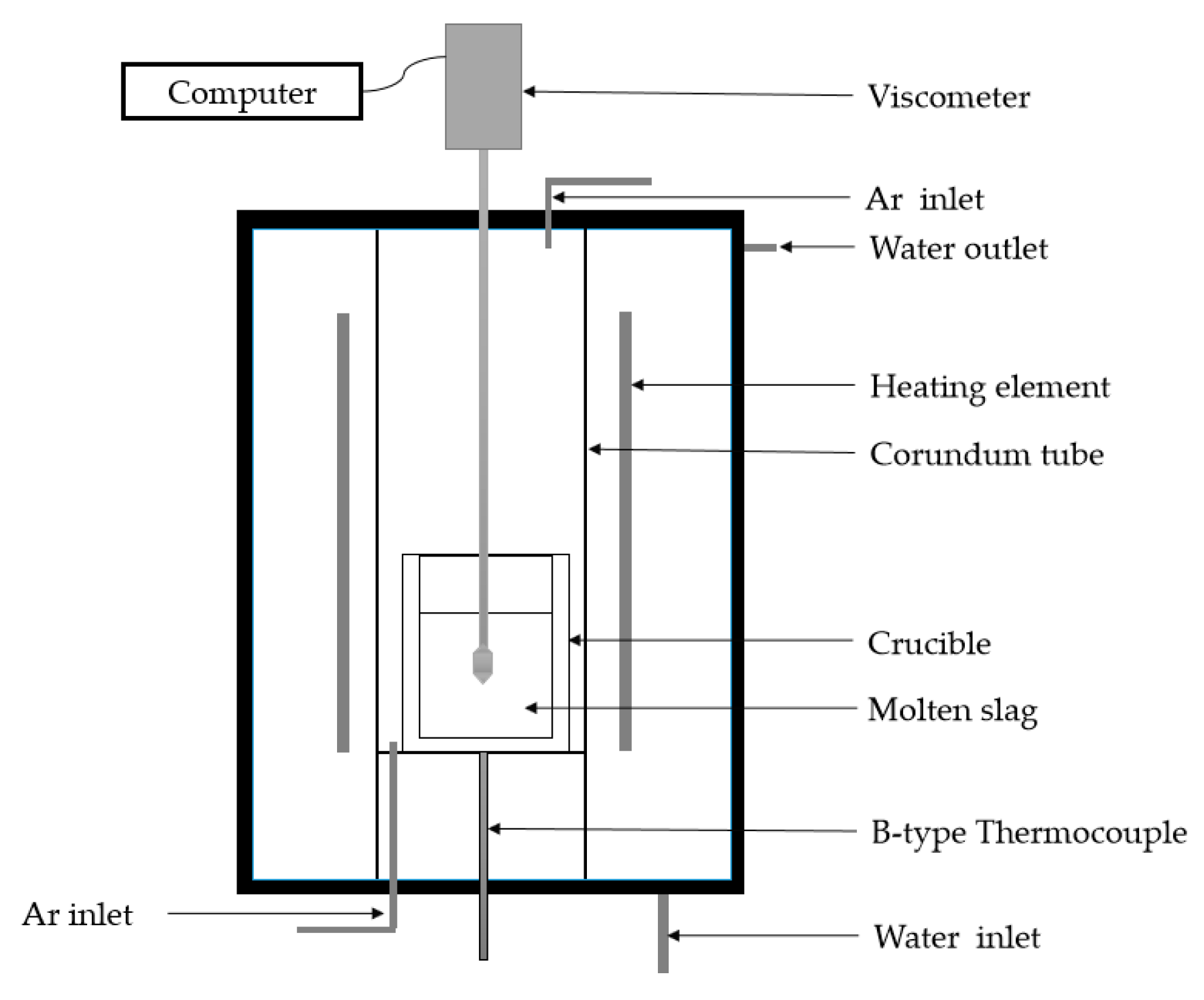
2.2.3. Measurement of the Viscosity
2.2.4. Preparation of the Water Quenching Slag Sample
2.3. Calculation Method by FactSage.
3. Results and Discussion
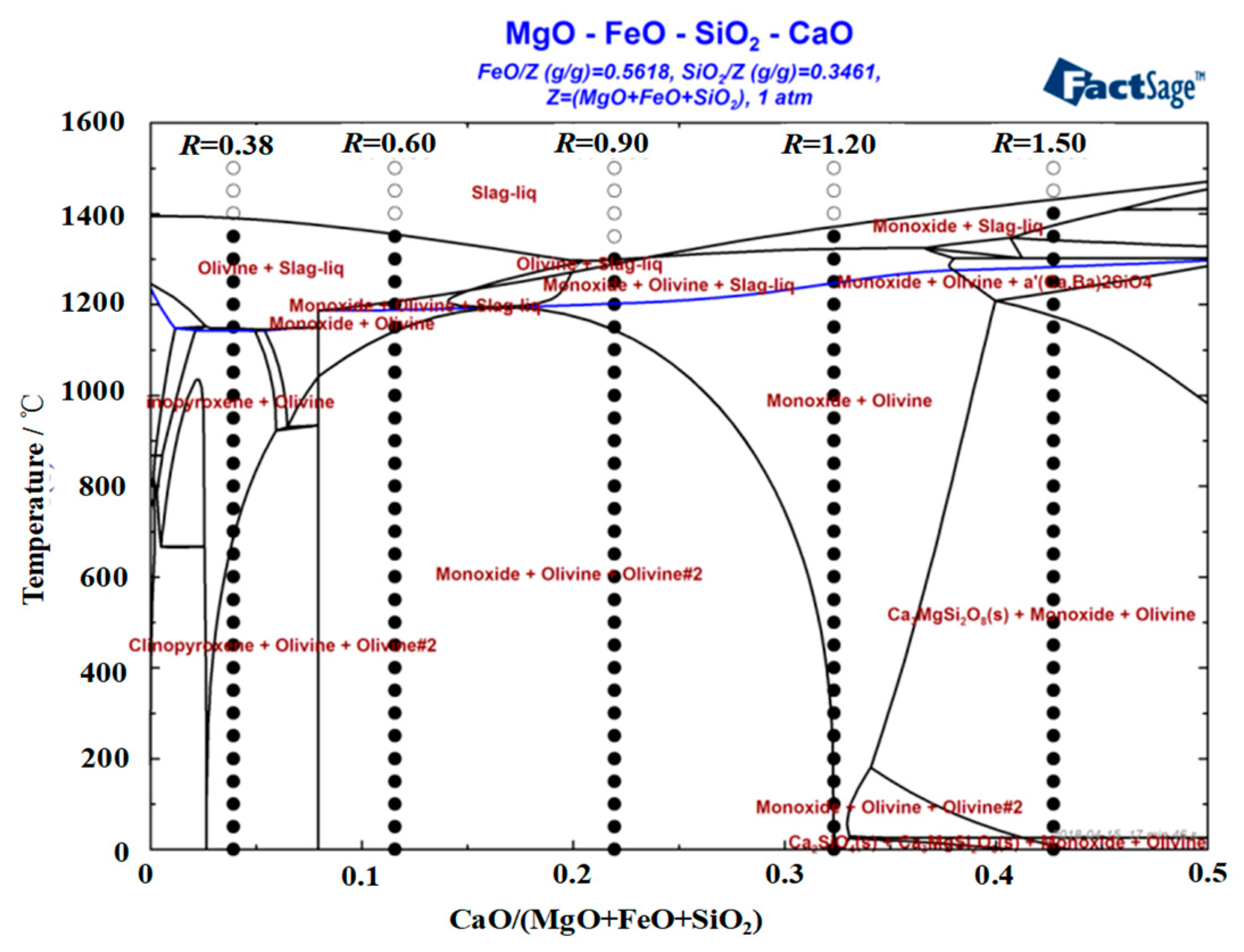
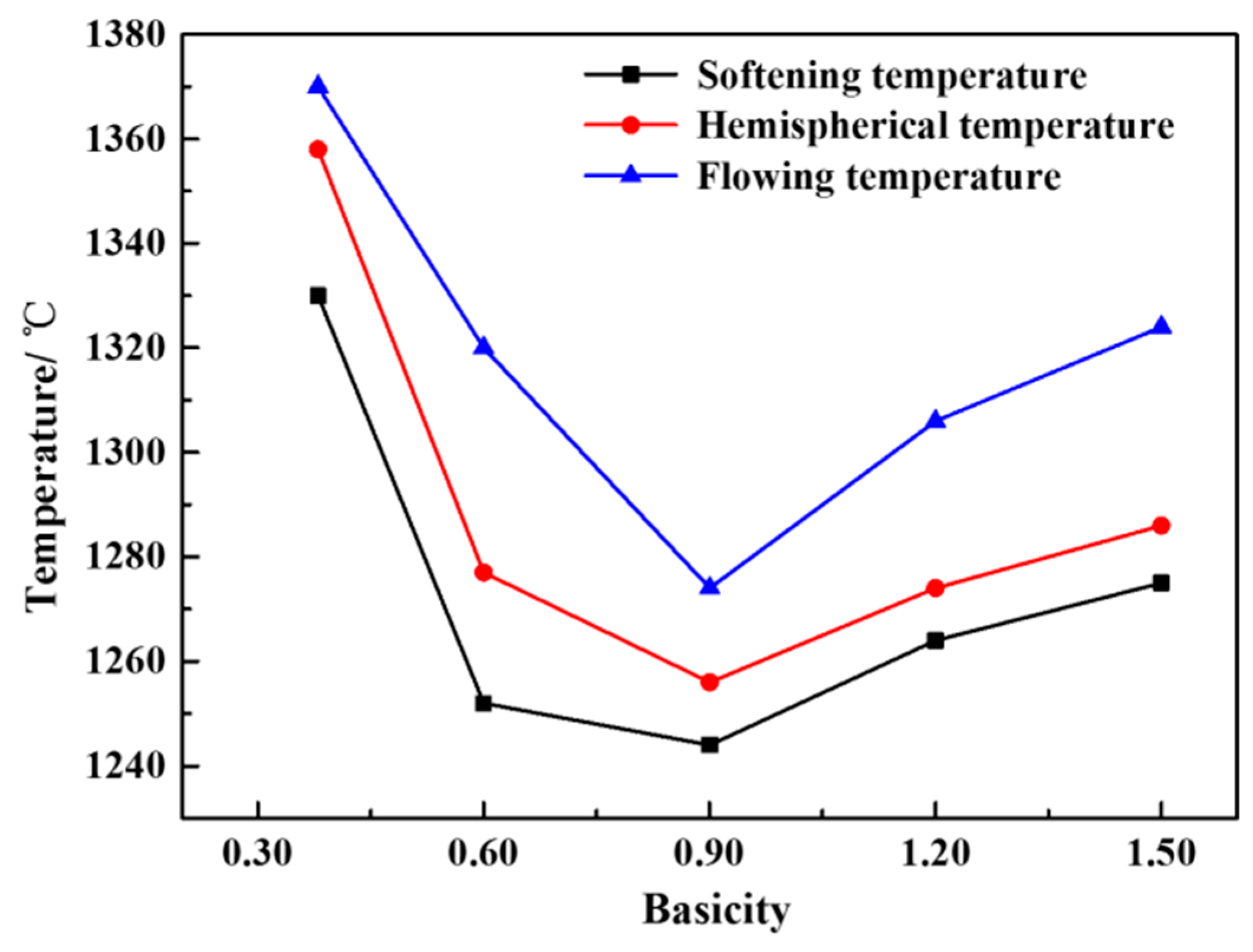
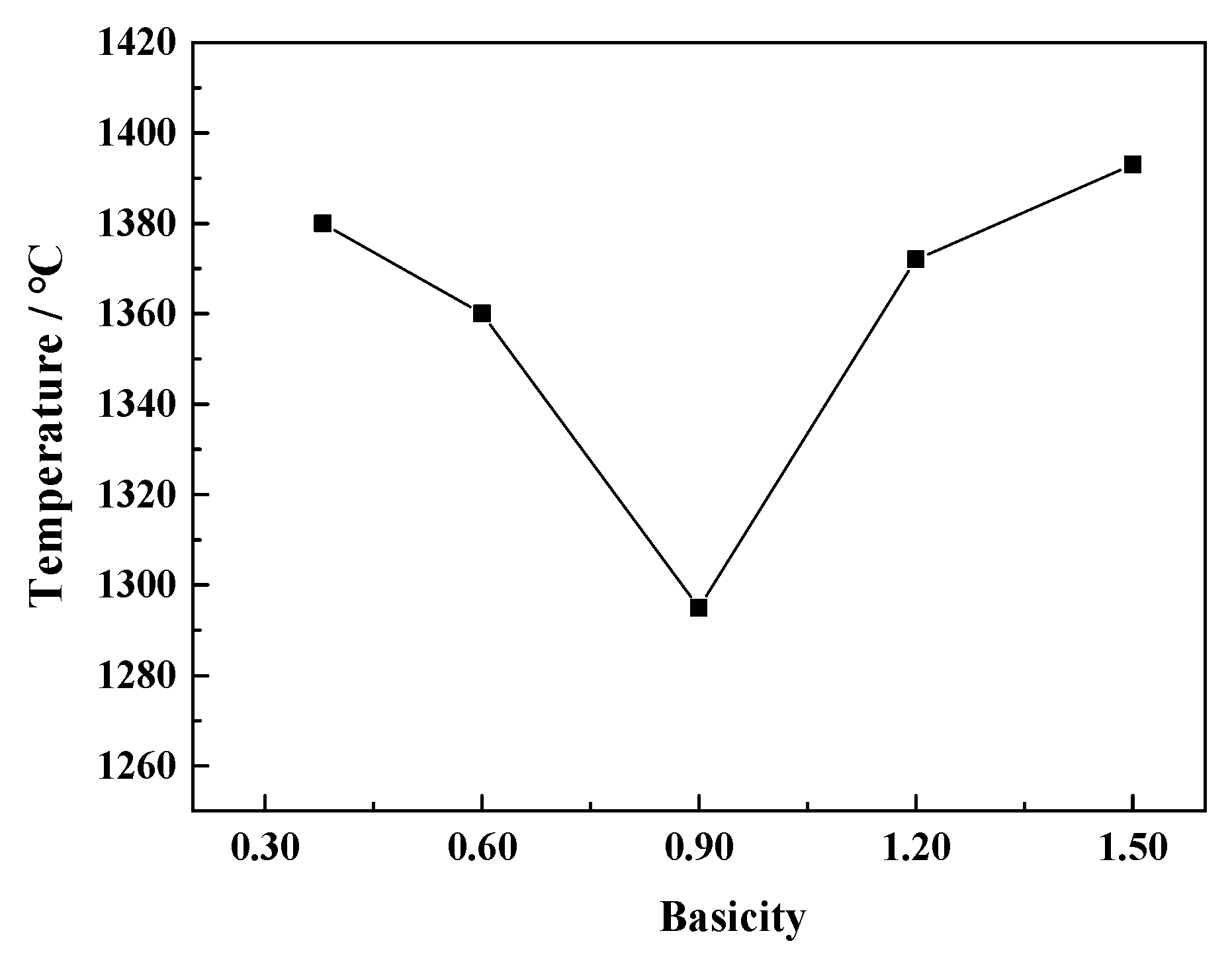
3.1. Effect of Basicity on the Melting Characteristics of the CaO-SiO2-FeO-MgO System
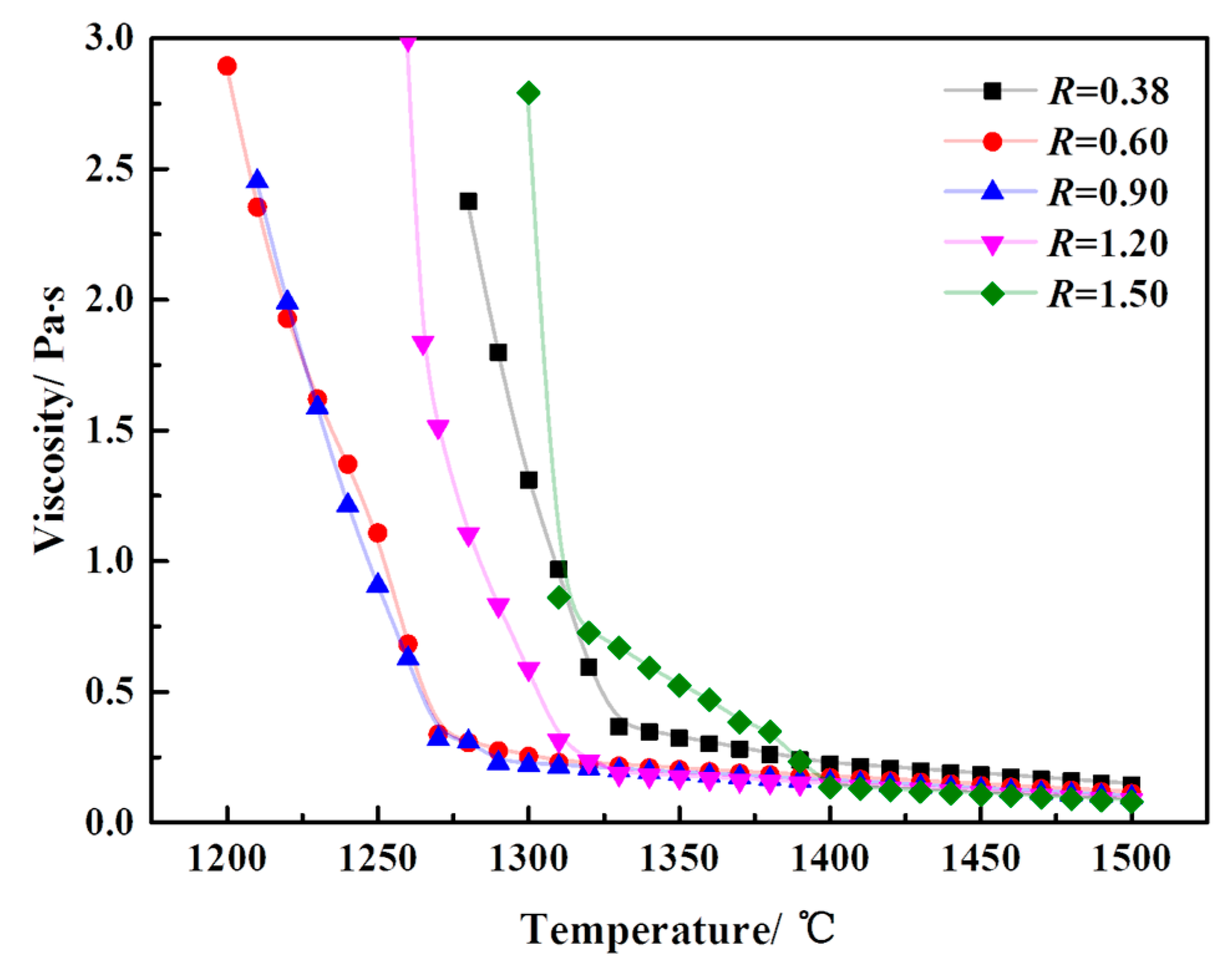
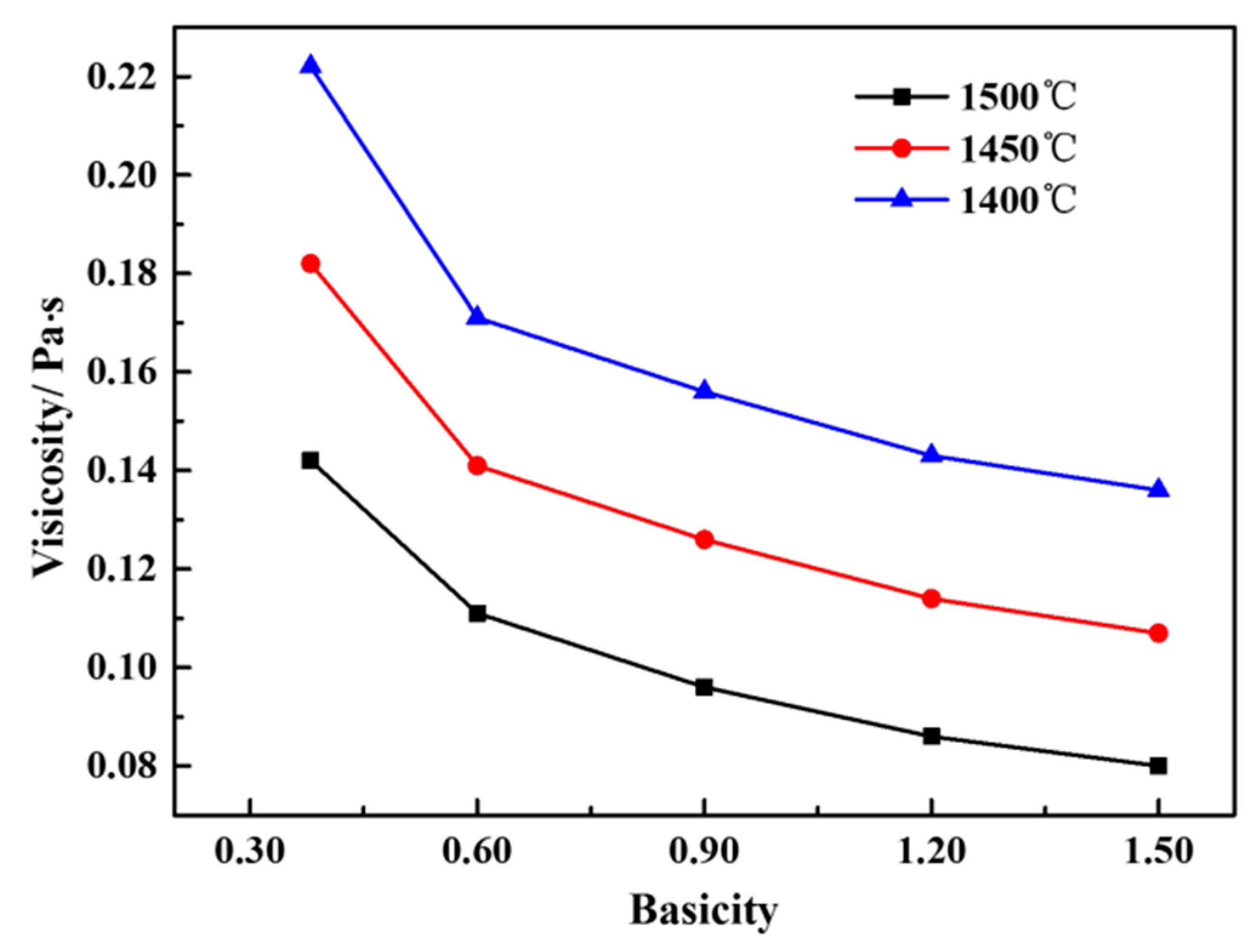
3.2. Effect of Basicity on the Viscosity of a CaO-SiO2-FeO-MgO System
3.2.1. Effect of Basicity on the Viscosity-Temperature Curve of a CaO-SiO2-FeO-MgO System
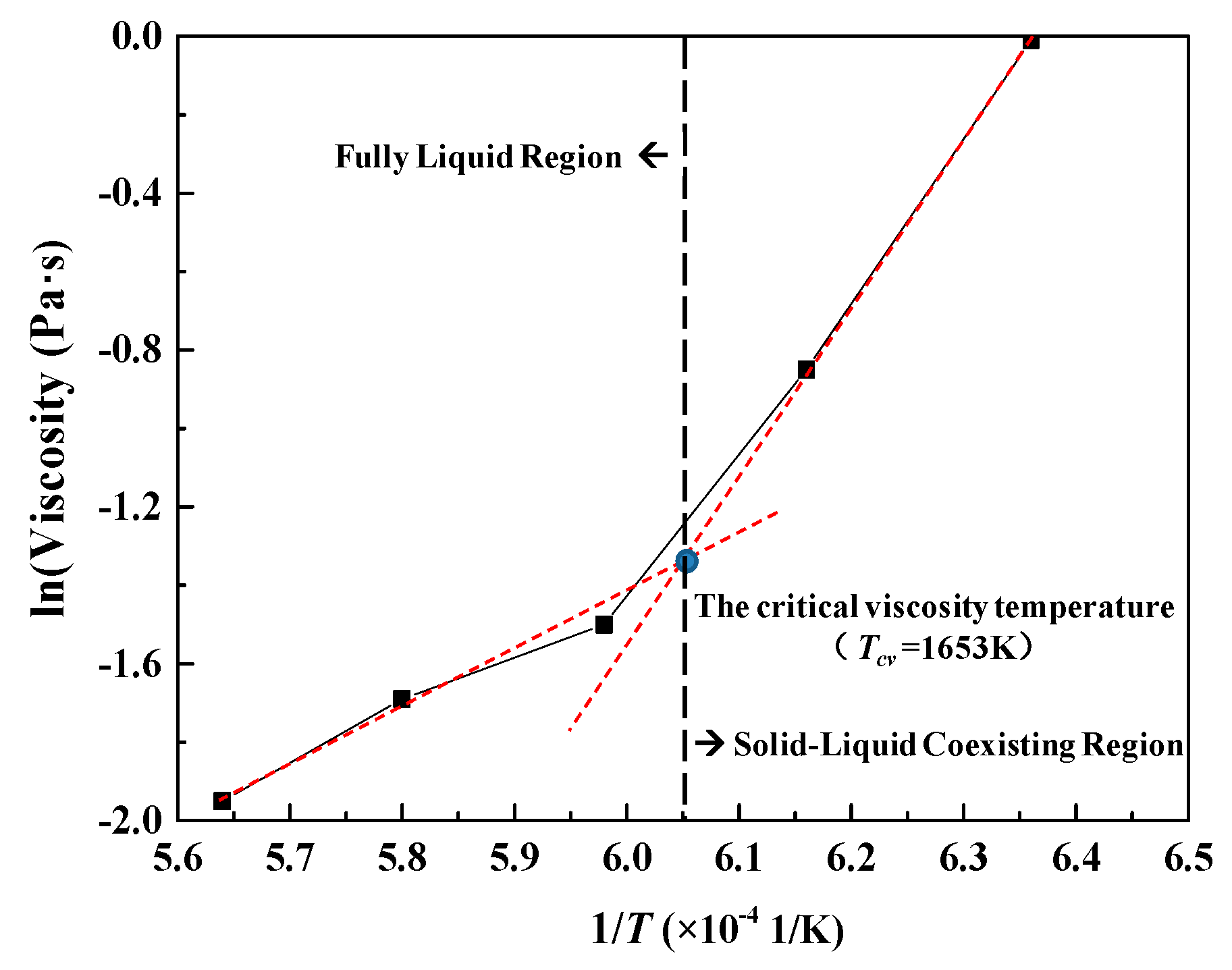
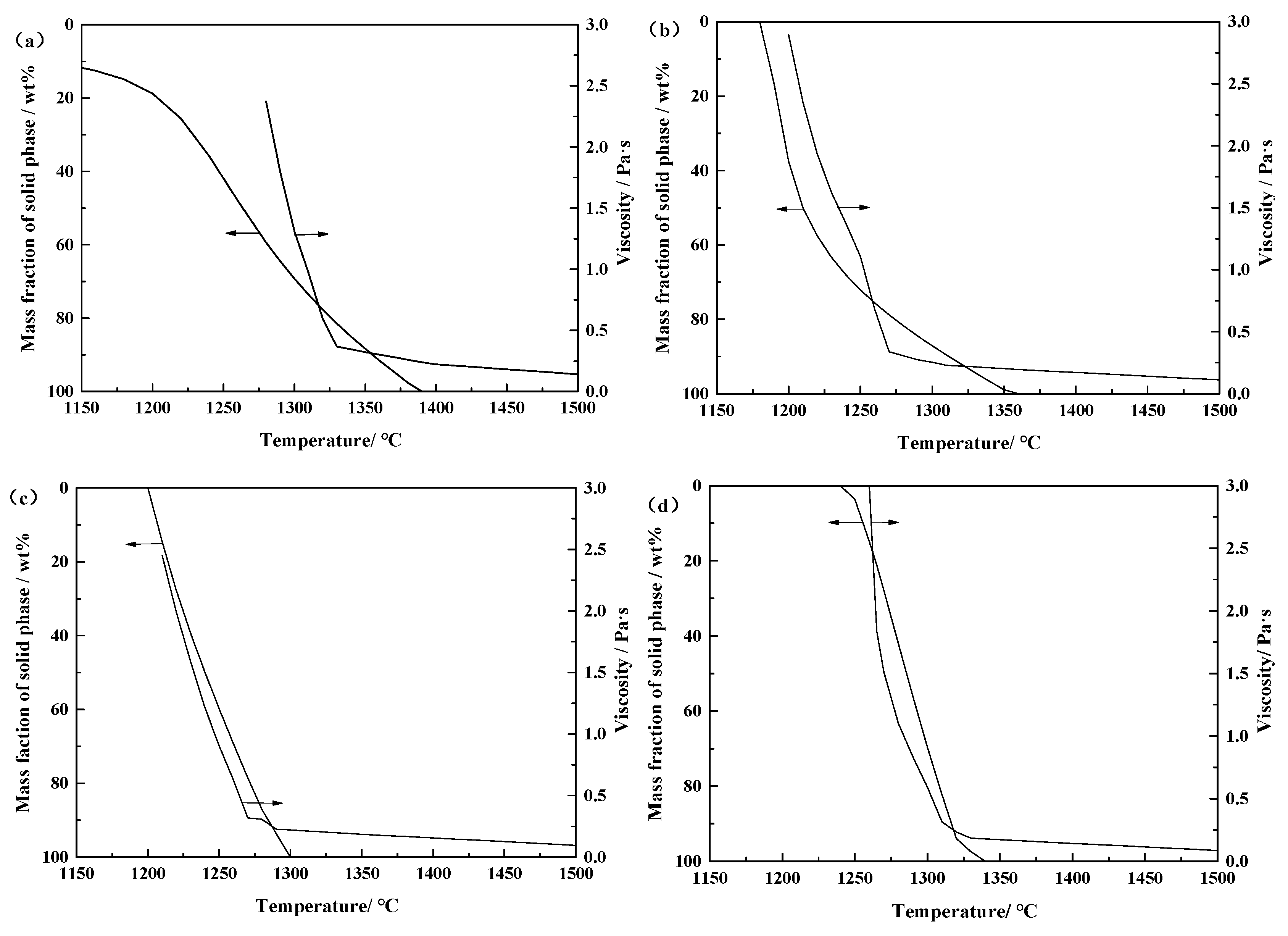
3.2.2. Effect of Basicity on Critical Viscosity Temperature of a CaO-SiO2-FeO-MgO System
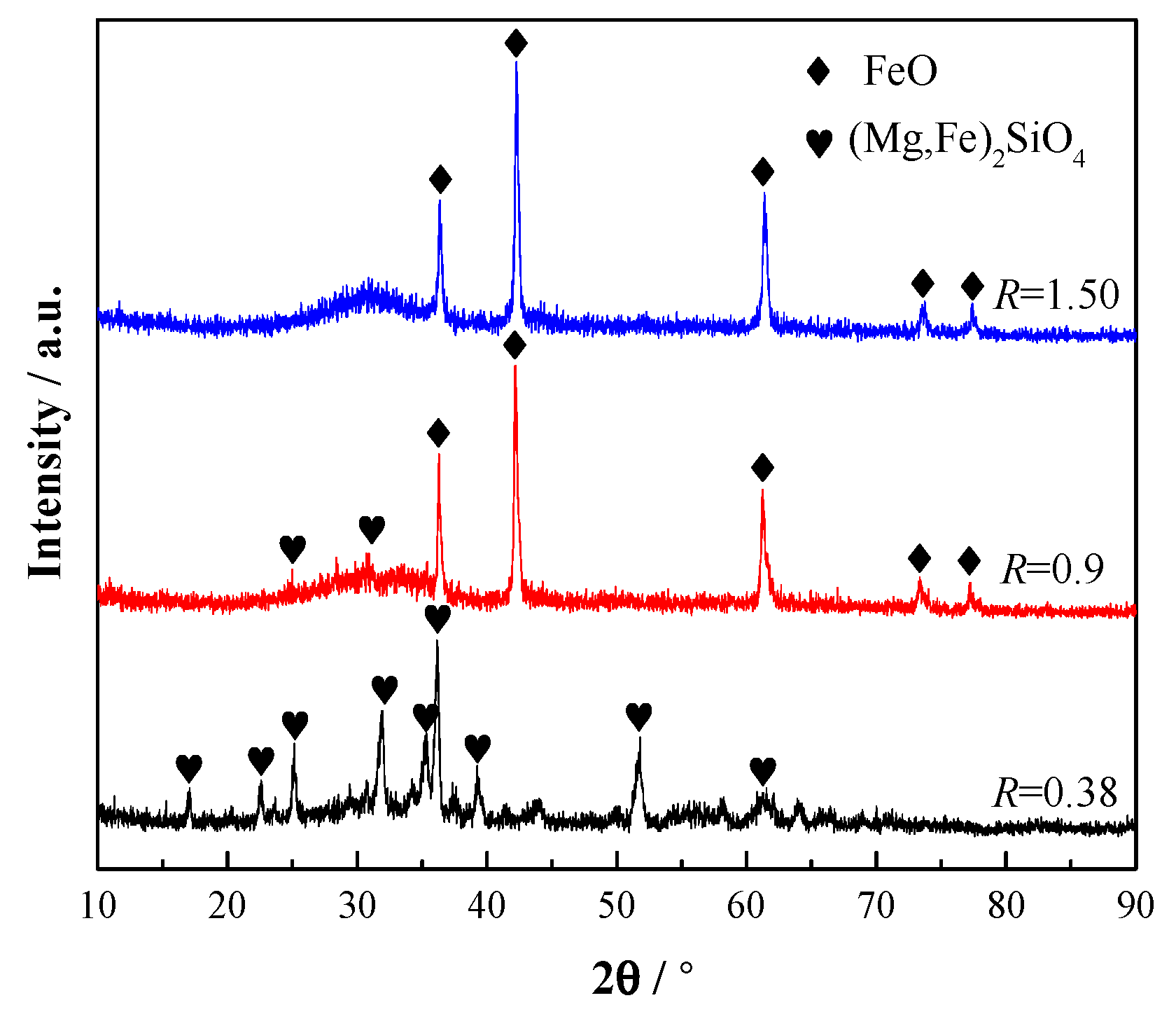
3.2.3. Effect of Phase Precipitation on the Viscosity of the CaO-SiO2-FeO-MgO System
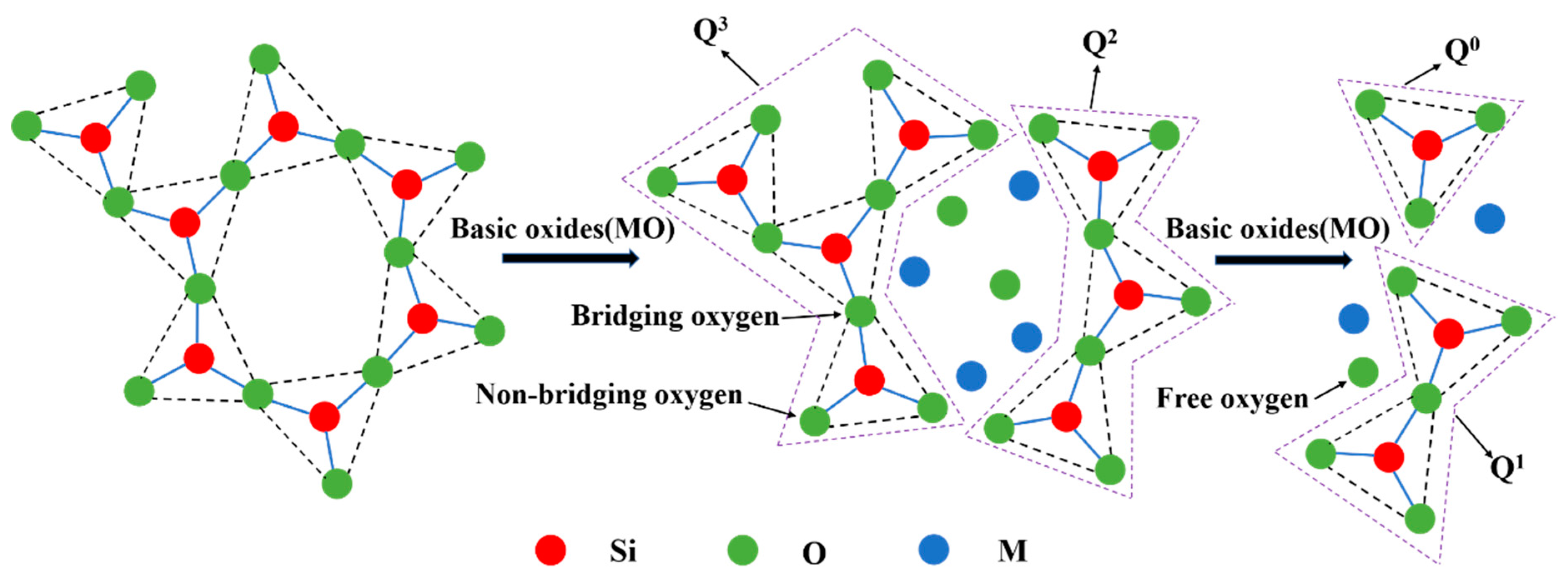
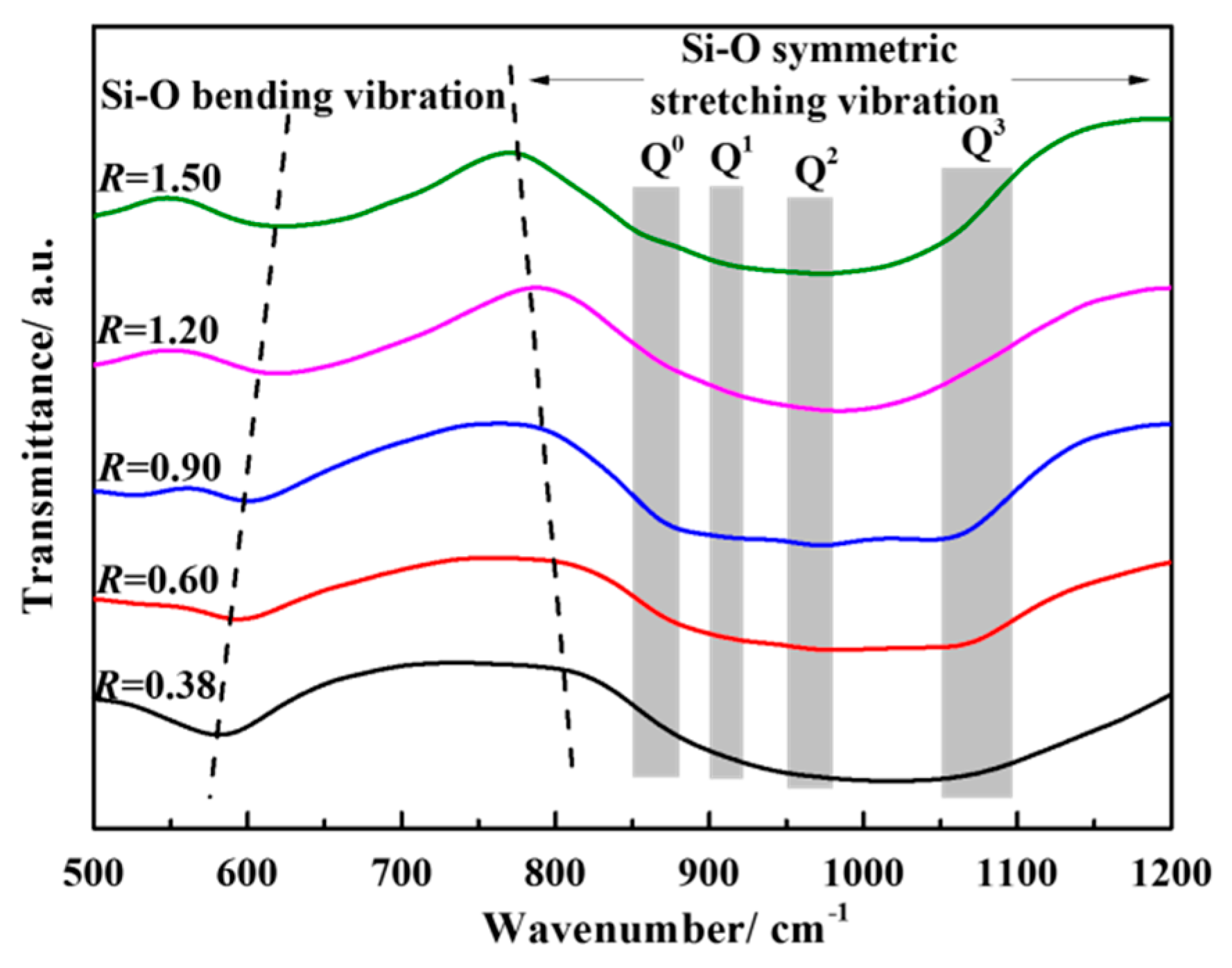
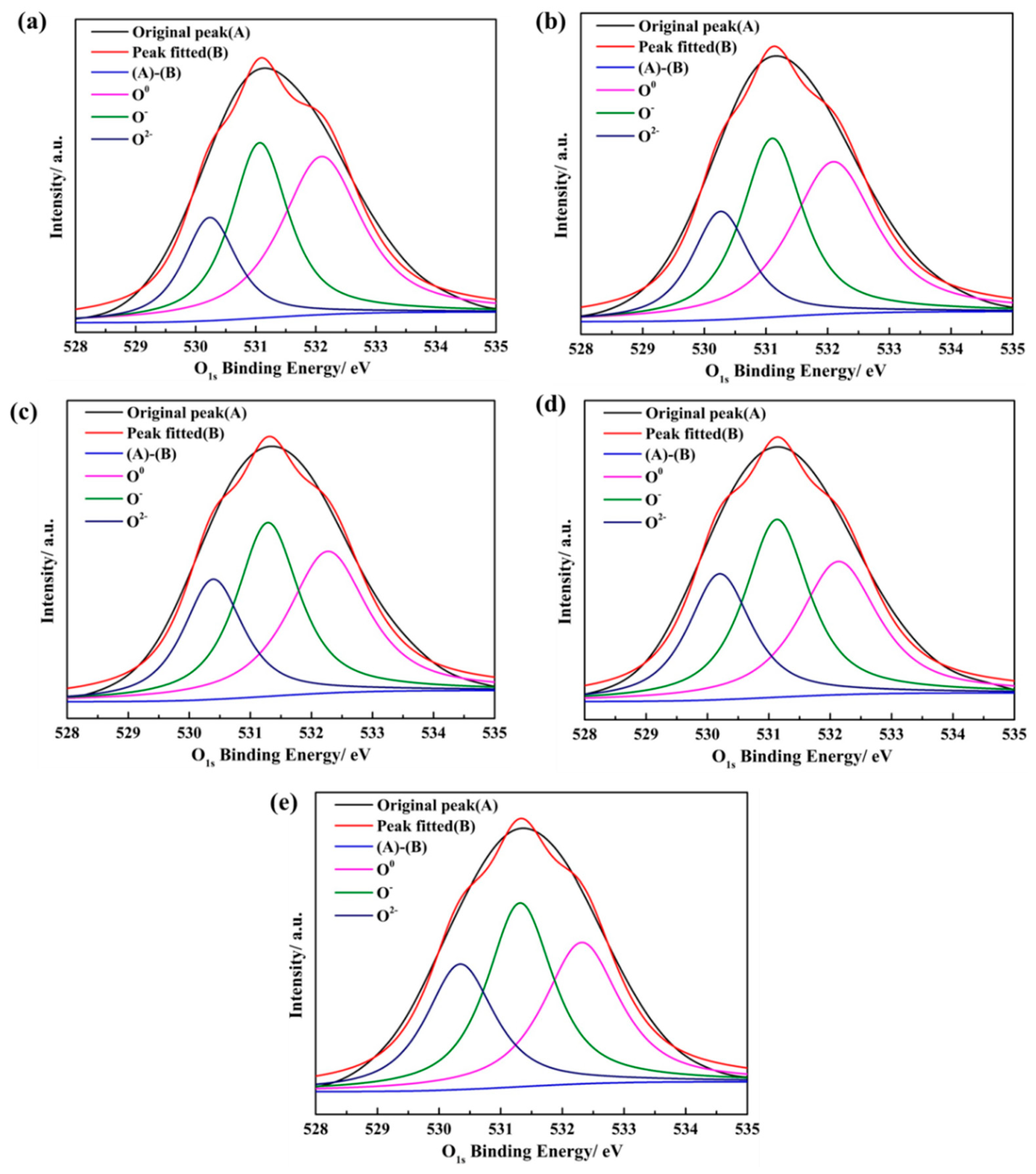
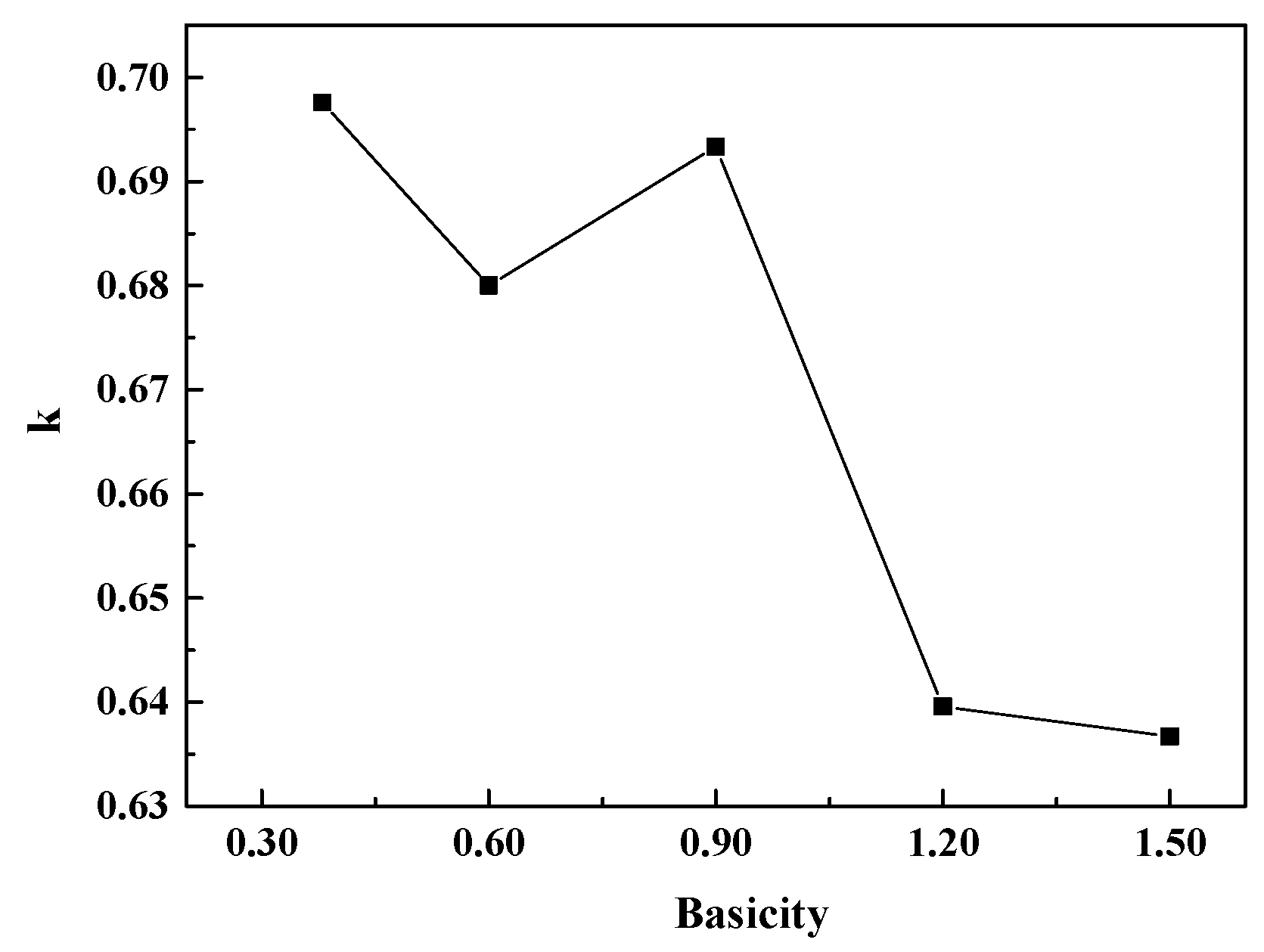
3.3. Effect of Basicity on the Structure of the CaO-SiO2-FeO-MgO System
4. Conclusions
- (1)
- When the basicity is lower than 0.90, the primary phase of the slag system is olivine phase. When the basicity is greater than 0.90, the primary phase of the slag system transforms into monoxide. When the basicity is 0.90, olivine and monoxide precipitate together as the temperature continues to decrease. At the same time, the liquidus temperature, softening temperature, hemispherical temperature, and flow temperature all reach the lowest value.
- (2)
- With the increase of basicity, critical viscosity temperature of the CaO-SiO2-FeO-MgO system decreases first and then increases. Critical viscosity temperature is the lowest at the basicity of 0.90, which is 1295 °C.
- (3)
- When the slag system is heterogeneous, the viscosity of the molten slag increases rapidity because of the quantity of solid phase precipitated from the CaO-SiO2-FeO-MgO system.
- (4)
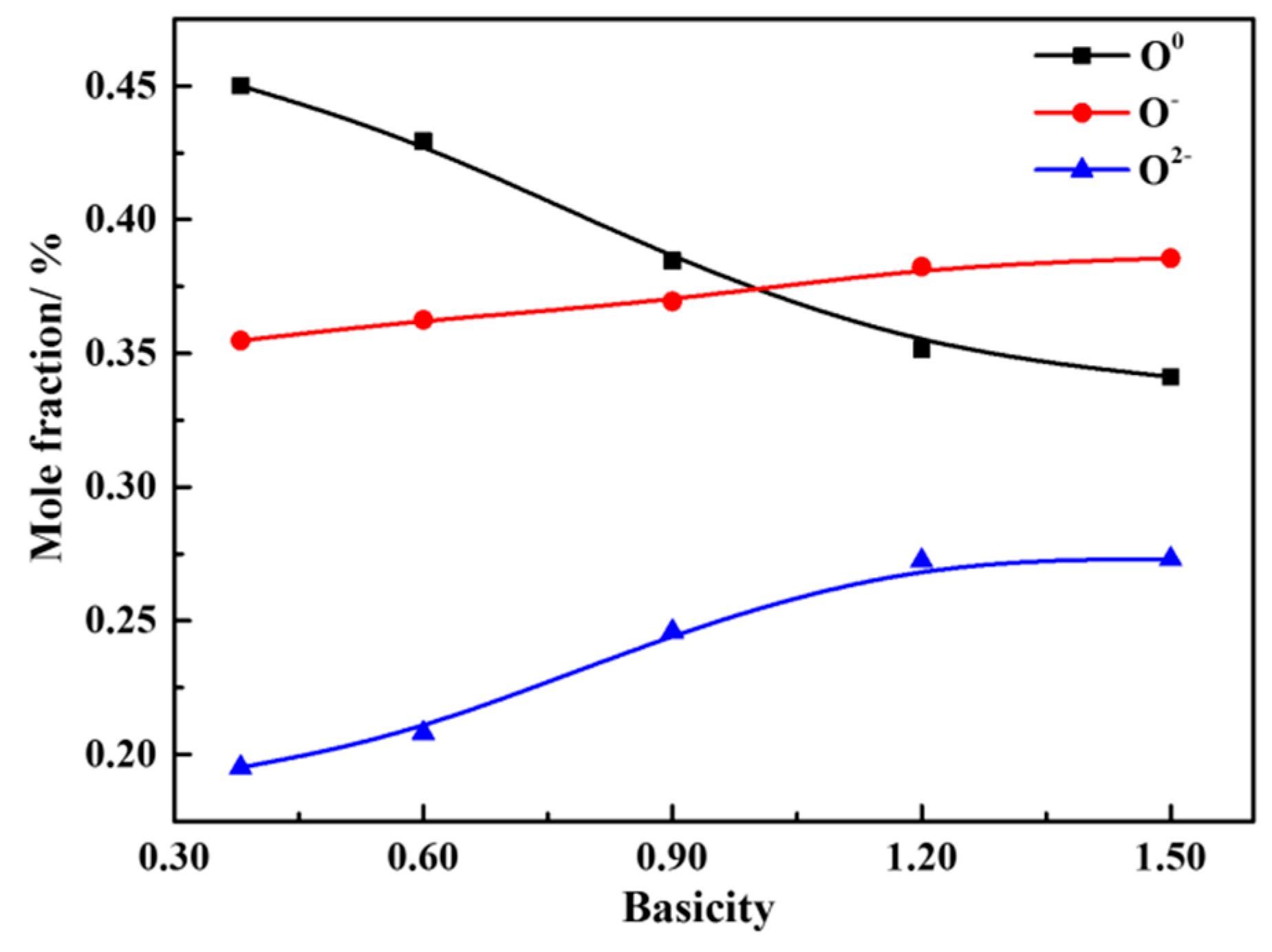
- When the slag system is in a homogeneous liquid phase, the molar fraction of O0 decreases with the increase of basicity and the mole fraction of O−and O2− increases continuously at the basicity of 0.38~1.50. The silicate network structure is gradually depolymerized into simple monomers, resulting in the degree of polymerization being reduced and the viscosity being reduced as well. The mole fraction of different kinds of oxygen atoms is converged to a constant value when the basicity is above 1.20.
Author Contributions
Funding
Conflicts of Interest
References
- Ni, W.; Jia, Y.; Zheng, F.; Wang, Z.J.; Zheng, M.J. Comprehensive utilization of iron recovery from Jinchuan nickel residue. Chin. J. Eng. 2010, 32, 975–980. [Google Scholar]
- Zhao, J.X.; Zhao, Z.Y.; Cui, Y.R.; Shi, R.M.; Tang, W.D.; Li, X.M.; Shang, N. New slag for nickel matte smelting process and subsequent Fe extraction. Metall. Mater. Trans. B 2018, 49, 304–310. [Google Scholar] [CrossRef]
- Ni, W.; Ma, M.S.; Wang, Y.L.; Wang, Z.J.; Liu, F.M. Thermodynamic and kinetic in recovery of iron from nickel residue. J. Univ. Sci. Technol. Beijing 2009, 31, 163–168. [Google Scholar]
- Wang, Z.J.; Ni, W.; Jia, Y.; Zhu, L.P.; Huang, X.Y. Crystallization behavior of glass ceramics prepared from the mixture of nickel slag, blast furnace slag and quartz sand. J. Non-Cryst. Solids 2010, 356, 1554–1558. [Google Scholar] [CrossRef]
- Lv, F.L. Direct Reduction and Magnetic Separating Experiment Study of Nickel Slag. Jiugang Technol. 2014, 3, 1–6. [Google Scholar]
- Shen, Y.Y.; Chen, M.; Zhang, Y.Y.; Xu, X.Q.; Li, G.Z.; Du, X.Y. Influence of temperature control system on the crystallisation behavior of magnetite phases in nickel Slags. Steel Res. Int. 2018, 89, 1700300. [Google Scholar] [CrossRef]
- Lv, X.M.; Lv, X.W.; Wang, L.W.; Qiu, J.; Liu, M. Viscosity and structure evolution of the SiO2-MgO-FeO-CaO-Al2O3 slag in ferronickel smelting process from laterite. J. Min. Metall. 2017, 53, 147–154. [Google Scholar] [CrossRef]
- Talapaneni, T.; Yedla, N.; Pal, S.; Sarkar, S. Experimental and Theoretical Studies on the Viscosity-Structure Correlation for High Alumina-Silicate Melts. Metall. Mater. Trans. B 2017, 48, 1450–1462. [Google Scholar] [CrossRef]
- Shen, Y.Y.; Huang, Z.N.; Zhang, Y.Y.; Zhong, J.K.; Zhang, W.J.; Yang, Y.; Chen, M.; Du, X.Y. Transfer behavior of Fe element in nickel slag during molten oxidation and magnetic separation processes. Mater. Trans. 2018, 59, 1659–1664. [Google Scholar] [CrossRef]
- Gao, Y.; Duan, C.; Yang, Y. Effect of applied voltage on electroreduction with controlled oxygen flow of molten slag containing FeO at 1723 K. ISIJ International 2015, 55, 2273–2282. [Google Scholar] [CrossRef]
- Gao, Y.M.; Wang, B.; Wang, S.B. Study on electrolytic reduction with controlled oxygen flow for iron from molten oxide slag containing FeO. J. Min. Metall. B: Metall. 2013, 49, 49–55. [Google Scholar] [CrossRef]
- Sun, C.Y.; Guo, X.M. Electrical conductivity of MO (MO = FeO, NiO)-containing CaO-MgO-SiO2-Al2O3 slag with low basicity. Trans. Nonferrous Met. Soc. China 2011, 21, 1648–1654. [Google Scholar] [CrossRef]
- Zhang, G.H.; Chou, K.C. Influence of Al2O3/SiO2 ratio on viscosities of CaO-Al2O3-SiO2 melt. Isij Int. 2013, 53, 177–180. [Google Scholar] [CrossRef]
- Park, J.H.; Kim, H.; Min, D.J. Novel approach to link between viscosity and structure of silicate melts via Darken’s excess stability function: Focus on the amphoteric behavior of alumina. Metall. Mater. Trans. B 2008, 39B, 150–153. [Google Scholar] [CrossRef]
- Li, J.L.; Shu, Q.F.; Chou, K. Effect of Al2O3/SiO2 mass ratio on viscosity of CaO-Al2O3-SiO2-CaF2 slag. Ironmak. Steelmak. 2015, 42, 154–160. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, C.; Cai, D.; Zhang, J.; Sasaki, Y.; Ostovski, O. Effects of CaO/SiO2 ratio and Na2O content on melting properties and viscosity of SiO2-CaO-Al2O3-B2O3-Na2O mold fluxes. Metall. Mater. Trans. B 2017, 48, 516–526. [Google Scholar] [CrossRef]
- Xu, J.F.; Zhang, J.Y.; Jie, C.; Tang, L.; Chou, K.C. Melting temperature of a selected area in CaO-MgO-Al2O3-SiO2 slag system. Adv. Mater. Res. 2011, 194–196, 169–174. [Google Scholar] [CrossRef]
- Wen, G.H.; Sridhar, S.; Tang, P.; Qi, X.; Liu, Y. Development of fluoride-free mold powders for peritectic steel slab casting. Isij Int. 2007, 47, 1117–1125. [Google Scholar] [CrossRef]
- Lee, S.; Min, D.J. Viscous behavior of FeO-Bearing slag melts considering structure of slag. Steel Res. Int. 2018, 89, 1800055. [Google Scholar] [CrossRef]
- Sohn, I.; Min, D.J. A review of the relationship between viscosity and the structure of calcium-silicate-based slags in ironmaking. Steel Res. Int. 2012, 83, 611–630. [Google Scholar] [CrossRef]
- Fu, G.Q.; Ju, H.X.; Xue, X.; Sun, J.; Wang, C.Y.; Zhu, M.Y. Study on Viscosity and Temperature of Acidic Vanadium-titanium-containing Slag. Chin. J. Process Eng. 2008, 8, 276–279. [Google Scholar]
- Wen, Y.C.; Zhou, J.Z.; Yang, S.B.; Zhang, Y.D.; Tang, T.Y.; Xu, L.Z.; Luan, Q.S.; Dong, L.R. Study of melting properties of converter slag containing vanadium and titanium oxides. Iron Steel Vanadium Titan. 2001, 22, 32–36. [Google Scholar]
- Kondretiev, A.; Jak, E. Predicting coal ash slag flow characteristics (viscosity model for the Al2O3-CaO-FeO-SiO2, system). Fuel 2001, 80, 1989–2000. [Google Scholar] [CrossRef]
- Nicholls, P.; Reid, W.T. Viscosity of coal-ash slag. Trans. Asme 1944, 66, 83–97. [Google Scholar]
- Park, H.S.; Park, S.S.; Sohn, I. The viscous behavior of FeOt-Al2O3-SiO2 copper smelting slags. Metall. Mater. Trans. B 2011, 42, 692–699. [Google Scholar] [CrossRef]
- Song, W.J.; Sun, Y.M.; Wu, Y.Q.; Zhu, Z.B.; Koyama, S. Measurement and simulation of flow properties of coal ash slag in coal gasification. Aiche Journel 2011, 57, 801–818. [Google Scholar] [CrossRef]
- Fincham, C.J.B.; Richardson, F.D. The Behaviour of Sulphur in Silicate and Aluminate Melts. Proc. R. Soc. A 1954, 223, 40–62. [Google Scholar]
- Park, H.; Park, J.Y.; Kim, G.H.; Sohn, I. Effect of TiO2 on the Viscosity and Slag Structure in Blast Furnace Type Slags. Steel Res. Int. 2012, 83, 150–156. [Google Scholar] [CrossRef]
- Björkman, B.O.; Eriksson, G.; Rosén, E. A generalized approach to the flood-knapp structure based model for binary liquid silicates: Application and update for the PbO-SiO2 System. Metall. Trans. B 1984, 15, 511–516. [Google Scholar] [CrossRef]
- Park, J.H.; Rhee, P.C.-H. Ionic properties of oxygen in slag. J. Noncryst. Solids 2001, 282, 7–14. [Google Scholar] [CrossRef]
- Yamashita, Y.; Hayes, P. Analysis of XPS spectra of Fe2+ and Fe3+ ions in oxide materials. Appl. Surf. Sci. 2008, 254, 2441–2449. [Google Scholar] [CrossRef]
- Toop, G.W.; Samis, C.S. Some new ionic concepts of silicate slags. Can. Metall. Q. 1962, 1, 129–152. [Google Scholar] [CrossRef]

| Slag System | CaO | MgO | SiO2 | FeO | Basicity |
|---|---|---|---|---|---|
| S1 | 3.77 | 8.86 | 33.31 | 54.06 | 0.38 |
| S2 | 10.36 | 8.26 | 31.03 | 50.35 | 0.60 |
| S3 | 17.99 | 7.75 | 28.39 | 46.07 | 0.90 |
| S4 | 24.43 | 6.96 | 26.15 | 42.46 | 1.20 |
| S5 | 29.93 | 6.45 | 24.25 | 39.37 | 1.50 |
| Slag System | CaO | MgO | SiO2 | FeO | Al2O3 |
|---|---|---|---|---|---|
| S1 | 3.72 | 8.68 | 32.78 | 51.54 | 3.28 |
| S2 | 10.28 | 8.14 | 30.01 | 47.51 | 4.06 |
| S3 | 16.72 | 7.65 | 28.10 | 43.40 | 4.13 |
| S4 | 22.41 | 6.82 | 25.11 | 41.48 | 4.18 |
| S5 | 29.84 | 6.16 | 24.17 | 35.31 | 4.52 |
| Basicity | O0 | O− | O2− | |||
|---|---|---|---|---|---|---|
| Position (eV) | Mole Fraction | Position (eV) | Mole Fraction | Position (eV) | Mole Fraction | |
| R = 0.38 | 532.10 | 45.01% | 531.07 | 35.48% | 530.24 | 19.51% |
| R = 0.60 | 532.09 | 42.94% | 531.10 | 36.25% | 530.27 | 20.81% |
| R = 0.90 | 532.27 | 38.46% | 531.29 | 36.94% | 530.39 | 24.60% |
| R = 1.20 | 532.14 | 35.16% | 531.13 | 38.24% | 530.20 | 26.60% |
| R = 1.50 | 532.31 | 34.12% | 531.31 | 38.56% | 530.34 | 27.31% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, Y.; Chong, J.; Huang, Z.; Tian, J.; Zhang, W.; Tang, X.; Ding, W.; Du, X. Viscosity and Structure of a CaO-SiO2-FeO-MgO System during a Modified Process from Nickel Slag by CaO. Materials 2019, 12, 2562. https://doi.org/10.3390/ma12162562
Shen Y, Chong J, Huang Z, Tian J, Zhang W, Tang X, Ding W, Du X. Viscosity and Structure of a CaO-SiO2-FeO-MgO System during a Modified Process from Nickel Slag by CaO. Materials. 2019; 12(16):2562. https://doi.org/10.3390/ma12162562
Chicago/Turabian StyleShen, Yingying, Junkai Chong, Ziniu Huang, Jianke Tian, Wenjuan Zhang, Xingchang Tang, Wanwu Ding, and Xueyan Du. 2019. "Viscosity and Structure of a CaO-SiO2-FeO-MgO System during a Modified Process from Nickel Slag by CaO" Materials 12, no. 16: 2562. https://doi.org/10.3390/ma12162562
APA StyleShen, Y., Chong, J., Huang, Z., Tian, J., Zhang, W., Tang, X., Ding, W., & Du, X. (2019). Viscosity and Structure of a CaO-SiO2-FeO-MgO System during a Modified Process from Nickel Slag by CaO. Materials, 12(16), 2562. https://doi.org/10.3390/ma12162562





