A New Bioactive Glass/Collagen Hybrid Composite for Applications in Dentistry
Abstract
:1. Introduction
2. Materials and Methods
2.1. Composites’ Preparation
- -
- BGMS/C: 31 wt.%BGMS10, 43 wt.% collagen, 26 wt.% PEG;
- -
- 45S5/C: 31 wt.% 45S5, 43 wt.% collagen, 26 wt.% PEG.
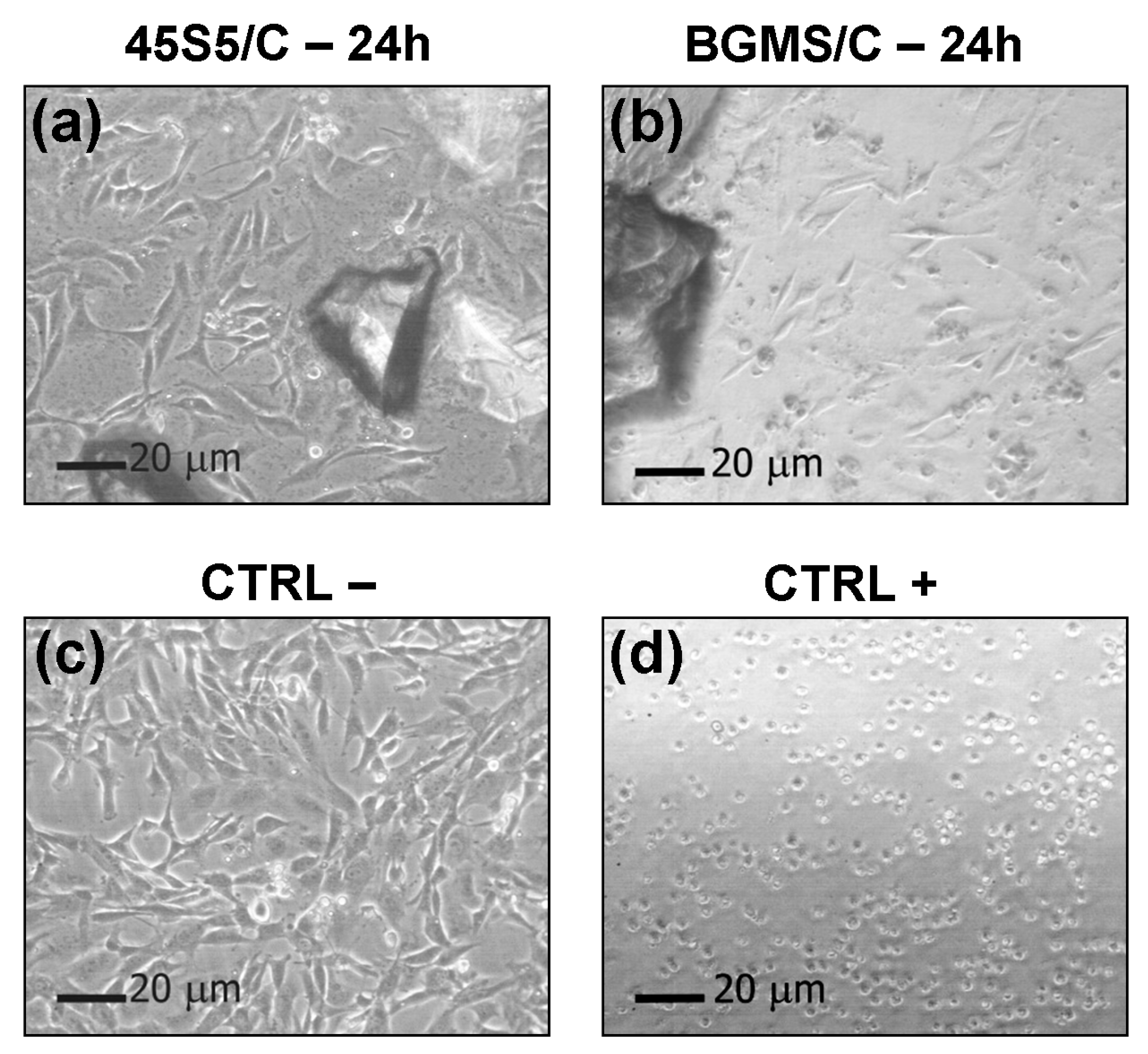
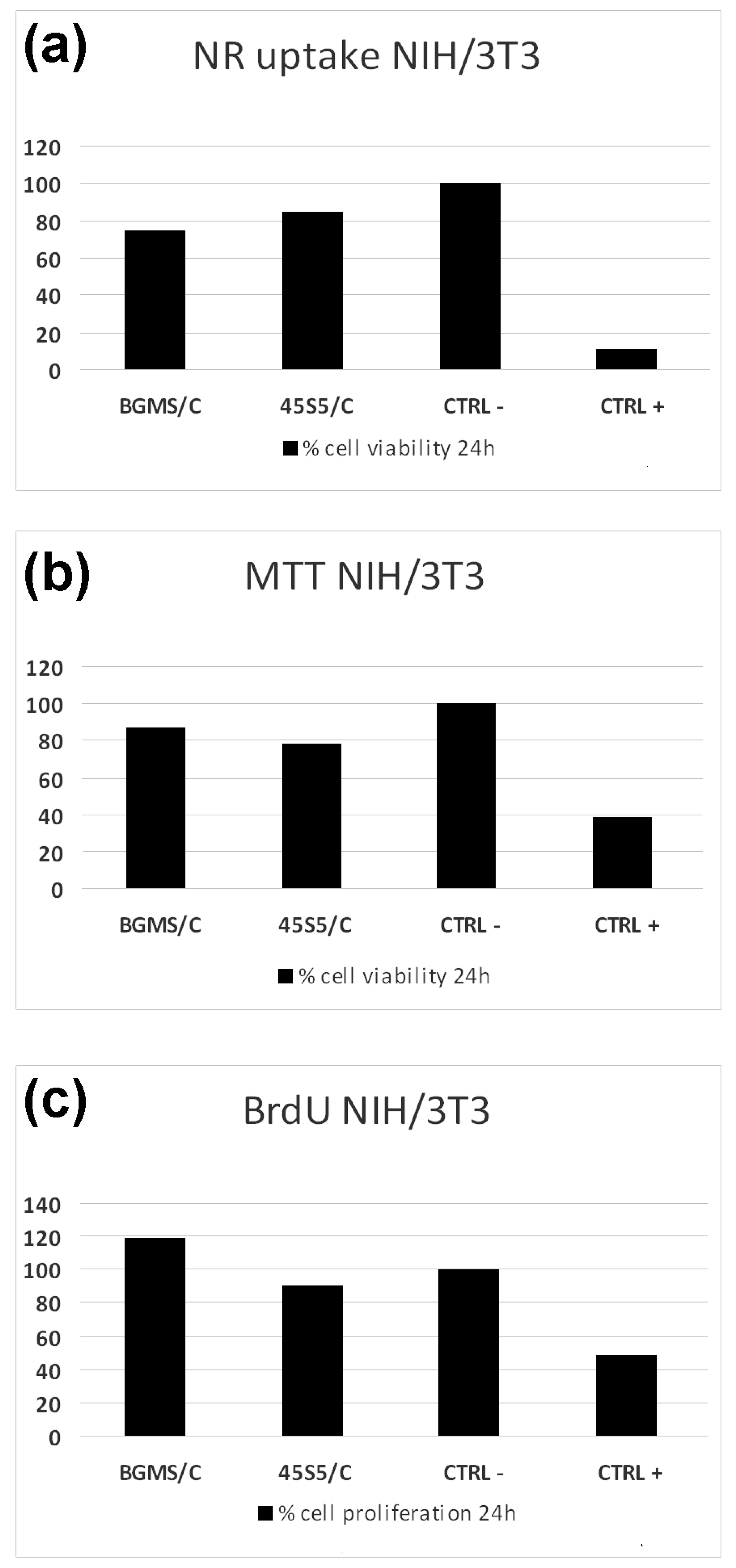
2.2. Biocompatibility Tests
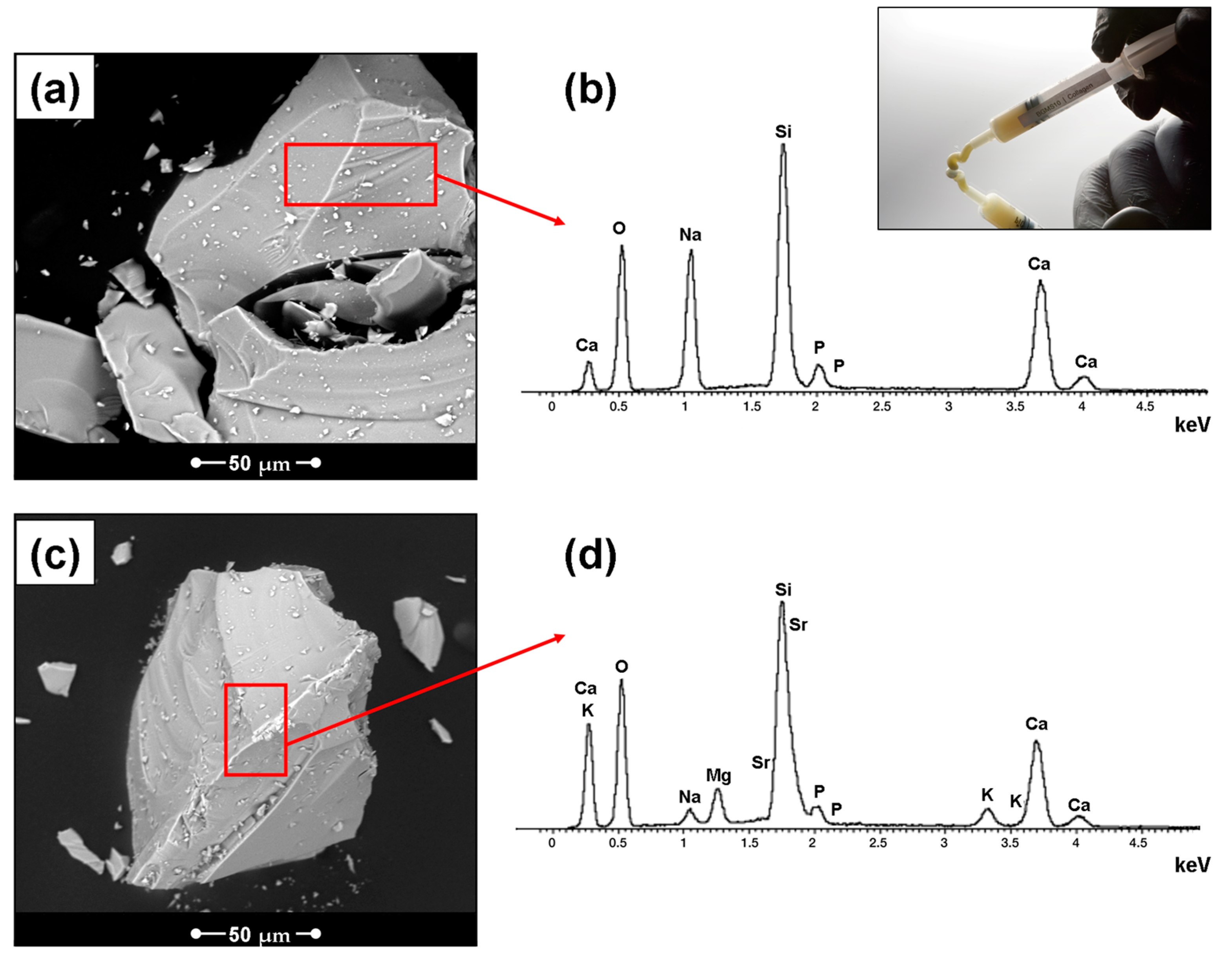
3. Results
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Jones, J.R. Reprint of: Review of bioactive glass: From Hench to hybrids. Acta Biomater. 2015, 23, S53–S82. [Google Scholar] [CrossRef] [PubMed]
- Baino, F.; Novajra, G.; Miguez-Pacheco, V.; Boccaccini, A.R.; Vitale-Brovarone, C. Bioactive glasses: Special applications outside the skeletal system. J. Non-Cryst. Solids 2016, 432, 15–30. [Google Scholar] [CrossRef]
- Miguez-Pacheco, V.; Hench, L.L.; Boccaccini, A.R. Bioactive glasses beyond bone and teeth: Emerging applications in contact with soft tissues. Acta Biomater. 2015, 13, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Kargozar, S.; Mozafari, M.; Hill, R.G.; Milan, P.B.; Joghataei, M.T.; Hamzehlou, S.; Baino, F. Synergistic combination of bioactive glasses and polymers for enhanced bone tissue regeneration. Mater. Today Proc. 2018, 5, 15532–15539. [Google Scholar] [CrossRef]
- Sarker, B.; Hum, J.; Nazhat, S.N.; Boccaccini, A.R. Combining collagen and bioactive glasses for bone tissue engineering: A review. Adv. Healthc. Mater. 2015, 4, 176–194. [Google Scholar] [CrossRef] [PubMed]
- Fruijtier-Polloth, C. Safety assessment on polyethylene glycols (PEGs) and their derivatives as used in cosmetic products. Toxicology 2005, 214, 1–38. [Google Scholar] [CrossRef] [PubMed]
- Bellucci, D.; Cannillo, V. A novel bioactive glass containing strontium and magnesium with ultra-high crystallization temperature. Mater. Lett. 2018, 213, 67–70. [Google Scholar] [CrossRef]
- Bellucci, D.; Cannillo, V.; Ciardelli, G.; Gentile, P.; Sola, A. Potassium based bioactive glass for bone tissue engineering. Ceram. Int. 2010, 36, 2449–2453. [Google Scholar] [CrossRef]
- Tests for Cytotoxicity: In Vitro Methods; ISO 10993–5; International Organization for Standardization: Geneva, Switzerland, 2009.
- Sample Preparation and Reference Materials; ISO 10993–12; International Organization for Standardization: Geneva, Switzerland, 2007.
- Bellucci, D.; Sola, A.; Salvatori, R.; Anesi, A.; Chiarini, L.; Cannillo, V. Role of magnesium oxide and strontium oxide as modifiers in silicate-based bioactive glasses: Effects on thermal behaviour, mechanical properties and in-vitro bioactivity. Mater. Sci. Eng. C 2017, 72, 566–575. [Google Scholar] [CrossRef] [PubMed]
- Berridge, M.V.; Herst, P.M.; Tan, A.S. Tetrazolium dyes as tools in cell biology: New insights into their cellular reduction. Biotechnol. Annu. Rev. 2005, 11, 127–152. [Google Scholar] [PubMed]
- Mahesh, L.; Kurtzman, G.M.; Shukla, S. Regeneration in Periodontics: Collagen-A Review of Its Properties and Applications in Dentistry. Compend. Contin. Educ. Dent. 2015, 36, 358–363. [Google Scholar] [PubMed]
- Nardone, V.; Zonefrati, R.; Mavilia, C.; Romagnoli, C.; Ciuffi, S.; Fabbri, S.; Palmini, G.; Galli, G.; Tanini, A.; Brandi, M.L. In vitro effects of strontium on proliferation and osteoinduction of human preadipocytes. Stem Cells Int. 2015, 2015, 871863. [Google Scholar] [CrossRef] [PubMed]
- Capuccini, C.; Torricelli, P.; Sima, F.; Boanini, E.; Ristoscu, C.; Bracci, B.; Socol, G.; Fini, M.; Mihailescu, I.N.; Bigi, A. Strontium-substituted hydroxyapatite coatings synthesized by pulsed-laser deposition: In vitro osteoblast and osteoclast response. Acta Biomater. 2008, 4, 1885–1893. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bellucci, D.; Salvatori, R.; Giannatiempo, J.; Anesi, A.; Bortolini, S.; Cannillo, V. A New Bioactive Glass/Collagen Hybrid Composite for Applications in Dentistry. Materials 2019, 12, 2079. https://doi.org/10.3390/ma12132079
Bellucci D, Salvatori R, Giannatiempo J, Anesi A, Bortolini S, Cannillo V. A New Bioactive Glass/Collagen Hybrid Composite for Applications in Dentistry. Materials. 2019; 12(13):2079. https://doi.org/10.3390/ma12132079
Chicago/Turabian StyleBellucci, Devis, Roberta Salvatori, Jessica Giannatiempo, Alexandre Anesi, Sergio Bortolini, and Valeria Cannillo. 2019. "A New Bioactive Glass/Collagen Hybrid Composite for Applications in Dentistry" Materials 12, no. 13: 2079. https://doi.org/10.3390/ma12132079
APA StyleBellucci, D., Salvatori, R., Giannatiempo, J., Anesi, A., Bortolini, S., & Cannillo, V. (2019). A New Bioactive Glass/Collagen Hybrid Composite for Applications in Dentistry. Materials, 12(13), 2079. https://doi.org/10.3390/ma12132079








