Nanostructured Polymethylsiloxane/Fumed Silica Blends
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
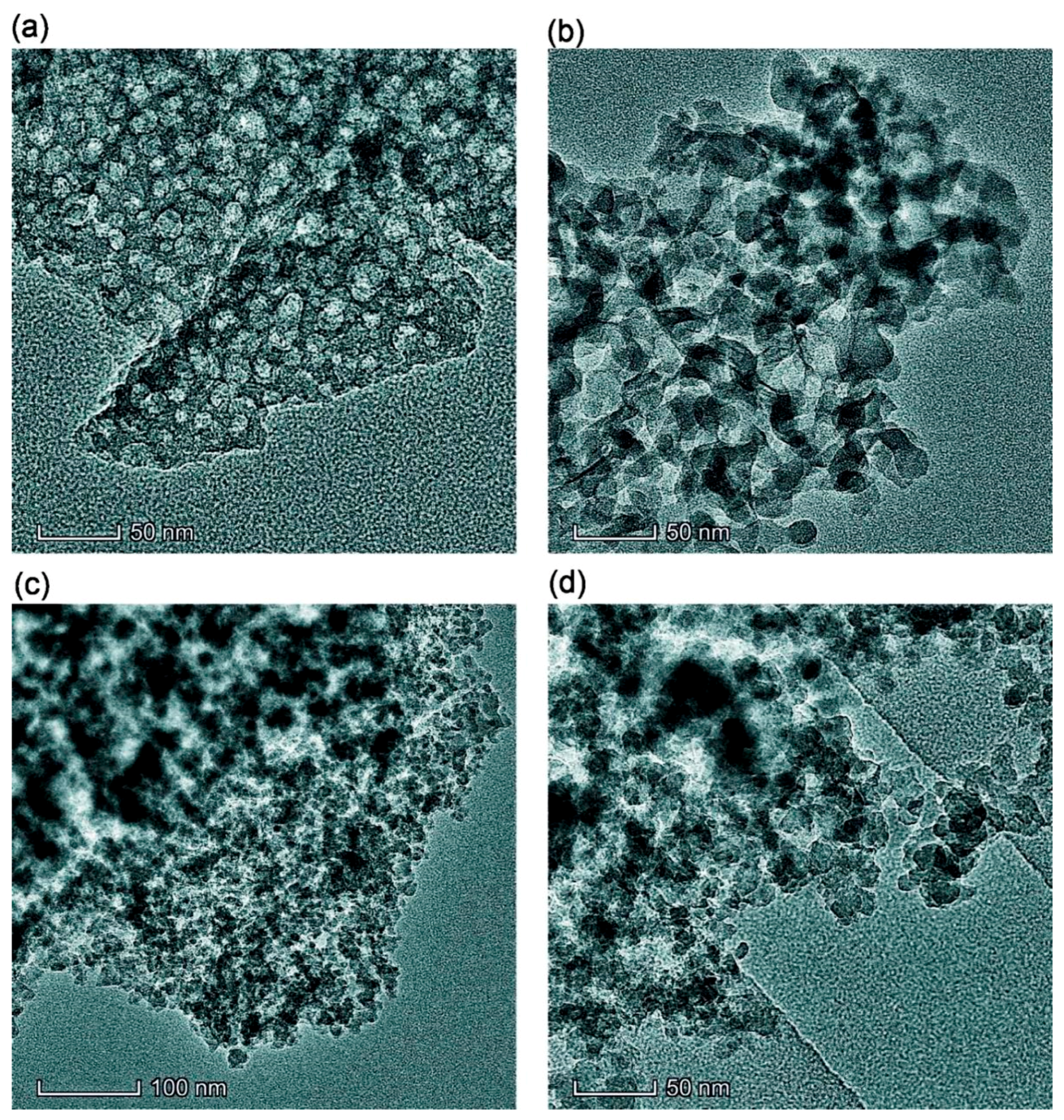
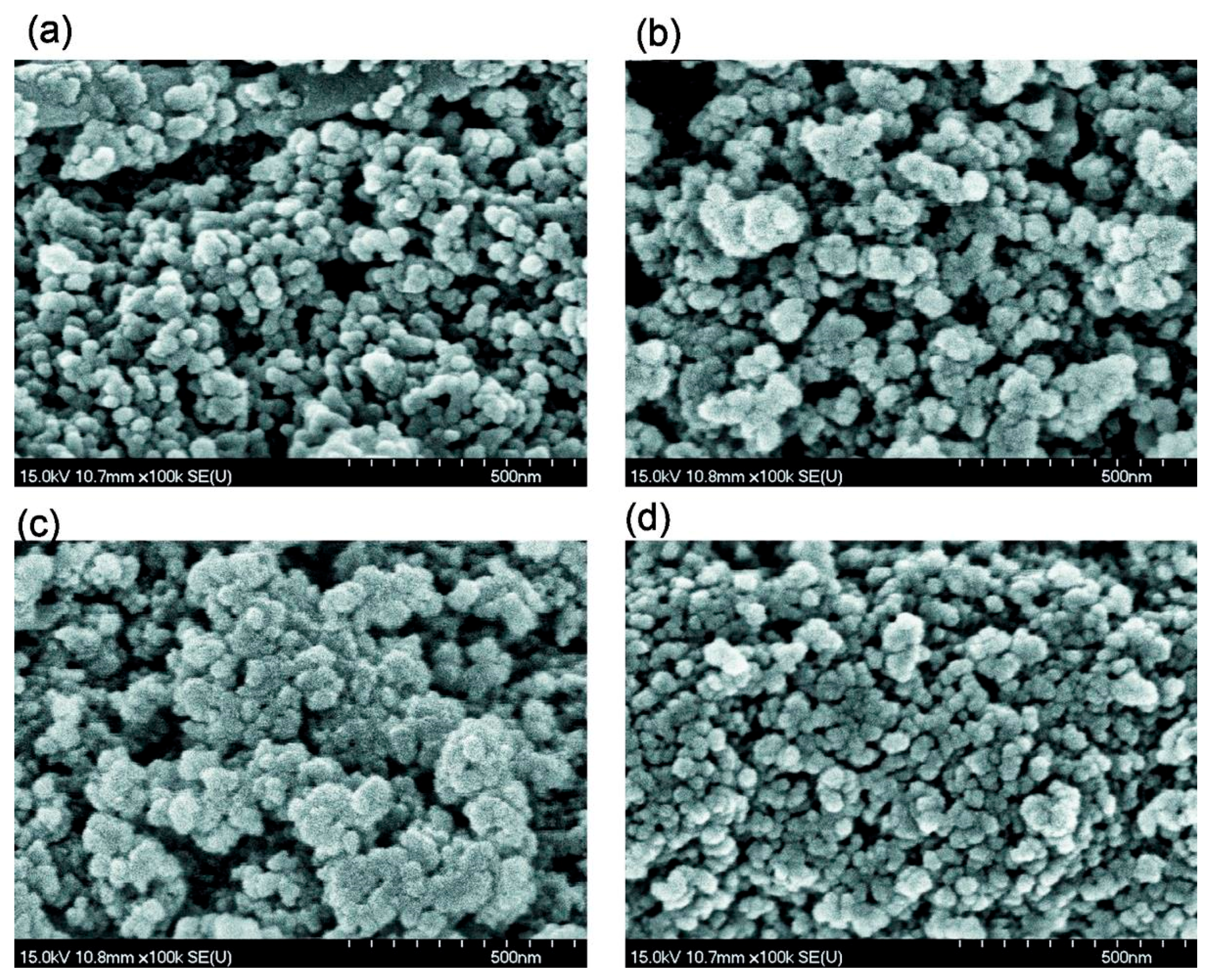
2.2. Transmission (TEM) and Scanning (SEM) Electron Microscopy
2.3. Textural Characteristics
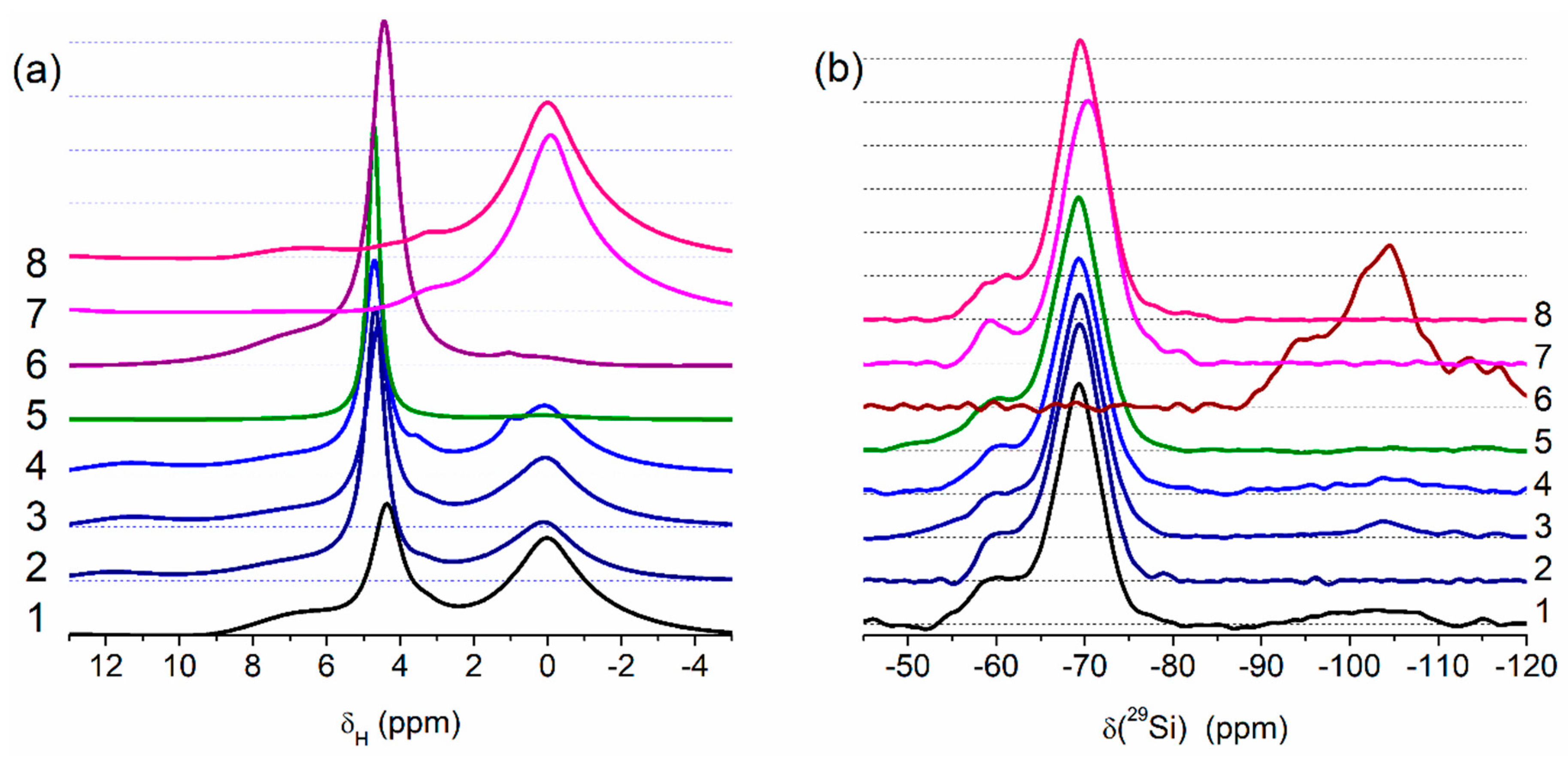
2.4. 1H MAS and 29Si CP/MAS NMR Spectroscopy
2.5. Infrared Spectroscopy
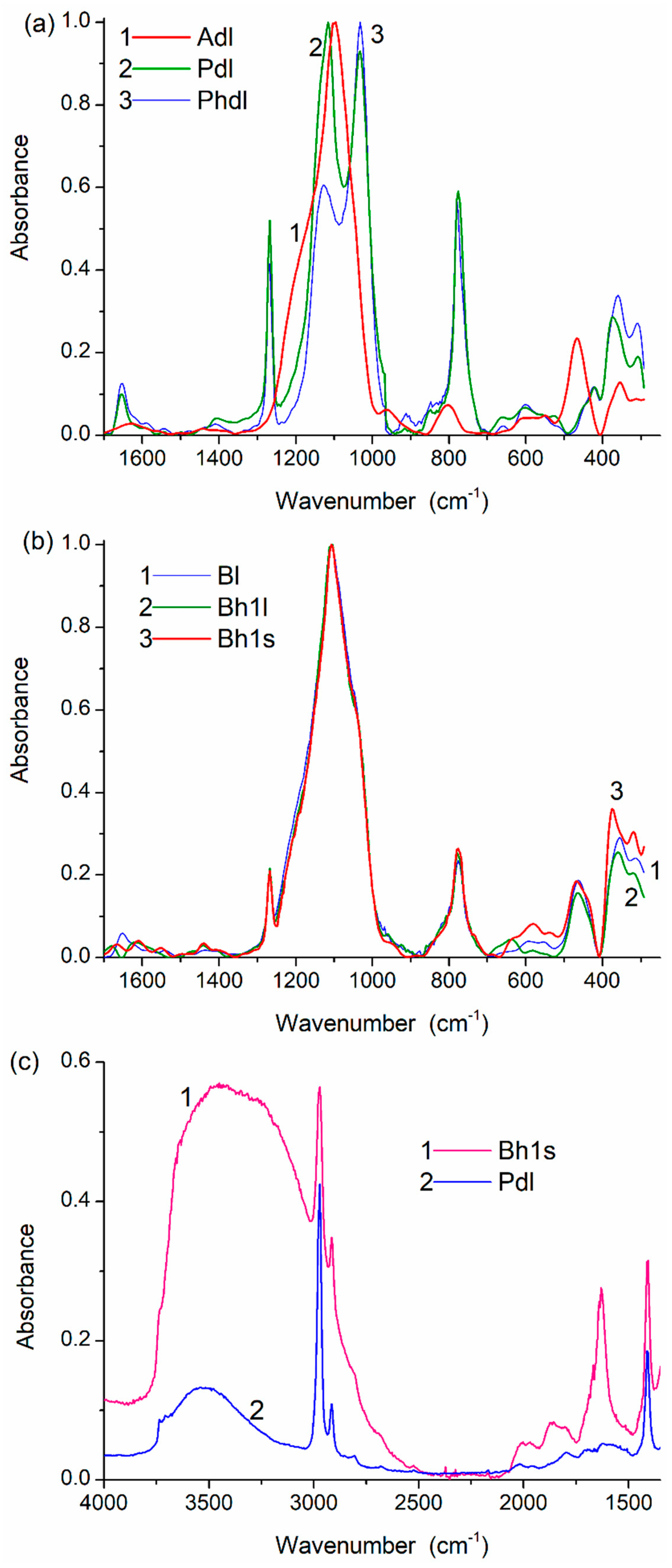
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Availability of Data and Materials
Conflicts of Interest
Notations
| Abbreviation | Full Name |
| SiO2 | Silica |
| PDMS | Poly(dimethylsiloxane) |
| PMS | Polymethylsiloxane |
| SBET | Surface area |
| δ | Chemical shift |
| CP/MAS NMR | Cross Polarization Magic-Angle Spinning Nuclear Magnetic Resonance |
| hPMS | hydrated polymethylsiloxane |
| dPMS | dehydrated polymethylsiloxane |
| ρb | bulk density |
| Bl | dPMS and dry cA-300 (1:1 w/w) mixed without strong mechanical loading |
| Bh1l | dPMS and dry cA-300 (1:1 w/w) mixed with an addition of distilled water without strong mechanical loading |
| Bh1s | dPMS and dry cA-300 (1:1 w/w) mixed with an addition of distilled water with strong mechanical loading |
| Bh2l | Water (h = 0.2 g/g) was added to S1 sample; and the sample was stirred without strong mechanical loading |
| Bh2s | Bh2l was additionally stirred with strong mechanical loading for 10 min; Adl, dried and stirred cA-300 |
| Pdl | Stirred dehydrated PMS |
| R | Pore radius |
| <RV> and <RS> | The average pore radii with respect to the pore volume and specific surface area, respectively |
| PSD | Pore size distributions |
| Vp | Pore volume |
| Vnano | Volume of nanopores (R < 1 nm) |
| Vmeso | Volume of mesopores (1 nm < R < 25 nm) |
| Vmacro | volume of macropores (25 nm < R < 100 nm) |
| Snano | Specific surface area of nanopores (R < 1 nm) |
| Smeso | Specific surface area of mesopores (1 nm < R < 25 nm) |
| Smacro | Specific surface area of macropores (25 nm < R < 100 nm) |
| SDFT/cyl | NLDFT specific surface area with a model of cylindrical pores in silica |
| cslit, ccyl, and cvoid | contributions of slit-shaped, cylindrical pores and voids between nonporous nanoparticles, the weight constants calculated using the SCV/SCR method |
| NLDFT | nonlocal density functional theory |
| TEM and SEM | Transmission and scanning electron microscopy; IR, infrared spectroscopy. |
References
- Iler, R.K. The Chemistry of Silica: Solubility, Polymerization, Colloid and Surface Properties and Biochemistry of Silica; Wiley: Chichester, UK, 1979; ISBN 978-0-471-02404-0. [Google Scholar]
- Legrand, A.P. The Surface Properties of Silicas, 1st ed.; Wiley: New York, NY, USA, 1998; ISBN 978-0471953326. [Google Scholar]
- Auner, N.; Weis, J. (Eds.) Oganosilicon Chemistry VI: From Molecules to Materials, 1st ed.; Wiley-VCH: Weinheim, Germany, 2005; ISBN 978-3527312146. [Google Scholar]
- Protsak, I.S.; Tertykh, V.A.; Pakhlov, E.M.; Derylo-Marczewska, A. Modification of fumed silica surface with mixtures of polyorganosiloxanes and dialkyl carbonates. Prog. Org. Coat. 2017, 106, 163–169. [Google Scholar] [CrossRef]
- Rao, A.V.; Kulkarni, M.; Amalnerkar, D.P.; Seth, T. Surface chemical modification of silica aerogels using various alkyl-alkoxy/chloro silanes. Appl. Surf. Sci. 2003, 206, 262–270. [Google Scholar] [CrossRef]
- Park, S.E.; Prasetyanto, E.A. Morphosynthesis and Catalysis by Organofunctionalized Mesoporous Materials. In Organosilanes Properties Performance and Applications; Wyman, E.B., Skief, M.C., Eds.; Nova Science Publishers: New York, NY, USA, 2010; pp. 101–131. ISBN 978-1-60876-452-5. [Google Scholar]
- Daoud, W.A.; Xin, J.H.; Xiaoming, T. Synthesis and characterization of hydrophobic silica nanocomposites. Appl. Surf. Sci. 2006, 252, 5368–5371. [Google Scholar] [CrossRef]
- Bernardoni, F.; Kouba, M.; Fadeev, A.Y. Effect of curvature on the packing and ordering of organosilane monolayers supported on solids. Chem. Mater. 2008, 20, 382–387. [Google Scholar] [CrossRef]
- Moitra, N.; Ichii, S.; Kamei, T.; Kanamori, K.; Zhu, Y.; Takeda, K.; Nakanishi, K.; Shimada, T. Surface Functionalization of Silica by Si–H Activation of Hydrosilanes. J. Am. Chem. Soc. 2014, 136, 11570–11573. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-F.; Xia, Y.-X.; Xu, D.-P.; Li, G.-L. Surface Reaction of Particulate Silica with Polydimethylsiloxanes. J. Polym. Sci. 1981, 19, 3069–3079. [Google Scholar] [CrossRef]
- Xiao, D.; Zhang, H.; Wirth, M. Chemical Modification of the Surface of Poly(dimethylsiloxane) by Atom-Transfer Radical Polymerization of Acrylamide. Langmuir 2002, 18, 9971–9976. [Google Scholar] [CrossRef]
- Protsak, I.S.; Kuzema, P.O.; Tertykh, V.A.; Bolbukh, Y.M.; Kozakevich, R.B. Thermogravimetric analysis of silicas chemically modified with products of deoligomerization of polydimethylsiloxane. J. Therm. Anal. Calorim. 2015, 121, 547–557. [Google Scholar] [CrossRef]
- Barandeh, F.; Nguyen, P.; Kumar, R.; Iacobucci, G.J.; Kuznicki, M.L.; Kosterman, A.; Bergey, E.J.; Prasad, P.N.; Gunawardena, S. Organically modified silica nanoparticles are biocompatible and can be targeted to neurons in vivo. PLoS ONE 2012, 7, e29424. [Google Scholar] [CrossRef]
- Dash, S.; Mishra, S.; Patel, S.; Mishra, B.K. Organically modified silica: Synthesis and applications due to its surface interaction with organic molecules. Adv. Colloid Interface Sci. 2008, 140, 77–94. [Google Scholar] [CrossRef]
- Peng, P.; Lan, Y.; Luo, J. Modified silica incorporating into PDMS polymeric membranes for bioethanol selection. Adv. Polym. Technol. 2019, 2019, 5610282. [Google Scholar] [CrossRef]
- Jung, H.S.; Moon, D.S.; Lee, J.K. Quantitative analysis and efficient surface modification of silica nanoparticles. J. Nanomater. 2012, 2012, 593471. [Google Scholar] [CrossRef]
- Blitz, J.P.; Gun’ko, V.M. (Eds.) Surface Chemistry in Biomedical and Environmental Science; NATO Science Series II: Mathematics, Physics and Chemistry; Springer: Dordrecht, The Netherlands, 2006. [Google Scholar]
- Finiels, B.; Alonso, M.; Bousmina, D.; Brunel, A.; Kadib, E. Periodic mesoporous organosilicas derived from amphiphilic bulky polymethylsiloxane. New J. Chem. 2016, 40, 4132–4135. [Google Scholar] [CrossRef]
- Sharaf, M.A.; Mark, J.E. Modulus of randomly crosslinked polymethysiloxane networks. PMSE 1993, 68, 180–181. [Google Scholar] [CrossRef]
- Zhang, H.; Fidelis, L.C.; Serva, A.L.T.; Michaela, M.; Rezwan, K. Water-based freeze casting: Adjusting hydrophobic polymethylsiloxane for obtaining hierarchically ordered porous SiOC. J. Am. Ceram. Soc. 2017, 100, 1907–1918. [Google Scholar] [CrossRef]
- Clarson, S.J.; Semlyen, J.A. Studies of Cyclic and Linear Poly(dimethylsiloxanes): 21. High Temperature Thermal Behavior. Polymer 1986, 27, 91–95. [Google Scholar] [CrossRef]
- Krumpfer, J.W.; McCarthy, T.J. Rediscovering Silicones: “Unreactive” Silicones React with Inorganic Surfaces. Langmuir 2011, 27, 11514–11519. [Google Scholar] [CrossRef]
- Barthel, H.; Nikitina, E. INS and IR study of Intermolecular Interactions at the Fumed Silica-Polydimethylsiloxane Interphase, Part 3. Silica-Siloxane Adsorption Complexes. Silicon Chem. 2004, 1, 261–279. [Google Scholar] [CrossRef]
- Gun’ko, V.M.; Turov, V.V.; Zarko, V.I.; Goncharuk, E.V.; Gerashchenko, I.I.; Turova, A.A.; Mironyuk, I.F.; Leboda, R.; Skubiszewska-Zięba, J.; Janusz, W. Comparative characterization of polymethylsiloxane hydrogel and silylated fumed silica and silica gel. J. Colloid Interface Sci. 2007, 308, 142–156. [Google Scholar] [CrossRef]
- Gun’ko, V.M.; Turov, V.V.; Krupska, T.V.; Protsak, I.S.; Borysenko, M.V.; Pakhlov, E.M. Polymethylsiloxane alone and in composition with nanosilica under various conditions. J. Colloid Interface Sci. 2019, 541, 213–225. [Google Scholar] [CrossRef]
- Gun’ko, V.M.; Pakhlov, E.M.; Goncharuk, O.V.; Andriyko, L.S.; Marynin, A.I.; Ukrainets, A.I.; Charmas, B.; Skubiszewska-Zięba, J.; Blitz, J.P. Influence of hydrophobization of fumed oxides on interactions with polar and nonpolar adsorbates. Appl. Surf. Sci. 2017, 423, 855–868. [Google Scholar] [CrossRef]
- Protsak, I.S.; Henderson, I.M.; Tertykh, V.A.; Dong, W.; Le, Z. Cleavage of organosiloxanes with dimethyl carbonate: A mild approach to graft-to-surface modification. Langmuir 2018, 34, 9719–9730. [Google Scholar] [CrossRef] [PubMed]
- Protsak, I.S.; Morozov, Y.M.; Dong, W.; Le, Z.; Zhang, D.; Henderson, I.M. A 29Si, 1H, and 13C Solid-State NMR Study on the Surface Species of Various Depolymerized Organosiloxanes at Silica Surface. Nanoscale Res. Lett. 2019, 14, 160. [Google Scholar] [CrossRef] [PubMed]
- Nikolaev, V.G. Enterosgel: A Novel Organosilicon Enterosorbent with a Wide Range of Medical Applications; Mikhalovsky, S., Khajibaev, A., Eds.; Springer: Berlin, Germany, 2011; pp. 199–221. [Google Scholar]
- Shevchenko, Y.N.; Dushanin, B.M.; Yashinina, N.I. New Silicon Compounds Porous Organosilicon Matrices for Technology and Medicine. In Silicon for the Chemistry Industry III; The Norwegian University of Science and Technology: Sandefjord, Norway, 1996; ISBN 9788290265194. [Google Scholar]
- Chaplin, M. Water Structure and Science. Available online: http://www1.lsbu.ac.uk/water/ (accessed on 26 May 2019).
- Mikheev, Y.A.; Guseva, L.N.; Davydov, E.Y.; Ershov, Y.A. The hydration of hydrophobic substances. Russ. J. Phys. Chem. A 2007, 81, 1897–1913. [Google Scholar] [CrossRef]
- Yaminsky, V.V.; Vogler, E.A. Hydrophobic hydration. Curr. Opin. Colloid Interface Sci. 2001, 6, 342–349. [Google Scholar] [CrossRef]
- Widom, B.; Bhimalapuram, P.; Koga, K. The hydrophobic effect. PCCP 2003, 5, 3085–3093. [Google Scholar] [CrossRef]
- Chandler, D. Interfaces and the driving force of hydrophobic assembly. Nature 2005, 437, 640–647. [Google Scholar] [CrossRef]
- Gun’ko, V.M.; Turov, V.V. Nuclear Magnetic Resonance Studies of Interfacial Phenomena; CRC Press: Boca Raton, FL, USA, 2013; ISBN 9781466551688. [Google Scholar]
- Gun’ko, V.M.; Zarko, V.I.; Turov, V.V.; Leboda, R.; Chibowski, E. Distribution effect of the second phase in disperse silica/X oxides (X=Al2O3, TiO2, GeO2) on their surface properties. Langmuir 1999, 15, 5694–5702. [Google Scholar] [CrossRef]
- Gun’ko, V.M.; Turov, V.V.; Pakhlov, E.M.; Krupska, T.V.; Borysenko, M.V.; Kartel, M.T.; Charmas, B. Water interactions with hydrophobic versus hydrophilic nanosilica. Langmuir 2018, 34, 12145–12153. [Google Scholar] [CrossRef]
- Gun’ko, V.M.; Turov, V.V.; Protsak, I.S.; Krupska, T.V.; Pakhlov, E.M.; Tsapko, M.D. Effects of pre-adsorbed water on methane adsorption onto blends with hydrophobic and hydrophilic nanosilicas. Colloids Surf. A Physicochem. Eng. Asp. 2019, 570, 471–480. [Google Scholar] [CrossRef]
- Chuiko, A.A. (Ed.) Medical Chemistry and Clinical Application of Silicon Dioxide; Naukova Dumka: Kiev, Ukraine, 2003; ISBN 966-00-0185-1. (In Russian) [Google Scholar]
- Gun’ko, V.M.; Turov, V.V.; Pakhlov, E.M.; Krupska, T.V.; Charmas, B. Effect of water content on the characteristics of hydro-compacted nanosilica. Appl. Surf. Sci. 2018, 459, 171–178. [Google Scholar] [CrossRef]
- Gregg, S.; Sing, K.S.W. Adsorption, Surface Area and Porosity; Academic Press: London, UK, 1982; ISBN 9780123009562. [Google Scholar]
- Adamson, A.W.; Gast, A.P. Physical Chemistry of Surface; Wiley: New York, NY, USA, 1997; ISBN 978-0-471-14873-9. [Google Scholar]
- Gun’ko, V.M. Composite materials: Textural characteristics. Appl. Surf. Sci. 2014, 307, 444–454. [Google Scholar] [CrossRef]
- Neimark, A.V.; Ravikovitch, P.I. Capillary condensation in MMS and pore structure characterization. Microporous Mesoporous Mater. 2001, 44, 697–707. [Google Scholar] [CrossRef]
- Thommes, M.; Köhn, R.; Fröba, M. Sorption and pore condensation behavior of pure fluids in mesoporous MCM-48 silica, MCM-41 silica, SBA-15 silica and controlled-pore glass at temperatures above and below the bulk triple point. Appl. Surf. Sci. 2002, 196, 239–249. [Google Scholar] [CrossRef]
- Corminbœuf, C.; Heine, T.; Weber, J. 29Si NMR chemical shifts of silane derivatives. Chem. Phys. Lett. 2002, 357, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Kruk, M.; Jaroniec, M. Gas adsorption characterization of ordered organic-inorganic nanocomposite materials. Chem. Mater. 2001, 13, 3169–3183. [Google Scholar] [CrossRef]
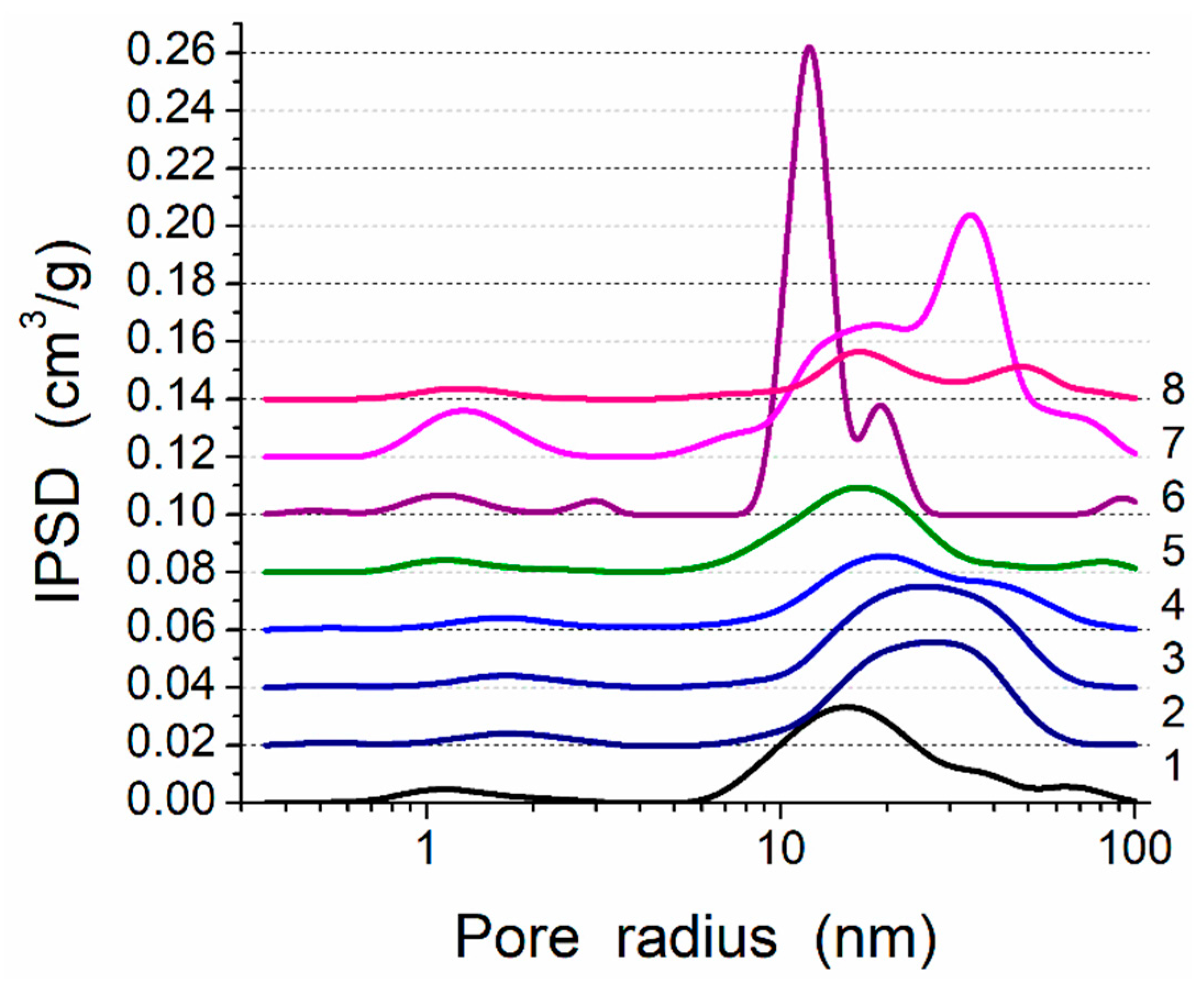
| No | Sample Composition | Sample Label | SBET (m2/g) | SDFT,cyl (m2/g) | Snano (m2/g) | Smeso (m2/g) | Smacro (m2/g) | Vp (cm3/g) | Vnano (cm3/g) | Vmeso (cm3/g) | Vmacro (cm3/g) | <RV> (nm) | <RS> (nm) | cslit | ccyl | cvoid |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | dPMS/cA-300 | Bl | 186 | 164 | 52 | 124 | 10 | 0.788 | 0.026 | 0.594 | 0.168 | 19.65 | 7.57 | 0.699 | 0.221 | 0.080 |
| 2 | dPMS/cA-300 | Bh1l | 166 | 152 | 38 | 107 | 22 | 0.827 | 0.012 | 0.463 | 0.352 | 24.17 | 9.69 | 0.162 | 0.798 | 0.041 |
| 3 | dPMS/cA-300 | Bh1s | 165 | 151 | 34 | 108 | 23 | 0.848 | 0.011 | 0.461 | 0.376 | 25.13 | 10.27 | 0.171 | 0.774 | 0.055 |
| 4 | dPMS/cA-300 | Bh2l | 159 | 150 | 31 | 113 | 14 | 0.691 | 0.011 | 0.433 | 0.247 | 23.51 | 8.44 | 0.200 | 0.740 | 0.060 |
| 5 | dPMS/cA-300 | Bh2s | 134 | 127 | 32 | 97 | 5 | 0.613 | 0.018 | 0.506 | 0.089 | 18.37 | 7.60 | 0.775 | 0.136 | 0.089 |
| 6 | cA-300 | Adl | 278 | 278 | 77 | 201 | 1 | 1.304 | 0.038 | 1.243 | 0.024 | 13.39 | 7.19 | 0.794 | 0.190 | 0.016 |
| 7 | dPMS | Pdl | 453 | 459 | 83 | 319 | 52 | 1.808 | 0.049 | 0.916 | 0.843 | 25.26 | 7.86 | 0.396 | 0.302 | 0.302 |
| 8 | PMS initial | Phdl | 98 | 99 | 19 | 71 | 8 | 0.390 | 0.011 | 0.234 | 0.145 | 25.41 | 7.62 | 0.437 | 0.249 | 0.315 |
| 9 | A-300 initial | A-300 | 294 | 289 | 44 | 229 | 16 | 0.850 | 0.023 | 0.567 | 0.259 | 20.41 | 6.14 | 0.379 | 0.280 | 0.341 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Protsak, I.; Gun’ko, V.M.; Turov, V.V.; Krupska, T.V.; Pakhlov, E.M.; Zhang, D.; Dong, W.; Le, Z. Nanostructured Polymethylsiloxane/Fumed Silica Blends. Materials 2019, 12, 2409. https://doi.org/10.3390/ma12152409
Protsak I, Gun’ko VM, Turov VV, Krupska TV, Pakhlov EM, Zhang D, Dong W, Le Z. Nanostructured Polymethylsiloxane/Fumed Silica Blends. Materials. 2019; 12(15):2409. https://doi.org/10.3390/ma12152409
Chicago/Turabian StyleProtsak, Iryna, Volodymyr M. Gun’ko, Volodymyr V. Turov, Tetyana V. Krupska, Eugeniy M. Pakhlov, Dong Zhang, Wen Dong, and Zichun Le. 2019. "Nanostructured Polymethylsiloxane/Fumed Silica Blends" Materials 12, no. 15: 2409. https://doi.org/10.3390/ma12152409
APA StyleProtsak, I., Gun’ko, V. M., Turov, V. V., Krupska, T. V., Pakhlov, E. M., Zhang, D., Dong, W., & Le, Z. (2019). Nanostructured Polymethylsiloxane/Fumed Silica Blends. Materials, 12(15), 2409. https://doi.org/10.3390/ma12152409







