Effectiveness of Low-Level Laser Therapy during Tooth Movement: A Randomized Clinical Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Sample
2.3. Power Sample Analysis
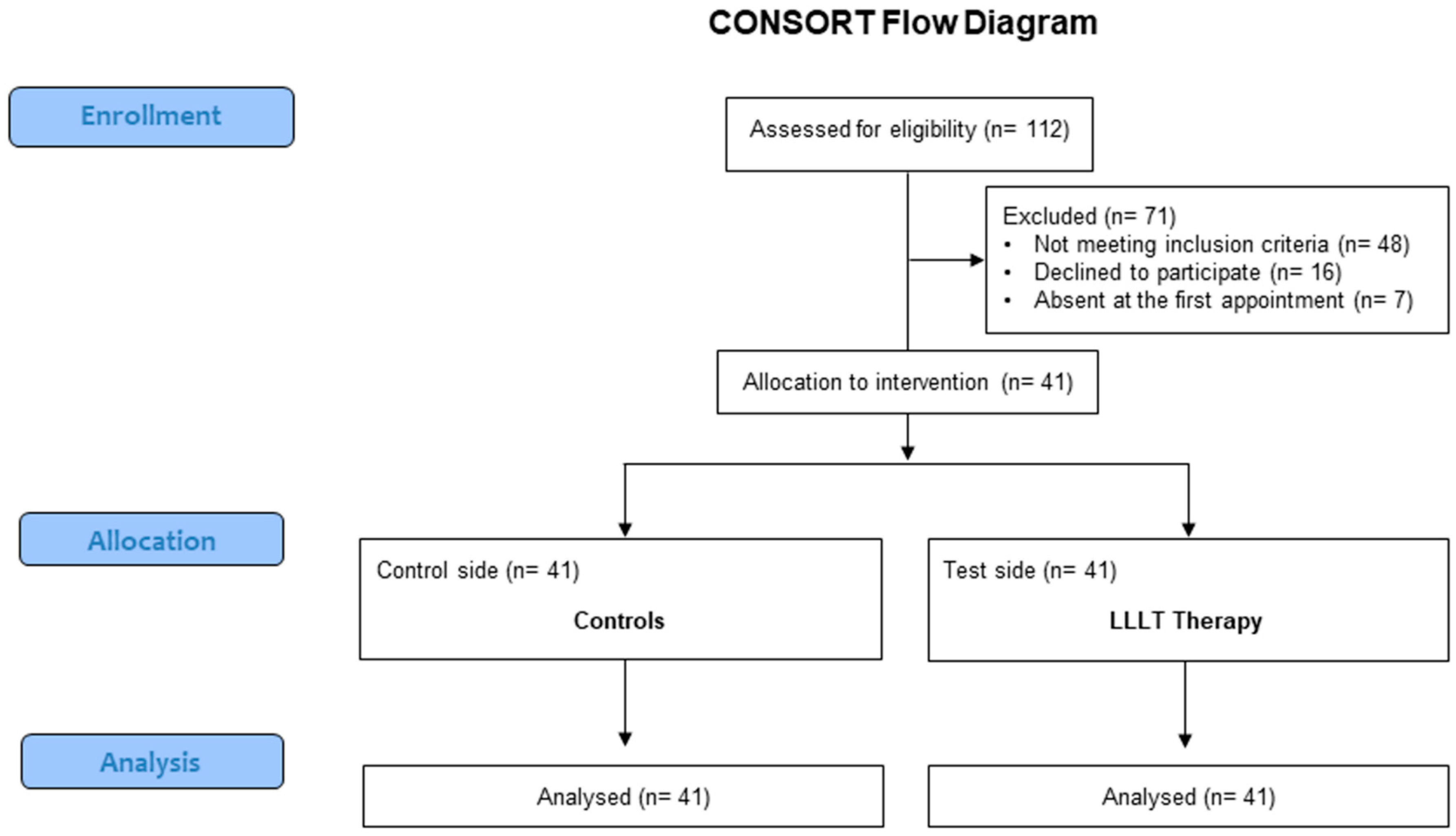
2.4. Randomization
2.5. Treatment
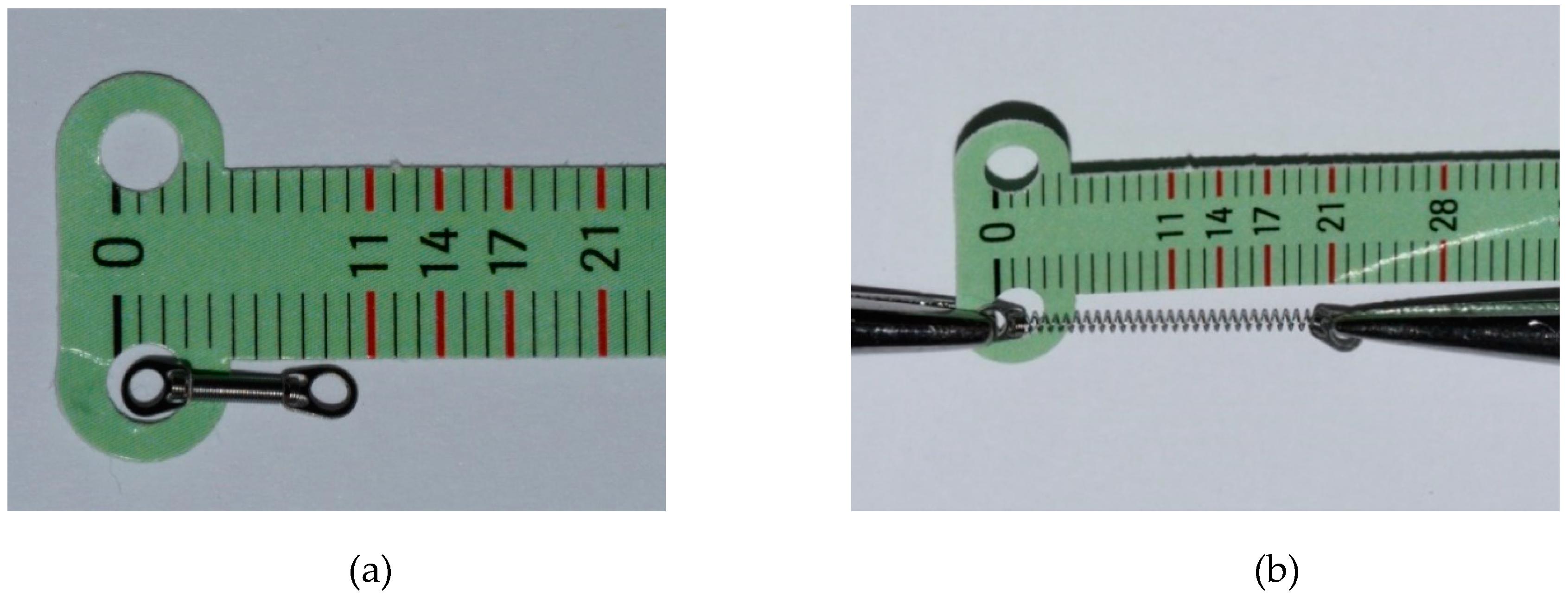
2.6. Outcomes
2.7. Statistical Analysis
3. Results
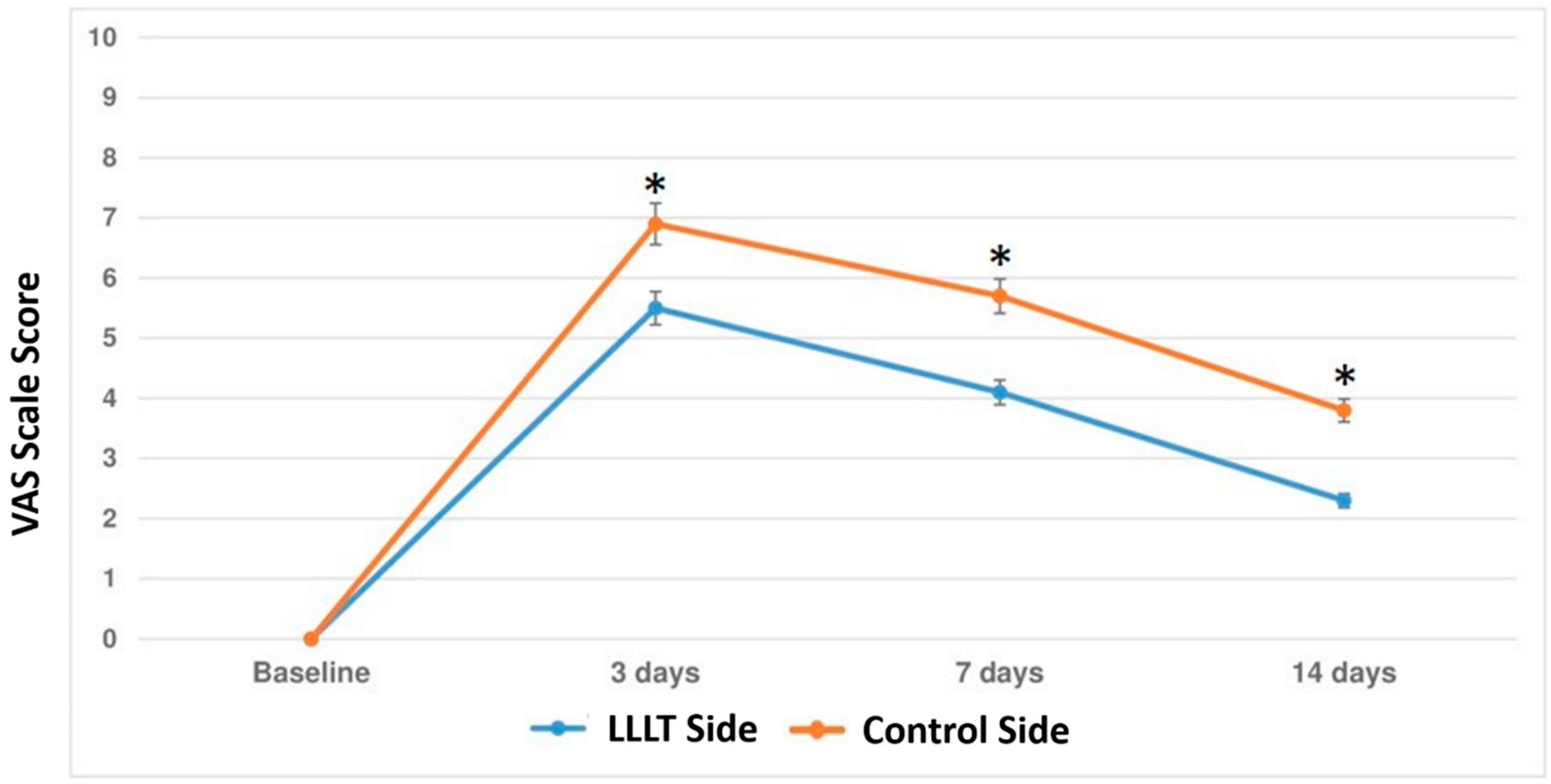
3.1. Primary Outcome
3.2. Secondary Outcome
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Florez-Moreno, G.A.; Martin-Restrepo, L.M.; Isaza-Guzman, D.M.; Tobon-Arroyave, S.I. Screening for salivary levels of deoxypyridinoline and bone-specific alkaline phosphatase during orthodontic tooth movement: A pilot study. Eur. J. Orthod. 2013, 35, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Isola, G.; Perillo, L.; Migliorati, M.; Matarese, M.; Dalessandri, D.; Grassia, V.; Alibrandi, A.; Matarese, G. The impact of temporomandibular joint arthritis on functional disability and global health in patients with juvenile idiopathic arthritis. Eur. J. Orthod. 2019, 41, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Cianetti, S.; Lombardo, G.; Lupatelli, E.; Pagano, S.; Abraha, I.; Montedori, A.; Caruso, S.; Gatto, R.; Degiorgio, S.; Salvato, R. Dental fear/anxiety among children and adolescents. A systematic review. Eur. J. Paedr. Dent. 2017, 18, 121–130. [Google Scholar]
- Verna, C.; Cattaneo, P.M.; Dalstra, M. Corticotomy affects both the modus and magnitude of orthodontic tooth movement. Eur. J. Orthod. 2018, 40, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Thilander, B.; Rygh, P.; Reitan, K. Tissue reactions in orthodontics. In Orthodontics. Current Principles and Techniques, 3rd ed.; Graber, T.M., Varnarsdall, R.L., Eds.; Mosby: St Louis, MO, USA, 2000; pp. 117–192. [Google Scholar]
- Navet, B.; Vargas-Franco, J.W.; Gama, A.; Amiaud, J.; Choi, Y.; Yagita, H.; Mueller, C.G.; Rédini, F.; Heymann, D.; Castaneda, B.; et al. Maternal RANKL Reduces the Osteopetrotic Phenotype of Null Mutant Mouse Pups. J. Clin. Med. 2018, 7, E426. [Google Scholar] [CrossRef] [PubMed]
- Zymperdikas, V.F.; Yavropoulou, M.P.; Kaklamanos, E.G.; Papadopoulos, M.A. Effects of systematic bisphosphonate use in patients under orthodontic treatment: A systematic review. Eur. J. Orthod. 2019. [Google Scholar] [CrossRef] [PubMed]
- Antonarakis, G.S.; Joss, C.U.; Triaca, A.; Kuijpers-Jagtman, A.M.; Kiliaridis, S. Gingival recessions of lower incisors after proclination by orthodontics alone or in combination with anterior mandibular alveolar process distraction osteogenesis. Clin. Oral Investig. 2017, 21, 2569–2579. [Google Scholar] [CrossRef] [PubMed]
- Bock, N.; Ruehl, J.; Ruf, S. Orthodontic Class II:1 treatment-efficiency and outcome quality of Herbst-multibracket appliance therapy. Clin. Oral Investig. 2018, 22, 2005–2011. [Google Scholar] [CrossRef] [PubMed]
- Pereira, S.; Nogueira, L.; Canova, F.; Lopez, M.; Silva, H.C. IRAK1 variant is protective for orthodontic-induced external apical root resorption. Oral Dis. 2016, 22, 658–664. [Google Scholar] [CrossRef] [PubMed]
- Matarese, G.; Isola, G.; Anastasi, G.P.; Cutroneo, G.; Favaloro, A.; Vita, G.; Cordasco, G.; Milardi, D.; Zizzari, V.L.; Tetè, S.; et al. Transforming Growth Factor Beta 1 and Vascular Endothelial Growth Factor levels in the pathogenesis of periodontal disease. Eur. J. Inflamm. 2013, 11, 479–488. [Google Scholar] [CrossRef]
- Schell, T.G. Avoiding and Managing Oral Appliance Therapy Side Effects. Sleep Med. Clin. 2018, 13, 503–512. [Google Scholar] [CrossRef] [PubMed]
- Pavlin, D.; Anthony, R.; Raj, V.; Gakunga, P.T. Cyclic loading (vibration) accelerates tooth movement in orthodontic patients: A doubleblind, randomized controlled trial. Semin. Orthod. 2015, 21, 187–194. [Google Scholar] [CrossRef]
- Wilcko, M.T.; Wilcko, W.M.; Bissada, N.F. An evidence-based analysis of periodontally accelerated orthodontic and osteogenic techniques: A synthesis of scientific perspectives. Semin. Orthod. 2008, 14, 305–316. [Google Scholar] [CrossRef]
- Ramaglia, L.; Cicciù, M.; Fiorentino, E.; Saviano, R.; Blasi, A.; Cervino, G.; Isola, G. Effectiveness of a piezoelectric-assisted distraction osteogenesis procedure for the treatment of ankylosed permanent front teeth. J. Craniofac. Surg. 2019, 30, e356–e359. [Google Scholar] [CrossRef] [PubMed]
- Camacho, A.D.; Velásquez Cujar, S.A. Dental movement acceleration: Literature review by an alternative scientific evidence method. World J. Methodol. 2014, 4, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Patterson, B.M.; Dalci, O.; Papadopoulou, A.K.; Madukuri, S.; Mahon, J.; Petocz, P.; Spahr, A.; Darendeliler, M.A. Effect of piezocision on root resorption associated with orthodontic force: A microcomputed tomography study. Am. J. Orthod. Dentofac. Orthop. 2017, 151, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Isola, G.; Matarese, G.; Lo Giudice, G.; Briguglio, F.; Alibrandi, A.; Crupi, A.; Cordasco, G.; Ramaglia, L. A new approach for the treatment of lateral periodontal cysts with an 810-nm diode laser. Int. J. Periodontics Restor. Dent. 2017, 37, e120–e129. [Google Scholar] [CrossRef] [PubMed]
- Borzabadi-Farahani, A. The adjunctive soft-tissue diode laser in orthodontics. Compend. Contin. Educ. Dent. 2017, 38, e18–e31. [Google Scholar] [PubMed]
- Borzabadi-Farahani, A.; Cronshaw, M. Lasers in orthodontics. In Lasers in Dentistry—Current Concepts. Textbooks in Contemporary Dentistry; Coluzzi, D., Parker, S., Eds.; Springer: Cham, Switzerland, 2017. [Google Scholar]
- Lopes, L.P.B.; Herkrath, F.J.; Vianna, E.C.B.; Gualberto Júnior, E.C.; Marques, A.A.F.; Sponchiado Júnior, E.C. Effect of photobiomodulation therapy on postoperative pain after endodontic treatment: A randomized, controlled, clinical study. Clin. Oral Investig. 2018. [Google Scholar] [CrossRef]
- Kanaguchi Arita, A.; Yonemitsu, I.; Ikeda, Y.; Miyazaki, M.; Ono, T. Low-intensity pulsed ultrasound stimulation for mandibular condyle osteoarthritis lesions in rats. Oral Dis. 2018, 24, 600–610. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Hayashi, M.; Fujita, S.; Yoshida, T.; Utsunomiya, T.; Yamamoto, H.; Yamamoto, H.; Kasai, K. Low-energy laser irradiation facilitates the velocity of tooth movement and the expressions of matrix metalloproteinase-9, cathepsin K, and alpha(v) beta(3) integrin in rats. Eur. J. Orthod. 2010, 32, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, K.; Shimizu, N. Effects of low-energy laser irradiation on bone remodeling during experimental tooth movement in rats. Lasers Surg. Med. 2000, 26, 282–291. [Google Scholar] [CrossRef]
- Fujita, S.; Yamaguchi, M.; Utsunomiya, T.; Yamamoto, H.; Kasai, K. Lowenergy laser stimulates tooth movement velocity via expression of RANK and RANKL. Orthod. Craniofac. Res. 2008, 11, 143–155. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, M.; Fujita, S.; Yoshida, T.; Oikawa, K.; Utsunomiya, T.; Yamamoto, H.; Kasai, K. Low-energy laser irradiation stimulates the tooth movement velocity via expression of M-CSF and c-fms. Orthod. Waves 2007, 66, 139–148. [Google Scholar] [CrossRef]
- Yassaei, S.; Aghili, H.; Afshari, J.T.; Bagherpour, A.; Eslami, F. Effects of diode laser (980 nm) on orthodontic tooth movement and interleukin 6 levels in gingival crevicular fluid in female subjects. Lasers Med Sci. 2016, 31, 1751–1759. [Google Scholar] [CrossRef] [PubMed]
- Varella, A.M.; Revankar, A.V.; Patil, A.K. Low-level laser therapy increases interleukin-1β in gingival crevicular fluid and enhances the rate of orthodontic tooth movement. Am. J. Orthod. Dentofac. Orthop. 2018, 154, 535–544. [Google Scholar] [CrossRef] [PubMed]
- Montedori, A.; Abraha, I.; Orso, M.; D’Errico, P.; Pagano, S.; Lombardo, G. Laser for caries removal in deciduous and permanent teeth. Cochrane Database Syst. Rev. 2016, 9, CD010229. [Google Scholar] [CrossRef] [PubMed]
- Isola, G.; Alibrandi, A.; Pedullà, E.; Grassia, V.; Ferlito, S.; Perillo, L.; Rapisarda, E. Analysis of the Effectiveness of Lornoxicam and Flurbiprofen on Management of Pain and Sequelae Following Third Molar Surgery: A Randomized, Controlled, Clinical Trial. J. Clin. Med. 2019, 8, E325. [Google Scholar] [CrossRef] [PubMed]
- Zeppieri, M.; Salvetat, M.L.; Beltrami, A.; Cesselli, D.; Russo, R.; Alcalde, I.; Merayo-Lloves, J.; Brusini, P.; Parodi, P.C. Adipose derived stem cells for corneal wound healing after laser induced corneal lesions in mice. J. Clin. Med. 2017, 6, E115. [Google Scholar] [CrossRef] [PubMed]
- Schulz, K.F.; Altman, D.G.; Moher, D.; CONSORT Group. CONSORT 2010 statement: Updated guidelines for reporting parallel group randomized trials. Ann. Intern. Med. 2010, 152, 726–732. [Google Scholar] [CrossRef]
- Doshi-Mehta, G.; Bhad-Patil, W.A. Efficacy of low-intensity laser therapy in reducing treatment time and orthodontic pain: A clinical investigation. Am. J. Orthod. Dentofac. Orthop. 2012, 141, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Luppanapornlarp, S.; Kajii, T.S.; Surarit, R.; Iida, J. Interleukin-1beta levels, pain intensity, and tooth movement using two different magnitudes of continuous orthodontic force. Eur. J. Orthod. 2010, 32, 596–601. [Google Scholar] [CrossRef] [PubMed]
- Isola, G.; Ramaglia, L.; Cordasco, G.; Lucchese, A.; Fiorillo, L.; Matarese, G. The effect of a functional appliance in the management of temporomandibular joint disorders in patients with juvenile idiopathic arthritis. Minerva Stomatol. 2017, 66, 1–8. [Google Scholar] [PubMed]
- Üretürk, S.E.; Saraç, M.; Fıratlı, S.; Can, Ş.B.; Güven, Y.; Fıratlı, E. The effect of low-level laser therapy on tooth movement during canine distalization. Lasers Med. Sci. 2017, 32, 757–764. [Google Scholar] [CrossRef] [PubMed]
- Spadari, G.S.; Zaniboni, E.; Vedovello, S.A.; Santamaria, M.P.; Do Amaral, M.E.; Dos Santos, G.M.; Esquisatto, M.A.; Mendonca, F.A.; Santamaria, M., Jr. Electrical stimulation enhances tissue reorganization during orthodontic tooth movement in rats. Clin. Oral Investig. 2017, 21, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Lui, J.; Corbet, E.F.; Jin, L. Combined photodynamic and lowlevel laser therapies as an adjunct to nonsurgical treatment of chronic periodontitis. J. Periodont. Res. 2011, 46, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Calderin, S.; Garcıa Nunez, J.A.; Gomez, C. Short-term clinical and osteoimmunological effects of scaling. Lasers Med. Sci. 2013, 28, 157–166. [Google Scholar] [PubMed]
- Kim, S.J.; Paek, J.H.; Park, K.H.; Kang, S.G.; Park, Y.G. Laser-aided supracrestal circumferential fiberotomy and low-level laser therapy effects on relapse of rotated teeth in beagles. Angle Orthod. 2010, 80, 385–390. [Google Scholar] [CrossRef]
- Kim, S.J.; Kang, Y.G.; Park, J.H.; Kim, E.C.; Park, Y.G. Effects of low-intensity laser therapy on periodontal tissue remodeling during relapse and retention of orthodontically moved teeth. Lasers Med. Sci. 2013, 28, 325–333. [Google Scholar] [CrossRef]
- Sadowsky, C.; Schneider, B.J.; BeGole, E.A.; Tahir, E. Long-term stability after orthodontic treatment: Nonextraction with prolonged retention. Am. J. Orthod. Dentofac. Orthop. 1994, 106, 243–249. [Google Scholar] [CrossRef]
- Isola, G.; Matarese, G.; Cordasco, G.; Rotondo, F.; Crupi, A.; Ramaglia, L. Anticoagulant therapy in patients undergoing dental interventions: A critical review of the literature and current perspectives. Minerva Stomatol. 2015, 64, 21–46. [Google Scholar] [PubMed]
- Fujioka-Kobayashi, M.; Schaller, B.; Zhang, Y.; Kandalam, U.; Hernandez, M.; Miron, R.J. Recombinant human bone morphogenetic protein (rhBMP)9 induces osteoblast differentiation when combined with demineralized freeze-dried bone allografts (DFDBAs) or biphasic calcium phosphate (BCP). Clin. Oral Investig. 2017, 21, 1883–1893. [Google Scholar] [CrossRef] [PubMed]
- Saito, S.; Shimizu, N. Stimulatory effects of low-power laser irradiation on bone regeneration in midpalatal suture during expansion in the rat. Am. J. Orthod. Dentofac. Orthop. 1997, 111, 525–532. [Google Scholar] [CrossRef]
- Ferlazzo, N.; Currò, M.; Zinellu, A.; Caccamo, D.; Isola, G.; Ventura, V.; Carru, C.; Matarese, G.; Ientile, R. Influence of MTHFR genetic background on p16 and MGMT Methylation in ORAL squamous cell cancer. Int. J. Mol. Sci. 2017, 18, E724. [Google Scholar] [CrossRef] [PubMed]
- Tao, X.; Yao, J.W.; Wang, H.L.; Huang, C. Comparison of gingival troughing by laser and retraction cord. Int. J. Periodontics Restor. Dent. 2018, 38, 527–532. [Google Scholar] [CrossRef] [PubMed]
- Benetti, F.; Lemos, C.A.A.; de Oliveira Gallinari, M.; Terayama, A.M.; Briso, A.L.F.; de Castilho Jacinto, R.; Sivieri-Araújo, G.; Cintra, L.T.A. Influence of different types of light on the response of the pulp tissue in dental bleaching: a systematic review. Clin. Oral Investig. 2018, 22, 1825–1837. [Google Scholar] [CrossRef] [PubMed]
- Weißmann, V.; Drescher, P.; Seitz, H.; Hansmann, H.; Bader, R.; Seyfarth, A.; Klinder, A.; Jonitz-Heincke, A. Effects of build orientation on surface morphology and bone cell activity of additively manufactured Ti6Al4V specimens. Materials 2018, 11, 915. [Google Scholar] [CrossRef] [PubMed]
- Matarese, G.; Isola, G.; Anastasi, G.P.; Favaloro, A.; Milardi, D.; Vermiglio, G.; Vita, G.; Cordasco, G.; Cutroneo, G. Immunohistochemical analysis of TGF-β1 and VEGF in gingival and periodontal tissues: A role of these biomarkers in the pathogenesis of scleroderma and periodontal disease. Int. J. Mol. Med. 2012, 30, 502–508. [Google Scholar] [CrossRef]
- Luger, E.J.; Rochkind, S.; Wollman, Y.; Kogan, G.; Dekel, S. Effect of low-power laser irradiation on the mechanical properties of bone fracture healing in rats. Lasers Surg. Med. 1998, 22, 97–102. [Google Scholar] [CrossRef]
- Matarese, G.; Ramaglia, L.; Fiorillo, L.; Cervino, G.; Lauritano, F.; Isola, G. Implantology and periodontal disease: The panacea to problem solving? Open Dent. J. 2017, 11, 460–465. [Google Scholar] [CrossRef]
- Shimizu, N.; Yamaguchi, M.; Goseki, T.; Shibata, Y.; Takiguchi, H.; Iwasawa, T.; Abiko, Y. Inhibition of prostaglandin E2 and interleukin 1-beta production by low-power laser irradiation in stretched human periodontal ligament cells. J. Dent. Res. 1995, 74, 1382–1388. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, T.; Bernardello, F.; Berton, F.; Porrelli, D.; Rapani, A.; Camurri Piloni, A.; Fiorillo, L.; Di Lenarda, R.; Stacchi, C. Efficacy of Alveolar Ridge Preservation after Maxillary Molar Extraction in Reducing Crestal Bone Resorption and Sinus Pneumatization: A Multicenter Prospective Case-Control Study. Biomed Res. Int. 2018, 2018, 9352130. [Google Scholar] [CrossRef] [PubMed]
- Harazaki, M.; Takahashi, H.; Ito, A.; Isshiki, Y. Soft laser irradiation induced pain reduction in orthodontic treatment. Bull. Tokyo Dent. Coll. 1998, 39, 95–101. [Google Scholar] [PubMed]
- Ren, C.; McGrath, C.; Yang, Y. The effectiveness of low-level diode laser therapy on orthodontic pain management: A systematic review and meta-analysis. Lasers Med Sci. 2015, 30, 1881–1893. [Google Scholar] [CrossRef] [PubMed]
- Turhani, D.; Scheriau, M.; Kapral, D.; Benesch, T.; Jonke, E.; Bantleon, H.P. Pain relief by single low-level laser irradiation in orthodontic patients undergoing fixed appliance therapy. Am. J. Orthod. Dentofac. Orthop. 2006, 130, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.T.; Bayome, M.; Park, J.B.; Park, J.H.; Baek, S.H.; Kook, Y.A. Effect of frequent laser irradiation on orthodontic pain. A single-blind randomized clinical trial. Angle Orthod. 2013, 83, 611–616. [Google Scholar] [CrossRef] [PubMed]
- Mizutani, K.; Musya, Y.; Wakae, K.; Kobayashi, T.; Tobe, M.; Taira, K.; Harada, T. A clinical study on serum prostaglandin E2 with low-level laser therapy. Photomed. Laser Surg. 2004, 22, 537–539. [Google Scholar] [CrossRef] [PubMed]
- Eslamian, L.; Borzabadi-Farahani, A.; Edini, H.Z.; Badiee, M.R.; Lynch, E.; Mortazavi, A. The analgesic effect of benzocaine mucoadhesive patches on orthodontic pain caused by elastomeric separators, a preliminary study. Acta Odontol. Scand. 2013, 71, 1168–1173. [Google Scholar] [CrossRef]
- Lo Giudice, G.; Lo Giudice, R.; Matarese, G.; Isola, G.; Cicciù, M.; Terranova, A.; Palaia, G.; Romeo, U. Evaluation of magnification systems in restorative dentistry. An in-vitro study. Dent. Cadmos 2015, 83, 296–305. [Google Scholar] [CrossRef]
- Eslamian, L.; Borzabadi-Farahani, A.; Gholami, H. The effect of benzocaine and ketoprofen gels on pain during fixed orthodontic appliance treatment: A randomised, double-blind, crossover trial. Aust. Orthod. J. 2016, 32, 64–72. [Google Scholar]
- Cannavale, R.; Matarese, G.; Isola, G.; Grassia, V.; Perillo, L. Early treatment of an ectopic premolar to prevent molar-premolar transposition. Am. J. Orthod. Dentofacial Orthop. 2013, 143, 559–569. [Google Scholar] [CrossRef] [PubMed]
- Briguglio, F.; Briguglio, E.; Briguglio, R.; Cafiero, C.; Isola, G. Treatment of infrabony periodontal defects using a resorbable biopolymer of hyaluronic acid: A randomized clinical trial. Quintessence Int. 2013, 44, 231–240. [Google Scholar] [PubMed]
- Matarese, G.; Isola, G.; Alibrandi, A.; Lo Gullo, A.; Bagnato, G.; Cordasco, G.; Perillo, L. Occlusal and MRI characterizations in systemic sclerosis patients: A prospective study from Southern Italian cohort. Jt. Bone Spine 2016, 83, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Isola, G.; Matarese, M.; Ramaglia, L.; Cicciù, M.; Matarese, G. Evaluation of the efficacy of celecoxib and ibuprofen on postoperative pain, swelling, and mouth opening after surgical removal of impacted third molars: A randomized, controlled clinical trial. Int. J. Oral Maxillofac. Surg. 2019. [Google Scholar] [CrossRef] [PubMed]
- Isola, G.; Matarese, M.; Ramaglia, L.; Iorio-Siciliano, V.; Cordasco, G.; Matarese, G. Efficacy of a drug composed of herbal extracts on postoperative discomfort after surgical removal of impacted mandibular third molar: A randomized, triple-blind, controlled clinical trial. Clin. Oral Investig. 2019, 23, 2443–2453. [Google Scholar] [CrossRef] [PubMed]
- Eslamian, L.; Borzabadi-Farahani, A.; Hassanzadeh-Azhiri, A.; Badiee, M.R.; Fekrazad, R. The effect of 810-nm low-level laser therapy on pain caused by orthodontic elastomeric separators. Lasers Med. Sci. 2014, 29, 559–564. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Chen, Y.; Zhang, J.; Chen, W.; Shao, S.; Shen, H.; Zhu, L.; Ye, P.; Svensson, P.; Wang, K. Effect of low-level laser therapy on tooth-related pain and somatosensory function evoked by orthodontic treatment. Int. J. Oral Sci. 2018, 10, 22. [Google Scholar] [CrossRef] [PubMed]
- Qamruddin, I.; Alam, M.K.; Mahroof, V.; Fida, M.; Khamis, M.F.; Husein, A. Effects of low-level laser irradiation on the rate of orthodontic tooth movement and associated pain with self-ligating brackets. Am. J. Orthod. Dentofac. Orthop. 2017, 152, 622–630. [Google Scholar] [CrossRef]
| Group | N°of Analyzed Teeth | Mean ± SD (Days) (95% CI) | p-Value |
|---|---|---|---|
| Test side | 41 | 84.35 ± 12.34 (79.3–86.9) | <0.001 |
| Control side | 41 | 97.49 ±11.44 (91.7–102.3) |
| T1 | T2 | T3 | |
|---|---|---|---|
| Mean ± SD (95% CI) | Mean ± SD (95% CI) | Mean ± SD (95% CI) | |
| Test side | 65.36 ± 11.39 (59.9–67.4) | 89.42 ± 7.16 (78.2–95.2) | 91.1 ± 5.56 (87.3–94.4) |
| Control side | 44.39 ± 15.51 (41.3–48.7) | 68.66 ± 15.12 (62.1–71.3) | 92.3 6.69 (86.5–95.6) |
| p-value | 0.003 | <0.001 | 0.878 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Isola, G.; Matarese, M.; Briguglio, F.; Grassia, V.; Picciolo, G.; Fiorillo, L.; Matarese, G. Effectiveness of Low-Level Laser Therapy during Tooth Movement: A Randomized Clinical Trial. Materials 2019, 12, 2187. https://doi.org/10.3390/ma12132187
Isola G, Matarese M, Briguglio F, Grassia V, Picciolo G, Fiorillo L, Matarese G. Effectiveness of Low-Level Laser Therapy during Tooth Movement: A Randomized Clinical Trial. Materials. 2019; 12(13):2187. https://doi.org/10.3390/ma12132187
Chicago/Turabian StyleIsola, Gaetano, Marco Matarese, Francesco Briguglio, Vincenzo Grassia, Giacomo Picciolo, Luca Fiorillo, and Giovanni Matarese. 2019. "Effectiveness of Low-Level Laser Therapy during Tooth Movement: A Randomized Clinical Trial" Materials 12, no. 13: 2187. https://doi.org/10.3390/ma12132187
APA StyleIsola, G., Matarese, M., Briguglio, F., Grassia, V., Picciolo, G., Fiorillo, L., & Matarese, G. (2019). Effectiveness of Low-Level Laser Therapy during Tooth Movement: A Randomized Clinical Trial. Materials, 12(13), 2187. https://doi.org/10.3390/ma12132187









