Bioinspired Materials: From Living Systems to New Concepts in Materials Chemistry
Abstract
1. Introduction
2. From Creatures to Concepts
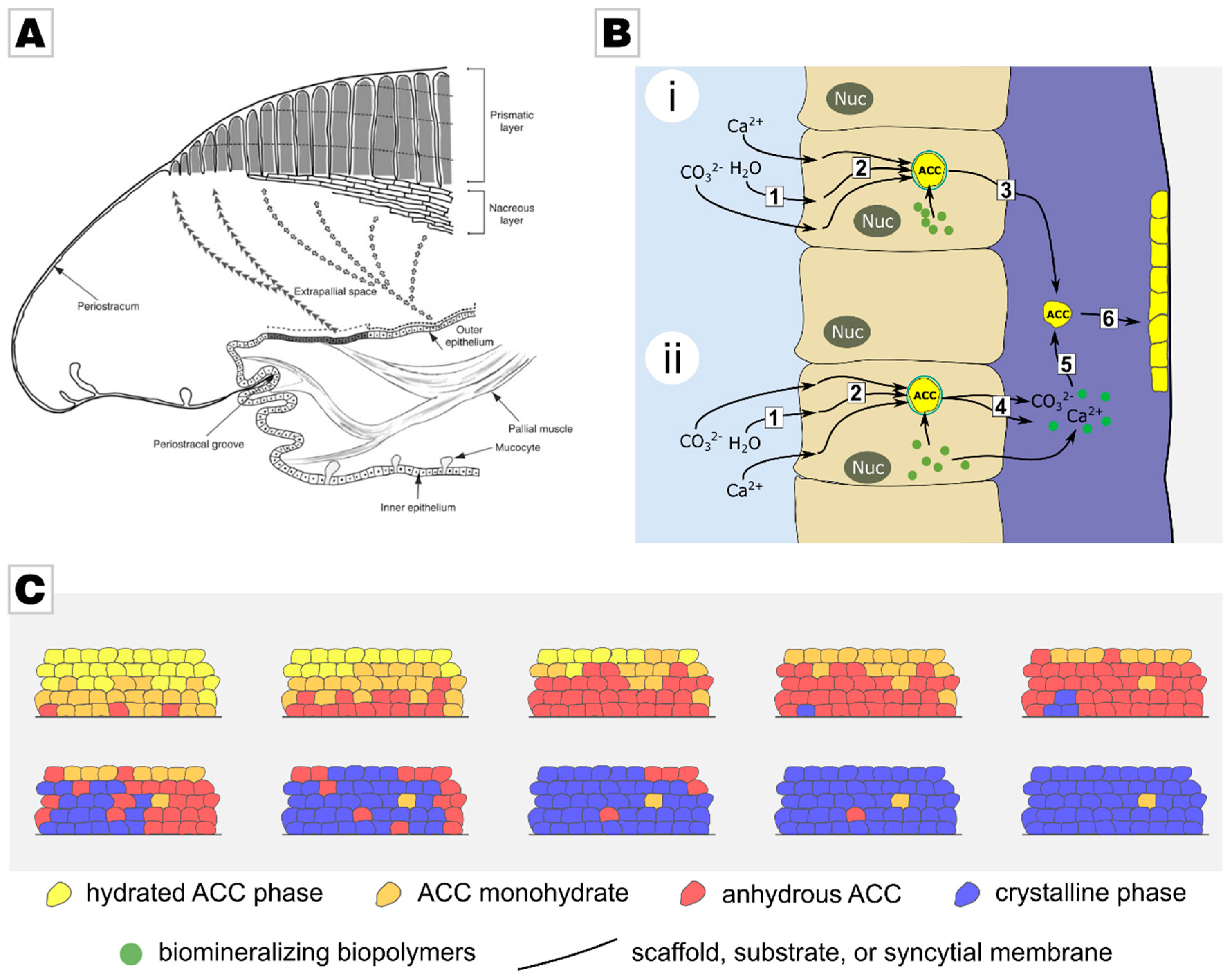
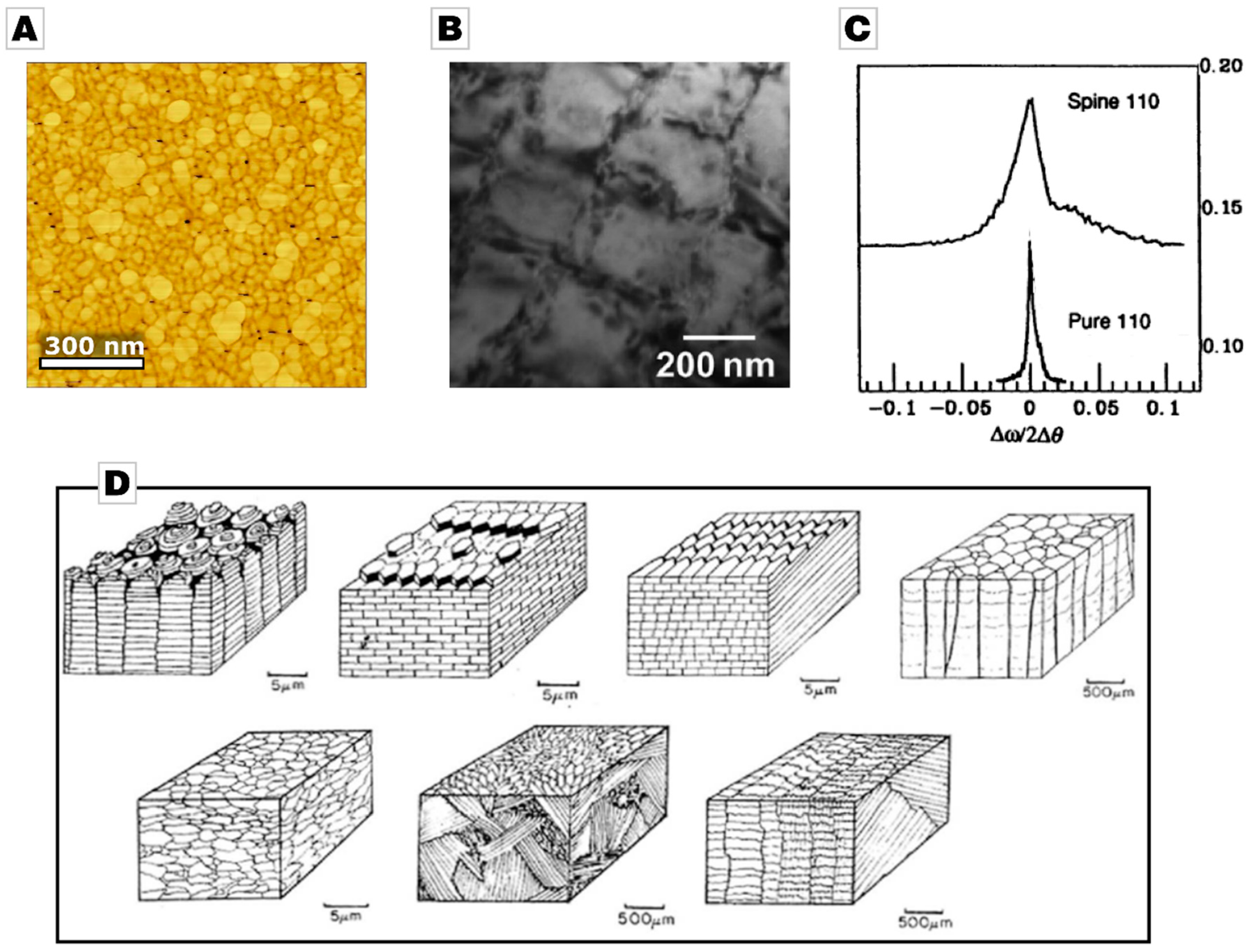
2.1. From Calcareous Creatures to New Concepts in Crystallization
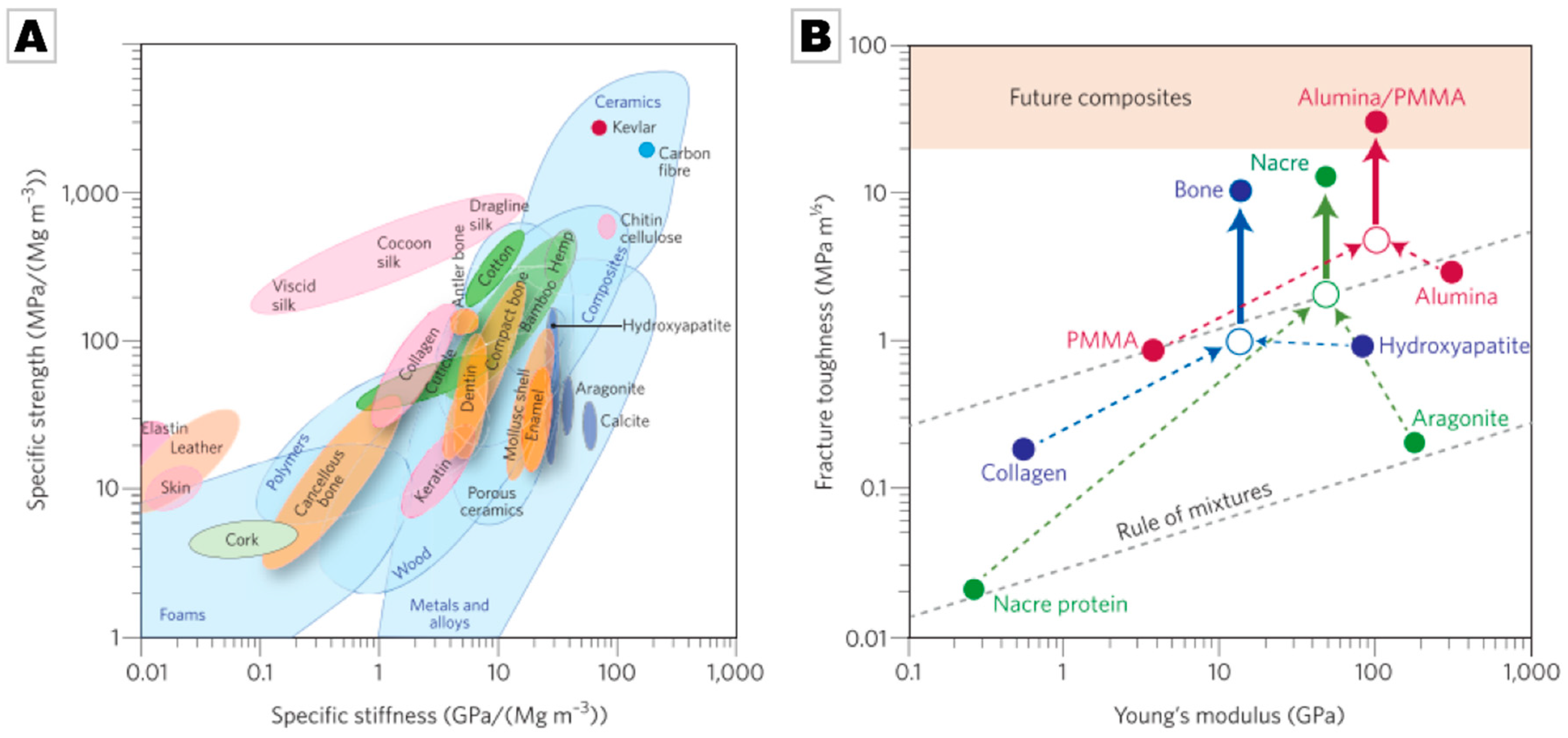
2.2. Biominerals as an Evolutionarily-Tested Archive of Functional Material Design Motifs
2.3. Biogenic Silica Formation Demonstrates How to Mildly Drive Metal Oxide Formation
3. Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Mann, S. Biomineralization: Principles and Concepts in Bioinorganic Materials Chemistry; Compton, R., Davies, S.G., Evans, J., Eds.; Oxford University Press: Oxford, UK, 2001. [Google Scholar]
- Meldrum, F.C.; Cölfen, H.; Cölfen, H. Controlling Mineral Morphologies and Structures in Biological and Synthetic Systems. Chem. Rev. 2008, 108, 4332–4432. [Google Scholar] [CrossRef] [PubMed]
- Espinosa, H.D.; Rim, J.E.; Barthelat, F.F.; Buehler, M.J. Merger of Structure and Material in Nacre and Bone—Perspectives on de Novo Biomimetic Materials. Prog. Mater. Sci. 2009, 54, 1059–1100. [Google Scholar] [CrossRef]
- Barthelat, F.; Rabiei, R. Toughness Amplification in Natural Composites. J. Mech. Phys. Solids 2011, 59, 829–840. [Google Scholar] [CrossRef]
- Wegst, U.G.K.; Bai, H.; Saiz, E.; Tomsia, A.P.; Ritchie, R.O. Bioinspired Structural Materials. Nat. Mater. 2014, 14, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Marin, F.; Luquet, G.; Marie, B.; Medakovic, D. Molluscan Shell Proteins: Primary Structure, Origin, and Evolution. Curr. Top. Dev. Biol. 2008, 80, 209–276. [Google Scholar] [CrossRef] [PubMed]
- Wilbur, K.M.; Saleuddin, A.S.M. (Eds.) Shell Formation. In The Mollusca, Volume 4; Academic Press: New York, NY, USA, 1983; pp. 235–287. [Google Scholar]
- Marie, B.; Joubert, C.; Tayalé, A.; Zanella-Cléon, I.; Belliard, C.; Piquemal, D.; Cochennec-Laureau, N.; Marin, F.; Gueguen, Y.; Montagnani, C. Different Secretory Repertoires Control the Biomineralization Processes of Prism and Nacre Deposition of the Pearl Oyster Shell. Proc. Natl. Acad. Sci. USA 2012, 109, 20986–20991. [Google Scholar] [CrossRef] [PubMed]
- Mourea, G.; Vilarinho, L.; Santos, A.C.; Machado, J. Organic Compounds in the Extrapalial Fluid and Haemolymph of Anodonta Cygnea (L.) with Emphasis on the Seasonal Biomineralization Process. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2000, 125, 293–306. [Google Scholar] [CrossRef]
- Marin, F.; Narayanappa, P.; Motreuil, S. Acidic Shell Proteins of the Mediterranean Fan Mussel Pinna nobilis. In Molecular Biomineralization. Progress in Molecular and Subcellular Biology; Müller, W.E.G., Ed.; Springer: Berlin/Heidelberg, Germany, 2011; Volume 52, pp. 353–396. [Google Scholar]
- Thula, T.T.; Svedlund, F.; Rodriguez, D.E.; Podschun, J.; Pendi, L.; Gower, L.B. Mimicking the Nanostructure of Bone: Comparison of Polymeric Process-Directing Agents. Polymers 2011, 3, 10–35. [Google Scholar] [CrossRef] [PubMed]
- Marin, F.; Amons, R.; Guichard, N.; Stigter, M.; Hecker, A.; Luquet, G.; Layrolle, P.; Alcaraz, G.; Riondet, C.; Westbroek, P. Caspartin and Calprismin, Two Proteins of the Shell Calcitic Prisms of the Mediterranean Fan Mussel Pinna nobilis. J. Biol. Chem. 2005, 280, 33895–33908. [Google Scholar] [CrossRef]
- Marin, F.; Luquet, G. Unusually Acidic Proteins in Biomineralization. In Handbook of Biomineralization: Biological Aspects and Structure Formation; Bäuerlein, E., Ed.; Wiley-VCH Verlag GmbH: Weinheim, Germany, 2007; Volume 1. [Google Scholar]
- Hovden, R.; Wolf, S.E.; Holtz, M.E.; Marin, F.; Muller, D.A.; Estroff, L.A. Nanoscale Assembly Processes Revealed in the Nacroprismatic Transition Zone of Pinna nobilis Mollusc Shells. Nat. Commun. 2015, 6, 10097. [Google Scholar] [CrossRef]
- Teng, H.H.; Dove, P.M.; De Yoreo, J.J. Kinetics of Calcite Growth: Surface Processes and Relationships to Macroscopic Rate Laws. Geochim. Cosmochim. Acta 2000, 64, 2255–2266. [Google Scholar] [CrossRef]
- Nielsen, A.E. Rate Laws and Rate Constants in Crystal Growth. Croat. Chim. Acta 1987, 60, 531–539. [Google Scholar]
- Vielzeuf, D.; Garrabou, J.; Baronnet, A.; Grauby, O.; Marschal, C. Nano to Macroscale Biomineral Architecture of Red Coral (Corallium Rubrum). Am. Mineral. 2008, 93, 1799–1815. [Google Scholar] [CrossRef]
- Addadi, L.; Joester, D.; Nudelman, F.; Weiner, S. Mollusk Shell Formation: A Source of New Concepts for Understanding Biomineralization Processes. Chem. Eur. J. 2006, 12, 980–987. [Google Scholar] [CrossRef] [PubMed]
- Wolf, S.E.; Böhm, C.F.; Harris, J.; Demmert, B.; Jacob, D.E.; Mondeshki, M.; Ruiz-Agudo, E.E.; Rodriguez-Navarro, C.; Rodríguez-Navarro, C. Nonclassical Crystallization in vivo et in vitro (I): Process-Structure-Property Relationships of Nanogranular Biominerals. J. Struct. Biol. 2016, 196, 260–287. [Google Scholar] [CrossRef] [PubMed]
- Weiner, S.; Addadi, L. Crystallization Pathways in Biomineralization. Annu. Rev. Mater. Res. 2011, 41, 21–40. [Google Scholar] [CrossRef]
- Watabe, N. Shell Repair. In The Mollusca, Physiology, Volume 4, Part 1; Wilbur, K.M., Saleuuddin, A., Eds.; Academic Press, Inc. (London) LTD: New York, NY, USA, 1983; pp. 289–310. [Google Scholar]
- Gower, L.B. Biomimetic Model Systems for Investigating the Amorphous Precursor Pathway and Its Role in Biomineralization. Chem. Rev. 2008, 108, 4551–4627. [Google Scholar] [CrossRef] [PubMed]
- Wolf, S.E.; Lieberwirth, I.; Natalio, F.; Bardeau, J.-F.; Delorme, N.; Emmerling, F.; Barrea, R.; Kappl, M.; Marin, F. Merging Models of Biomineralisation with Concepts of Nonclassical Crystallisation: Is a Liquid Amorphous Precursor Involved in the Formation of the Prismatic Layer of the Mediterranean Fan Mussel Pinna nobilis? Faraday Discuss. 2012, 159, 433–448. [Google Scholar] [CrossRef]
- Beniash, E.; Aizenberg, J.; Addadi, L.; Weiner, S. Amorphous calcium carbonate transforms into calcite during sea urchin larval spicule growth. Proc. R. Soc. Lond. B Biol. Sci. 1997, 264, 461–465. [Google Scholar] [CrossRef]
- Li, H.; Xin, H.L.; Kunitake, M.E.; Keene, E.C.; Muller, D.A.; Estroff, L.A.; Muller, A.; Estroff, L.A. Calcite Prisms from Mollusk Shells (Atrina Rigida): Swiss-Cheese-like Organic-Inorganic Single-Crystal Composites. Adv. Funct. Mater. 2011, 21, 2028–2034. [Google Scholar] [CrossRef]
- Falini, G.; Albeck, S.; Weiner, S.; Addadi, L.; Falini, G.; Albeck, S.; Weiner, S.; Addadit, L. Control of Aragonite or Calcite Polymorphism by Mollusk Shell Macromolecules. Science 1996, 271, 67–69. [Google Scholar] [CrossRef]
- Gong, Y.U.T.; Killian, C.E.; Olson, I.C.; Appathurai, N.P.; Amasino, A.L.; Martin, M.C.; Holt, L.J.; Wilt, F.H.; Gilbert, P.U.P.A. Phase Transitions in Biogenic Amorphous Calcium Carbonate. Proc. Natl. Acad. Sci. USA 2012, 109, 6088–6093. [Google Scholar] [CrossRef] [PubMed]
- Radha, A.V.; Forbes, T.Z.T.Z.; Killian, C.E.; Gilbert, P.U.P.A.; Navrotsky, A. Transformation and Crystallization Energetics of Synthetic and Biogenic Amorphous Calcium Carbonate. Proc. Natl. Acad. Sci. USA 2010, 107, 16438–16443. [Google Scholar] [CrossRef] [PubMed]
- De Yoreo, J.J.; Gilbert, P.U.P.A.; Sommerdijk, N.A.J.M.; Penn, R.L.; Whitelam, S.; Joester, D.; Zhang, H.; Rimer, J.D.; Navrotsky, A.; Banfield, J.F.; et al. Crystallization by Particle Attachment in Synthetic, Biogenic, and Geologic Environments. Science 2015, 349, aaa6760. [Google Scholar] [CrossRef] [PubMed]
- Albéric, M.; Bertinetti, L.; Zou, Z.; Fratzl, P.; Habraken, W.; Politi, Y. The Crystallization of Amorphous Calcium Carbonate Is Kinetically Governed by Ion Impurities and Water. Adv. Sci. 2017, 5, 1701000. [Google Scholar] [CrossRef]
- Killian, C.E.; Metzler, R.; Gong, Y.U.T.; Olson, I.C.; Aizenberg, J.; Politi, Y.; Wilt, F.H.; Scholl, A.; Young, A.; Doran, A.; et al. Mechanism of Calcite Co-Orientation in the Sea Urchin Tooth. J. Am. Chem. Soc. 2009, 131, 18404–18409. [Google Scholar] [CrossRef]
- Kim, Y.-Y.; Schenk, A.S.; Ihli, J.; Kulak, A.N.; Hetherington, N.B.J.; Tang, C.C.; Schmahl, W.W.; Griesshaber, E.; Hyett, G.; Meldrum, F.C. A Critical Analysis of Calcium Carbonate Mesocrystals. Nat. Commun. 2014, 5, 4341. [Google Scholar] [CrossRef]
- Gal, A.; Kahil, K.; Vidavsky, N.; DeVol, R.T.; Gilbert, P.U.P.A.; Fratzl, P.; Weiner, S.; Addadi, L. Particle Accretion Mechanism Underlies Biological Crystal Growth from an Amorphous Precursor Phase. Adv. Funct. Mater. 2014, 24, 5420–5426. [Google Scholar] [CrossRef]
- Gal, A.; Weiner, S.; Addadi, L. A Perspective on Underlying Crystal Growth Mechanisms in Biomineralization: Solution Mediated Growth versus Nanosphere Particle Accretion. CrystEngComm 2015, 17, 2606–2615. [Google Scholar] [CrossRef]
- Harris, J.; Mey, I.; Hajir, M.; Mondeshki, M.; Wolf, S.E. Pseudomorphic Transformation of Amorphous Calcium Carbonate Films Follows Spherulitic Growth Mechanisms and Can Give Rise to Crystal Lattice Tilting. CrystEngComm 2015, 17, 6831–6837. [Google Scholar] [CrossRef]
- Gower, L.B.; Odom, D. Deposition of Calcium Carbonate Films by a Polymer-Induced Liquid-Precursor (PILP) Process. J. Cryst. Growth 2000, 210, 719–734. [Google Scholar] [CrossRef]
- Politi, Y.; Metzler, R.A.; Abrecht, M.; Gilbert, B.; Wilt, F.H.; Sagi, I.; Addadi, L.; Weinfurter, H.; Gilbert, P.U.P.A. Transformation Mechanism of Amorphous Calcium Carbonate into Calcite in the Sea Urchin Larval Spicule. Proc. Natl. Acad. Sci. USA 2008, 105, 17362–17366. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, P.U.P.A.; Metzler, R.A.; Zhou, D.; Scholl, A.; Doran, A.; Young, A.; Kunz, M.; Tamura, N.; Coppersmith, S.N. Gradual Ordering in Red Abalone Nacre. J. Am. Chem. Soc. 2008, 130, 17519–17527. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Nielsen, M.H.; Freeman, C.L.; Hamm, L.M.; Tao, J.; Lee, J.R.I.; Han, T.Y.J.; Becker, U.; Harding, J.H.; Dove, P.M.; et al. The Thermodynamics of Calcite Nucleation at Organic Interfaces: Classical vs. Non-Classical Pathways. Faraday Discuss. 2012, 159, 509–523. [Google Scholar] [CrossRef]
- Cölfen, H.; Mann, S. Higher-Order Organization by Mesoscale Self-Assembly and Transformation of Hybrid Nanostructures. Angew. Chem. Int. Ed. 2003, 42, 2350–2365. [Google Scholar] [CrossRef] [PubMed]
- Bergström, L.; Sturm Née Rosseeva, E.V.; Salazar-Alvarez, G.; Cölfen, H. Mesocrystals in Biominerals and Colloidal Arrays. Acc. Chem. Res. 2015, 48, 1391–1402. [Google Scholar] [CrossRef] [PubMed]
- Cölfen, H. Bio-Inspired Mineralization Using Hydrophilic Polymers. Top. Curr. Chem. 2006, 271, 1–77. [Google Scholar] [CrossRef]
- Robb, D.T.; Privman, V. Model of Nanocrystal Formation in Solution by Burst Nucleation and Diffusional Growth. Langmuir 2008, 24, 26–35. [Google Scholar] [CrossRef]
- Gower, L.B.; Tirrell, D. Calcium Carbonate Films and Helices Grown in Solutions of Poly(Aspartate). J. Cryst. Growth 1998, 191, 153–160. [Google Scholar] [CrossRef]
- Rodríguez-Navarroa, C.; Ruiz-Agudo, E.; Harris, J.; Wolf, S.E. Nonclassical crystallization in vivo et in vitro (II): Nanogranular features in biomimetic minerals disclose a general colloid-mediated crystal growth mechanism. J. Struct. Biol. 2016, 196, 260–287. [Google Scholar] [CrossRef]
- Wolf, S.E.; Leiterer, J.; Kappl, M.; Emmerling, F.; Tremel, W. Early Homogenous Amorphous Precursor Stages of Calcium Carbonate and Subsequent Crystal Growth in Levitated Droplets. J. Am. Chem. Soc. 2008, 130, 12342–12347. [Google Scholar] [CrossRef] [PubMed]
- Tartaj, P.; Amarilla, J.M. Multifunctional Response of Anatase Nanostructures Based on 25 Nm Mesocrystal-Like Porous Assemblies. Adv. Mater. 2011, 23, 4904–4907. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.-Y.; Long, L.-L.; Li, W.-W.; Wang, W.-K.; Yu, H.-Q. Hexagonal Microrods of Anatase Tetragonal TiO2: Self-Directed Growth and Superior Photocatalytic Performance. Chem. Commun. 2013, 49, 6075. [Google Scholar] [CrossRef] [PubMed]
- Song, R.Q.; Krasia-Christoforou, T.; Debus, C.; Cölfen, H. Structure and Magnetic Property Control of Copper Hydroxide Acetate by Non-Classical Crystallization. Small 2017, 13, 1602702. [Google Scholar] [CrossRef] [PubMed]
- Song, R.-Q.; Cölfen, H. Mesocrystals-Ordered Nanoparticle Superstructures. Adv. Mater. 2010, 22, 1301–1330. [Google Scholar] [CrossRef] [PubMed]
- Song, R.-Q.; Cölfen, H.; Xu, A.-W.; Hartmann, J.; Antonietti, M. Polyelectrolyte-Directed Nanoparticle Aggregation: Systematic Morphogenesis of Calcium Carbonate by Nonclassical Crystallization. ACS Nano 2009, 3, 1966–1978. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Xie, T.; Peng, Q.; Li, Y. Ag, Ag2S, and Ag2Se Nanocrystals: Synthesis, Assembly, and Construction of Mesoporous Structures. J. Am. Chem. Soc. 2008, 130, 4016–4022. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Antonietti, M.; Cölfen, H. Calcite Mesocrystals: “Morphing” Crystals by a Polyelectrolyte. Chemistry 2006, 12, 5722–5730. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Cölfen, H.; Antonietti, M. Nonclassical Crystallization: Mesocrystals and Morphology Change of CaCO3 Crystals in the Presence of a Polyelectrolyte Additive. J. Am. Chem. Soc. 2005, 127, 3246–3247. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Yang, Y.; Wu, H.; Guo, S. Control of the Formation of Rod-like ZnO Mesocrystals and Their Photocatalytic Properties. CrystEngComm 2013, 15, 2608–2615. [Google Scholar] [CrossRef]
- Da Silva, R.O.; Gonçalves, R.H.; Stroppa, D.G.; Ramirez, A.J.; Leite, E.R. Synthesis of Recrystallized Anatase TiO2 Mesocrystals with Wulff Shape Assisted by Oriented Attachment. Nanoscale 2011, 3, 1910–1916. [Google Scholar] [CrossRef] [PubMed]
- Schenk, A.S.; Eiben, S.; Goll, M.; Reith, L.; Kulak, A.N.; Meldrum, F.C.; Jeske, H.; Wege, C.; Ludwigs, S. Virus-Directed Formation of Electrocatalytically Active Nanoparticle-Based Co3O4tubes. Nanoscale 2017, 9, 6334–6345. [Google Scholar] [CrossRef] [PubMed]
- Ross, M.B.; Ku, J.C.; Vaccarezza, V.M.; Schatz, G.C.; Mirkin, C.A. Nanoscale Form Dictates Mesoscale Function in Plasmonic DNA–Nanoparticle Superlattices. Nat. Nanotechnol. 2015, 10, 453–458. [Google Scholar] [CrossRef]
- Ortega, S.; Ibáñez, M.; Liu, Y.; Zhang, Y.; Kovalenko, M.V.; Cadavid, D.; Cabot, A. Bottom-up Engineering of Thermoelectric Nanomaterials and Devices from Solution-Processed Nanoparticle Building Blocks. Chem. Soc. Rev. 2017, 46, 3510–3528. [Google Scholar] [CrossRef] [PubMed]
- Sang, L.; Zhao, Y.; Burda, C. TiO2 Nanoparticles as Functional Building Blocks. Chem. Rev. 2014, 114, 9283–9318. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-W.; Christenson, H.K.; Meldrum, F.C. Confinement Leads to Control over Calcium Sulfate Polymorph. Adv. Funct. Mater. 2013, 23, 5615–5623. [Google Scholar] [CrossRef]
- Uebe, R.; Schüler, D. Magnetosome Biogenesis in Magnetotactic Bacteria. Nat. Rev. Microbiol. 2016, 14, 621–637. [Google Scholar] [CrossRef]
- Smeets, P.J.M.; Finney, A.R.; Habraken, W.J.E.M.; Nudelman, F.; Friedrich, H. A Classical View on Nonclassical Nucleation. Proc. Natl. Acad. Sci. USA 2017, 114, E7882–E7890. [Google Scholar] [CrossRef]
- Wolf, S.E.; Gower, L.B. Challenges and Perspectives of the Polymer-Induced Liquid-Precursor Process: The Pathway from Liquid-Condensed Mineral Precursors to Mesocrystalline Products. In New Perspectives on Mineral Nucleation and Growth; van Driessche, A.E.S., Kellermeier, M., Benning, L.G., Gebauer, D., Eds.; Springer: Cham, Switzerland, 2017; pp. 43–75. [Google Scholar]
- Cölfen, H.; Antonietti, M. Mesocrystals and Nonclassical Crystallization; Cölfen, H., Antonietti, M., Eds.; Wiley-VCH Verlag GmbH: Berlin, Germany, 2008. [Google Scholar]
- Niederberger, M.; Cölfen, H. Oriented Attachment and Mesocrystals: Non-Classical Crystallization Mechanisms Based on Nanoparticle Assembly. Phys. Chem. Chem. Phys. 2006, 8, 3271–3287. [Google Scholar] [CrossRef]
- Wallace, A.F.; Hedges, L.O.; Fernandez-Martinez, A.; Raiteri, P.; Gale, J.D.; Waychunas, G.A.; Whitelam, S.; Banfield, J.F.; De Yoreo, J.J. Microscopic Evidence for Liquid-Liquid Separation in Supersaturated CaCO3 Solutions. Science 2013, 341, 885–889. [Google Scholar] [CrossRef]
- Kim, Y.-Y.; Schenk, A.S.; Walsh, D.; Kulak, A.N.; Cespedes, O.; Meldrum, F.C. Bio-Inspired Formation of Functional Calcite/Metal Oxide Nanoparticle Composites. Nanoscale 2014, 6, 852–859. [Google Scholar] [CrossRef] [PubMed]
- Schenk, A.S.; Zlotnikov, I.; Pokroy, B.; Gierlinger, N.; Masic, A.; Zaslansky, P.; Fitch, A.N.; Paris, O.; Metzger, T.H.; Cölfen, H.; et al. Hierarchical Calcite Crystals with Occlusions of a Simple Polyelectrolyte Mimic Complex Biomineral Structures. Adv. Funct. Mater. 2012, 22, 4668–4676. [Google Scholar] [CrossRef]
- Barthelat, F.; Rim, J.E.; Espinosa, H.D. A Review on the Structure and Mechanical Properties of Mollusk Shells—Perspectives on Synthetic Biomimetic Materials. In Applied Scanning Probe Methods XIII, Biomimetics and Industrial Applications; Bhushan, B., Fuchs, H., Eds.; Springer: Berlin, Germany, 2009; pp. 17–41. [Google Scholar]
- Villanova, J.; Kozachkevich, S.; Zaslansky, P.; Kundanati, L.; Bracha, A.A.; Polishchuk, I.; Bloch, L.; Levy, D.; Katsman, A.; Giacobbe, C.; et al. Coherently Aligned Nanoparticles within a Biogenic Single Crystal: A Biological Prestressing Strategy. Science 2017, 358, 1294–1298. [Google Scholar] [CrossRef]
- Sethmann, I.; Hinrichs, R.; Wörheide, G.; Putnis, A. Nano-Cluster Composite Structure of Calcitic Sponge Spicules—A Case Study of Basic Characteristics of Biominerals. J. Inorg. Biochem. 2006, 100, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Addadi, L.; Weiner, S. Biomineralization: Mineral Formation by Organisms. Phys. Scr. 2014, 89, 098003. [Google Scholar] [CrossRef]
- Weiner, S.; Addadi, L. Design Strategies in Mineralized Biological Materials. J. Mater. Chem. 1997, 7, 689–702. [Google Scholar] [CrossRef]
- Gebauer, D.; Wolf, S.E. Designing Solid Materials from their Solute State: a Shift in Paradigms toward a Holistic Approach in Functional Materials Chemistry. J. Am. Chem. Soc. 2019, 141, 4490–4504. [Google Scholar] [CrossRef]
- Wegst, U.G.K.; Ashby, M.F. The Mechanical Efficiency of Natural Materials. Philos. Mag. 2004, 84, 2167–2186. [Google Scholar] [CrossRef]
- Meyers, M.A.; Chen, P.-Y.; Lin, A.Y.-M.; Seki, Y. Biological Materials: Structure and Mechanical Properties. Prog. Mater. Sci. 2008, 53, 1–206. [Google Scholar] [CrossRef]
- Pokroy, B.; Zolotoyabko, E. Microstructure of Natural Plywood-like Ceramics: A Study by High-Resolution Electron Microscopy and Energy-Variable X-Ray Diffraction. J. Mater. Chem. 2003, 13, 682–688. [Google Scholar] [CrossRef]
- Tai, K.; Ulm, F.J.; Ortiz, C. Nanogranular Origins of the Strength of Bone. Nano Lett. 2006, 6, 2520–2525. [Google Scholar] [CrossRef] [PubMed]
- Ryall, R.L.; Fleming, D.E.; Doyle, I.R.; Evans, N.A.; Dean, C.J.; Marshall, V.R. Intracrystalline Proteins and the Hidden Ultrastructure of Calcium Oxalate Urinary Crystals: Implications for Kidney Stone Formation. J. Struct. Biol. 2001, 134, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Böhm, C.F.; Demmert, B.; Harris, J.; Fey, T.; Marin, F.; Wolf, S.E. Structural Commonalities and Deviations in the Hierarchical Organization of Crossed-Lamellar Shells: A Case Study on the Shell of the Bivalve Glycymeris Glycymeris. J. Mater. Res. 2016, 31, 536–546. [Google Scholar] [CrossRef]
- Schenk, A.S.; Kim, Y.Y. Unraveling the Internal Microstructure of Biogenic and Bioinspired Calcite Single Crystals. MRS Bull. 2015, 40, 499–508. [Google Scholar] [CrossRef]
- Mutvei, H.; Dunca, E. Crystalline Structure, Orientation and Nucleation of the Nacreous Tablets in the Cephalopod Nautilus. Paläontologische Zeitschrift 2010, 84, 457–465. [Google Scholar] [CrossRef]
- Checa, A.G.; Mutvei, H.; Osuna-Mascaró, A.J.; Bonarski, J.T.; Faryna, M.; Berent, K.; Pina, C.M.; Rousseau, M.; Macías-Sánchez, E. Crystallographic Control on the Substructure of Nacre Tablets. J. Struct. Biol. 2013, 183, 368–376. [Google Scholar] [CrossRef] [PubMed]
- Athanasiadou, D.; Jiang, W.; Goldbaum, D.; Saleem, A.; Basu, K.; Pacella, M.S.; Böhm, C.F.; Chromik, R.R.; Hincke, M.T.; Rodríguez-Navarro, A.B.; et al. Nanostructure, Osteopontin, and Mechanical Properties of Calcitic Avian Eggshell. Sci. Adv. 2018, 4, eaar3219. [Google Scholar] [CrossRef]
- Rodríguez-Navarro, A.B.; Marie, P.; Nys, Y.; Hincke, M.T.; Gautron, J. Amorphous Calcium Carbonate Controls Avian Eggshell Mineralization: A New Paradigm for Understanding Rapid Eggshell Calcification. J. Struct. Biol. 2015, 190, 291–303. [Google Scholar] [CrossRef]
- Seto, J.; Ma, Y.; Davis, S.A.; Meldrum, F.C.; Gourrier, A.; Kim, Y.-Y.; Schilde, U.; Sztucki, M.; Burghammer, M.; Maltsev, S.; et al. Structure-Property Relationships of a Biological Mesocrystal in the Adult Sea Urchin Spines. Proc. Natl. Acad. Sci. USA 2012, 109, 3699–3704. [Google Scholar] [CrossRef]
- Dauphin, Y.; Cuif, J.P.; Doucet, J.; Salomé, M.; Susini, J.; Willams, C.T. In Situ Chemical Speciation of Sulfur in Calcitic Biominerals and the Simple Prism Concept. J. Struct. Biol. 2003, 142, 272–280. [Google Scholar] [CrossRef]
- Dauphin, Y. The Nanostructural Unity of Mollusc Shells. Mineral. Mag. 2008, 72, 243–246. [Google Scholar] [CrossRef]
- Jacob, D.E.; Soldati, A.; Wirth, R.; Huth, J.; Wehrmeister, U.; Hofmeister, W. Nanostructure, Composition and Mechanisms of Bivalve Shell Growth. Geochim. Cosmochim. Acta 2008, 72, 5401–5415. [Google Scholar] [CrossRef]
- Checa, A.G.; Bonarski, J.T.; Willinger, M.G.; Faryna, M.; Berent, K.; Kania, B.; González-Segura, A.; Pina, C.M.; Pospiech, J.; Morawiec, A. Crystallographic Orientation Inhomogeneity and Crystal Splitting in Biogenic Calcite. J. R. Soc. Interface 2013, 10, 20130425. [Google Scholar] [CrossRef]
- Okumura, T.; Suzuki, M.; Nagasawa, H.; Kogure, T. Microstructural Variation of Biogenic Calcite with Intracrystalline Organic Macromolecules. Cryst. Growth Des. 2012, 12, 224–230. [Google Scholar] [CrossRef]
- Suzuki, M.; Kameda, J.; Sasaki, T.; Saruwatari, K.; Nagasawa, H.; Kogure, T. Characterization of the Multilayered Shell of a Limpet, Lottia Kogamogai (Mollusca: Patellogastropoda), Using SEM–EBSD and FIB–TEM Techniques. J. Struct. Biol. 2010, 171, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Aizenberg, J.; Hanson, J.; Koetzle, T.F.; Weinfurter, H.; Addadi, L.; York, N.; August, R.V. Control of Macromolecule Distribution within Synthetic and Biogenic Single Calcite Crystals. J. Am. Chem. Soc. 1997, 119, 881–886. [Google Scholar] [CrossRef]
- Berman, A.; Addadi, L.; Kvick, A.Y.E.; Leiserowitz, L.; Nelson, M.; Weinfurter, H. Intercalation of Sea Urchin Proteins in Calcite: Study of a Crystalline Composite Material. Science 1990, 250, 664–667. [Google Scholar] [CrossRef]
- Pisklak, D.M.; Szeleszczuk, Ł.; Wawer, I. 1H and 13C Magic-Angle Spinning Nuclear Magnetic Resonance Studies of the Chicken Eggshell. J. Agric. Food Chem. 2012, 60, 12254–12259. [Google Scholar] [CrossRef]
- Ben Shir, I.; Kababya, S.; Katz, I.; Pokroy, B.; Schmidt, A. Exposed and Buried Biomineral Interfaces in the Aragonitic Shell of Perna canaliculus Revealed by Solid-State NMR. Chem. Mater. 2013, 25, 4595–4602. [Google Scholar] [CrossRef]
- Lee, D.; Leroy, C.; Crevant, C.; Bonhomme-Coury, L.; Babonneau, F.; Laurencin, D.; Bonhomme, C.; De Paëpe, G.; Bryce, D.L.; Laurencin, D.; et al. Interfacial Ca2+ Environments in Nanocrystalline Apatites Revealed by Dynamic Nuclear Polarization Enhanced 43Ca NMR Spectroscopy. Nat. Commun. 2017, 8, 14104. [Google Scholar] [CrossRef]
- Agbaje, O.B.A.; Ben Shir, I.; Zax, D.B.; Schmidt, A.; Jacob, D.E. Biomacromolecules within Bivalve Shells: Is Chitin Abundant? Acta Biomater. 2018, 80, 176–187. [Google Scholar] [CrossRef] [PubMed]
- Bar-On, B.; Wagner, H.D. Structural Motifs and Elastic Properties of Hierarchical Biological Tissues—A Review. J. Struct. Biol. 2013, 183, 149–164. [Google Scholar] [CrossRef] [PubMed]
- Meyers, M.A.; Lin, A.; Chen, P.; Muyco, J. Mechanical Strength of Abalone Nacre: Role of the Soft Organic Layer. J. Mech. Behav. Biomed. Mater. 2008, 1, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Meyers, M.A.; Zhang, Z.; Ritchie, R.O. Functional Gradients and Heterogeneities in Biological Materials: Design Principles, Functions, and Bioinspired Applications. Prog. Mater. Sci. 2017, 88, 467–498. [Google Scholar] [CrossRef]
- Currey, J.D.; Taylor, J.D. The Mechanical Behaviour of Some Molluscan Hard Tissues. J. Zool. 1974, 173, 395–406. [Google Scholar] [CrossRef]
- Chen, P.-Y.; McKittrick, J.; Meyers, M.A. Biological Materials: Functional Adaptations and Bioinspired Designs. Prog. Mater. Sci. 2012, 57, 1492–1704. [Google Scholar] [CrossRef]
- Bayerlein, B.; Bertinetti, L.; Bar-on, B.; Blumtritt, H.; Fratzl, P. Inherent Role of Water in Damage Tolerance of the Prismatic Mineral-Organic Biocomposite in the Shell of Pinna nobilis. Adv. Fcunt. Mater. 2016, 26, 3663–3669. [Google Scholar] [CrossRef]
- Cuif, J.-P.; Burghammer, M.; Chamard, V.; Dauphin, Y.; Godard, P.; Moullac, G.; Nehrke, G.; Perez-Huerta, A. Evidence of a Biological Control over Origin, Growth and End of the Calcite Prisms in the Shells of Pinctada Margaritifera (Pelecypod, Pterioidea). Minerals 2014, 4, 815–834. [Google Scholar] [CrossRef]
- Nakahara, H.; Kakei, M.; Bevelander, G. Fine Structure and Amino Acid Composition of the Organic “Envelope” in the Prismatic Layer of Some Bivlave Shells. Jpn. J. Malacol. 1980, 39, 167–177. [Google Scholar]
- Gilbert, P.U.P.A.; Young, A.; Coppersmith, S.N. Measurement of C-Axis Angular Orientation in Calcite (CaCO3) Nanocrystals Using X-Ray Absorption Spectroscopy. Proc. Natl. Acad. Sci. USA 2011, 108, 11350–11355. [Google Scholar] [CrossRef]
- Kunitake, M.E.; Mangano, L.M.; Peloquin, J.M.; Baker, S.P.; Estroff, L.A. Evaluation of Strengthening Mechanisms in Calcite Single Crystals from Mollusk Shells. Acta Biomater. 2013, 9, 5353–5359. [Google Scholar] [CrossRef] [PubMed]
- Kuhn-Spearing, L.T.; Kessler, H.; Chateau, E.; Ballarini, R.; Heuer, A.H.; Spearing, S.M. Fracture Mechanisms of the Strombus Gigas Conch Shell: Implications for the Design of Brittle Laminates. J. Mater. Sci. 1996, 31, 6583–6594. [Google Scholar] [CrossRef]
- Kamat, S.; Su, X.; Ballarini, R.; Heuer, A. Structural Basis for the Fracture Toughness of the Shell of the Conch Strombus Gigas. Nature 2000, 405, 1036–1040. [Google Scholar] [CrossRef] [PubMed]
- Weaver, J.C.; Milliron, G.W.; Miserez, A.; Evans-Lutterodt, K.; Herrera, S.; Gallana, I.; Mershon, W.J.; Swanson, B.; Zavattieri, P.; DiMasi, E.; et al. The Stomatopod Dactyl Club: A Formidable Damage-Tolerant Biological Hammer. Science 2012, 336, 1275–1280. [Google Scholar] [CrossRef] [PubMed]
- Grunenfelder, L.K.; Suksangpanya, N.; Salinas, C.; Milliron, G.; Yaraghi, N.; Herrera, S.; Evans-Lutterodt, K.; Nutt, S.R.; Zavattieri, P.; Kisailus, D. Bio-Inspired Impact-Resistant Composites. Acta Biomater. 2014, 10, 3997–4008. [Google Scholar] [CrossRef] [PubMed]
- Guarín-Zapata, N.; Gomez, J.; Yaraghi, N.; Kisailus, D.; Zavattieri, P.D. Shear Wave Filtering in Naturally-Occurring Bouligand Structures. Acta Biomater. 2015, 23, 11–20. [Google Scholar] [CrossRef] [PubMed]
- de Obaldia, E.E.; Jeong, C.; Grunenfelder, L.K.; Kisailus, D.; Zavattieri, P. Analysis of the Mechanical Response of Biomimetic Materials with Highly Oriented Microstructures through 3D Printing, Mechanical Testing and Modeling. J. Mech. Behav. Biomed. Mater. 2015, 48, 70–85. [Google Scholar] [CrossRef] [PubMed]
- Mirkhalaf, M.; Dastjerdi, A.K.; Barthelat, F. Overcoming the Brittleness of Glass through Bio-Inspiration and Micro-Architecture. Nat. Commun. 2014, 5, 3166. [Google Scholar] [CrossRef]
- Ritchie, R.O. The Conflicts between Strength and Toughness. Nat. Mater. 2011, 10, 817–822. [Google Scholar] [CrossRef]
- Weißbach, W.; Dahms, M.; Jaroschek, C. Werkstoffkunde; Springer: Wiesbaden, Germany, 2015. [Google Scholar]
- Turner, F.J.; Griffs, D.T.; Heard, H. Experimental Deformation of Calcite Crystals. Geol. Soc. Am. Bull. 1954, 65, 883–934. [Google Scholar] [CrossRef]
- Carlton, C.E.; Ferreira, P.J. What Is behind the Inverse Hall-Petch Effect in Nanocrystalline Materials? Acta Mater. 2007, 55, 3749–3756. [Google Scholar] [CrossRef]
- Wiederhorn, S.M. Brittle Fracture and Toughening Mechanisms in Ceramics. Annu. Rev. Mater. Sci. 1984, 14, 373–403. [Google Scholar] [CrossRef]
- Salmang, H.; Scholze, H. Keramik; Springer-Verlag: Berlin/Heidelberg, Germany, 2007. [Google Scholar] [CrossRef]
- Schamel, M.; Barralet, J.E.; Gelinsky, M.; Groll, J.; Gbureck, U. Intrinsic 3D Prestressing: A New Route for Increasing Strength and Improving Toughness of Hybrid Inorganic Biocements. Adv. Mater. 2017, 29, 1701035. [Google Scholar] [CrossRef] [PubMed]
- Barthelat, F.; Tang, H.; Zavattieri, P.; Li, C.; Espinosa, H. On the Mechanics of Mother-of-Pearl: A Key Feature in the Material Hierarchical Structure. J. Mech. Phys. Solids 2007, 55, 306–337. [Google Scholar] [CrossRef]
- Fantner, G.E.; Hassenkam, T.; Kindt, J.H.; Weaver, J.C.; Birkedal, H.; Pechenik, L.; Cutroni, J.A.; Cidade, G.A.G.; Stucky, G.D.; Morse, D.E.; et al. Sacrificial Bonds and Hidden Length Dissipate Energy as Mineralized Fibrils Separate during Bone Fracture. Nat. Mater. 2005, 4, 612–616. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xu, Z.-H.; Wang, R. In Situ Observation of Nanograin Rotation and Deformation in Nacre. Nano Lett. 2006, 6, 2301–2304. [Google Scholar] [CrossRef]
- Gordon, L.M.; Joester, D. Nanoscale Chemical Tomography of Buried Organic-Inorganic Interfaces in the Chiton Tooth. Nature 2011, 469, 194–197. [Google Scholar] [CrossRef]
- Barthelat, F.F. Biomimetics for next Generation Materials. Philos. Trans. A Math. Phys. Eng. Sci. 2007, 365, 2907–2919. [Google Scholar] [CrossRef]
- Taylor, J.D.; Layman, M. The Mechanical Properties of Bivalve (Mollusca) Shell Structure. Paleontology 1972, 15, 73–87. [Google Scholar]
- Marin, F.; Luquet, G. Molluscan Shell Proteins. C. R. Palevol 2004, 3, 469–492. [Google Scholar] [CrossRef]
- Checa, A.G.; Cartwright, J.H.E.; Willinger, M.G. Mineral Bridges in Nacre. J. Struct. Biol. 2011, 176, 330–339. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.Z.; Suo, Z.; Evans, A.G.; Yao, N.; Aksay, I.A. Deformation Mechanisms in Nacre. J. Mater. Res. 2011, 16, 2485–2493. [Google Scholar] [CrossRef]
- Heinemann, F.; Launspach, M.; Gries, K.; Fritz, M. Gastropod Nacre: Structure, Properties and Growth—Biological, Chemical and Physical Basics. Biophys. Chem. 2011, 153, 126–153. [Google Scholar] [CrossRef] [PubMed]
- Barthelat, F.; Li, C.-M.; Comi, C.; Espinosa, H.D. Mechanical Properties of Nacre Constituents and Their Impact on Mechanical Performance. J. Mater. Res. 2006, 21, 1977–1986. [Google Scholar] [CrossRef]
- Weber, E.; Pokroy, B. Intracrystalline Inclusions within Single Crystalline Hosts: From Biomineralization to Bio-Inspired Crystal Growth. CrystEngComm 2015, 17, 5873–5883. [Google Scholar] [CrossRef]
- Levi-Kalisman, Y.; Falini, G.; Addadi, L.; Weinfurter, H. Structure of the Nacreous Organic Matrix of a Bivalve Mollusk Shell Examined in the Hydrated State Using Cryo-TEM. J. Struct. Biol. 2001, 135, 8–17. [Google Scholar] [CrossRef]
- Younis, S.; Kauffmann, Y.; Bloch, L.; Zolotoyabko, E. Inhomogeneity of Nacre Lamellae on the Nanometer Length Scale. Cryst. Growth Des. 2012, 12, 4574–4579. [Google Scholar] [CrossRef]
- Kim, Y.-Y.; Carloni, J.D.; Demarchi, B.; Sparks, D.; Reid, D.G.; Kunitake, M.E.; Tang, C.C.; Duer, M.J.; Freeman, C.L.; Pokroy, B.; et al. Tuning Hardness in Calcite by Incorporation of Amino Acids. Nat. Mater. 2016, 15, 903–910. [Google Scholar] [CrossRef]
- Koyama, M.; Zhang, Z.; Wang, M.; Ponge, D.; Raabe, D.; Tsuzaki, K.; Noguchi, H.; Tasan, C.C. Bone-like Crack Resistance in Hierarchical Metastable Nanolaminate Steels. Science 2017, 355, 1055–1057. [Google Scholar] [CrossRef]
- Cao, S.C.; Liu, J.; Zhu, L.; Li, L.; Dao, M.; Lu, J.; Ritchie, R.O. Nature-Inspired Hierarchical Steels. Sci. Rep. 2018, 8, 5088. [Google Scholar] [CrossRef]
- Wat, A.; Lee, J.I.; Ryu, C.W.; Gludovatz, B.; Kim, J.; Tomsia, A.P.; Ishikawa, T.; Schmitz, J.; Meyer, A.; Alfreider, M.; et al. Bioinspired Nacre-like Alumina with a Bulk-Metallic Glass-Forming Alloy as a Compliant Phase. Nat. Commun. 2019, 10, 961. [Google Scholar] [CrossRef] [PubMed]
- Kilper, S.; Facey, S.J.; Burghard, Z.; Hauer, B.; Rothenstein, D.; Bill, J. Macroscopic Properties of Biomimetic Ceramics Are Governed by the Molecular Recognition at the Bioorganic—Inorganic Interface. Adv. Funct. Mater. 2018, 28, 1705842. [Google Scholar] [CrossRef]
- Eder, M.; Amini, S.; Fratzl, P. Biological Composites—Complex Structures for Functional Diversity. Science 2018, 362, 543–547. [Google Scholar] [CrossRef] [PubMed]
- Aizenberg, J.; Weiner, S.; Tkachenko, A.; Addadi, L.; Hendler, G. Calcitic Microlenses as Part of the Photoreceptor System in Brittlestars. Nature 2001, 412, 819–822. [Google Scholar] [CrossRef] [PubMed]
- Nys, Y.; Gautron, J. Structure and Formation of the Eggshell. In Bioactive Egg Compounds; Anton, M., Schade, R., Huopalahti, R., López-Fandino, R., Eds.; Springer: Berlin/Heidelberg, Germany, 2007; pp. 1–4. [Google Scholar]
- Aizenberg, J.; Weaver, J.C.; Thanawala, M.S.; Sundar, V.C.; Morse, D.E.; Fratzl, P. Skeleton of Euplectella sp.: Structural Hierarchy from the Nanoscale to the Macroscale. Science 2005, 309, 275–278. [Google Scholar] [CrossRef] [PubMed]
- Weaver, J.C.; Aizenberg, J.; Fantner, G.E.; Kisailus, D.; Woesz, A.; Allen, P.; Fields, K.; Porter, M.J.; Zok, F.W.; Hansma, P.K.; et al. Hierarchical Assembly of the Siliceous Skeletal Lattice of the Hexactinellid Sponge Euplectella Aspergillum. J. Struct. Biol. 2007, 158, 93–106. [Google Scholar] [CrossRef]
- Ehrlich, H.; Deutzmann, R.; Brunner, E.; Cappellini, E.; Koon, H.; Solazzo, C.; Yang, Y.; Ashford, D.; Thomas-Oates, J.; Lubeck, M.; et al. Mineralization of the Metre-Long Biosilica Structures of Glass Sponges Is Templated on Hydroxylated Collagen. Nat. Chem. 2010, 2, 1084–1088. [Google Scholar] [CrossRef]
- Ehrlich, H. Chitin and Collagen as Universal and Alternative Templates in Biomineralization. Int. Geol. Rev. 2010, 52, 661–699. [Google Scholar] [CrossRef]
- Kröger, N.; Deutzmann, R.; Sumper, M. Polycationic Peptides from Diatom Biosilica That Direct Silica Nanosphere Formation. Science 1999, 286, 1129–1132. [Google Scholar]
- Matsunaga, S.; Sakai, R.; Jimbo, M.; Kamiya, H. Long-Chain Polyamines (LCPAs) from Marine Sponge: Possible Implication in Spicule Formation. Chembiochem 2007, 8, 1729–1735. [Google Scholar] [CrossRef]
- Poulsen, N.; Kröger, N. Silica Morphogenesis by Alternative Processing of Silaffins in the Diatom Thalassiosira Pseudonana. J. Chem. Phys. 2004, 279, 42993–42999. [Google Scholar] [CrossRef]
- Shimizu, K.; Cha, J.; Stucky, G.D. Silicatein: Cathepsin L-like Protein in Sponge Biosilica. Proc. Natl. Acad. Sci. USA 1998, 95, 6234–6238. [Google Scholar] [CrossRef] [PubMed]
- Sundar, V.C.; Yablon, A.D.; Grazul, J.L.; Ilan, M.; Aizenberg, J. Fibre-Optical Features of a Glass Sponge. Nature 2003, 424, 899–900. [Google Scholar] [CrossRef] [PubMed]
- Müller, W.E.G.; Wolf, S.E.; Schlossmacher, U.; Wang, X.-H.; Boreiko, A.; Brandt, D.; Tremel, W.; Schröder, H.-C. Poly(Silicate)-Metabolizing Silicatein in Siliceous Spicules and Silicasomes of Demosponges Comprises Dual Enzymatic Activities (Silica Polymerase and Silica Esterase). FEBS J. 2008, 275, 362–370. [Google Scholar] [CrossRef] [PubMed]
- Cha, J.N.; Shimizu, K.; Zhou, Y.; Christiansen, S.C.; Chmelka, B.F.; Stucky, G.D.; Morse, D.E. Silicatein Filaments and Subunits from a Marine Sponge Direct the Polymerization of Silica and Silicones in Vitro. Proc. Natl. Acad. Sci. USA 1999, 96, 361–365. [Google Scholar] [CrossRef]
- Zhou, Y.; Shimizu, K.; Cha, J.N.; Stucky, G.D.; Morse, D.E. Efficient Catalysis of Polysiloxane Synthesis by Silicatein α Requires Specific Hydroxy and Imidazole Functionalities. Angew. Chem. Int. Ed. 1999, 38, 780–782. [Google Scholar] [CrossRef]
- Shimizu, K.; Morse, D.E. Silicatein: A Unique Silica-Synthesizing Catalytic Triad Hydrolase From Marine Sponge Skeletons and Its Multiple Applications. Methods Enzymol. 2018, 605, 429–455. [Google Scholar] [CrossRef]
- Brutchey, R.L.; Morse, D.E. Silicatein and the Translation of Its Molecular Mechanism of Biosilicification into Low Temperature Nanomaterial Synthesis. Chem. Rev. 2008, 108, 4915–4934. [Google Scholar] [CrossRef]
- Kisailus, D.; Choi, J.H.; Weaver, J.C.; Yang, W.; Morse, D.E. Enzymatic Synthesis and Nanostructural Control of Gallium Oxide at Low Temperature. Adv. Mater. 2005, 17, 314–318. [Google Scholar] [CrossRef]
- Sumerel, J.L.; Yang, W.J.; Kisailus, D.; Weaver, J.C.; Choi, J.H.; Morse, D.E. Biocatalytically Templated Synthesis of Titanium Dioxide. Chem. Mater. 2003, 15, 4804–4809. [Google Scholar] [CrossRef]
- Curnow, P.; Bessette, P.H.; Kisailus, D.; Murr, M.M.; Daugherty, P.S.; Morse, D.E. Enzymatic Synthesis of Layered Titanium Phosphates at Low Temperature and Neutral PH by Cell-Surface Display of Silicatein-α. J. Am. Chem. Soc. 2005, 127, 15749–15755. [Google Scholar] [CrossRef]
- Wolf, S.E.; Schlossmacher, U.; Pietuch, A.; Mathiasch, B.; Schröder, H.-C.; Müller, W.E.G.; Tremel, W. Formation of Silicones Mediated by the Sponge Enzyme Silicatein-α. Dalton Trans. 2010, 39, 9245–9249. [Google Scholar] [CrossRef]
- O’Leary, P.; van Walree, C.A.; Mehendale, N.C.; Sumerel, J.; Morse, D.E.; Kaska, W.C.; van Koten, G.; Gebbink, R.J.M. Enzymatic Immobilization of Organometallic Species: Biosilification of NCN- and PCP-Pincer Metal Species Using Demosponge Axial Filaments. Dalton Trans. 2009, 22, 4289–4291. [Google Scholar] [CrossRef]
- Tahir, M.N.; Théato, P.; Müller, W.E.G.; Schröder, H.-C.; Janshoff, A.; Zhang, J.; Huth, J.; Tremel, W. Monitoring the Formation of Biosilica Catalysed by Histidine-Tagged Silicatein. Chem. Commun. 2004, 2848–2849. [Google Scholar] [CrossRef]
- Tahir, M.N.; Théato, P.; Müller, W.E.G.; Schröder, H.C.; Borejko, A.; Faiß, S.; Janshoff, A.; Huth, J.; Tremel, W. Formation of Layered Titania and Zirconia Catalysed by Surface-Bound Silicatein. Chem. Commun. 2005, 5533–5535. [Google Scholar] [CrossRef]
- Tahir, M.N.; Eberhardt, M.; Therese, H.A.; Kolb, U.; Theato, P.; Müller, W.E.G.; Schröder, H.-C.; Tremel, W. From Single Molecules to Nanoscopically Structured Functional Materials: Au Nanocrystal Growth on TiO2 Nanowires Controlled by Surface-Bound Silicatein. Angew. Chem. Int. Ed. 2006, 45, 4803–4809. [Google Scholar] [CrossRef]
- Shukoor, M.I.; Natalio, F.; Therese, H.A.; Tahir, M.N.; Ksenofontov, V.; Panthöfer, M.; Eberhardt, M.; Theato, P.; Schröder, H.C.; Müller, W.E.G.; et al. Fabrication of a Silica Coating on Magnetic γ-Fe2O3 Nanoparticles by an Immobilized Enzyme. Chem. Mater. 2008, 20, 3567–3573. [Google Scholar] [CrossRef]
- Natalio, F.; Link, T.; Müller, W.E.G.; Schröder, H.C.; Cui, F.-Z.; Wang, X.; Wiens, M. Bioengineering of the Silica-Polymerizing Enzyme Silicatein-α for a Targeted Application to Hydroxyapatite. Acta Biomater. 2010, 6, 3720–3728. [Google Scholar] [CrossRef]
- Natalio, F.; Corrales, T.P.; Panthofer, M.; Schollmeyer, D.; Lieberwirth, I.; Muller, W.E.G.; Kappl, M.; Butt, H.-J.; Tremel, W. Flexible Minerals: Self-Assembled Calcite Spicules with Extreme Bending Strength. Science 2013, 339, 1298–1302. [Google Scholar] [CrossRef]
- Cha, J.N.; Stucky, G.D.; Morse, D.E.; Deming, T.J. Biomimetic Synthesis of Ordered Silica Structures Mediated by Block Copolypeptides. Nature 2000, 403, 289–292. [Google Scholar] [CrossRef]
- Adamson, D.H.; Dabbs, D.M.; Pacheco, C.R.; Giotto, M.V.; Morse, D.E.; Aksay, I.A. Non-Peptide Polymeric Silicatein α Mimic for Neutral PH Catalysis in the Formation of Silica. Macromolecules 2007, 40, 5710–5717. [Google Scholar] [CrossRef]
- Robinson, D.B.; Rognlien, J.L.; Bauer, C.A.; Simmons, B.A. Dependence of Amine-Accelerated Silicate Condensation on Amine Structure. J. Mater. Chem. 2007, 17, 2113–2119. [Google Scholar] [CrossRef]
- Kisailus, D.; Najarian, M.; Weaver, J.C.; Morse, D.E. Functionalized Gold Nanoparticles Mimic Catalytic Activity of a Polysiloxane-Synthesizing Enzyme. Adv. Mater. 2005, 17, 1234–1239. [Google Scholar] [CrossRef]
- Roth, K.M.; Zhou, Y.; Yang, W.; Morse, D.E. Bifunctional Small Molecules Are Biomimetic Catalysts for Silica Synthesis at Neutral PH. J. Am. Chem. Soc. 2005, 127, 325–330. [Google Scholar] [CrossRef]
- Luckarift, H.R.; Spain, J.C.; Naik, R.R.; Stone, M.O. Enzyme Immobilization in a Biomimetic Silica Support. Nat. Biotechnol. 2004, 22, 211–213. [Google Scholar] [CrossRef]
- Corma, A.; Díaz-Cabañas, M.J.; Moliner, M.; Rodríguez, G. Synthesis of Micro- and Mesoporous Molecular Sieves at Room Temperature and Neutral PH Catalyzed by Functional Analogues of Silicatein. Chem. Commun. 2006, 3137–3139. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Böhm, C.F.; Harris, J.; Schodder, P.I.; Wolf, S.E. Bioinspired Materials: From Living Systems to New Concepts in Materials Chemistry. Materials 2019, 12, 2117. https://doi.org/10.3390/ma12132117
Böhm CF, Harris J, Schodder PI, Wolf SE. Bioinspired Materials: From Living Systems to New Concepts in Materials Chemistry. Materials. 2019; 12(13):2117. https://doi.org/10.3390/ma12132117
Chicago/Turabian StyleBöhm, Corinna F., Joe Harris, Philipp I. Schodder, and Stephan E. Wolf. 2019. "Bioinspired Materials: From Living Systems to New Concepts in Materials Chemistry" Materials 12, no. 13: 2117. https://doi.org/10.3390/ma12132117
APA StyleBöhm, C. F., Harris, J., Schodder, P. I., & Wolf, S. E. (2019). Bioinspired Materials: From Living Systems to New Concepts in Materials Chemistry. Materials, 12(13), 2117. https://doi.org/10.3390/ma12132117




