3.2.2. ATR-FTIR Spectroscopy
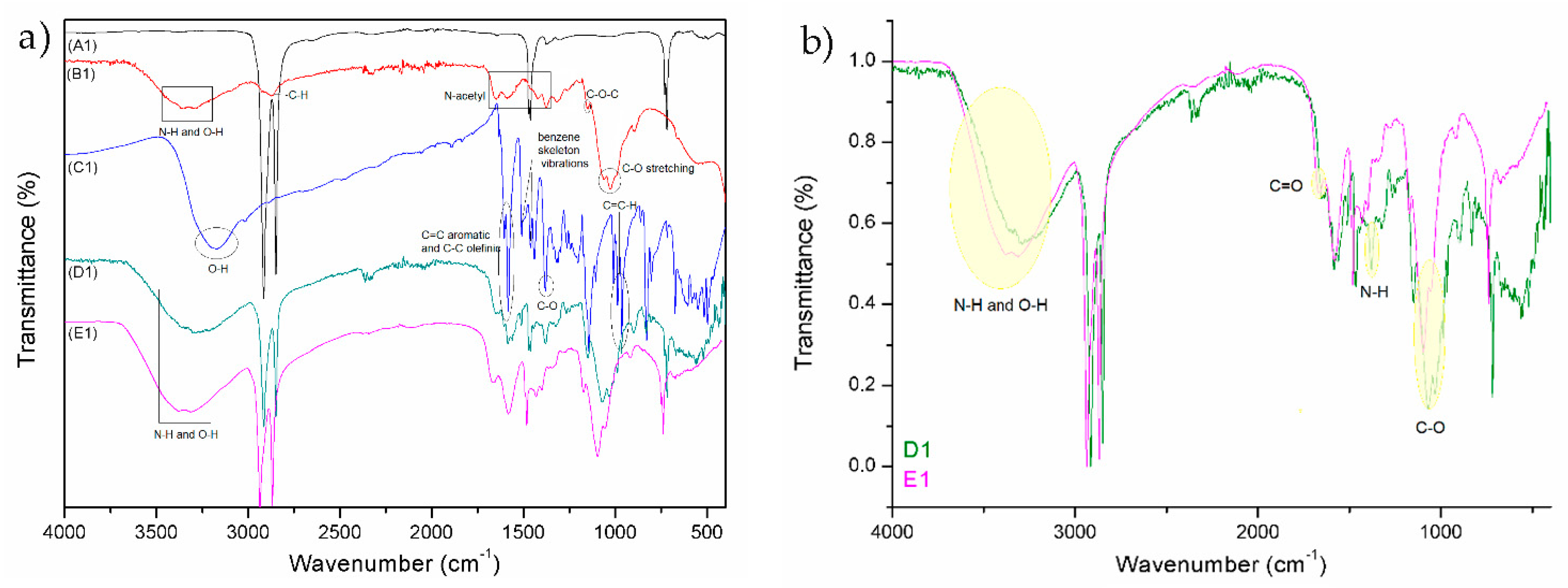
Typical peaks for PE and PP can be seen from the FTIR spectrum A1 and A2 (reference samples in
Figure 2 and
Figure 3). Details on FTIR spectrum of PE foil, labelled as A1, are shown in
Figure 2. The wave numbers of FTIR spectra of PE at 2915, 2848, 1463, and 718.2 cm
−1 were assigned to CH
2 asymmetric stretching, CH
2 symmetric stretching, bending deformation, and rocking deformation, respectively. Details of FTIR spectrum of PP foil (A2), are shown in
Figure 2. The wave number of FTIR spectra of PP at 2950, 2917, 2867, 2842, 1458, 1376, 1167, 997, and 973 cm
−1 were assigned to CH
3 stretching, CH
2 asymmetric stretching, CH
2 symmetric stretching, CH
2 symmetric stretching, CH
2 bending vibration, CH
3 bending vibration, CH
3 symmetric deformation vibration, CH
3 rocking vibration, and CH
2 rocking vibration, respectively. The presence of mainly C–H vibrational groups clearly shows on the materials’ hydrophobicity.
Spectrum B1 (
Figure 2 and
Figure 3) represents the chitosan powder. A strong band in the region 3303–3347 cm
−1 corresponds to N–H and O–H stretching, as well as the intramolecular hydrogen bonds. The absorption bands at around 2921 and 2877 cm
−1 can be attributed to C–H symmetric and asymmetric stretching, respectively. The presence of residual
N-acetyl groups was confirmed by the bands at around 1654 cm
−1 (C=O stretching of amide I) and 1581 cm
−1 (N–H bending of amide II); 1419 cm
−1 belongs to the N–H stretching of the amide and the ether bonds and N–H stretching at 1375 cm
−1 (amide III band), respectively. The CH
2 bending and CH
3 symmetrical deformations were confirmed by the presence of bands at around 1423 and 1375 cm
−1, respectively. The absorption band at 1150 cm
−1 can be attributed to asymmetric stretching of the C–O–C bridge. The bands at 1066 and 1026 cm
−1 correspond to C–O stretching [
45,
46].
The FTIR spectrum of resveratrol showed a typical OH stretching band at 3182 cm
−1 and intense bands at 1604 and 1583 cm
-1 corresponding to C–C aromatic double bond stretching and C–C olefinic stretching, respectively. The peaks at 1513 and 1462 cm
−1 reflect the benzene skeleton vibrations, while C–O stretching vibrations were seen by the peak at 1381 cm
−1. The peaks at 986 and 964 cm
−1 were ascribed to the bending vibration of C=C–H [
47].
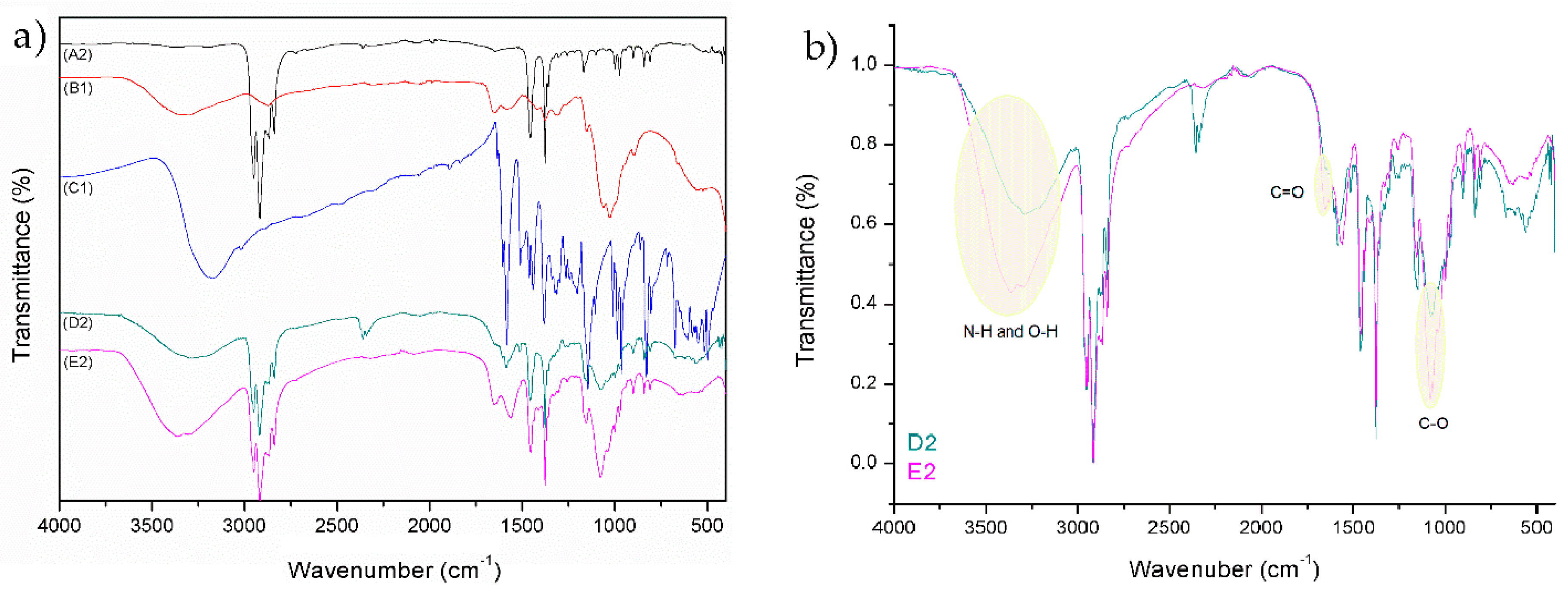
By comparing the peaks of reference foils (A1 and A2) with functionalized foils (D1, D2, E1, and E2), a successful application of coatings is seen. Spectrum D1 and D2 represent untreated PE and PP foils, which were firstly functionalized with solution of chitosan (2% w/v) and secondly with the chitosan–resveratrol nano dispersion (layer-by-layer). There are peaks on FTIR spectrum, which could confirm the presence of chitosan and resveratrol. The typical peaks of chitosan and resveratrol are smaller, but there are visible bonds such as N–H and O–H bond, all three amide bonds, C=C alkene, C=C aromatic, C–O phenols, and =C–H aromatic bonds. It must be pointed out that the coating on the films (spectra D1 and D2) were not homogeneous, due to the hydrophobic character of foils and the hydrophilic nature of chitosan and chitosan–resveratrol formulation. Due to the difference in polarity, the adhesion of applied formulations is lowered and not uniform.
All these peaks can also be observed on the spectra E1 and E2, but with the significant higher intensity. For all the coated PE and PP films, the characteristic peaks of chitosan were observed (N–H and O–H stretching; amide I, amide II, and amide III; C–O stretching). Comparison of the FTIR spectra between D1 and E1 (by normalizing) is shown in the
Figure 2b. The bands corresponding to C–O stretching (glycoside bonds) and C=O stretching (amide I) are shifted towards higher wavenumber (1096 and 1675 cm
−1) implying the linkage of CSNPs and CSPSs RES with the oxygen-rich foil with respect to unmodified foil (plasma-non activated). In addition, the vibration frequencies of N–H and O–H bands are also shifted towards higher wavenumber (3310 and 3383 cm
−1) and the peak is more pronounced with larger intensity for O
2 modified foil with CSNPs RES applied formulation. The increased band vibrations frequency of N–H and O–H bonds suggest that the interactions between the chitosan groups and foils surface are present in larger amount. It might be concluded that with O
2 plasma treatment the new binding sites (the appearance of oxygen-rich groups on PE surface such as CO, COOH, OH) are yielded onto foils surface, which are available for chemically binding with amino groups of chitosan and, consequently, more chitosan–resveratrol nano dispersion could be attached on this first bounded layer during a second coating step. When FTIR spectra between D2 and E2 for PP foils are compared (
Figure 3b), a similar trend was obtained, i.e., the occurrence of oxygen containing groups with O
2 plasma treatment led to better adsorption of chitosan as well as further attachment of chitosan nanoparticles with embedded resveratrol.
3.2.3. XPS Analysis
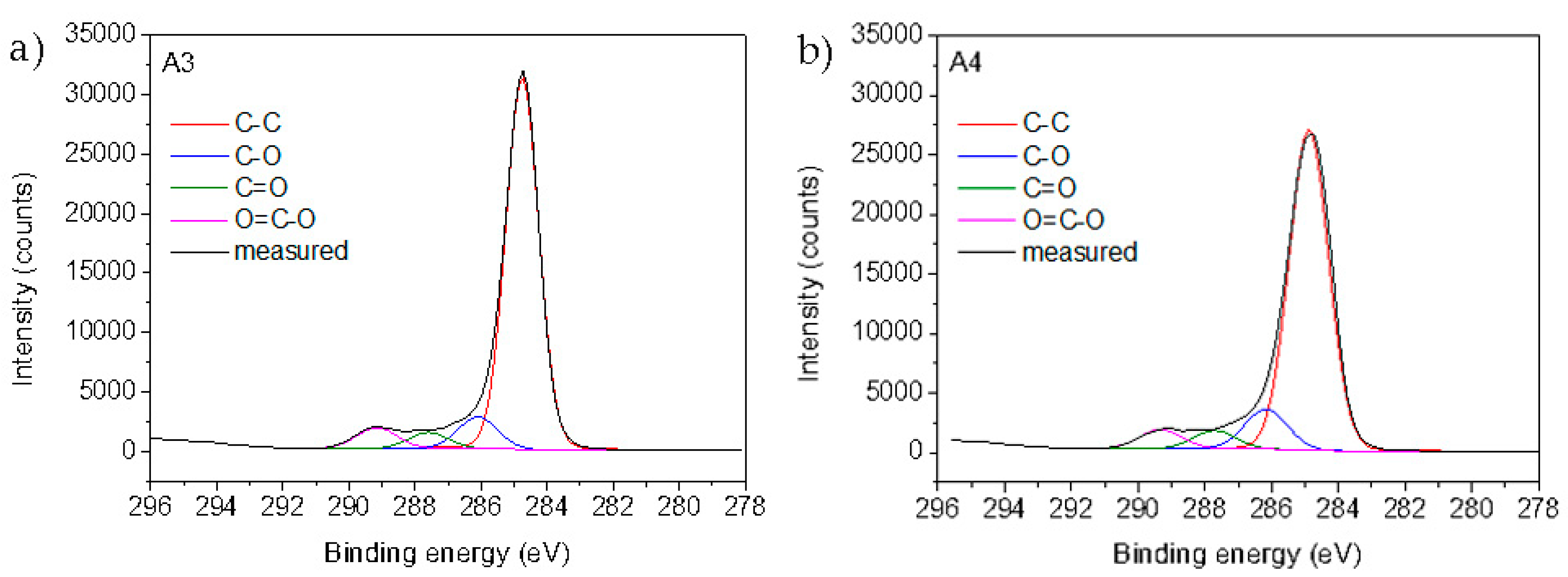
The surface chemical composition of the reference foils and plasma-functionalized foils determined by XPS is presented in
Table 6. For the untreated PE and PP foils (A1 and A2), mostly carbon was detected, which is in the agreement with chemical composition of the virgin foils. Small amounts of oxygen (approximately 1 at.%) are due to the surface contamination as often observed in XPS spectra measured on pure polymer foils. After oxygen plasma treatment, the oxygen concentration significantly increased to 13.7 ± 0.3 and 14.77 ± 0.1 at.% for PE and PP, respectively (samples A3 and A4 in
Table 6). Slightly higher oxygen concentration on PP sample can be explained by oxidation of the side methyl group, which is absent on PE. Increased oxygen concentration clearly indicates successful surface plasma activation of both polymer foils with introduction of oxygen functional groups that may act as a binding place for chitosan. This is further proved by
Figure 4 showing formation of hydroxyl, carbonyl, and carboxyl groups on the surface of the PE and PP foils.
When untreated (plasma none activated) foils were coated with chitosan and chitosan–resveratrol nano dispersion, nitrogen appears on the surface of the foils; i.e., 3.3 at.% for PE (D1) and 2.1 at.% for PP (D2). According to chemical structure, only chitosan contains nitrogen whereas resveratrol should not possess any nitrogen; therefore, nitrogen is explained by the presence of amino groups originating from chitosan because of its attachment on the foils. It can also be observed, that the carbon content is reduced, whereas the oxygen content is increased by 8% for PE (D1) and 7.4% for PP (D2) in comparison to the untreated foils, which is explained by a high oxygen content in both organic substances i.e., chitosan and resveratrol.
Functionalized foils that were previously treated with oxygen plasma show the highest nitrogen content, indicating a higher amount of adsorbed chitosan on the surface as compared to the untreated foils. This may be due to the chemisorption of prepared chitosan dispersion onto foils. As it has been shown, oxygen plasma introduced carbonyl and carboxyl group that may form binding places for chitosan amino groups [
16]. Oxygen functional groups formed by plasma treatment also raised hydrophilicity of the foils causing better wetting of the surface with chitosan solution. All these may improve the ability for chitosan adsorption, i.e., higher amount of chitosan could be bounded, which was clearly indicated in our case by a higher concentration of nitrogen; i.e., nitrogen concentration significantly increased for 6.7% on PE surface (E1) and for 6.7% on PP surface (E2). The carbon content considerably decreased by as much as 25.7% on PE (E1) and 25.8% on PP (E2), and the oxygen content significantly increased by as much as 18% on PE (E1) and PP (E2) indicating attachment of chitosan and resveratrol, which are both rich with oxygen. (E2). All these observations are in a good agreement with the results, obtained from the infrared spectroscopy.
Summarizing, when all plasma-functionalized samples are compared to those that were previously not activated by plasma via reference ones, it is clearly observed that the amount of nitrogen, originating from the coating, is significantly increased, which means that chitosan has adsorbed onto plasma-treated foil to a much higher extent.
3.2.4. Oxygen Permeability
Table 7 contains the results of oxygen permeability for the reference foils compared to the functionalized foils. Oxygen permeability after coatings of foils has been reduced in all cases. The permeability decrease for the plasma untreated PE and PP foils in comparison with reference samples was lower than for the same samples, which were previously activated by O
2 plasma. This may be attributed to the higher and uniform amount of attached chitosan macromolecular solution as the first layer and, thus, further better affinity for chitosan-RES nanoparticles as a second layer. The statistical analysis showed that the highest significant decrease in permeability was observed for O
2 plasma-treated PE foil with a layer of chitosan and a layer of chitosan–resveratrol nano dispersion (E1). For this sample, the oxygen permeability was reduced from 3226 ± 62 cm
3/m
2d by as much as 202 ± 16 cm
3/m
2d. Significantly different results were also obtained for the plasma-functionalized PP foil (E2), as oxygen permeability decreased from 1078 ± 36 cm
3/m
2d to 195 ± 14 cm
3/m
2d. For plasma-untreated PE and PP foils coated with a layer of chitosan and a second layer of chitosan–resveratrol nano dispersion, the maximum permeability of oxygen has non-significantly reduced from 3226 ± 62 cm
3/m
2d to 2417 ± 104 cm
3/m
2d for sample D1 and from 1078 ± 36 cm
3/m
2d to 968 ± 19 cm
3/m
2d for sample D2. The latest may be attributed to the lower, and especially homogenous, adsorption of chitosan colloidal systems onto both plasma non-activated foils, as will be discussed in
Section 3.2.6.
3.2.5. Goniometry
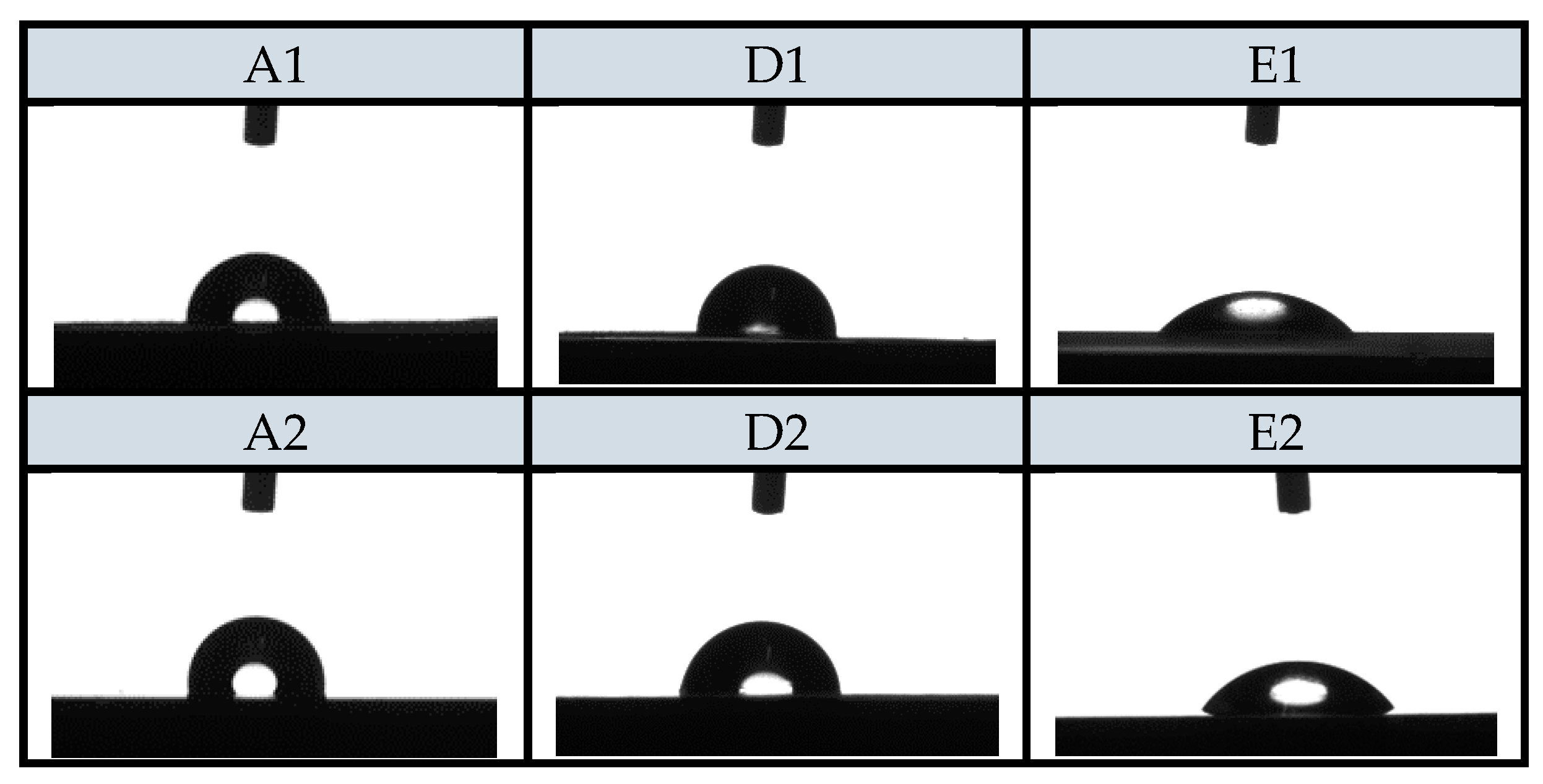
Static contact angle (SCA) measurements, which provides an inverse measure of wettability, have also been performed. SCA of various samples are also shown in
Figure 5. On the basis of SCA results, hydrophilicity/hydrophobicity of PE and PP surfaces was determined. Reduction of contact angle is of a great importance for practical use as the hydrophilic surface of the foils reduces the potential process of dew condensation on the surface of the foils (anti-fog efficiency) in contact with food, which worsens the packaging conditions and thus increases the food contamination [
48]. It is, thus, extremely important to avoid this process.
The results of SCA measurements are presented in
Table 8. The reference PE and PP foils had contact angles of 108.2° and 109.3°, which pointed out foils hydrophobicity. Statistically, all samples are significantly different compared to A1 or A2 (control sample). The treatment with oxygen plasma was successful as expected and resulted into contact angle decrease for around three to four times (A3, A4). With the functionalization of the foils using chitosan systems, the SCA significantly decreased in comparison to reference foils (A1 and A2). The contact angles slightly decreased for the samples D1, D2; however, the biggest significant difference in the decrease of the contact angle was observed for the samples E1 and E2, i.e., contact angle decreased down to 42.4° and 45.3°. It can be concluded that when foils (PE or PP) are previously treated with O
2 plasma, the higher and more homogeneous adsorption of chitosan macromolecular solution and chitosan–resveratrol nano dispersion occurred. Because of the adsorbate foils polarity and hydrophilicity, it is obvious that coatings on the plasma-activated PE and PP surface has better wettability and are thus more homogeneous after attachment.
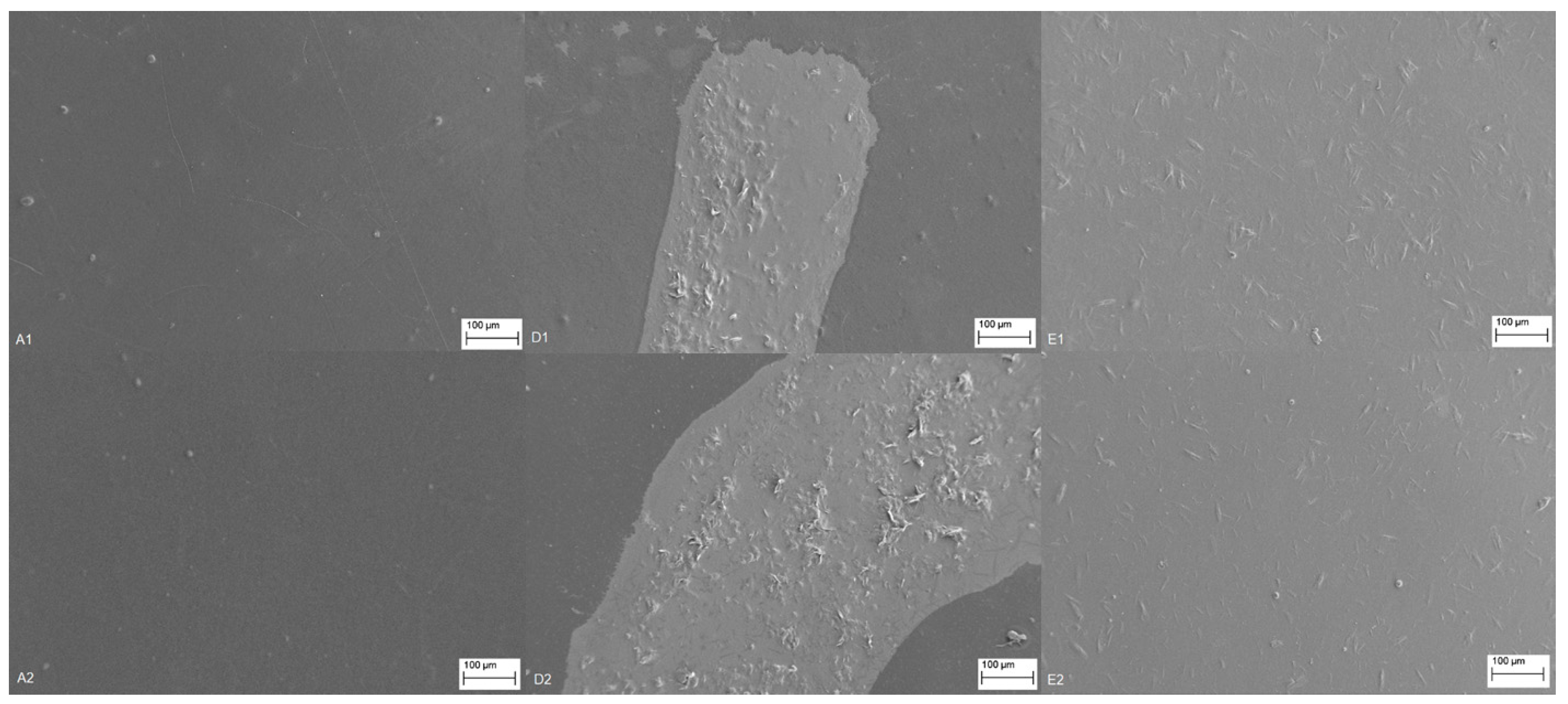
3.2.6. Morphology: Scanning Electron Microscopy—SEM
Figure 6 presents SEM images of the surface morphology and the adhesion uniformity of the uncoated (reference samples A1 and A2) and coated foils with chitosan in the first layer and CSNPs RES formulation in the second (upper) layer. For easier comparison, all images were taken at the comparable magnification (300×) and at the same imaging parameters. For samples D1 and D2, the coating is visible on the surface of the foils with significantly higher contrast together with the deposited agglomerates. However, the coating was rough and did not completely cover the foil as it can be seen in comparison to the reference foils. This can be explained by the hydrophobicity of the two polymers (the hydrophobicity of the reference PE and PP foils were demonstrated by measuring the contact angle as well as surface techniques that suggested the presence of nonpolar groups) that lowers the adhesion of applied formulations due to the difference in polarity.
With O
2 plasma treatment/foils surface modification before coating, the surface of the PE and PP foils were modified. The latter significantly improved the interaction between the two polymers (PE/PP foil and chitosan). When the reference foils are compared with the samples E1 and E2 (
Figure 6), a very successful application is obtained as the uniform hydrophilic surface, where the coating is homogenous, smoother, and thinner, uniformly covering the foil with less observed agglomerates. It was found by surface analyses (i.e., infrared spectroscopy and XPS) that the occurrence of oxygen-containing functional groups (C═O, C–O, and –OH) of the O
2 plasma-treated PE and PP foils increased from those of the untreated one, indicating that the O
2 plasma enhanced hydrophilicity of the PE and PP foils (E1 and E2). The SEM analysis also supported the oxygen permeability results, where the homogeneous formulation coating (samples E1 and E2) also showed higher reduced permeability with respect to samples D1 and D2. This can be in part explained by the more homogenous adhesion of the applied layer-by-layer coating, as evident from SEM images.