Ionic Liquid-Assisted Hydrothermal Synthesis of a Biocompatible Filler for Photo-Curable Dental Composite: From Theory to Experiment
Abstract
:1. Introduction
2. Experimental Procedure
2.1. Materials Synthesis
2.2. Models and Computational Details
2.3. Materials Characterization
2.4. Cell Viability Measurements
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Turon, P.; del Valle, L.J.; Alemán, C.; Puiggalí, J. Biodegradable and biocompatible Systems Based on Hydroxyapatite Nanoparticles. Appl. Sci. 2017, 7, 60. [Google Scholar] [CrossRef]
- Wahl, D.A.; Czernuszka, J.T. Collagen-hydroxyapatite composites for hard tissue repair. Eur. Cell. Mater. 2006, 11, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi Hafshejani, T.; Zamanian, A.; Venugopal, J.R.; Rezvani, Z.; Sefat, F.; Saeb, M.R.; Vahabi, H.; Zarrintaj, P.; Mozafari, M. Antibacterial glass-ionomer cement restorative materials: A critical review on the current status of extended release formulations. J. Control. Release 2017, 262, 317. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, S.; Bandyopadhyay, A.; Bose, S. Zn and Mg Doped Hydroxyapatite Nanoparticles for Controlled Release of Protein. Langmuir 2010, 26, 4958–4964. [Google Scholar] [CrossRef] [PubMed]
- Fakharzadeh, A.; Ebrahimi-Kahrizsangi, R.; Nasiri-Tabrizi, B.; Basirun, W.J. Effect of dopant loading on the structural features of silver-doped hydroxyapatite obtained by mechanochemical method. Ceram. Int. 2017, 43, 12588–12598. [Google Scholar] [CrossRef]
- Ciobanu, C.S.; Popa, C.L.; Predoi, D. Cerium doped hydroxyapatite nanoparticles synthesized by coprecipitation method. J. Serb. Chem. Soc. 2016, 81, 1–14. [Google Scholar] [CrossRef]
- Tayebi, L.; Moharamzadeh, K. Biomaterials for Oral and Dental Tissue Engineering; Woodhead Publishing: Shaston, UK, 2017; Chapter 5; pp. 65–84. [Google Scholar]
- Özbek, Y.Y.; Baştan, F.E.; Üstel, F. Synthesis and characterization of strontium-doped hydroxyapatite for biomedical applications. J. Therm. Anal. Calorim. 2016, 125, 745–750. [Google Scholar] [CrossRef]
- Terra, J.; Dourado, E.R.; Eon, J.G.; Ellis, D.E.; Gonzalez, G.; Rossi, A.M. The structure of strontium-doped hydroxyapatite: An experimental and theoretical study. Phys. Chem. Chem. Phys. 2009, 11, 568–577. [Google Scholar] [CrossRef]
- Mardziah, C.M.; Sopyan, I.; Ramesh, S. Strontium-doped hydroxyapatite nanopowder via sol-gel method: Effect of strontium concentration and calcination temperature on phase behavior. Trends Biomater. Artif. Organs. 2009, 23, 105–113. [Google Scholar]
- Li, Y.; Luo, E.; Zhu, S.; Li, J.; Zhang, L.; Hu, J. Cancellous Bone Response to Strontium-Doped Hydroxyapatite in Osteoporotic Rats. J. Appl. Biomater. Funct. Mater. 2015, 13, 28–34. [Google Scholar] [CrossRef]
- Ferreira, M.M.; Brito, A.F.; Ferreira, J.M.F. Doping β-TCP as a Strategy for Enhancing the Regenerative Potential of Composite-TCP—Alkali-Free Bioactive Glass Bone Grafts. Experimental Study in Rats Materials. Materials 2019, 12, 4. [Google Scholar] [CrossRef] [PubMed]
- Capuccini, C.; Torricelli, P.; Boanini, E.; Gazzano, M.; Giardino, R.; Bigi, A. Interaction of Sr-doped hydroxyapatite nanocrystals with osteoclast and osteoblast-like cells. J. Biomed. Mater. Res. Part A 2009, 89, 594–600. [Google Scholar] [CrossRef] [PubMed]
- Thuy, T.T.; Nakagaki, H.; Kato, K.; Hung, P.A.; Inukai, J.; Tsuboi, S.; Nakagaki, H.; Hirose, M.N.; Igarashi, S.; Robinson, C. Effect of strontium in combination with fluoride on enamel remineralisation in vitro. Arch. Oral Biol. 2008, 3, 1017–1022. [Google Scholar] [CrossRef] [PubMed]
- Kannan, S.; Lemos, I.A.F.; Rocha, J.H.G.; Ferreira, J.M.F. Synthesis and characterization of magnesium substituted biphasic mixtures of controlled hydroxyapatite/â-tricalcium phosphate ratios. J Solid State Chem. 2005, 178, 3190–3196. [Google Scholar] [CrossRef]
- Xiao, W.; Gao, H.; Qu, M.; Liu, X.; Zhang, J.; Li, H.; Yang, X.; Li, B.; Liao, X. Rapid microwave synthesis of hydroxyapatite phosphate microspheres with hierarchical porous structure. Ceram. Int. 2018, 44, 6144–6151. [Google Scholar] [CrossRef]
- Hassan, M.N.; Mahmoud, M.M.; Abd El-Fattah, A.; Kandil, S. Microwave-assisted preparation of Nano-hydroxyapatite for bone substitutes. Ceram. Int. 2016, 42, 3725–3744. [Google Scholar] [CrossRef]
- Chaikina, M.V.; Bulina, N.V.; Ishchenko, A.V.; Prosanov, I.Y. Mechanochemical synthesis of hydroxyapatite and its modifications: Composition, structure, and properties. Russ. Phys. J. 2014, 56, 1176–1182. [Google Scholar] [CrossRef]
- Ma, X.; Chen, Y.; Qian, J.; Yuan, Y.; Liu, C. Controllable synthesis of spherical hydroxyapatite nanoparticles using inverse microemulsion method. Mater. Chem. Phys. 2016, 183, 220–229. [Google Scholar] [CrossRef]
- Padmanabhan, S.K.; Balakrishnan, A.; Chu, M.C.; Lee, Y.J.; Kim, T.N.; Cho, S.J. Sol–gel synthesis and characterization of hydroxyapatite nanorods. Particuology 2009, 7, 466–470. [Google Scholar] [CrossRef]
- Yang, Y.; Wu, Q.; Wang, M.; Long, J.; Mao, Z.; Chen, X. Hydrothermal synthesis of hydroxyapatite with different morphologies: Influence of supersaturation of the reaction system. Cryst. Growth. Des. 2014, 14, 4864–4871. [Google Scholar] [CrossRef]
- Zhang, X.; Vecchio, K.S. Hydrothermal synthesis of hydroxyapatite rods. J. Cryst. Growth 2007, 308, 133–140. [Google Scholar] [CrossRef]
- Khalid, M.; Mujahid, M.; Amin, S.; Rawat, R.S.; Nusair, A.; Deen, G.R. Effect of surfactant and heat treatment on morphology, surface area and crystallinity in hydroxyapatite nanocrystals. Ceram. Int. 2013, 39, 39–50. [Google Scholar] [CrossRef]
- Hernández Ortiz, G.M.; Parra, R.; Fanovich, M.A. Comparative hydrothermal synthesis of hydroxyapatite by using cetyltrimethylammonium bromide and hexamethylenetetramine as additives. Ceram. Int. 2018, 44, 3658–3663. [Google Scholar] [CrossRef]
- Li, Z.; Jia, Z.; Luan, Y.; Mu, T. Ionic liquids for synthesis of inorganic nanomaterials. Curr. Opin. Solid State Mater. Sci. 2008, 12, 1–8. [Google Scholar] [CrossRef]
- Luczak, J.; Paszkiewicz, M.; Krukowska, A.; Malankowska, A.; Zaleska-Medynska, A. Ionic liquids for nano- and microstructures preparation. Part 1: Properties and multifunctional role. Adv. Colloid Interface Sci. 2016, 230, 13–28. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y. Recent advances in ionic liquids for synthesis of inorganic nanomaterials. Curr. Nanosci. 2005, 1, 35–42. [Google Scholar] [CrossRef]
- Bernardi, F.; Scholten, J.D.; Fecher, G.H.; Dupont, J.; Morais, J. Probing the chemical interaction between iridium nanoparticles and ionic liquid by XPS analysis. Chem. Phys. Lett. 2009, 479, 113–116. [Google Scholar] [CrossRef]
- Łuczak, J.; Hupka, J.; Thöming, J.; Jungnickel, C. Self-organization of imidazolium ionic liquids in aqueous solution, Colloids Surf. A: Physicochem. Eng. Asp. 2008, 329, 125–133. [Google Scholar] [CrossRef]
- Ren, J.F.; Li, J.Z.; Song, Z.W. Ionic liquid-assisted synthesis of Bi12TiO20 nanostructures and their visible-light photocatalytic performance. Mater. Technol. 2016, 31, 557–561. [Google Scholar] [CrossRef]
- Paszkiewicz, M.; Łuczak, J.; Lisowski, W.; Patyk, P.; Zaleska-Medynska, A. The ILs-assisted solvothermal synthesis of TiO2 spheres: The effect of ionic liquids on morphology and photoactivity of TiO2. Appl. Catal. B Environ. 2016, 184, 223–237. [Google Scholar] [CrossRef]
- Manukumar, K.N.; Nagaraju, G.; Kishore, B.; Madhu, C.; Munichandraiah, N. Ionic liquid-assisted hydrothermal synthesis of SnS nanoparticles: Electrode materials for lithium batteries, photoluminescence and photocatalytic activities. J. Energy Chem. 2018, 27, 806–812. [Google Scholar] [CrossRef] [Green Version]
- Manjunath, K.; Yadav, L.S.; Jayalakshmi, T.; Reddy, V.; Rajanaika, H.; Nagaraju, G. Ionic liquid assisted hydrothermal synthesis of TiO2 nanoparticles: Photocatalytic and antibacterial activity. J. Mater. Res. Tech. 2018, 7, 7–13. [Google Scholar] [CrossRef]
- Paszkiewicz-Gawron, M.; Długokȩcka, M.; Lisowski, W.; Łuczak, J. Dependence between ionic liquid Structure and mechanism of visible-light-induced activity of TiO2 obtained by ionic-liquid-assisted solvothermal synthesis. ACS Sustain. Chem. Eng. 2018, 6, 3927–3937. [Google Scholar] [CrossRef]
- Clark, S.J.; Segall, M.D.; Pickard, C.J.; Hasnip, P.J.; Refson, M.I.J.P.; Payne, M.C. First principles methods using CASTEP. Z. Krist. Cryst. Mater. 2005, 220, 567–570. [Google Scholar] [CrossRef] [Green Version]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed]
- Mostafa, N.Y.; Brown, P.W. Computer simulation of stoichiometric hydroxyapatite: Structure and substitutions. J. Phys. Chem. Solids 2007, 68, 431–437. [Google Scholar] [CrossRef]
- Sameie, H.; Sabbagh Alvani, A.A.; Naseri, N.; Du, S.; Rosei, F. First-principles study on ZnV2O6 and Zn2V2O7: Two new photoanode candidates for photoelectrochemical water oxidation. Ceram. Int. 2018, 44, 6607–6613. [Google Scholar] [CrossRef]
- Pack, J.D.; Monkhorst, H.J. Special points for Brillouin-zone integrations—A reply. Phys. Rev. B 1977, 16, 1748–1749. [Google Scholar] [CrossRef]
- Sameie, H.; Sabbagh Alvani, A.A.; Naseri, N.; Rosei, F.; Mul, G.; Mei, B.T. Photocatalytic Activity of ZnV2O6/Reduced Graphene Oxide Nanocomposite: From Theory to Experiment. J. Electrochem. Soc. 2018, 165, H353–H359. [Google Scholar] [CrossRef]
- Salimi, R.; Sabbagh Alvani, A.A.; Mei, B.T.; Naseri, N.; Du, S.F. Ag-Functionalized CuWO4/WO3 nanocomposites for solar water splitting. New J. Chem. 2019, 43, 2196–2203. [Google Scholar] [CrossRef]
- ISO EN. 10993-5. Biological Evaluation of Medical Devices. Part 5: Tests for In Vitro Cytotoxicity; International Organization for Standardization: Geneva, Switzerland, 2009. [Google Scholar]
- Taherian, M.; Sabbagh Alvani, A.; AShokrgozar, M.A.; Salimi, R.; Moosakhani, S.; Sameie, H.; Tabatabaee, F. Surface-Treated Biocompatible ZnS Quantum Dots: Synthesis, Photo-Physical and Microstructural Properties. Electron. Mater. Lett. 2014, 10, 393–400. [Google Scholar] [CrossRef]
- Matsunaga, K.; Kuwabara, A. First-principles study of vacancy formation in hydroxyapatite. Phys. Rev. B 2007, 75, 014102. [Google Scholar] [CrossRef] [Green Version]
- Yuan, Z.; Li, S.; Liu, J.; Kong, X.; Gao, T. Structural, electronic, dynamical and thermodynamic properties of Ca10(PO4)6(OH)2 and Sr10(PO4)6(OH)2: First-principles study. Int. J. Hydrogen Energy 2018, 43, 13639–13648. [Google Scholar] [CrossRef]
- Bhat, S.S.; Waghmare, U.V.; Ramamurty, U. First-principles study of structure, vibrational, and elastic properties of stoichiometric and calcium-deficient hydroxyapatite. Cryst. Growth Des. 2014, 14, 3131–3141. [Google Scholar] [CrossRef]
- Hughes, J.M.; Cameron, M.; Crowley, K.D. Structural variations in natural F, OH, and Cl apatites. Am. Mineral. 1989, 74, 870–876. [Google Scholar]
- Yeganeh Shad, M.; Nouri, M.; Salmasifar, A.; Sameie, H.; Salimi, R.; Eivaz Mohammadloo, H.; Sabbagh Alvani, A.A.; Ashuri, M.; Tahriri, M. Wet-Chemical Synthesis and Electrochemical Properties of Ce-Doped FeVO4 for Use as New Anode Material in Li-ion Batteries. J. Inorg. Organomet. Polym. Mater. 2013, 23, 1226–1232. [Google Scholar] [CrossRef]
- Paschotta, R. Encyclopedia of Laser Physics and Technology, 1st ed.; Wiley-VCH: New York, NY, USA, 2008. [Google Scholar]
- Li, P.; Fan, W.; Li, Y.; Sun, H.; Cheng, X.; Zhao, X.; Jiang, M. First-principles study of the electronic, optical properties and lattice dynamics of tantalum oxynitride. Inorg. Chem. 2010, 49, 6917–6924. [Google Scholar] [CrossRef] [PubMed]
- Salimi, R.; Sabbagh Alvani, A.A.; Naseri, N.; Du, S.F.; Poelman, D. Visible-enhanced photocatalytic performance of CuWO4/WO3 hetero-structures: Incorporation of plasmonic Ag nanostructures. New J. Chem. 2018, 42, 11109–11116. [Google Scholar] [CrossRef]
- Patterson, A.L. The Scherrer Formula for X-Ray Particle Size Determination. Phys. Rev. 1939, 56, 978–982. [Google Scholar] [CrossRef]
- Rodrigues, L.R.; Avila, M.A.; Monteiro, F.J.M.; Zavaglia, C.A.C. Synthesis and Characterization of Nanocrystalline Hydroxyapatite Gel and Its Application as Scaffold Aggregation. Mater. Res. 2012, 15, 974–980. [Google Scholar] [CrossRef]
- Sameie, H.; Salimi, R.; Sabbagh Alvani, A.A.; Sarabi, A.A.; Moztarzadeh, F.; Mokhtari Farsi, M.A.; Eivaz Mohammadloo, H.; Sabbagh Alvani, M.; Tahriri, M. A nanostructure phosphor: Effect of process parameters on the photoluminescence properties for near-UV WLED applications. J. Inorg. Organomet. Polym. Mater. 2012, 22, 737–743. [Google Scholar] [CrossRef]
- Klee, W.E.; Engel, G. Infrared spectra of the phosphate ions in various apatites. J. Inorg. Nucl. Chem. 1970, 32, 1837–1843. [Google Scholar] [CrossRef]
- Joris, S.J.; Amberg, C.H. Nature of deficiency in nonstoichiometric hydroxyapatites. II. Spectroscopic studies of calcium and strontium hydroxyapatites. J. Phys. Chem. 1971, 75, 3172–3178. [Google Scholar] [CrossRef]
- Fower, B.O. Infrared studies of apatites. I. Vibrational assignments for calcium, strontium, and barium hydroxyapatites utilizing isotopic substitution. Inorg. Chem. 1974, 13, 194–207. [Google Scholar] [CrossRef]
- Koutsopoulos, S. Synthesis and characterization of hydroxyapatite crystals: A review study on the analytical methods. J. Biomed. Mater. Res. 2002, 62, 600–612. [Google Scholar] [CrossRef] [PubMed]
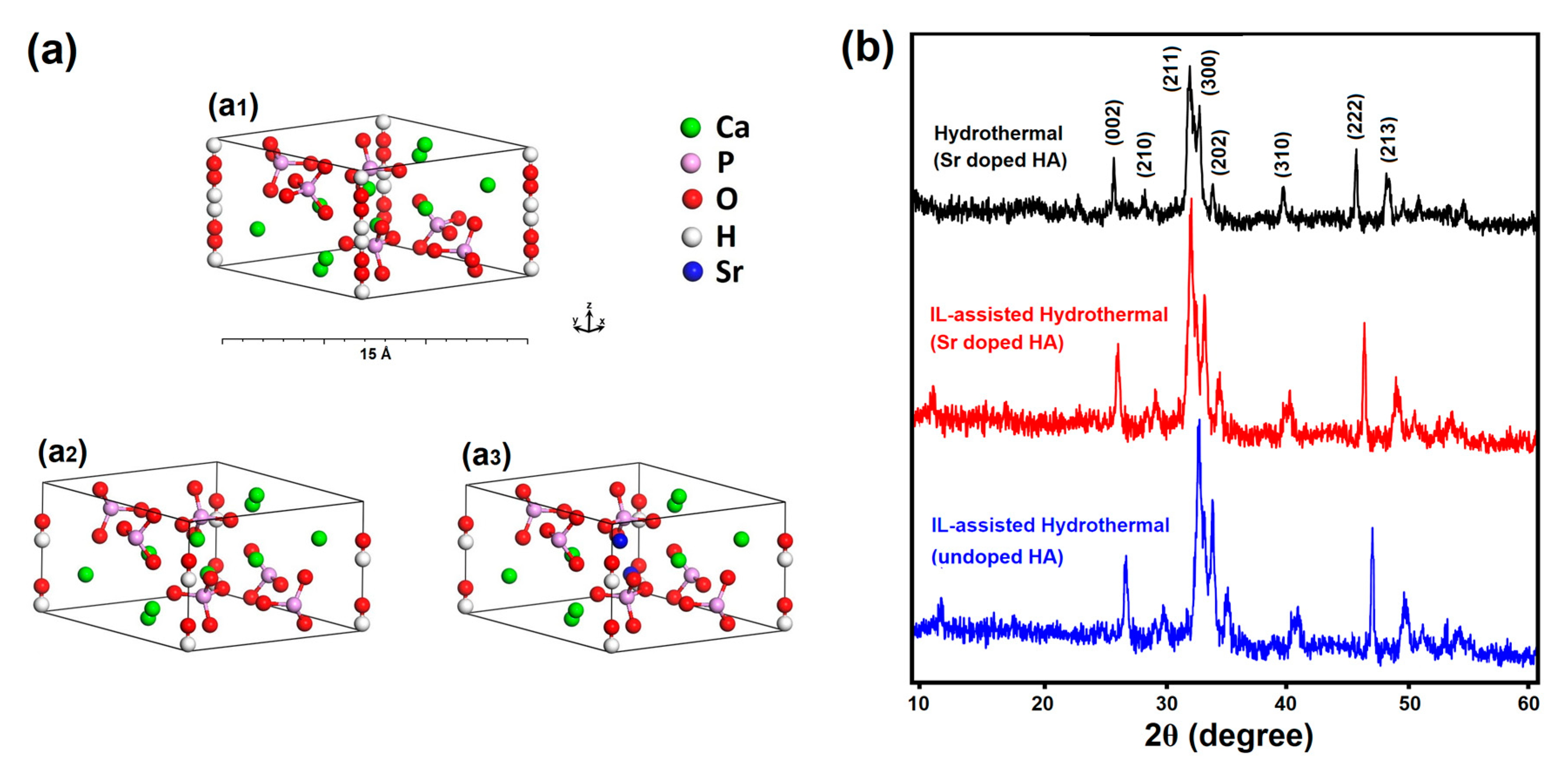


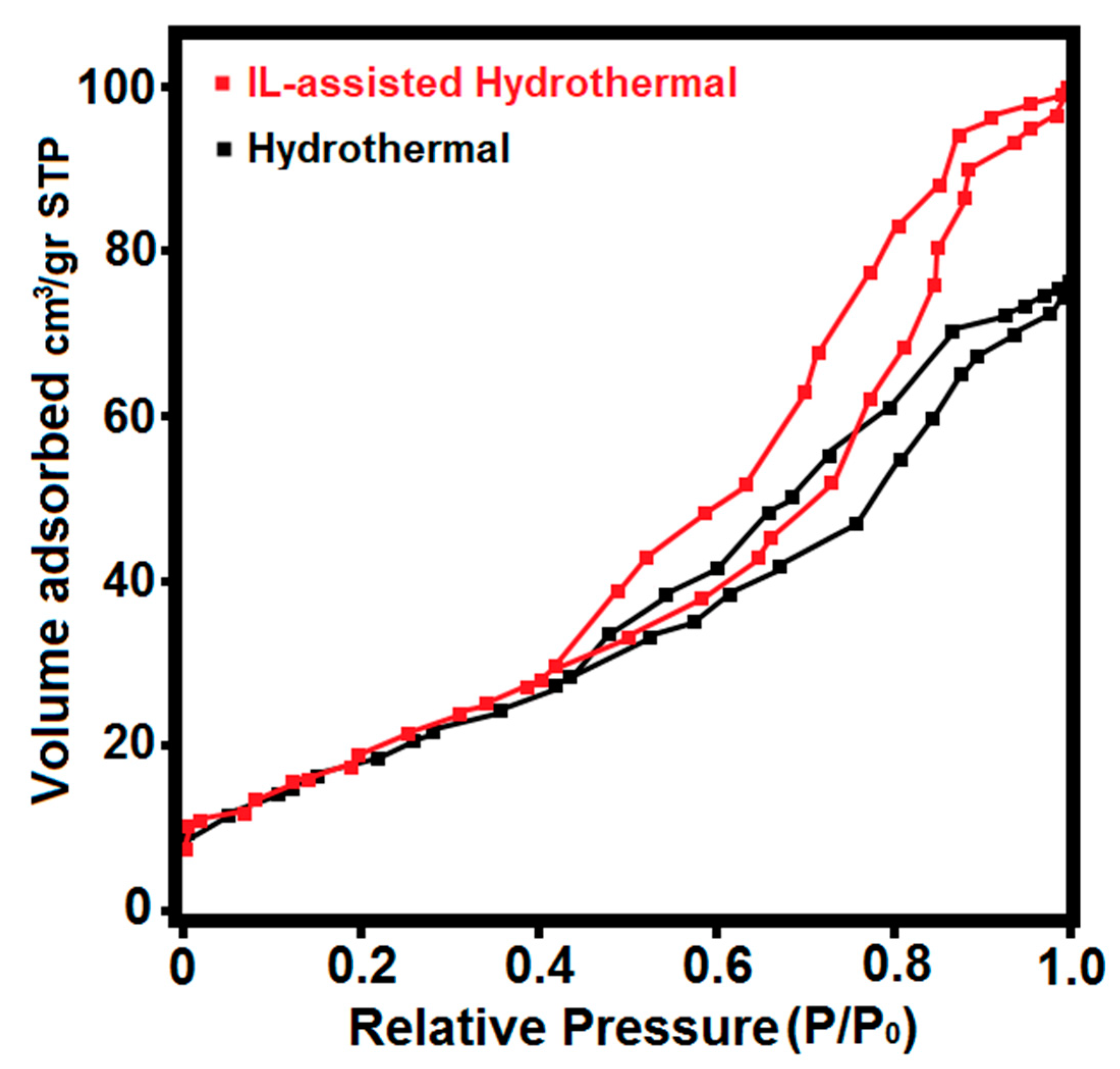
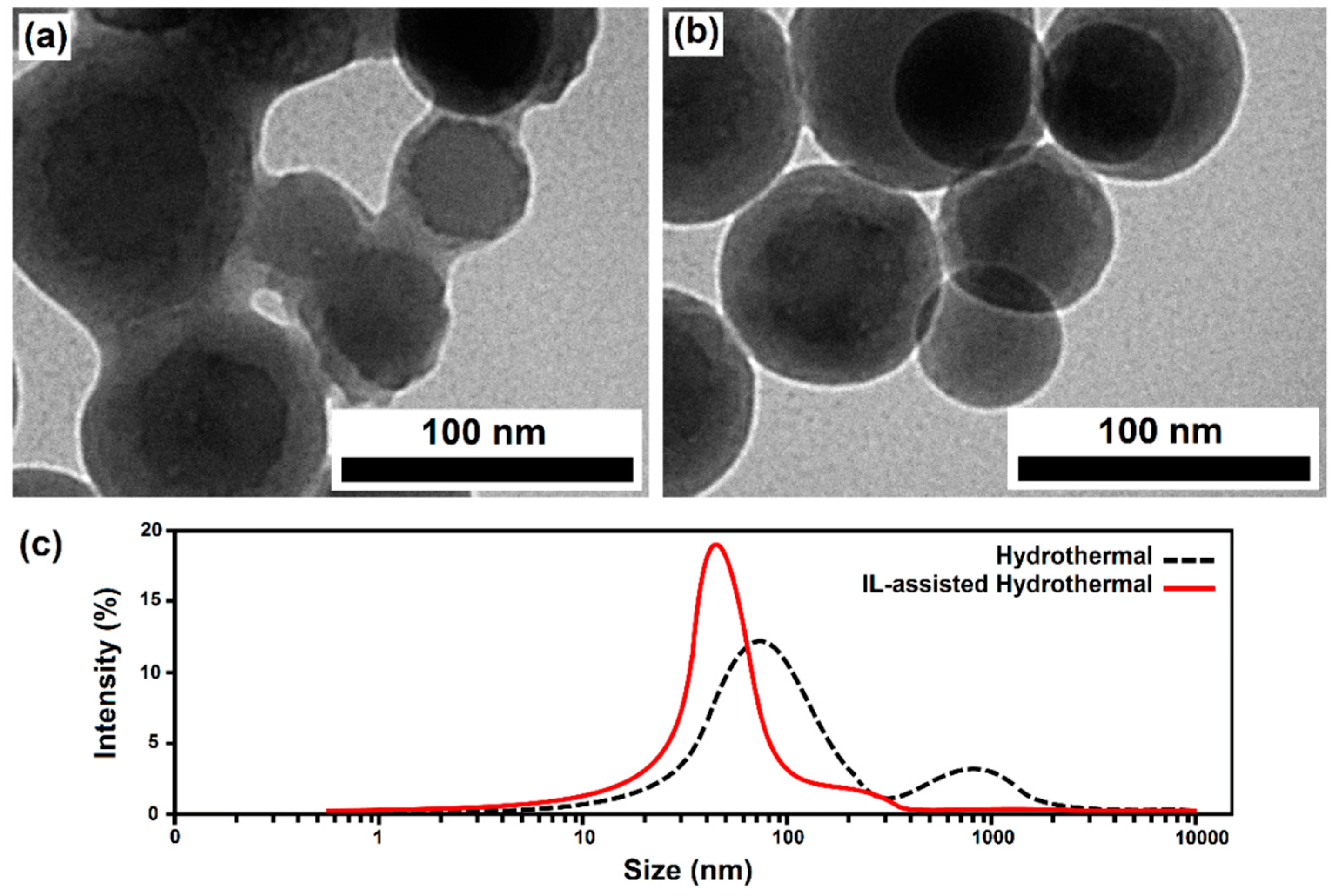


| Cell Parameters | a = b (Å) | c (Å) | |
|---|---|---|---|
| Calculated Data | |||
| Sr-doped HA (IL-assisted HT) | 9.5704 | 6.9980 | |
| Sr-doped HA (Conventional HT) | 9.5812 | 7.0502 | |
| Pure HA (IL-assisted HT) | 9.402 | 6.850 | |
| Sr-doped HA (Computational method) | 9.6590 | 7.1521 | |
| Pure HA (Computational method) | 9.6893 | 6.9762 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moradi, K.; Sabbagh Alvani, A.A.; Poelman, D. Ionic Liquid-Assisted Hydrothermal Synthesis of a Biocompatible Filler for Photo-Curable Dental Composite: From Theory to Experiment. Materials 2019, 12, 2339. https://doi.org/10.3390/ma12142339
Moradi K, Sabbagh Alvani AA, Poelman D. Ionic Liquid-Assisted Hydrothermal Synthesis of a Biocompatible Filler for Photo-Curable Dental Composite: From Theory to Experiment. Materials. 2019; 12(14):2339. https://doi.org/10.3390/ma12142339
Chicago/Turabian StyleMoradi, Kh., A.A. Sabbagh Alvani, and D. Poelman. 2019. "Ionic Liquid-Assisted Hydrothermal Synthesis of a Biocompatible Filler for Photo-Curable Dental Composite: From Theory to Experiment" Materials 12, no. 14: 2339. https://doi.org/10.3390/ma12142339
APA StyleMoradi, K., Sabbagh Alvani, A. A., & Poelman, D. (2019). Ionic Liquid-Assisted Hydrothermal Synthesis of a Biocompatible Filler for Photo-Curable Dental Composite: From Theory to Experiment. Materials, 12(14), 2339. https://doi.org/10.3390/ma12142339







