Plasma-Sprayed Bioactive Ceramic Coatings with High Resorption Resistance Based on Transition Metal-Substituted Calcium Hexaorthophosphates
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemical Stability of Precursor Powders
2.2. Plasma Spraying of Powders
2.3. Coating Characterization
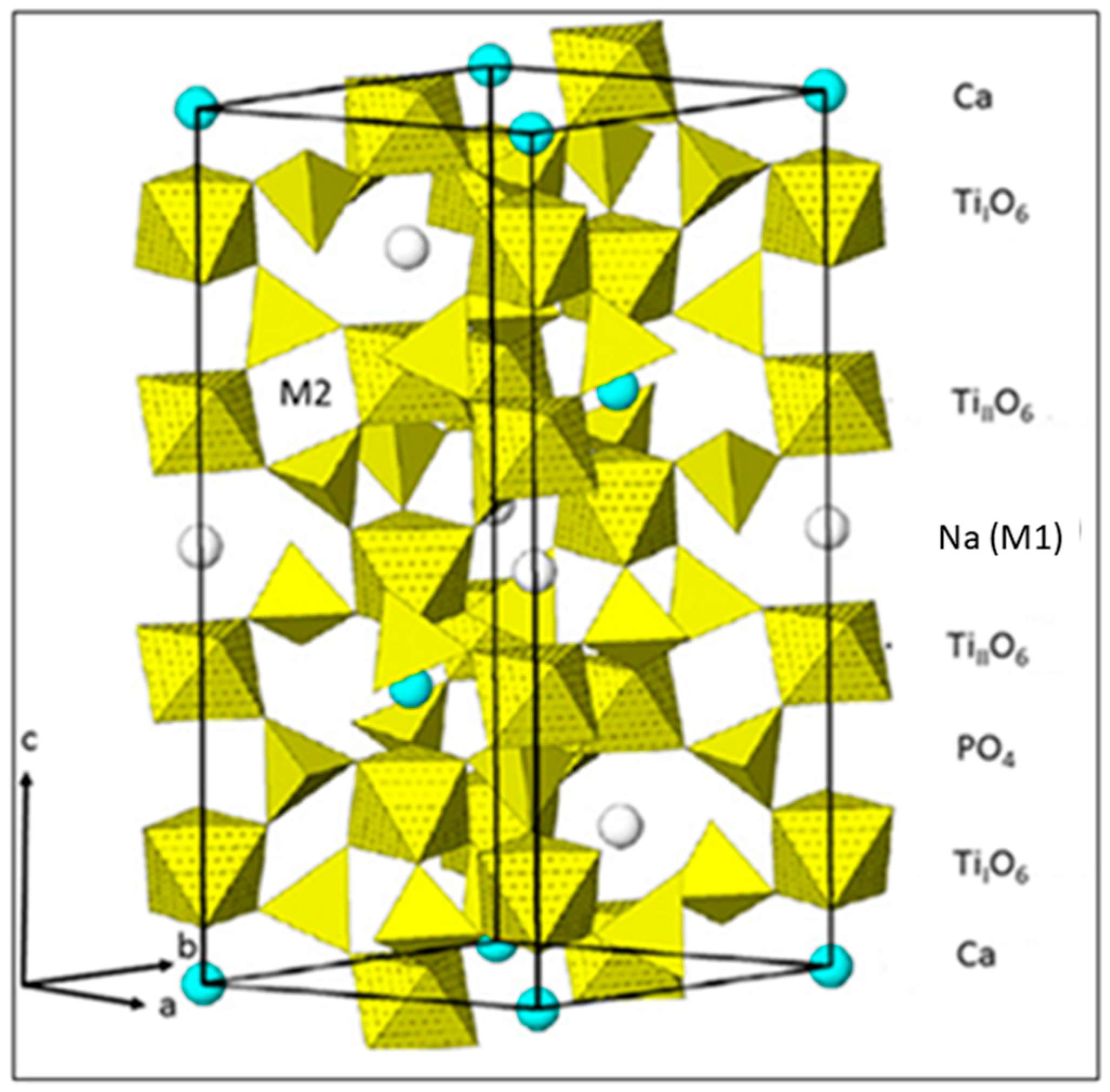
3. Results and Discussion
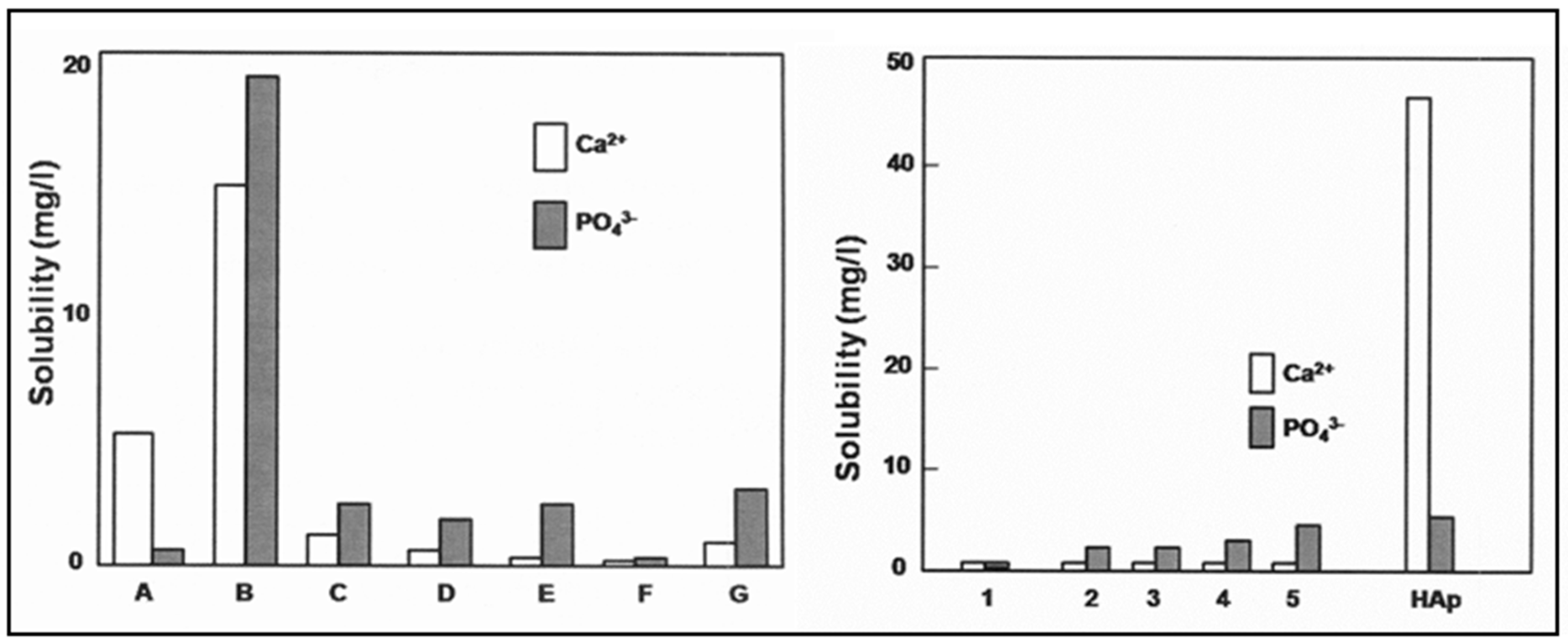
3.1. Solubility
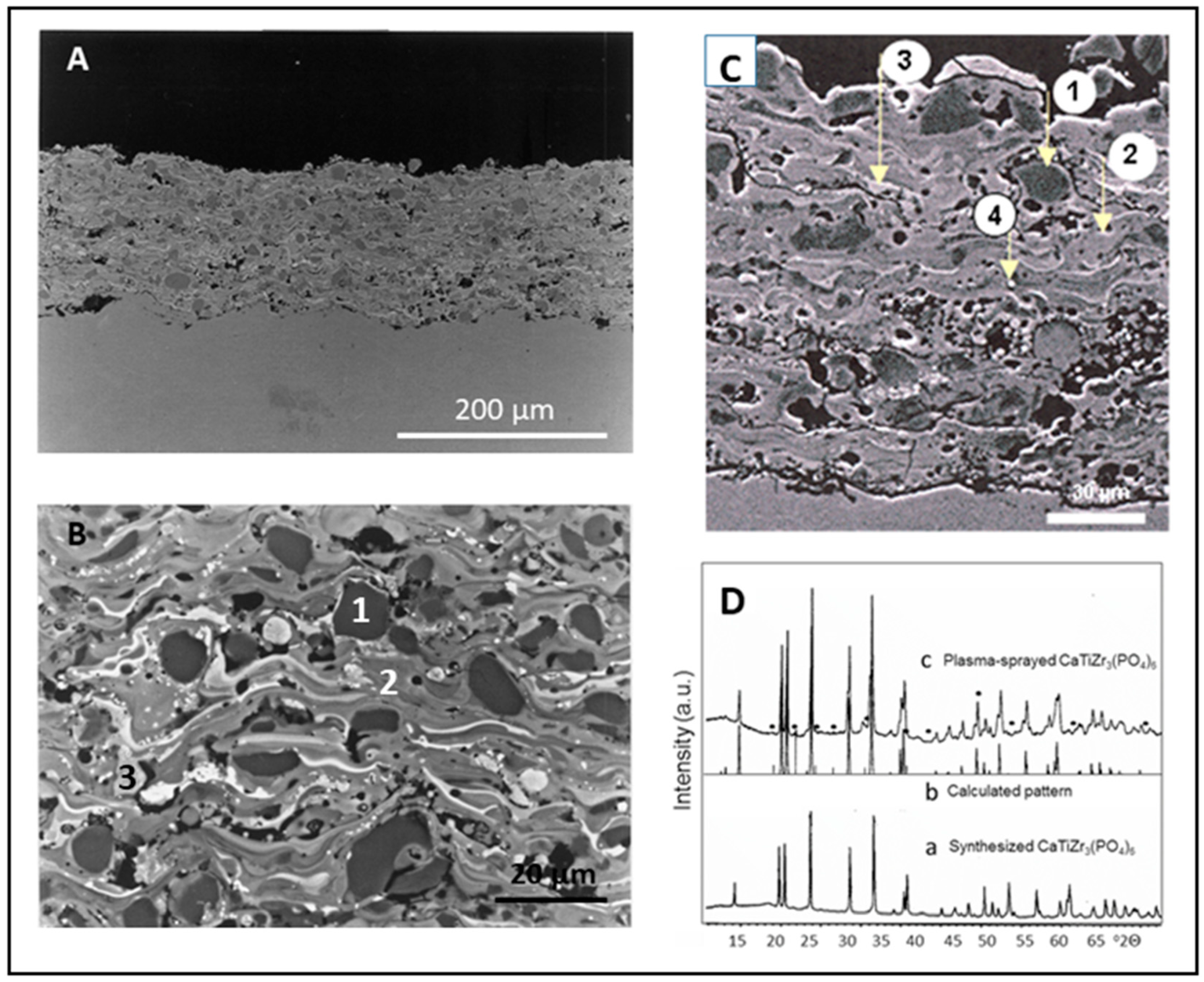
3.2. Coating Microstructure
3.3. Coating Porosity
3.4. Coating Adhesion
3.5. Mechanical Coating Properties
3.6. Electrical Properties
3.7. In Vitro Biocompatibility of Coatings
3.8. Osteoblast Cell Culture Tests
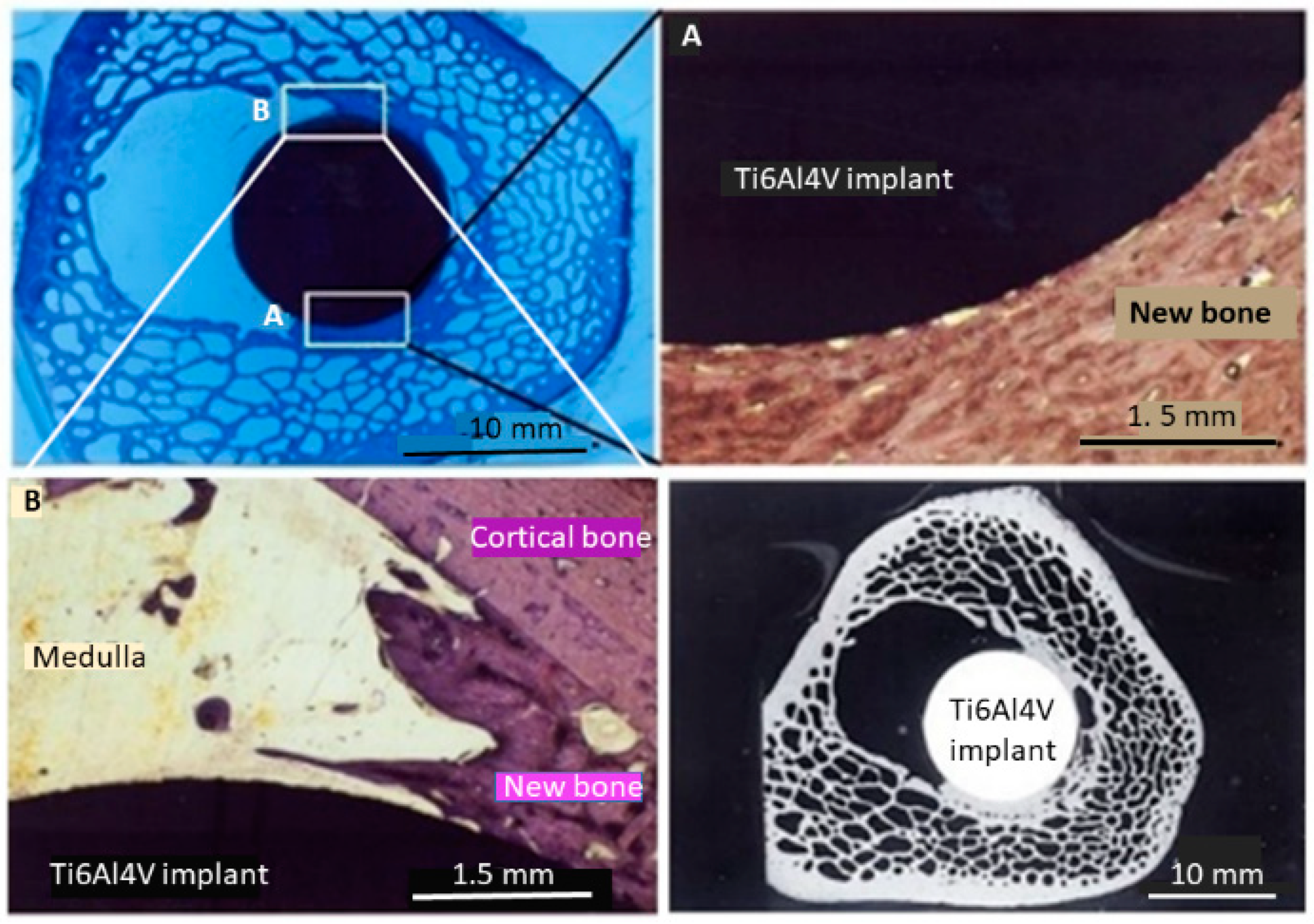
3.9. Animal Model Tests
4. Conclusions and Implications
Funding
Acknowledgments
Conflicts of Interest
References
- Biasetto, L.; Elsayed, H.; Bonollo, F.; Colombo, P. Polymer-derived sphene biocoatings on cpTi substrates for orthopedic and dental implants. Surf. Coat. Technol. 2016, 301, 140–147. [Google Scholar] [CrossRef]
- Elsayed, H.; Brunello, G.; Gardin, C.; Ferroni, L.; Badocco, D.; Pastore, P.; Sivolella, S.; Zavan, B.; Biasetto, L. Bioactive sphene-based ceramic coatings on cpTi substrates for dental implants: An In Vitro study. Materials 2018, 11, 2234. [Google Scholar] [CrossRef]
- Garcia, E.; Miranzo, P.; Sainz, M.A. Thermally sprayed wollastonite and wollastonite-diopside compositions as new modulated bioactive coatings for metal implants. Ceram. Int. 2018, 44, 12896–12904. [Google Scholar] [CrossRef]
- Buga, C.; Hunyadi, M.; Gácsi, Z.; Hegedüs, C.; Hakl, J.; Schmidt, U.; Ding, S.J.; Csik, A. Calcium silicate layer on titanium fabricated by electrospray deposition. Mater. Sci. Eng. C 2019, 98, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Sainz, M.A.; Pena, P.; Serena, P.; Caballero, A. Influence of design on bioactivity of novel CaSiO3-CaMg(SiO3)2 bioceramics: In Vitro simulated body fluid test and thermodynamic simulation. Acta Biomater. 2010, 6, 2797–2807. [Google Scholar] [CrossRef]
- Ardakani, M.H.; Moztarzadeh, F.; Rabiee, M.; Talebi, A.R. Synthesis and characterization of nanocrystalline merwinite (Ca3Mg(SiO4)2) via sol-gel method. Ceram. Int. 2011, 37, 175–180. [Google Scholar] [CrossRef]
- Kalantari, E.; Naghib, S.M. A comparative study on biological properties of novel nanostructured monticellite-based composites with hydroxyapatite bioceramic. Mater. Sci. Eng. C 2019, 98, 1087–1096. [Google Scholar] [CrossRef]
- Diba, M.; Goudouri, O.M.; Tapia, F.; Boccaccini, A.R. Magnesium-containing bioactive polycrystalline silicate-based ceramics and glass-ceramics for biomedical applications. Curr. Opin. Solid State Mater. Sci. 2014, 18, 147–167. [Google Scholar] [CrossRef]
- Huang, L.; Ji, H.; Liang, Y.; Xie, Y.; Zheng, X. Bone Replacing Material of Baghdadite Coated-Titanium Alloy, Useful for Inducing Formation of Bone-Like Apatite in Simulated Body Fluid, Comprises Titanium and Its Alloy as Matrix and Coating Is Deposited on Matrix by Plasma Spray Coating. Chinese Patent CN102049065-A, 11 May 2011. [Google Scholar]
- Wu, C.T.; Chang, J.; Zhai, W.Y. A novel hardystonite bioceramic: Preparation and characteristics. Ceram. Int. 2005, 31, 27–31. [Google Scholar] [CrossRef]
- Alamo, J. Chemistry and properties of solids with the [NZP] skeleton. Solid State Ion. 1993, 63, 547–561. [Google Scholar] [CrossRef]
- Heimann, R.B. Calcium (Ti, Zr) hexaorthophosphate bioceramics for electrically stimulated biomedical implant devices: A position paper. Am. Mineral. 2017, 102, 2170–2179. [Google Scholar] [CrossRef]
- Brownfield, M.E.; Foord, E.E.; Sutley, S.J.; Botinelly, T. Kosnarite, KZr2(PO4)3, a new mineral from Mount Mica and Black Mountain, Oxford County, Maine. Am. Mineral. 1993, 78, 653–656. [Google Scholar]
- Senbhagaraman, S.; Row, G.T.N.; Umarji, A.M. Structural refinement using high-resolution powder X-ray diffraction data of Ca0.5Ti2P3O12, a low-thermal-expansion material. J. Mater. Chem. 1993, 3, 309–314. [Google Scholar] [CrossRef]
- Alamo, J.; Roy, R. Crystal chemistry of the NaZr2(PO4)3, NZP or CTP, structure family. J. Mater. Sci. 1986, 21, 444–450. [Google Scholar] [CrossRef]
- Heimann, R.B. Transition metal-substituted calcium orthophosphates with NaSiCon structure: A novel type of bioceramics. In Calcium Phosphate. Structure, Synthesis, Properties, and Applications; Heimann, R.B., Ed.; Nova Science Publishers, Inc.: New York, NY, USA, 2012; pp. 363–379. [Google Scholar]
- Hung, T.F.; Lan, W.H.; Yeh, Y.W.; Chang, W.S.; Yang, C.C.; Lin, J.C. Hydrothermal synthesis of sodium titanium phosphate nanoparticles as efficient anode materials for aqueous sodium-ion batteries. ACS Sustain. Chem. Eng. 2016, 4, 7074–7079. [Google Scholar] [CrossRef]
- Scheetz, B.E.; Agrawal, D.K.; Breval, E.; Roy, R. Sodium zirconium phosphate (NZP) as a host structure for nuclear waste immobilization: A review. Waste Manag. 1994, 14, 489–505. [Google Scholar] [CrossRef]
- Vance, E.R.; Gregg, D.J. Calcium phosphate materials for radioactive waste immobilization. In Calcium Phosphate. Structure, Synthesis, Properties, and Applications; Heimann, R.B., Ed.; Nova Science Publishers, Inc.: New York, NY, USA, 2012; pp. 445–465. [Google Scholar]
- Agrawal, D.K.; Harshé, G.; Breval, E.; Roy, R. [NZP], NaZr2P3O12-type materials for protection of carbon-carbon composites. J. Mater. Res. 1996, 11, 3158–3163. [Google Scholar] [CrossRef]
- Heimann, R.B.; Schneider, K. Final report DFG project, grant number He 923/9-1/2. 1 December 2000. [Google Scholar]
- Heimann, R.B. In Vitro- und In Vivo-Verhalten von osteokonduktiven plasmagespritzten Ca-Ti-Zr-Phosphat-Beschichtungen auf Ti6Al4V-Substraten [In Vitro and In Vivo behavior of plasma-sprayed osseoconductive Ca-Ti-Zr phosphate coatings on Ti6Al4V substrates]. BIOmaterialien 2006, 7, 29–37. [Google Scholar]
- Berger, G.; Gildenhaar, R.; Ploska, U. Rapidly resorbable, glassy crystalline materials on the basis of calcium alkali orthophosphates. Biomaterials 1995, 16, 1241–1248. [Google Scholar] [CrossRef]
- Plackett, R.L.; Burman, J.P. The design of optimum multifactorial experiments. Biometrika 1946, 33, 305–325. [Google Scholar] [CrossRef]
- Heimann, R.B.; Lehmann, H.D. Biological performance testing of bioceramic coatings. In Bioceramic Coatings for Medical Implants. Trends and Techniques; Heimann, R.B., Lehmann, H.D., Eds.; Wiley-VCH: Weinheim, Germany, 2015; pp. 428–429. [Google Scholar]
- ASTM C373-88. Standard Test Method for Water Absorption, Bulk Density, Apparent Porosity, and Apparent Specific Gravity of Fired Whiteware Products; ASTM International: West Conshohocken, PA, USA, 2006. [Google Scholar]
- ASTM C633-13. Standard Test Method for Adhesion or Cohesion Strength of Thermal Spray Coatings; ASTM International: West Conshohocken, PA, USA, 2017. [Google Scholar]
- Heimann, R.B.; Schürmann, N.; Müller, R.T. In Vivo and In Vitro performance of Ti6Al4V implants with plasma-sprayed osteoconductive hydroxyapatite-bioinert bond coat ‘duplex’ systems: An experimental study in sheep. J. Mater. Sci. Mater. Med. 2004, 15, 1045–1052. [Google Scholar] [CrossRef] [PubMed]
- Ploska, U.; Berger, G. Solubility of compositions in the system CaTixZr4-x(PO4)6 with x = 0 − 4. Biomaterials 1997, 18, 1671–1675. [Google Scholar] [CrossRef]
- Reisel, G. Entwicklung von HVOF- und APS-Gespritzten Biokeramischen Schichten für die Endoprothetik [Development of HVOF- and APS-Sprayed Bioceramic Coatings for Endoprosthetic Uses]. Masters Thesis, Rheinisch-Westfälische Technische Hochschule (RWTH) Aachen, Aachen, Germany, September 1996. [Google Scholar]
- Schneider, K.; Heimann, R.B.; Berger, G. Plasma-sprayed coatings in the system CaO-TiO2-ZrO2-P2O5 for long-term stable endoprostheses. Mater. Werkst. 2001, 32, 166–171. [Google Scholar] [CrossRef]
- Wintermantel, E.; Ha, S.W. Biokompatible Werkstoffe und Bauweisen. Implantate für Medizin und Umwelt [Biocompabible Materials and Constructions. Implants for Medicine and Environment]; Springer: Berlin/Heidelberg, Germany; Tokyo, Japan, 1996; p. 225. [Google Scholar]
- Heimann, R.B. The third energy transfer process: Particle-substrate interaction. In Plasma Spray Coating. Principles and Applications, 2nd ed.; Wiley-VCH: Weinheim, Germany, 2008; p. 144. [Google Scholar]
- Willmann, G. Beschichtung von Implantaten mit Hydroxylapatite. Die Option auf eine stoffschlüssige Verbindung zwischen Knochen und Metall [Coating of implants with hydroxylapatite. The option of positive substance jointing of bone and metal]. Mater. Werkst. 1999, 30, 317–325. [Google Scholar] [CrossRef]
- Callahan, T.J.; Gantenberg, J.B.; Sands, B.E. Characterization and Performance of Calcium Phosphate Coatings for Implants; STP 1196; ASTM: Philadelphia, PA, USA, 1994; pp. 185–197. [Google Scholar]
- ASTM F1609–08. Standard Specification for Calcium Phosphate Coatings for Implantable Materials; ASTM International: West Conshohocken, PA, USA, 2014; Available online: www.astm.org (accessed on 29 April 2019).
- Gittings, J.P.; Bowen, C.R.; Dent, A.C.E.; Turner, I.G.; Baxter, F.R.; Chaudhuri, J.B. Electric characterization of hydroxyapatite-based bioceramics. Acta Biomater. 2009, 5, 743–754. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Li, Y.T.; Goodenough, J.B. NaSiCon-type Li1+2x Zr2−x Cax(PO4)3 with high ionic conductivity of room temperature. RSC Adv. 2011, 1, 1728–1731. [Google Scholar] [CrossRef]
- Ortiz, G.F.; López, M.C.; Lavela, P.; Tirado, J.L. Improved lithium-ion transport in NaSiCon-type lithium titanium phosphate by calcium and iron doping. Solid State Ion. 2014, 262, 573–577. [Google Scholar] [CrossRef]
- Jolley, A.G.; Cohn, G.; Hitz, G.T.; Wachsman, E.D. Improving the ionic conductivity of NaSiCon through aliovalent cation substitution of Na3Zr2Si2PO12. Ionics 2015, 21, 3031–3038. [Google Scholar] [CrossRef]
- Ning, C.Y.; Zhou, L.; Tan, G.X. Fourth-generation biomedical materials. Mater. Today 2016, 19, 2–3. [Google Scholar] [CrossRef]
- Szmukler-Moncler, S.; Daculsi, G.; Delécrin, J.; Passuti, N.; Deudon, C. Calcium-metallic phosphates: A new coating biomaterial? Adv. Biomater. 1992, 10, 377–383. [Google Scholar]
- Knabe, C.; Berger, G.; Gildenhaar, R.; Klar, F.; Zreiqat, H. The modulation of osteogenesis In Vitro by calcium titanium phosphate coatings. Biomaterials 2004, 25, 4911–4919. [Google Scholar] [CrossRef] [PubMed]
- Barrias, C.C.; Ribeiro, C.C.; Lamghari, M.; Miranda, C.S.; Barbosa, M.A. Proliferation, activity, and osteogenic differentiation of bone marrow stromal cells cultured on calcium titanium phosphate microspheres. J. Biomed. Mater. Res. A 2005, 72, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Maniatopoulos, C. Osteoblast cell cultures: A testing model for biomaterials. Cell Tissue Res. 1988, 254, 317–330. [Google Scholar] [PubMed]
- Bernstein, A.; Nöbel, D.; Mayr, H.; Göbel, T.; Berger, G.; Ploska, U.; Gildenhaar, R.; Brandt, J. Inhibition of mineralization by a calcium zirconium phosphate coating. J. Biomed. Mater. Res. Part B Appl. Biomater. 2008, 86, 422–429. [Google Scholar] [CrossRef] [PubMed]
- Gross, U.; Strunz, V. The interface of various glasses and glass ceramics with a bony implantation bed. J. Biomed. Mater. Res. 1985, 19, 251–271. [Google Scholar] [CrossRef] [PubMed]
- Gross, U.; Müller-Mai, C.; Berger, G.; Ploska, U. The tissue response to a novel calcium zirconium phosphate ceramics. Key Eng. Mater. 2003, 240, 629–632. [Google Scholar] [CrossRef]
- Gross, U.; Müller-Mai, C.; Berger, G.; Ploska, U. Do calcium zirconium phosphate ceramics inhibit mineralization? Key Eng. Mater. 2004, 254, 635–638. [Google Scholar] [CrossRef]
| Run # | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|---|---|---|---|---|---|---|
| 1 | 35 | 40 | 12 | 3 | 20/60 | 80 | +45–71 |
| 2 | 35 | 50 | 6 | 6 | 20/60 | 80 | +25–45 |
| 3 | 25 | 50 | 12 | 3 | 40/80 | 80 | +25–45 |
| 4 | 35 | 40 | 12 | 6 | 20/60 | 120 | +25–45 |
| 5 | 35 | 50 | 6 | 6 | 40/80 | 80 | +45–71 |
| 6 | 35 | 50 | 12 | 3 | 40/80 | 120 | +25–45 |
| 7 | 25 | 50 | 12 | 6 | 20/60 | 120 | +71–45 |
| 8 | 25 | 40 | 12 | 6 | 40/80 | 80 | +71–45 |
| 9 | 25 | 40 | 6 | 6 | 40/80 | 120 | +45–25 |
| 10 | 35 | 40 | 6 | 3 | 40/80 | 120 | +71–45 |
| 11 | 25 | 50 | 6 | 3 | 20/60 | 120 | +71–45 |
| 12 | 25 | 40 | 6 | 3 | 20/60 | 80 | +45–25 |
| Run # | Solubility (mg/l) | Porosity (vol%) | Adhesive Strength (MPa) | Surface Roughness (µm) | Shear Strength (MPa) | Thickness (µm) |
|---|---|---|---|---|---|---|
| 1 | 1.0 | 17 | 7.04 | 69.3 | 0.71 | 188 |
| 2 | 3.6 | 10 | 3.36 | 47.2 | 8.15 | 179 |
| 3 | 4.1 | 17 | 3.86 | 50.3 | 0.1 | 183 |
| 4 | 4.6 | 15 | 4.73 | 57.3 | 2.01 | 205 |
| 5 | 4.9 | 16 | 7.04 | 51.9 | 1,51 | 178 |
| 6 | 3.3 | 14 | 4.22 | 48.2 | 0.73 | 313 |
| 7 | 2.0 | 11 | 17.30 | 50.6 | 10.08 | 64 |
| 8 | 1.4 | 23 | 13.75 | 59.5 | 1.46 | 176 |
| 9 | 3.5 | 17 | 7.60 | 59.6 | 0.65 | 236 |
| 10 | 5.2 | 21 | 9.80 | 69.5 | 2.09 | 280 |
| 11 | 0.1 | 24 | 9.97 | 46.2 | 12.82 | 43 |
| 12 | 4.5 | 21 | 4.12 | 47.2 | 0.15 | 160 |
| Oxide | CaTiZr3(PO4)6 | Phase 1 | Phase 2 | Phase 3 | Phase 4 |
|---|---|---|---|---|---|
| CaO | 12.5 | 12.6 | 13.5 | 16.5 | 0 |
| ZrO2 | 37.5 | 39.5 | 43.6 | 49.7 | 100 |
| TiO2 | 12.5 | 12.9 | 18.0 | 19.8 | 0 |
| P2O5 | 37.5 | 35.0 | 24.9 | 14.0 | 0 |
| Property | Dimension | CTZ3P3 | HAp [32] | HAp [35] |
|---|---|---|---|---|
| Coating thickness range | µm | 43–312 | 50–200 | |
| Surface roughness Ra | µm | 46–70 | >75 | |
| Porosity | vol% | 10–24 | ||
| Pore diameter | µm | 10–40 | ||
| Adhesive strength (thickness > 200 µm) | MPa | ≤17 | ||
| Adhesive strength (thickness < 100 µm) | MPa | ≤32 | >35 | >51 |
| Shear strength | MPa | ≤13 | >22 | |
| Coefficient of thermal expansion | ppm | 1.14 [29] | 10–14 | |
| Solubility in pf-SBF | mg/l | <5 | ||
| Solubility in TRIS–HCl | mg/l | <1 |
| Cell Class | Sintered CTZ3P3 Disks | Thermanox™ Control |
|---|---|---|
| I | 1 | 7 |
| II | 24 | 27 |
| III | 27 | 24 |
| IV | 48 | 42 |
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heimann, R.B. Plasma-Sprayed Bioactive Ceramic Coatings with High Resorption Resistance Based on Transition Metal-Substituted Calcium Hexaorthophosphates. Materials 2019, 12, 2059. https://doi.org/10.3390/ma12132059
Heimann RB. Plasma-Sprayed Bioactive Ceramic Coatings with High Resorption Resistance Based on Transition Metal-Substituted Calcium Hexaorthophosphates. Materials. 2019; 12(13):2059. https://doi.org/10.3390/ma12132059
Chicago/Turabian StyleHeimann, Robert B. 2019. "Plasma-Sprayed Bioactive Ceramic Coatings with High Resorption Resistance Based on Transition Metal-Substituted Calcium Hexaorthophosphates" Materials 12, no. 13: 2059. https://doi.org/10.3390/ma12132059
APA StyleHeimann, R. B. (2019). Plasma-Sprayed Bioactive Ceramic Coatings with High Resorption Resistance Based on Transition Metal-Substituted Calcium Hexaorthophosphates. Materials, 12(13), 2059. https://doi.org/10.3390/ma12132059





