2.1. Thermal–Mechanical Model of Grinding
In grinding, the grinding force and heat are the causes of deformation, material removal, and phase transformation. The heat conduction satisfies the following differential equation:
where
,
, and
are the density, the specific heat, and the heat conductivity of the workpiece, respectively.
Table 1 shows the temperature-dependent
,
, and
used in the present work.
is the heat flux of the inner heat source. Considering the release or absorption of heat due to phase transformation,
can be estimated by [
15]:
where
is the changing latent heat per unit transformed volume, and
is the increment of transformed volume during the time increment
. Subscript
in
and
refers to the transformation type.
Table 2 shows
used in the present work [
16]. The subroutine *HETVAL provided in Abaqus is used and written to calculate the latent heat of phase transformation.
To solve Equation (1), it is necessary to define the initial and boundary conditions in grinding. The initial temperature of the workpiece is equal to the room temperature:
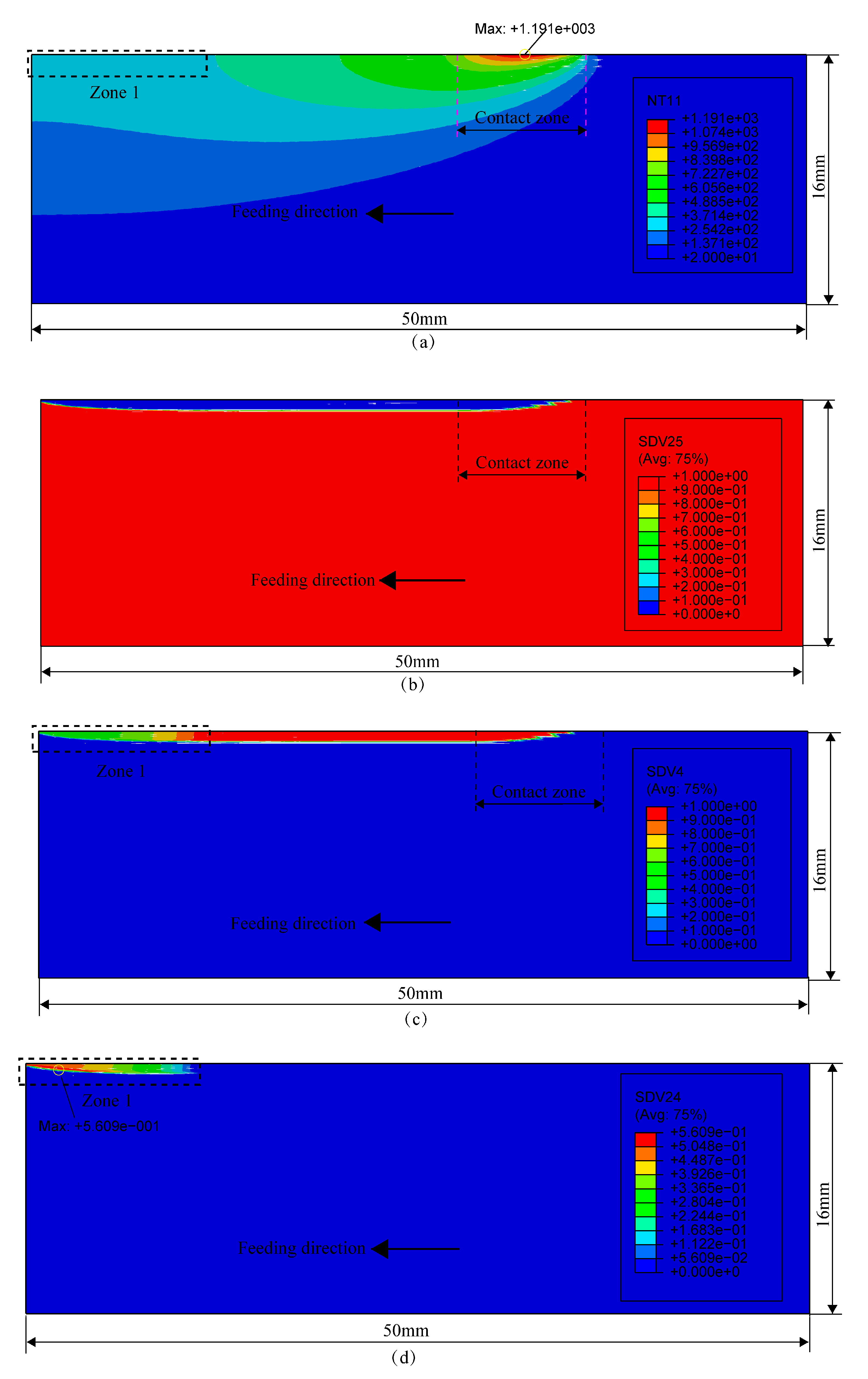
Figure 1a shows the diagram of the thermal boundary conditions in grinding. The distributed heat source moves and acts on the workpiece surface, and it is assumed that the heat source is distributed in a right triangle:
where
is the heat flux of the heat source,
is the feeding speed and
is the contact chord length, which can be estimated by
, where
is the wheel diameter and
is the grinding depth.
is the mean heat flux within the contact zone and can be expressed as:
where
is the tangential grinding force,
is the wheel speed, and
is the grinding width.
is the heat partition ratio transferred into the workpiece and can be estimated by [
17]:
where
and
are the thermal properties of the wheel and the workpiece, respectively.
and
are real and geometrical contact areas between the workpiece and the wheel, respectively. Furthermore, the heat convection and heat radiation occur on the workpiece surfaces, and the equivalent heat flux of the convection and radiation is:
where
is the convection coefficient,
is the surface temperature,
is the emissivity (0.21), and
is the Stefan–Boltzmann constant (5.67 × 10
−8 W/(m
2 4)).
In grinding, the surface materials have elastic–plastic deformation due to the actions of the grinding force and heat. According to Prandtl–Reuss plastic increment theory, the constitutive relationship within the elastic zone is:
and in the plastic zone is:
where
and
are the elastic matrix and plastic matrix, respectively, and
and
are, respectively, elastic vector and plastic vector related to the temperature, and
is the temperature increment.
and
are the total stress increment and strain increment, respectively. The major strain sources, such as mechanical strain, thermal strain, and the volume change strain caused by phase transformation, were considered, and some less important strain sources, such as stress relaxation and strain caused by transformation plasticity, were ignored in the present paper [
18,
19]. Then,
can be expressed as:
where
is the elastic strain, which can be calculated by Hooke’s law with the temperature-dependent elastic modulus
and constant Poisson’s ratio
(as shown in
Table 3).
is the plastic strain increment following Prandtl–Reuss flow rule, the von Mises yield criterion, and the Johnson-Cook model.
Table 4 shows the Johnson-Cook coefficients used in the present work [
20].
is the thermal strain increment and can be estimated by:
where
is the thermal expansion coefficient; the temperature-dependent
used in the present work is listed in
Table 3.
is the volume strain increment caused by phase transformation and can be estimated by [
21]:
where
is the volume change ratio caused by phase transformation and is listed in
Table 5 [
21].
was incorporated into the model by using and writing the subroutine *UEXPAN.
Figure 1b shows the mechanical boundary conditions in grinding. The upper surface is under the actions of moving normal grinding force and tangential grinding force. The grinding forces distribute in the contact zone in a right triangle and can be expressed as follows:
where
is the normal grinding force. The bottom surface is fixed on the worktable, and its displacements are set as 0.