Effect of Layer Charge Characteristics on the Distribution Characteristics of H2O and Ca2+ in Ca-Montmorillonites Interlayer Space: Molecular Dynamics Simulation
Abstract
1. Introduction
2. Simulation Details
2.1. Simulation Model Establishment
2.2. Simulation Parameters
3. Analysis and Discussion
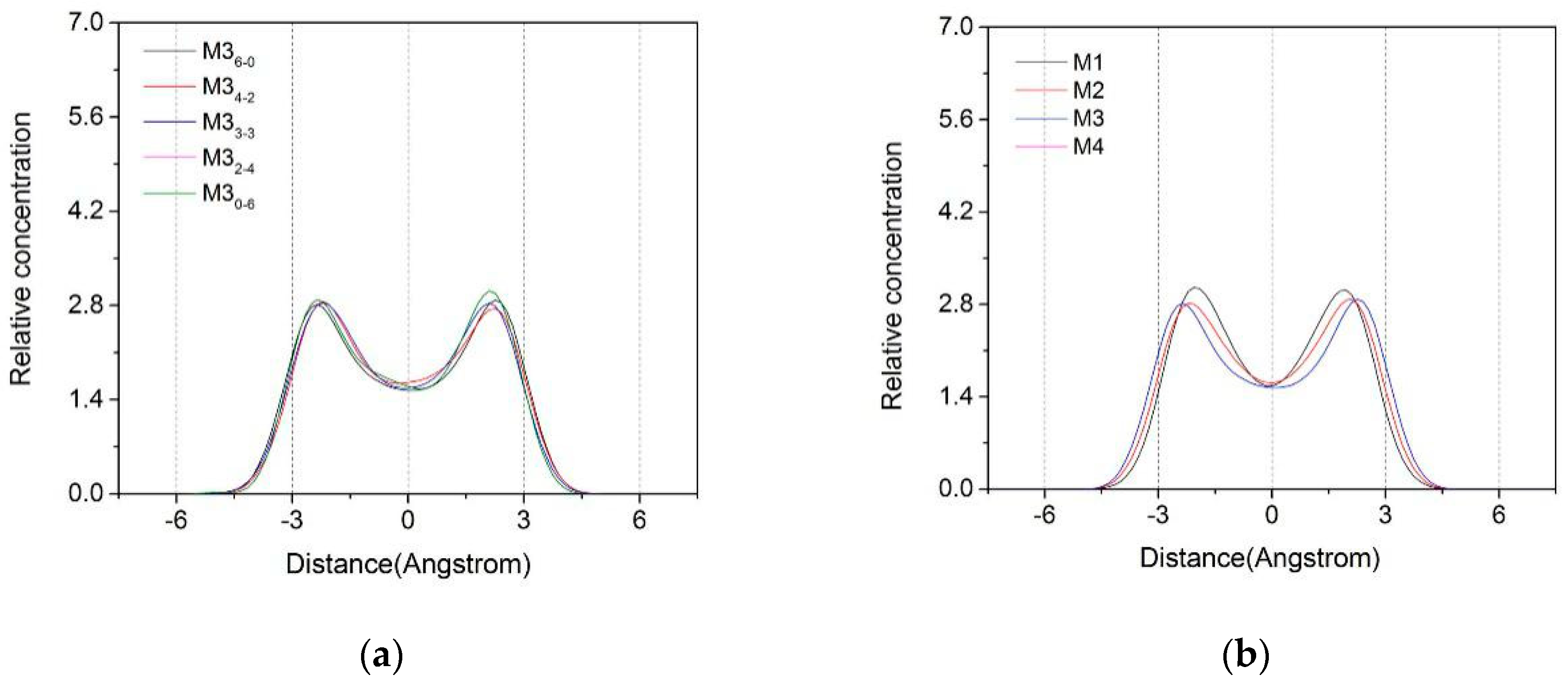
3.1. Z-Axis Density Distribution
3.1.1. Z-Axis Density Distribution Characteristics of Water Molecules
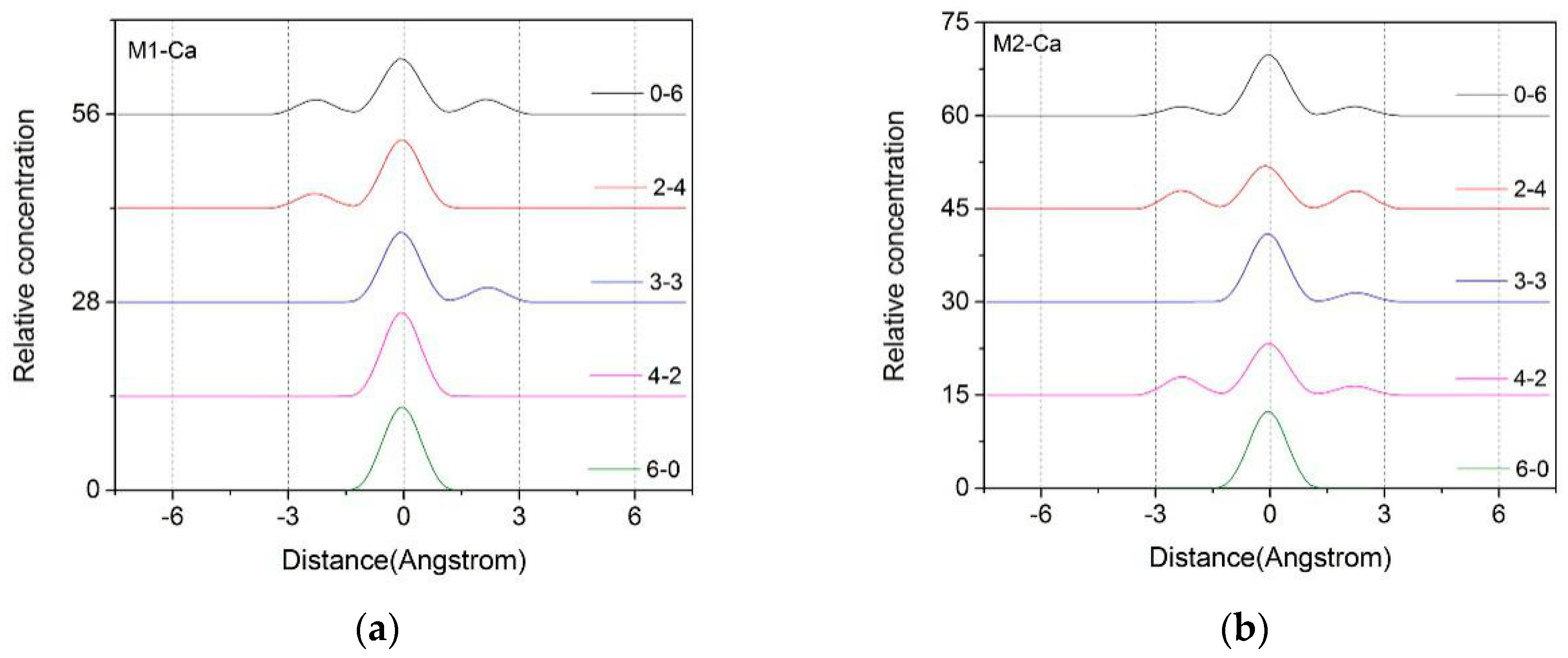
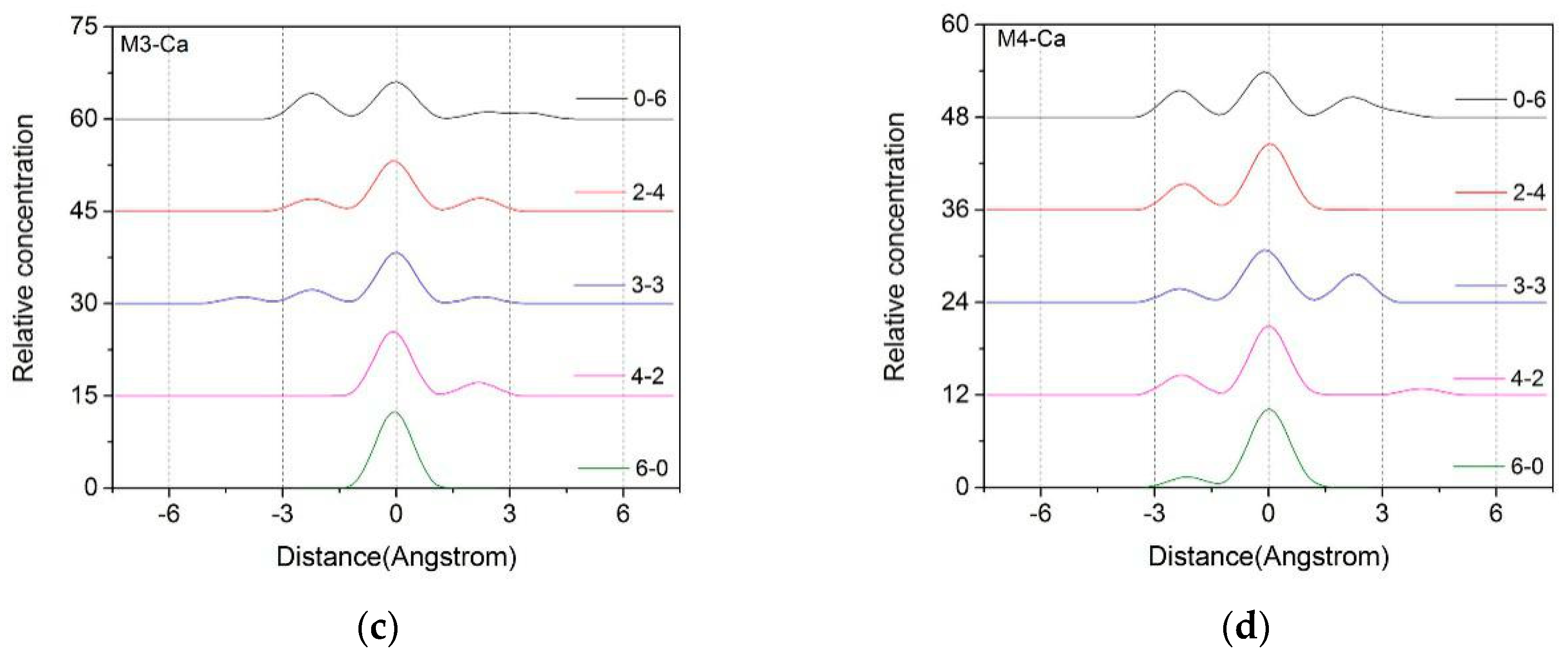
3.1.2. Z-Axis Density Distribution Characteristics of Ca2+
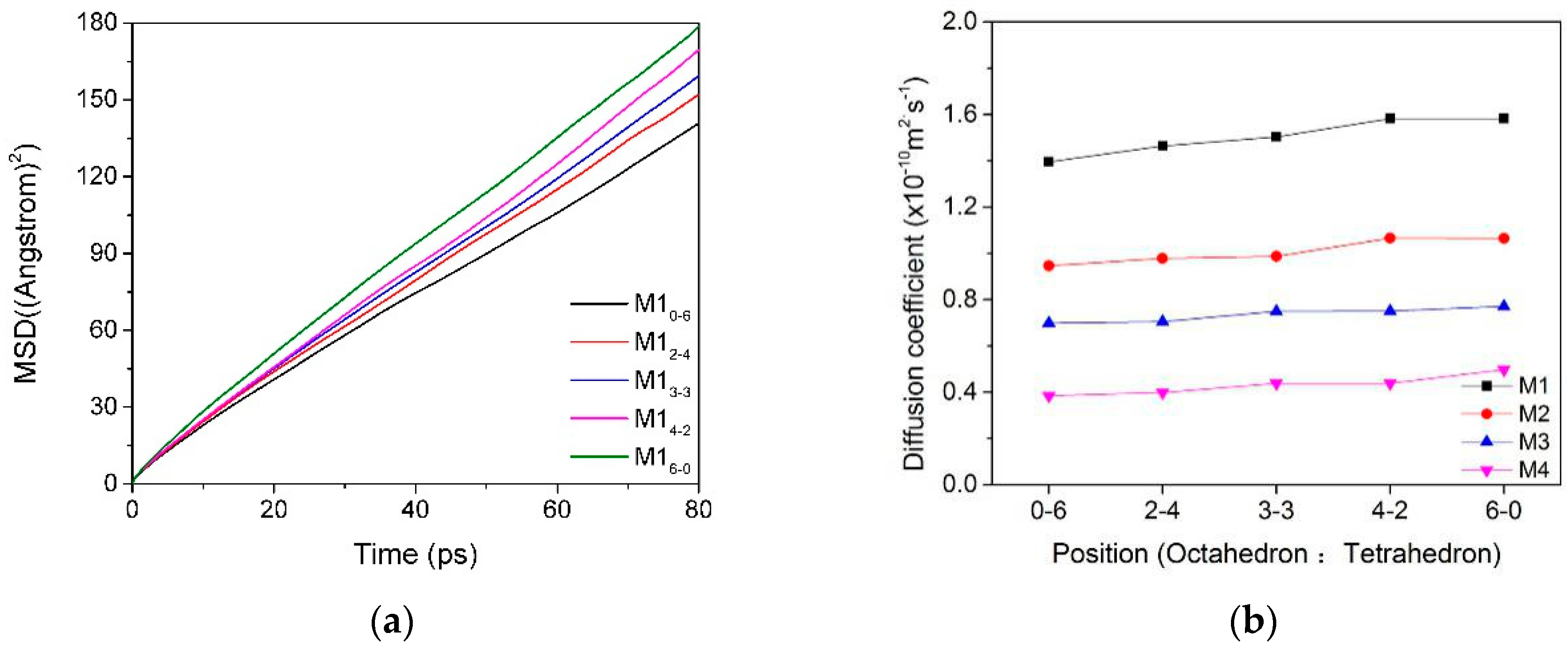
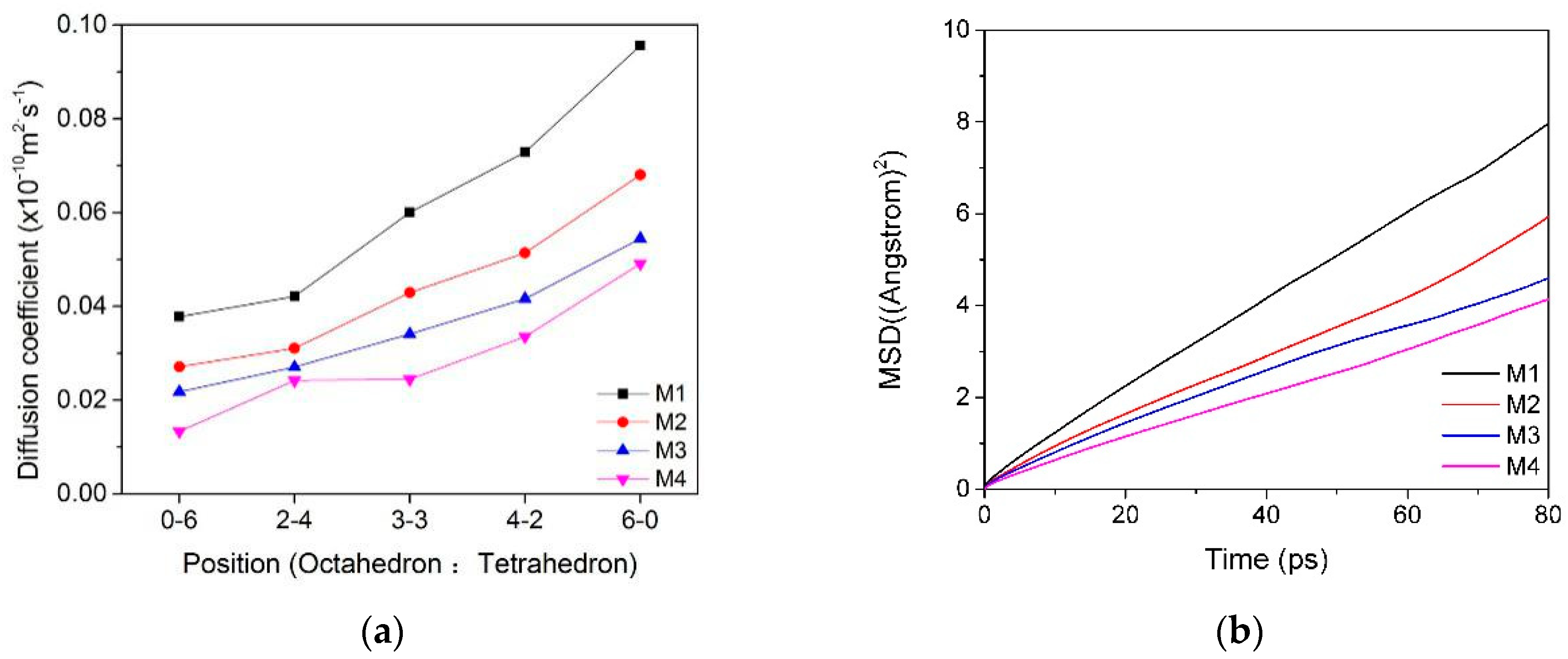
3.2. Mean Square Displacement (MSD) and Self-Diffusion Coefficient
3.2.1. MSD and Self-Diffusion Coefficient of Water Molecules
3.2.2. MSD and Self-Diffusion Coefficient of Ca2+
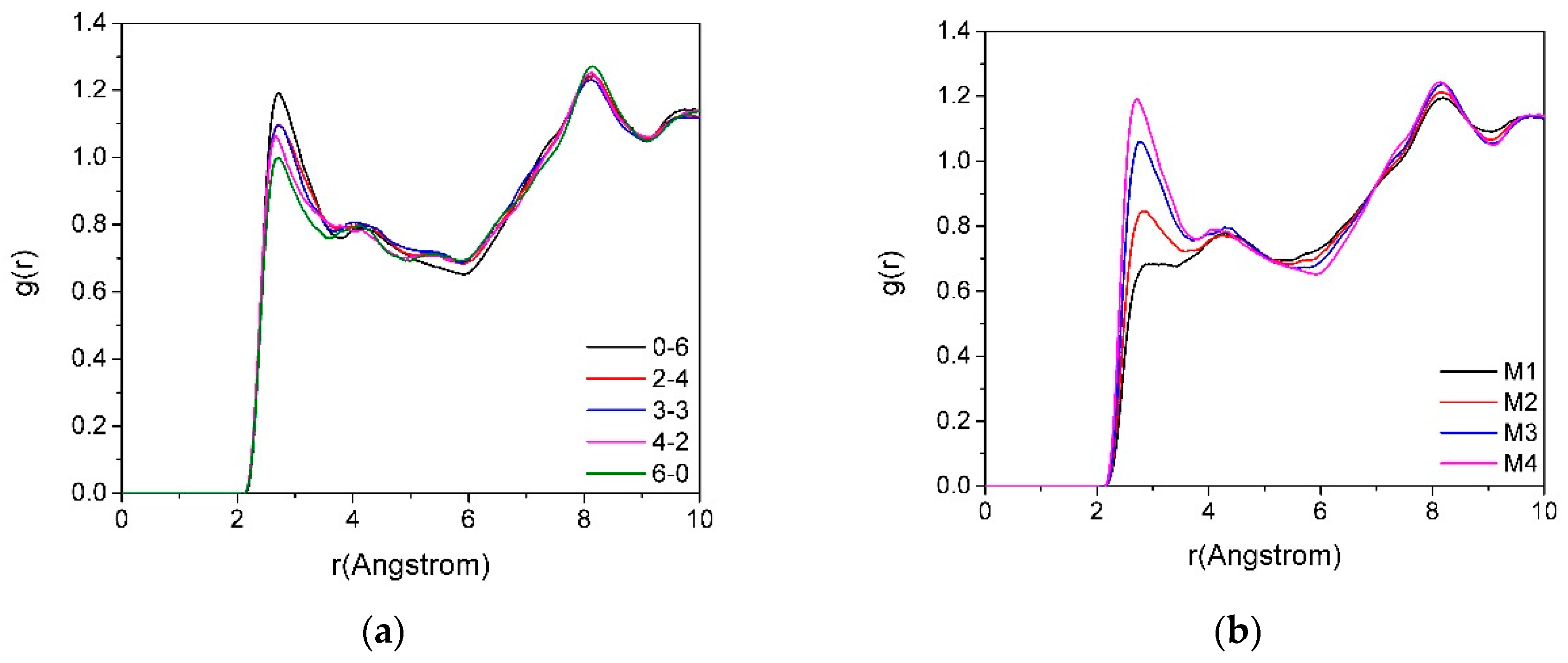
3.3. The Radial Distribution Function (RDF)
3.3.1. The RDF of Ot-Hw
3.3.2. The RDF of Ot-Ca
4. Conclusions
- (1)
- When the layer charge of the Ca-montmorillonite was mainly derived from the replacement of Si4+ with Al3+ in the tetrahedron, the Hw and the Ot were more easily combined to form a hydrogen bond in the interlayer. Due to the hydrogen bonding force, the water molecules were more compactly arranged, and the diffusion of water molecules among the layers was reduced. Due to the action of electrostatic attraction between the Ca2+ and the Si–O surface, the Ca2+ move to the Si–O surface on both sides and the distribution range was expanded.
- (2)
- With the increase of the charge density in the Ca-montmorillonite layer, the polarity of the Si–O surface was enhanced, and the polar force generated by the water molecules in the interlayers was stronger, the number of hydrogen bonds between the Hw and the Ot increased, and the self-diffusion coefficient of the water molecules decreased. For the Ca2+, the higher the layer charge density of montmorillonite, the larger the electrostatic force of the Ca2+ subjected to the negative point, and the larger the distribution range of Ca2+.
Author Contributions
Funding
Conflicts of Interest
References
- Mignon, P.; Ugliengo, P.; Sodupe, M.; Hernandez, E.R. Ab initio molecular dynamics study of the hydration of Li(+), Na(+) and K(+) in a montmorillonite model. Influence of isomorphic substitution. Phys. Chem. Chem. Phys. 2010, 12, 688–697. [Google Scholar] [CrossRef] [PubMed]
- Salles, F.; Douillard, J.-M.; Bildstein, O.; El Ghazi, S.; Prélot, B.; Zajac, J.; Van Damme, H. Diffusion of Interlayer Cations in Swelling Clays as a Function of Water Content: Case of Montmorillonites Saturated with Alkali Cations. J. Phys. Chem. C 2015, 119, 10370–10378. [Google Scholar] [CrossRef]
- Greathouse, J.A.; Cygan, R.T.; Fredrich, J.T.; Jerauld, G.R. Molecular Dynamics Simulation of Diffusion and Electrical Conductivity in Montmorillonite Interlayers. J. Phys. Chem. C 2016, 120, 1640–1649. [Google Scholar] [CrossRef]
- Park, J.H.; Shin, H.J.; Kim, M.H.; Kim, J.S.; Kang, N.; Lee, J.Y.; Kim, K.T.; Lee, J.I.; Kim, D.D. Application of montmorillonite in bentonite as a pharmaceutical excipient in drug delivery systems. J. Pharm. Investig. 2016, 46, 363–375. [Google Scholar] [CrossRef]
- Weng, Z.; Wang, J.; Senthil, T.; Wu, L. Mechanical and thermal properties of ABS/montmorillonite nanocomposites for fused deposition modeling 3D printing. Mater. Des. 2016, 102, 276–283. [Google Scholar] [CrossRef]
- Cygan, R.T.; Liang, J.-J.; Kalinichev, A.G. Molecular Models of Hydroxide, Oxyhydroxide, and Clay Phases and the Development of a General Force Field. J. Phys. Chem. B 2004, 108, 1255–1266. [Google Scholar] [CrossRef]
- Shahriyari, R.; Khosravi, A.; Ahmadzadeh, A. Nanoscale simulation of Na-Montmorillonite hydrate under basin conditions, application of CLAYFF force field in parallel GCMC. Mol. Phys. 2013, 111, 3156–3167. [Google Scholar] [CrossRef]
- Tangaraj, V.; Janot, J.M.; Jaber, M.; Bechelany, M.; Balme, S. Adsorption and photophysical properties of fluorescent dyes over montmorillonite and saponite modified by surfactant. Chemosphere 2017, 184, 1355–1361. [Google Scholar] [CrossRef]
- Anirudhan, T.S.; Ramachandran, M. Removal of 2,4,6-trichlorophenol from water and petroleum refinery industry effluents by surfactant-modified bentonite. J. Water Process Eng. 2014, 1, 46–53. [Google Scholar] [CrossRef]
- Skipper, N.T. Monte carlo simulation of interlayer molecular structure in swelling clay minerals. 2. Monolayer hydrates. Clays Clay Miner. 1995, 43, 285–293. [Google Scholar] [CrossRef]
- Boek, E.S.; Coveney, P.V.; Skipper, N.T. Molecular modeling of clay hydration: A study of hysteresis loops in the swelling curves of sodium montmorillonites. Langmuir 1995, 11, 4629–4631. [Google Scholar] [CrossRef]
- Chang, F.R.C.; Skipper, N.T.; Sposito, G. Computer simulation of interlayer molecular structure in sodium montmorillonite hydrates. Langmuir 1995, 11, 2734–2741. [Google Scholar] [CrossRef]
- Boek, E.S.; Coveney, P.V.; Skipper, N.T. Monte Carlo molecular modeling studies of hydrated Li-, Na-, and K-smectites_Understanding the role of potassium as a clay swelling inhibitor. J. Am. Chem. Soc. 1995, 117, 12608–12617. [Google Scholar] [CrossRef]
- Marry, V.; Turq, P.; Cartailler, T.; Levesque, D. Microscopic simulation of structure and dynamics of water and counterions in a monohydrated montmorillonite. J. Chem. Phys. 2002, 117, 3454–3463. [Google Scholar] [CrossRef]
- Kalinichev, A.; Liu, X.; Cygan, R. Introduction to a Special Issue on Molecular Computer Simulations of Clays and Clay–water Interfaces: Recent Progress, Challenges, and Opportunities. Clays Clay Miner. 2016, 64, 335–336. [Google Scholar] [CrossRef]
- Seppälä, A.; Puhakka, E.; Olin, M. Effect of layer charge on the crystalline swelling of Na+, K+ and Ca2+ montmorillonites: DFT and molecular dynamics studies. Clay Miner. 2018, 51, 197–211. [Google Scholar] [CrossRef]
- Miranda-Pascual, M.G.; Chávez-García, M.L. Monte Carlo molecular simulation of the Na-, Mg-, and mixtures of Na/Mg-montmorillonites systems, in function of the pressure. Mol. Phys. 2014, 113, 835–847. [Google Scholar] [CrossRef]
- Zhang, L.; Lu, X.; Liu, X.; Zhou, J.; Zhou, H. Hydration and Mobility of Interlayer Ions of (Nax, Cay)-Montmorillonite: A Molecular Dynamics Study. J. Phys. Chem. C 2014, 118, 29811–29821. [Google Scholar] [CrossRef]
- Dai, W.; Shui, Z.H.; Li, K.; Duan, P. Molecular Simulation on Modification of Structure and Thermodynamic Properties of Montmorillonite. Appl. Mech. Mater. 2010, 44–47, 2529–2533. [Google Scholar] [CrossRef]
- Shen, W.; Li, L.; Zhou, H.; Zhou, Q.; Chen, M.; Zhu, J. Effects of charge density on the hydration of siloxane surface of montmorillonite: A molecular dynamics simulation study. Appl. Clay Sci. 2018, 159, 10–15. [Google Scholar] [CrossRef]
- Marry, V.; Dubois, E.; Malikova, N.; Durand-Vidal, S.; Longeville, S.; Breu, J. Water dynamics in hectorite clays: Influence of temperature studied by coupling neutron spin echo and molecular dynamics. Environ. Sci. Technol. 2011, 45, 2850–2855. [Google Scholar] [CrossRef]
- Malikova, N.; Cadène, A.; Dubois, E.; Marry, V.; Durand-Vidal, S.; Turq, P.; Breu, J.; Longeville, S.; Zanotti, J.M. Water Diffusion in a Synthetic Hectorite Clay Studied by Quasi-elastic Neutron Scattering. J. Phys. Chem. C 2007, 111, 17603–17611. [Google Scholar] [CrossRef]
- Morodome, S.; Kawamura, K. In X-ray Diffraction Study of the Swelling of Montmorillonite as Affected by Exchangeable Cations and Temperature. Clays Clay Miner. 2011, 59, 165–175. [Google Scholar] [CrossRef]
- Shapley, T.V.; Molinari, M.; Zhu, R.; Parker, S.C. Atomistic Modeling of the Sorption Free Energy of Dioxins at Clay–Water Interfaces. J. Phys. Chem. C 2013, 117, 24975–24984. [Google Scholar] [CrossRef]
- Xu, J.; Camara, M.; Liu, J.; Peng, L.; Zhang, R.; Ding, T. Molecular dynamics study of the swelling patterns of Na/Cs-, Na/Mg-montmorillonites and hydration of interlayer cations. Mol. Simul. 2017, 43, 575–589. [Google Scholar] [CrossRef]
- Sun, L.; Ling, C.Y.; Lavikainen, L.P.; Hirvi, J.T.; Kasa, S.; Pakkanen, T.A. Influence of layer charge and charge location on the swelling pressure of dioctahedral smectites. Chem. Phys. 2016, 473, 40–45. [Google Scholar] [CrossRef]
- Dazas, B.; Lanson, B.; Delville, A.; Robert, J.-L.; Komarneni, S.; Michot, L.J.; Ferrage, E. Influence of Tetrahedral Layer Charge on the Organization of Interlayer Water and Ions in Synthetic Na-Saturated Smectites. J. Phys. Chem. C 2015, 119, 4158–4172. [Google Scholar] [CrossRef]
- Christidis, G.E.; Blum, A.E.; Eberl, D.D. Influence of layer charge and charge distribution of smectites on the flow behaviour and swelling of bentonites. Appl. Clay Sci. 2006, 34, 125–138. [Google Scholar] [CrossRef]
- Sun, L.; Tanskanen, J.T.; Hirvi, J.T.; Kasa, S.; Schatz, T.; Pakkanen, T.A. Molecular dynamics study of montmorillonite crystalline swelling: Roles of interlayer cation species and water content. Chem. Phys. 2015, 455, 23–31. [Google Scholar] [CrossRef]
- Yi, H.; Zhang, X.; Zhao, Y.; Liu, L.; Song, S. Molecular dynamics simulations of hydration shell on montmorillonite (001) in water. Surf. Interface Anal. 2016, 48, 976–980. [Google Scholar] [CrossRef]
- Liu, X.; Zhu, R.; Ma, J.; Ge, F.; Xu, Y.; Liu, Y. Molecular dynamics simulation study of benzene adsorption to montmorillonite: Influence of the hydration status. Colloids Surf. A Physicochem. Eng. Asp. 2013, 434, 200–206. [Google Scholar] [CrossRef]
- Qu, X.; Zhang, Y.; Li, H.; Zheng, S.; Zhu, D. Probing the specific sorption sites on montmorillonite using nitroaromatic compounds and hexafluorobenzene. Environ. Sci. Technol. 2011, 45, 2209–2216. [Google Scholar] [CrossRef]
- Berghout, A.; Tunega, D.; Zaoui, A. Density Functional Theory (DFT) Study of the Hydration Steps of Na+/Mg2+/Ca2+/Sr2+/Ba2+-Exchanged Montmorillonites. Clays Clay Miner. 2010, 58, 174–187. [Google Scholar] [CrossRef]
- Cygan, R.T.; Romanov, V.N.; Myshakin, E.M. Molecular Simulation of Carbon Dioxide Capture by Montmorillonite Using an Accurate and Flexible Force Field. J. Phys. Chem. C 2012, 116, 13079–13091. [Google Scholar] [CrossRef]
- Ferrage, E.; Lanson, B.; Sakharov, B.A.; Geoffroy, N.; Jacquot, E.; Drits, V.A. Investigation of dioctahedral smectite hydration properties by modeling of X-ray diffraction profiles: Influence of layer charge and charge location. Am. Mineral. 2007, 92, 1731–1743. [Google Scholar] [CrossRef]
- Zhu, R.; Chen, W.; Shapley, T.V.; Molinari, M.; Ge, F.; Parker, S.C. Sorptive characteristics of organomontmorillonite toward organic compounds: A combined LFERs and molecular dynamics simulation study. Environ. Sci. Technol. 2011, 45, 6504–6510. [Google Scholar] [CrossRef]
- Zhao, Q.; Burns, S.E. Molecular dynamics simulation of secondary sorption behavior of montmorillonite modified by single chain quaternary ammonium cations. Environ. Sci. Technol. 2012, 46, 3999–4007. [Google Scholar] [CrossRef]
- Casewit, C.J.C.K.S.; Rappe, A.K. Application of a Universal Force Field to Main Group Compounds. J. Am. Chem. Soc. 1992, 114, 10046–10053. [Google Scholar] [CrossRef]
- Zhou, J.; Lu, X.; Wang, Y.; Shi, J. Molecular dynamics study on ionic hydration. Fluid Phase Equilibria 2002, 194, 257–270. [Google Scholar] [CrossRef]
- Tambach, T.J.; Bolhuis, P.G.; Hensen, E.J.; Smit, B. Hysteresis in clay swelling induced by hydrogen bonding: Accurate prediction of swelling states. Langmuir 2006, 22, 1223–1234. [Google Scholar] [CrossRef]
- Tambach, T.J.; Hensen, E.J.M.; Smit, B. Molecular Simulations of Swelling Clay Minerals. J. Phys. Chem. B 2004, 108, 7586–7596. [Google Scholar] [CrossRef]
- Douillard, J.M.; Lantenois, S.; Prelot, B.; Zajac, J.; Henry, M. Study of the influence of location of substitutions on the surface energy of dioctahedral smectites. J. Colloid Interface Sci. 2008, 325, 275–281. [Google Scholar] [CrossRef]
- Mohammadi, M.; Mohammadiarani, H.; Shaw, V.S.; Neubig, R.R.; Vashisth, H. Interplay of cysteine exposure and global protein dynamics in small-molecule recognition by a regulator of G-protein signaling protein. Proteins 2019, 87, 146–156. [Google Scholar] [CrossRef]


| Atom | X | Y | Z |
|---|---|---|---|
| Al | 0.000 | 3.020 | 15.500 |
| Si | 0.472 | 1.510 | 12.580 |
| Oo | 0.122 | 0.000 | 12.040 |
| Oo | −0.686 | 2.615 | 12.240 |
| Ot | 0.772 | 5.510 | 14.200 |
| O(OH) | 0.808 | 4.530 | 14.250 |
| H(OH) | −0.103 | 4.530 | 13.812 |
| M1 | 0-6 | 2-4 | 3-3 | 4-2 | 6-0 |
| Volume/Å3 × 103 | 16.94 | 16.94 | 16.94 | 16.94 | 1.694 |
| c/Å | 15.41 | 15.42 | 15.42 | 15.41 | 15.42 |
| β/° | 104.9 | 105.0 | 105.0 | 105.0 | 105.0 |
| n(Hydrogen bonding) | 92 | 92 | 93 | 91 | 94 |
| M2 | 0-6 | 2-4 | 3-3 | 4-2 | 6-0 |
| Volume/Å3 × 103 | 16.94 | 16.94 | 16.94 | 16.94 | 16.94 |
| c/Å | 15.41 | 15.42 | 15.42 | 15.42 | 15.41 |
| β/° | 104.9 | 105.0 | 105.0 | 105.0 | 104.9 |
| n(Hydrogen bonding) | 100 | 99 | 100 | 99 | 99 |
| M3 | 0-6 | 2-4 | 3-3 | 4-2 | 6-0 |
| Volume/Å3 × 103 | 16.93 | 16.94 | 16.95 | 16.94 | 16.94 |
| c/Å | 15.41 | 15.42 | 15.42 | 15.42 | 15.42 |
| β/° | 105.0 | 105.0 | 105.0 | 104.9 | 105.0 |
| n(Hydrogen bonding) | 108 | 105 | 109 | 108 | 106 |
| M4 | 0-6 | 2-4 | 3-3 | 4-2 | 6-0 |
| Volume/Å3 × 103 | 16.93 | 16.94 | 16.94 | 16.94 | 16.94 |
| c/Å | 15.42 | 15.41 | 15.42 | 15.42 | 15.42 |
| β/° | 105.1 | 105.0 | 105.0 | 104.9 | 105.0 |
| n(Hydrogen bonding) | 117 | 117 | 115 | 117 | 118 |
| Type | 0-6 | 2-4 | 3-3 | 4-2 | 6-0 |
|---|---|---|---|---|---|
| M1 | 1.3959 | 1.4648 | 1.5043 | 1.5823 | 1.5823 |
| M2 | 0.9461 | 0.9780 | 0.9870 | 1.0659 | 1.0650 |
| M3 | 0.6987 | 0.7048 | 0.7496 | 0.7512 | 0.7717 |
| M4 | 0.3848 | 0.3994 | 0.4388 | 0.4386 | 0.4977 |
| Type | 0-6 | 2-4 | 3-3 | 4-2 | 6-0 |
|---|---|---|---|---|---|
| M1 | 0.0378 | 0.0421 | 0.0600 | 0.0729 | 0.0956 |
| M2 | 0.0331 | 0.0311 | 0.0430 | 0.0514 | 0.0681 |
| M3 | 0.0217 | 0.0270 | 0.0341 | 0.0347 | 0.0545 |
| M4 | 0.0133 | 0.0242 | 0.0244 | 0.0336 | 0.0492 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiu, J.; Li, G.; Liu, D.; Jiang, S.; Wang, G.; Chen, P.; Zhu, X.; Cao, X.; Lyu, X. Effect of Layer Charge Characteristics on the Distribution Characteristics of H2O and Ca2+ in Ca-Montmorillonites Interlayer Space: Molecular Dynamics Simulation. Materials 2019, 12, 2318. https://doi.org/10.3390/ma12142318
Qiu J, Li G, Liu D, Jiang S, Wang G, Chen P, Zhu X, Cao X, Lyu X. Effect of Layer Charge Characteristics on the Distribution Characteristics of H2O and Ca2+ in Ca-Montmorillonites Interlayer Space: Molecular Dynamics Simulation. Materials. 2019; 12(14):2318. https://doi.org/10.3390/ma12142318
Chicago/Turabian StyleQiu, Jun, Guoqing Li, Dongliang Liu, Shan Jiang, Guifang Wang, Ping Chen, Xiangnan Zhu, Xiaoqiang Cao, and Xianjun Lyu. 2019. "Effect of Layer Charge Characteristics on the Distribution Characteristics of H2O and Ca2+ in Ca-Montmorillonites Interlayer Space: Molecular Dynamics Simulation" Materials 12, no. 14: 2318. https://doi.org/10.3390/ma12142318
APA StyleQiu, J., Li, G., Liu, D., Jiang, S., Wang, G., Chen, P., Zhu, X., Cao, X., & Lyu, X. (2019). Effect of Layer Charge Characteristics on the Distribution Characteristics of H2O and Ca2+ in Ca-Montmorillonites Interlayer Space: Molecular Dynamics Simulation. Materials, 12(14), 2318. https://doi.org/10.3390/ma12142318




