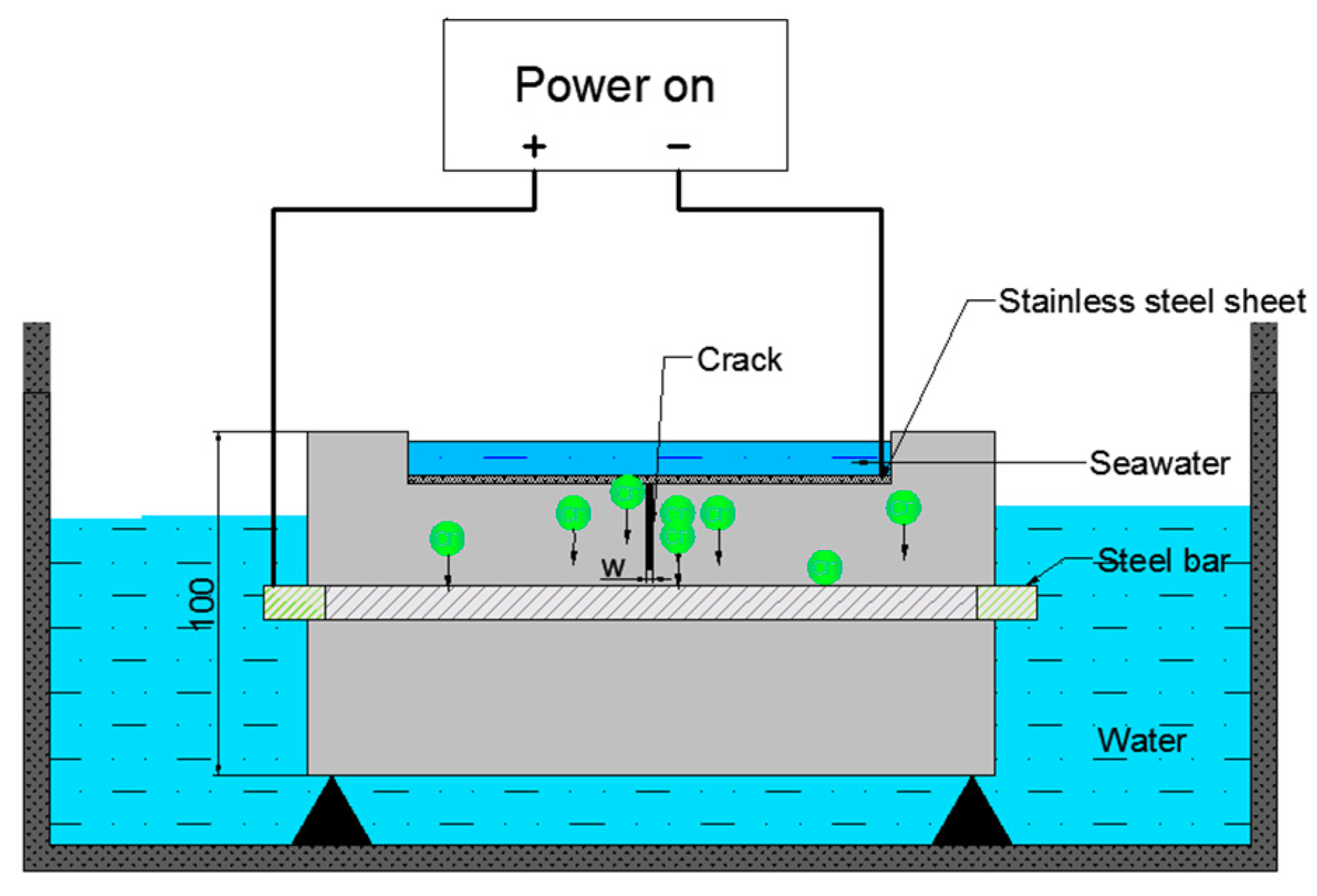
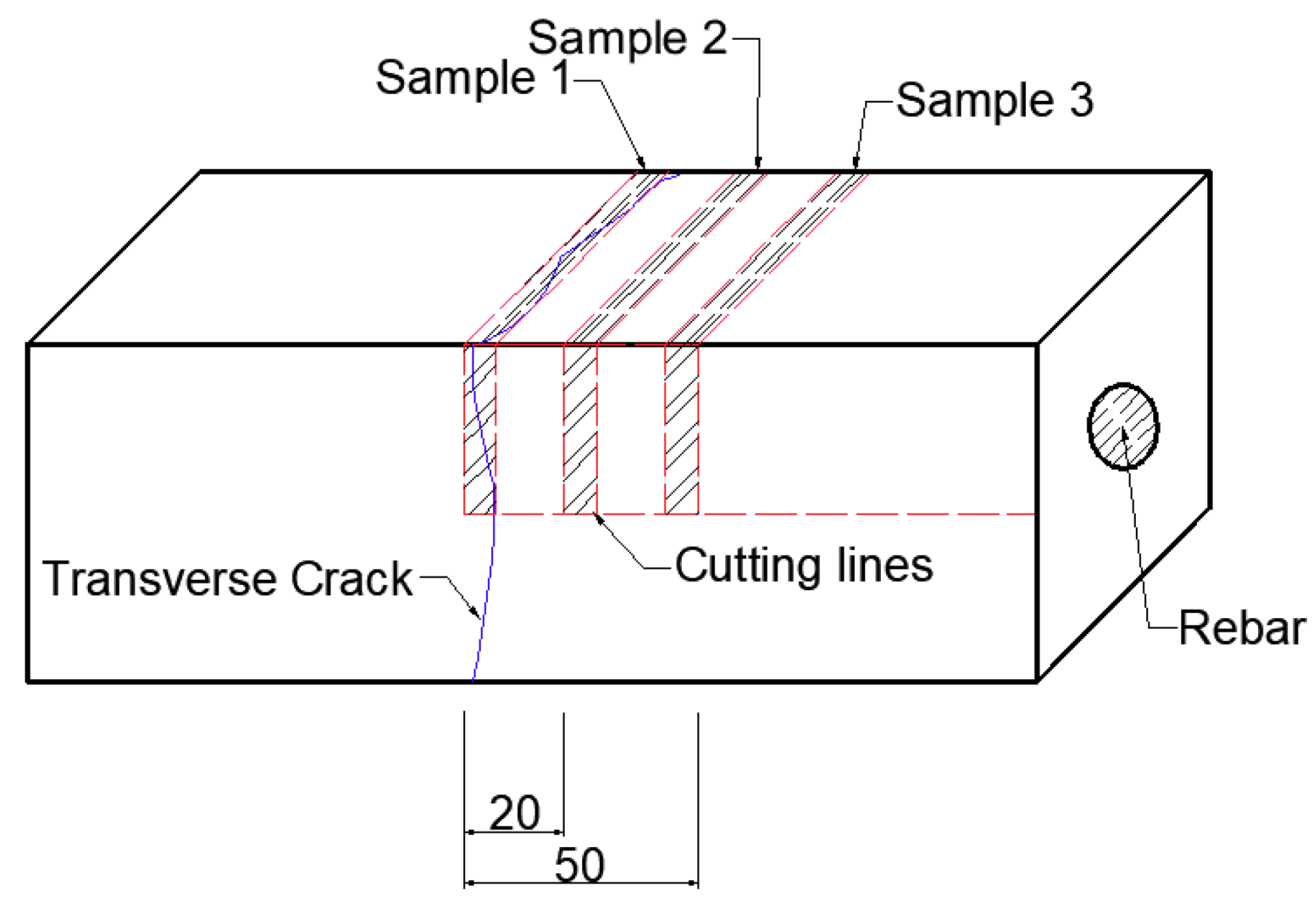
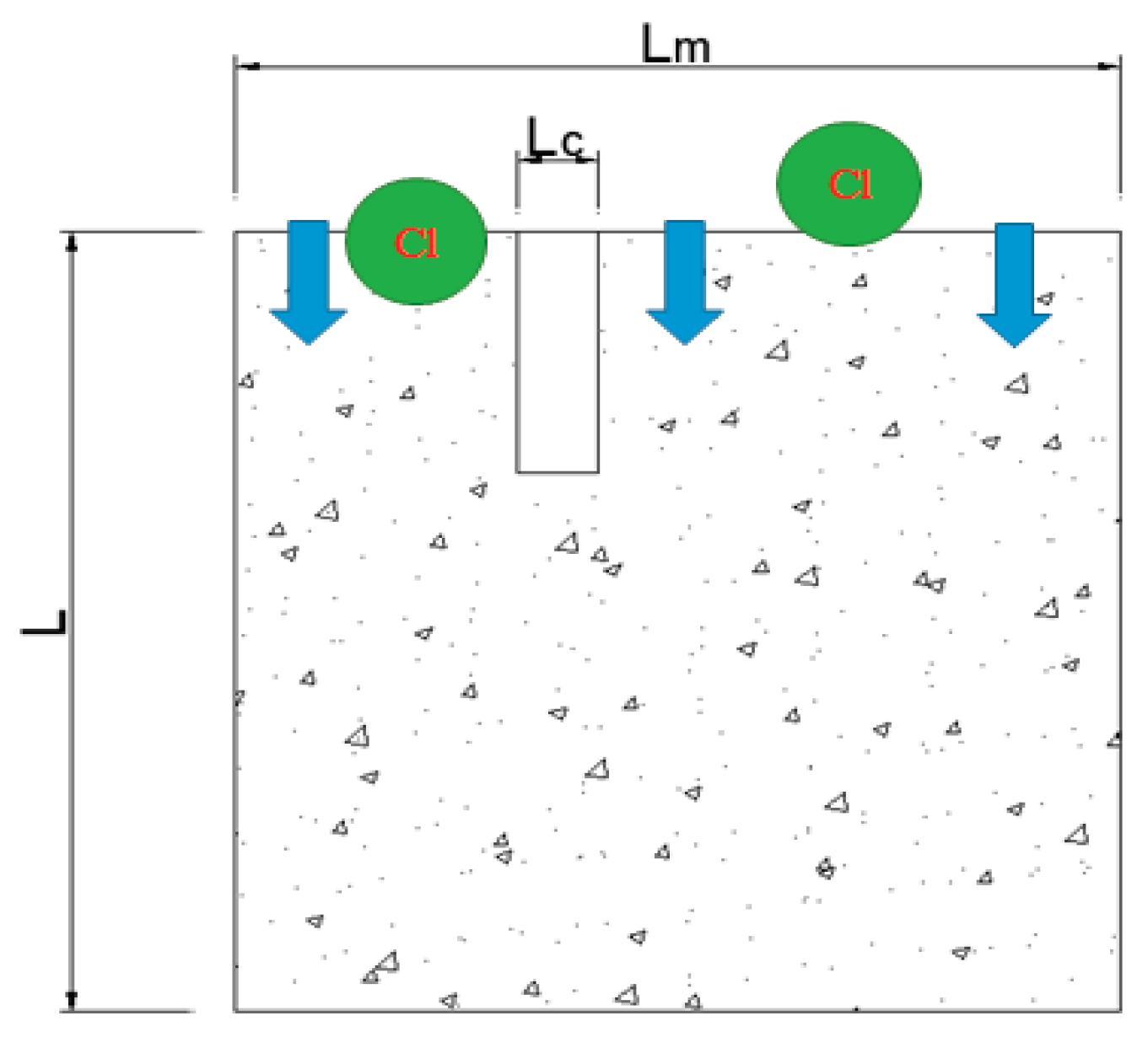
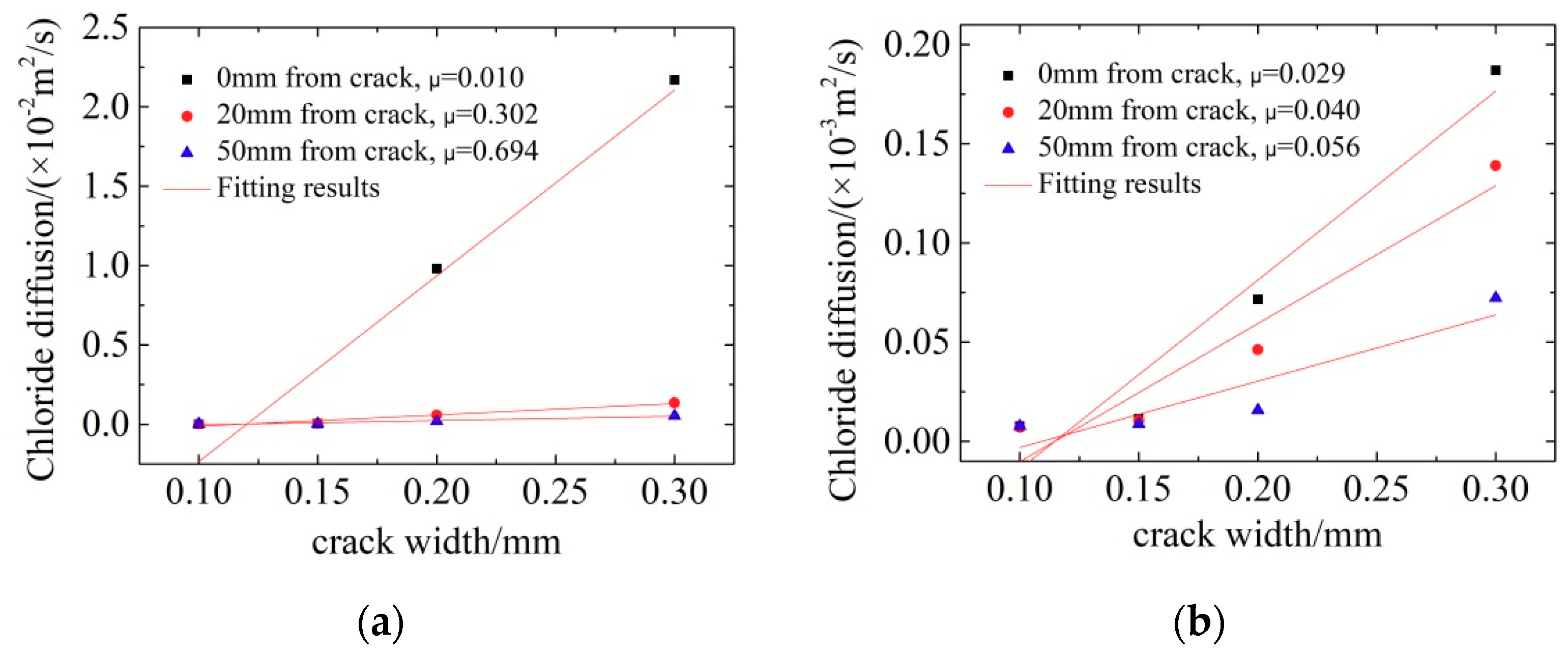
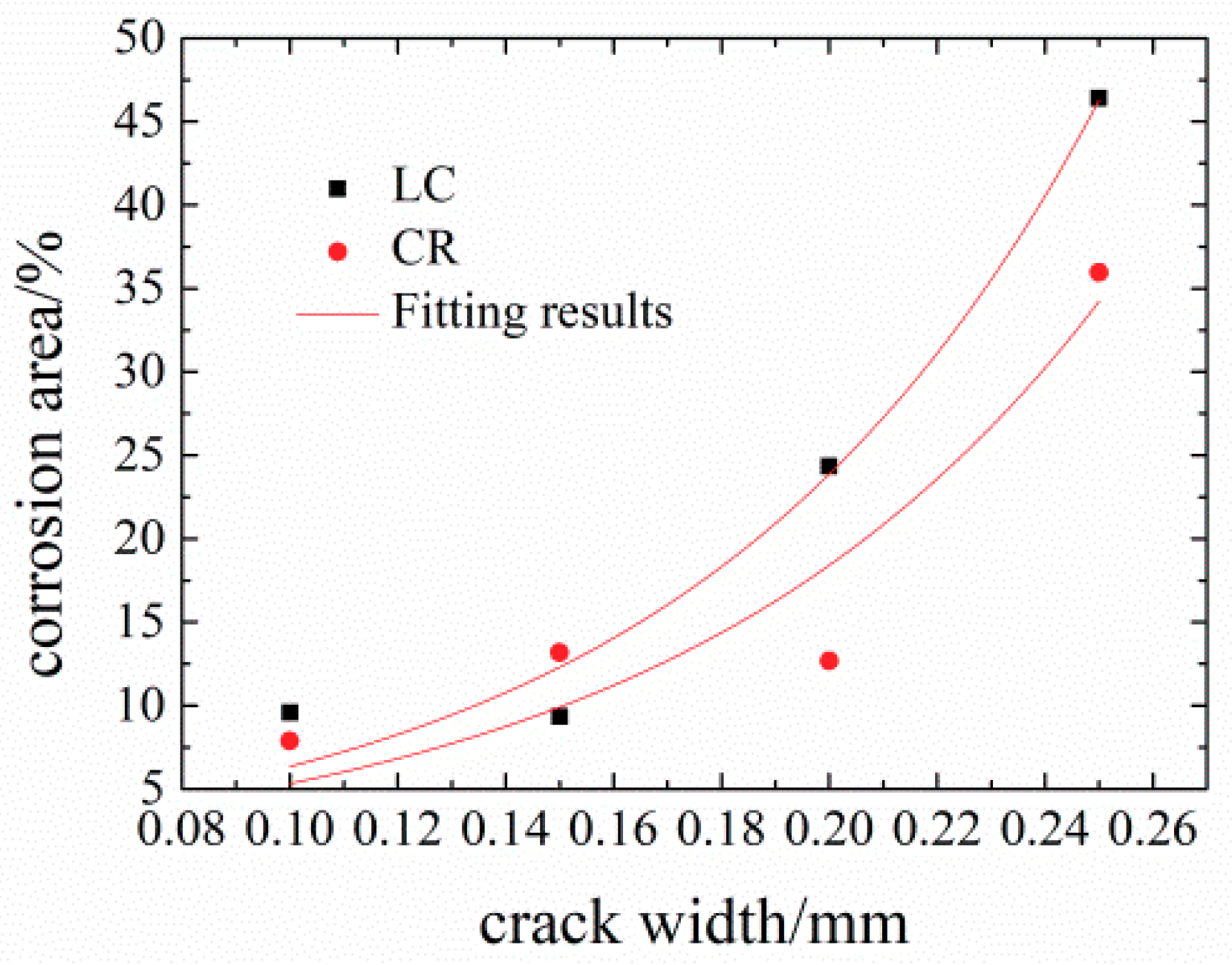
3.2.1. LC Steels
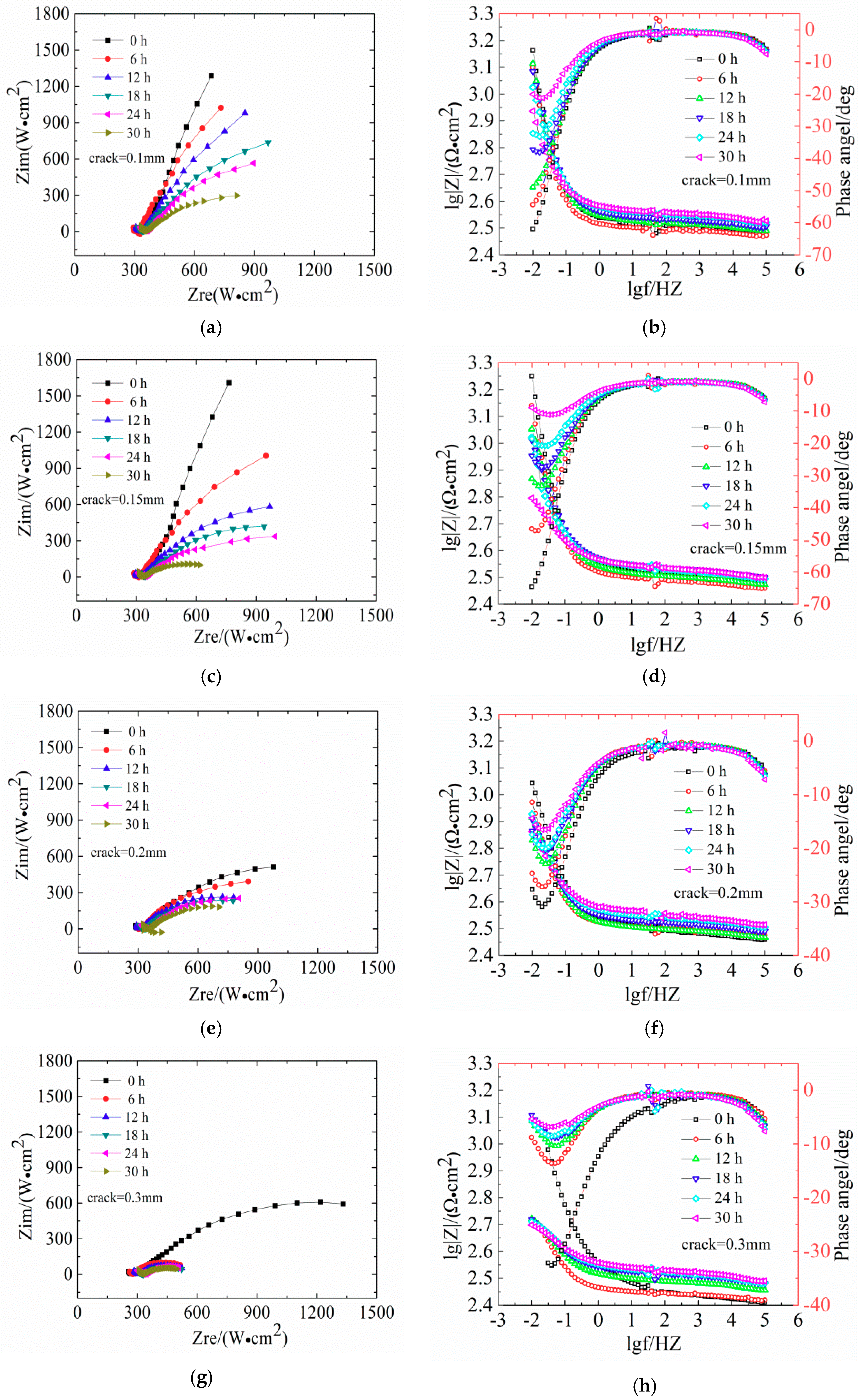
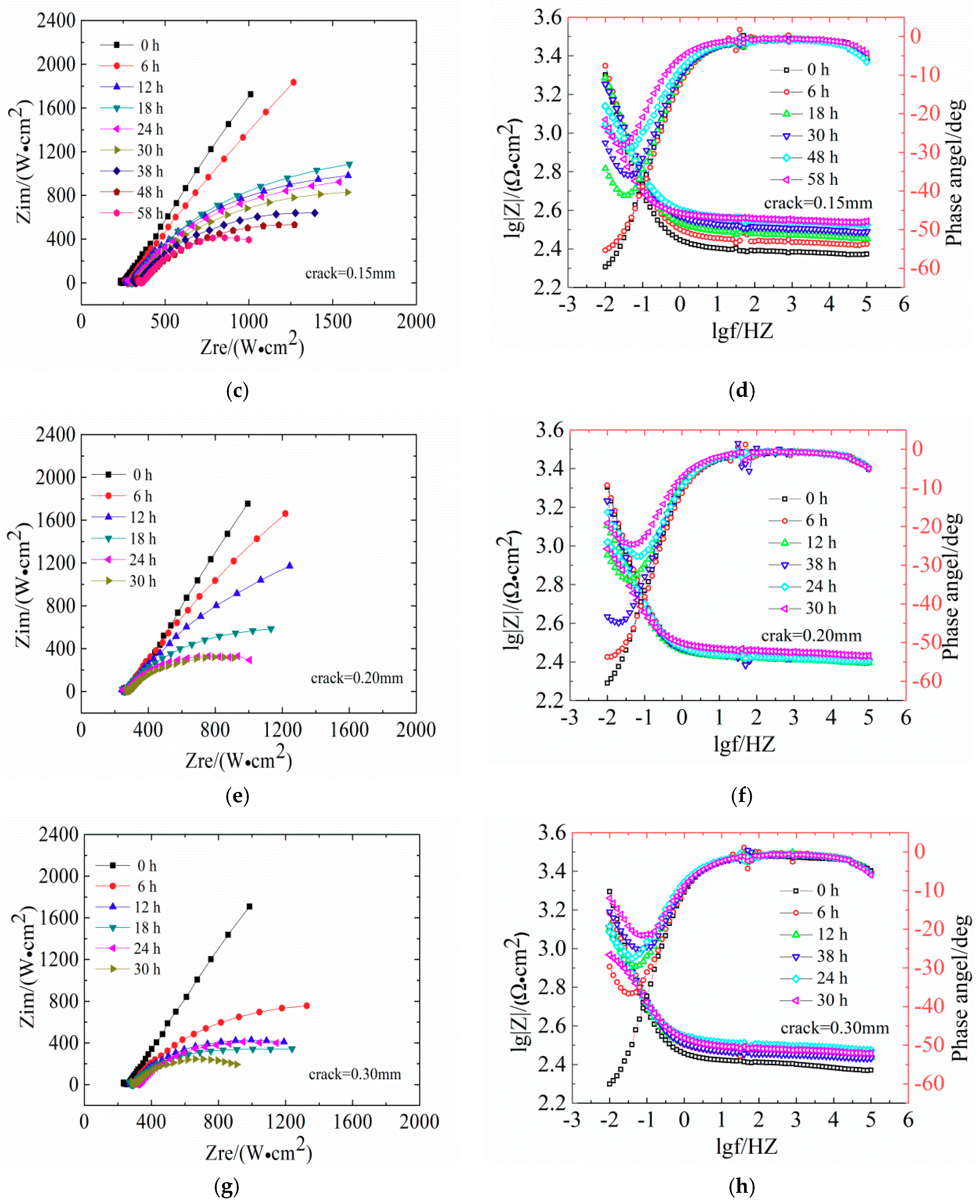
Figure 6 shows the evaluation of Nysquist plots and the Bode diagrams of LC steels in concrete samples.
Figure 6 shows the EIS diagrams of reinforcement steel specimens after 30 h of immersion in seawater with different crack widths (Nyquist plots and Bode diagrams). The Nyquist plots show a diameter of the capacitive loop, which reflects the resistance on the surface of the steels. The corrosion of steel in low-frequency reaction concrete in the impedance spectrum is mainly controlled by the material transfer process. In the Nyquist diagram, the slope of the curve in the low frequency region increases, indicating that the transmission resistance is large, which means that the steel bars are in a passivated state. With increasing exposure age, the capacitive arc is present in the low frequency region, and the radius of the capacitive arc is smaller; therefore, corrosive resistance is reduced. In the Bode phase, a larger absolute value of the maximum phase angle in the low frequency region indicates a more stable metal in the electrochemical system.
The diameter of the capacitive loop shrunk gradually with exposure time. In
Figure 6a,c, the Nyquist plots of reinforced concrete with crack widths of 0.10 mm and 0.15 mm show slight deviations. This indicates that the impedance gradually increased and the reinforced concrete was gradually compacting. The slope of low-frequency curve is not obvious, indicating that the high-alkaline environment exerts a beneficial effect on the steel bar passivation.
Figure 6e shows that the Nyquist plots of concrete with 0.20 mm shows a significant capacitive reactance arc, indicating the degeneration of steel bars. The Bode diagram in
Figure 6f shows that the absolute value of the maximum phase angle in the low-frequency area decreases from 31° at 0 h to 16° at 30 h, indicating the destruction of the steel passivation film.
Figure 6h shows that the capacitive reactance arc radius in Nyquist plots of the concrete with 0.30 mm is strongly decreasing and the absolute value of the maximum phase angle is 7° at 30 h, indicating the depassivation of the steel bars.
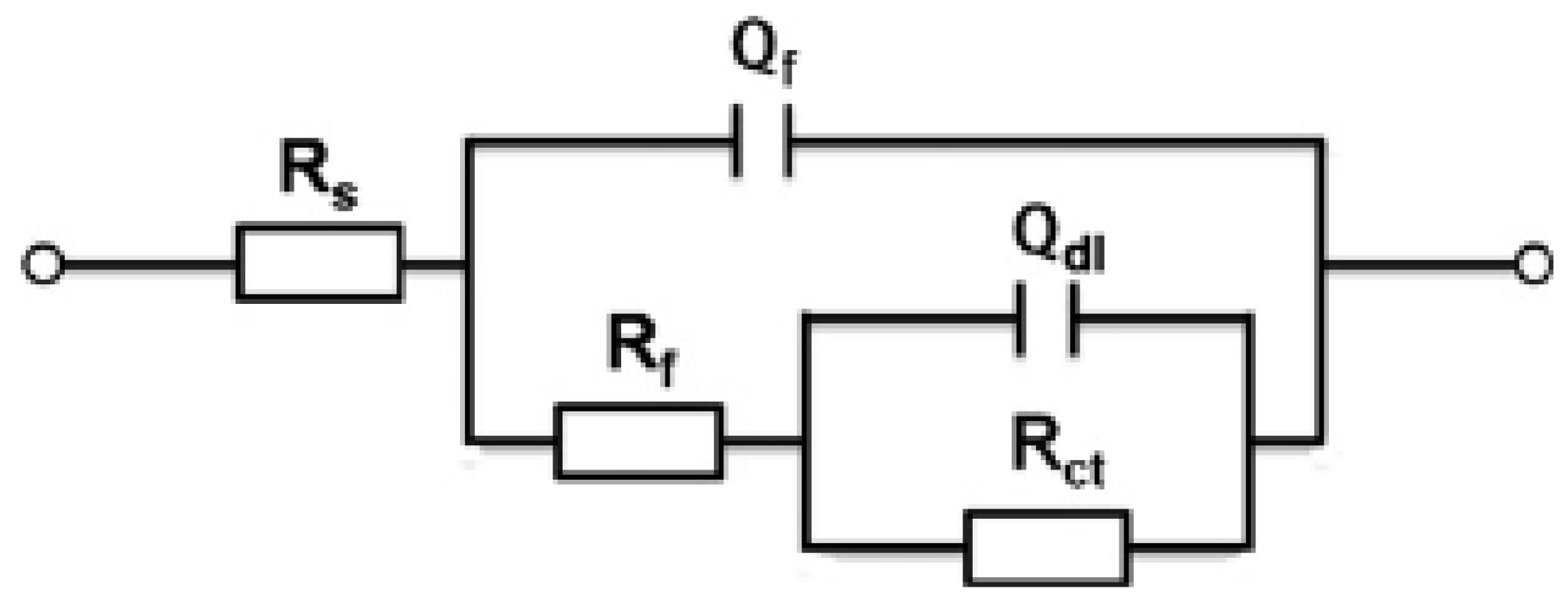
The Nyquist and Bode phase plots were only qualitatively analyzed by the graph topological structure of the corrosion of the steel bars. Therefore, the future parameters were obtained through the fitting software Zsimpwin and Stern–Geary formula. The software Zsimpin was used to fit impedance spectroscopy to obtain
Rp and Equation (12) [
41] was used to calculate the corrosion current density
icorr.
According to previous research [
41,
42,
43], equivalent circuits with two-time constant are generally selected for the fitting of reinforced concrete. As shown in
Figure 7, the R{R[Q(R(QW))]} equivalent circuit was selected in this experiment [
41,
42]. In this equivalent circuit, the passive film is considered to have a porous structure and show non-ideal capacitive behavior [
43].
Rs represents the resistance of the simulated solution,
Rct represents the charge transfer resistance of the utilized steel,
Qdl represents the double laver capacitance at the steel-solution interface,
Rf and
Qf represent the resistance and capacitance of the passive film, respectively. The constant phase element (CPE, expressed as
Q) was used as an alternative to the pure capacitor to represent non-homogeneity of the steel surface.
EIS measurement was conducted in the frequency range of 10
−2–10
5 Hz and an AC signal with an amplitude of 10 mV. The corrosion current density (
icorr) was based on the Stern–Geary relationship:
where,
Rp represents the polarization resistance and
B is a constant as a function of anodic and cathodic Tafel slopes. For steels in concrete, the
B value is normally equal to 26 mV for active state and 52 mV for passive state [
32,
44,
45]. In this work, a value of 26 mV was used to calculate the corrosion.
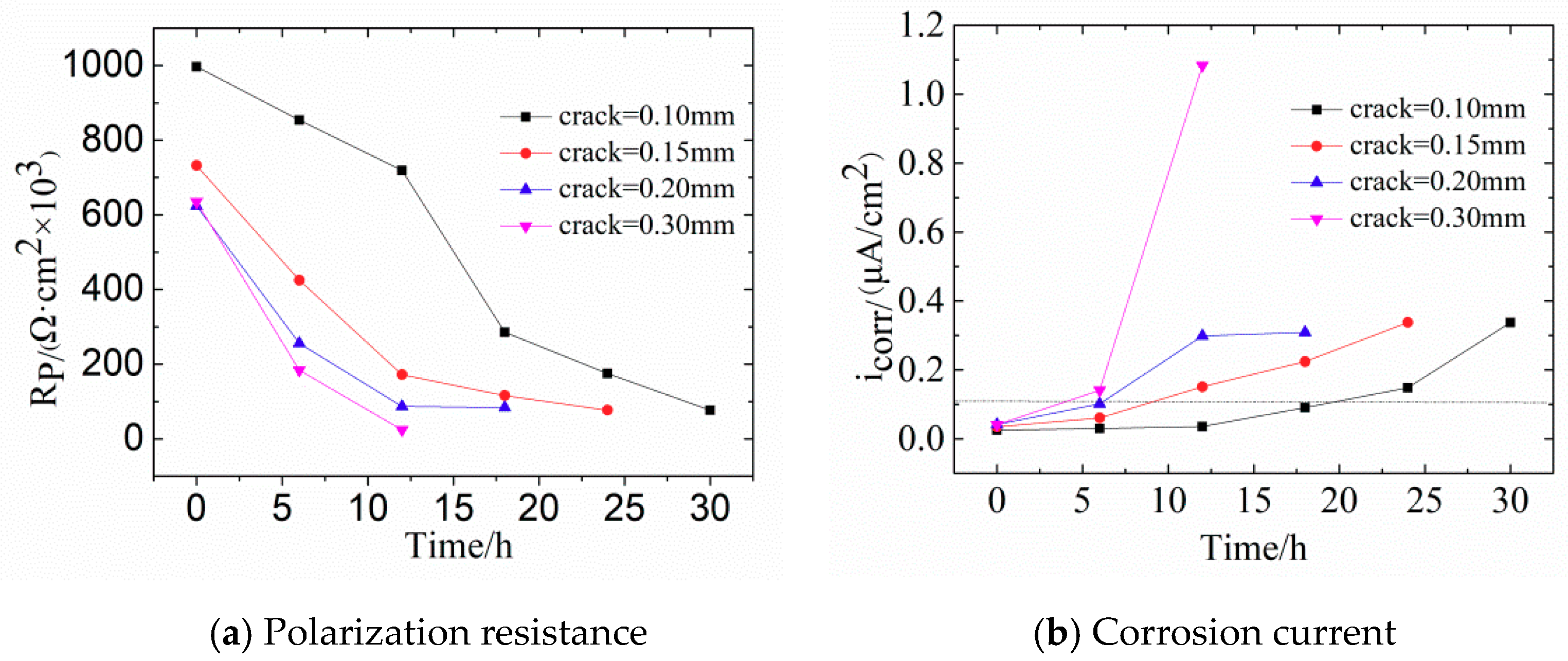
Figure 8 shows the changes of
Rp and
icorr in concrete with different crack widths. With increasing width of transverse cracks,
Rp becomes smaller. For crack widths of 0.10 mm and 0.15 mm,
Rp is relatively large, which means that the fine cracks slightly influence steel corrosion. Noteworthy, the value of
Rp changed slightly when the corrosive time was shorter than 12 h, indicating that steel bars are in the passive stage. The possible reason for this phenomenon is the self-healing of concretes with fine cracks. The main causes of this are: (1) The volcanic ash effect of fly ash produces Ca(OH)
2 and CSH gel, which exert a certain filling effect on internal micro-cracks [
46]; (2) planktonic microorganisms and fine sand particles in the seawater block the surface cracks so that oxygen cannot reach the surface of steel bars within a short time.
Figure 8b shows that the corrosion current density follows
icorr0.30 >
icorr0.20 >
icorr0.15 >
icorr0.10. For crack widths of 0.10 mm and 0.15 mm, more than 8 h are needed for the corrosion of steel bars. According to
Table 4, when
icorr exceeds 0.1 μA/cm
2, steel bars enter the low-speed corrosion stage.
The current density of steel bars is an important chemical parameter; however, it is difficult to evaluate the degree of corrosion in reinforced concrete. Therefore, it needs to be converted into the corrosion rate of steel bars. According to Faraday’s law, the corrosion velocity can be expressed as:
where,
represents the rate of polarization resistance values change;
t = time, h;
R0 represents the initial polarization resistance, KΩ·cm
2;
H represents the corrosive depth, μm/a;
M represents the molar mass of Fe, 56 g/mol;
icorr represents the corrosive current density, μA/cm
2;
t represents the corrosion time, s;
represents the density of Fe, 7.9 × 10
3 kg/m
3;
n represents the charge of reactive ion, (Fe→Fe
2+ + e
−);
F is the Faraday constant, 96,485 C/mol. This formula shows that 0.1 μA/cm
2 corrosive current density leads to 1.587 × 10
−3 μm loss depth of steel bars per 12 h.
Table 5 shows that the initial
Rp maintains around 600–1000 KΩ·cm
2. With increasing crack width, the rate of
Rp change gradually increases, which indicates that the larger crack width leads to a greater corrosion risk of steel bars. For a crack width of 0.10 mm and 0.15 mm, 19 h and 9 h are respectively needed for steels to enter the low-rate corrosion condition. With the increasing crack width and corrosion rate, the corrosion rates of steel bars in concrete with crack widths of 0.10 mm and 0.15 mm were 0.574 × 10
−3 μm/12 h and 2.399 × 10
−3 μm/12 h, respectively, compared to that of reinforced concrete with 0.30 mm crack width where corrosion rate was 17.136 × 10
−3 μm/12 h, indicating a severe corrosion condition.
3.2.2. Corrosion Resistant Steel Bar
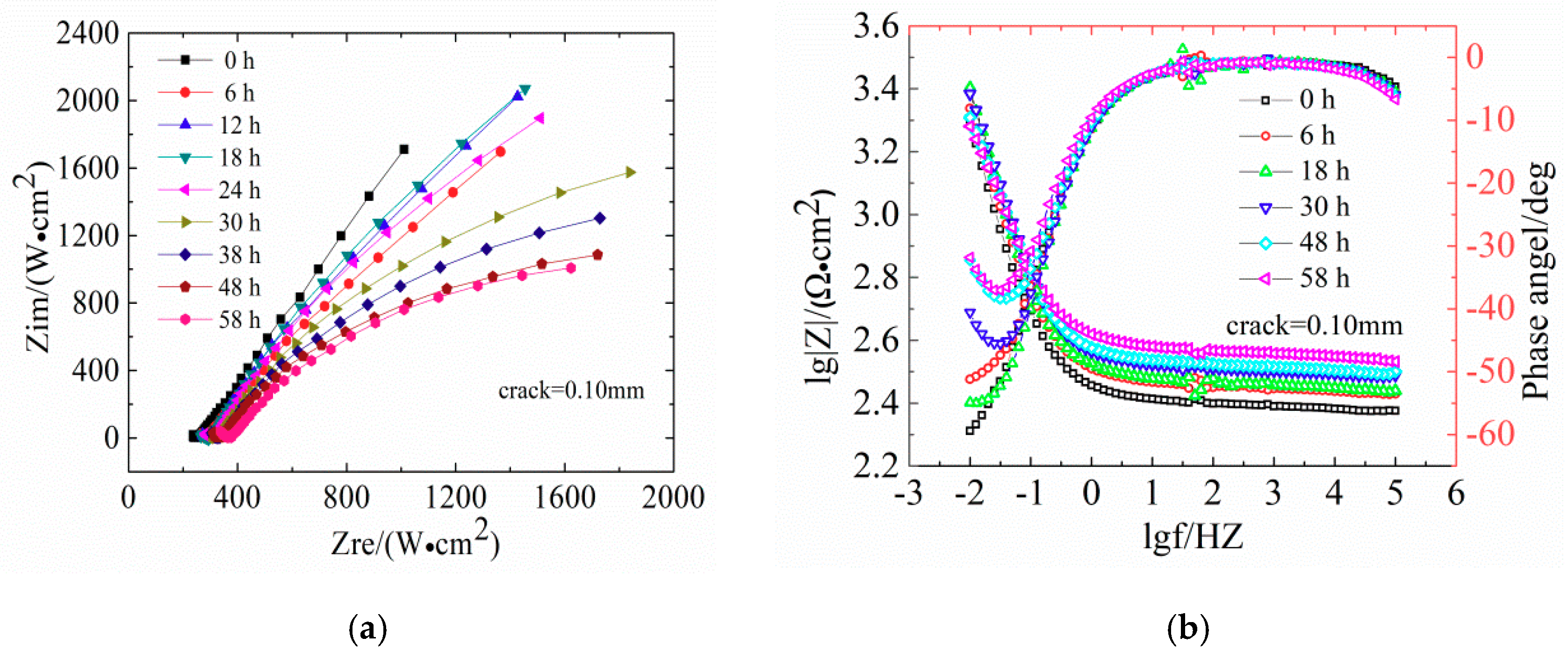
The same experiment was conducted for reinforced concrete specimens with corrosion resistant steel bars. The evolutions of Nyquist plots and the Bode diagrams of CR are shown in
Figure 9.
Comparison of the Nyquist plots between
Figure 7 and
Figure 9 clearly shows that the reduction of the capacitive arc radius of CR steel bars in concretes is significantly smaller than that of LC steel bars, indicating that the failure rate of the passivation film of the LC steels is faster than that of CR steels. Moreover, the Bode diagrams in
Figure 9 show that the maximum phase angle of concrete with low-frequency area decreased to 6°, indicating severe damage of steel bars.
Figure 10 shows the changes of R
p and i
corr of CR steel bars in concrete.
Comparison between
Figure 8 and
Figure 10 shows that the reduction rate of the
Rp value of CR steel bars is significantly smaller than that of LC steel bars. The smaller the crack width is, the slower the reduction rate will be. When the crack width is 0.10 mm, 48 h and 19 h are needed for CR steels and LC steels, respectively, indicating that CR steels have better corrosion resistance than LC steels.
Figure 10 shows that the time for concretes with 0.30 mm crack widths to be eroded is much shorter than that with 0.10 mm (4 h and 48 h, respectively). When the accelerated time was 12 h, the corrosive current density of concrete with 0.30 mm crack was 0.30 μA/cm
2.
The absolute value of the slope of
Rp of LC steels exceeds that of CR steels and the smaller the crack width is, the slower the slope change. In addition, for a crack width of 0.30 mm, the corrosion rate of CR steels is much smaller than that of LC steels, at 4.126 × 10
−3 μm/12 h and 17.136 × 10
−3 μm/12 h. Furthermore, the better corrosion resistance of CR steels is also indicated by
Table 6.