Development and Characterization of Glass-Ceramics from Combinations of Slag, Fly Ash, and Glass Cullet without Adding Nucleating Agents
Abstract
1. Introduction
2. Results
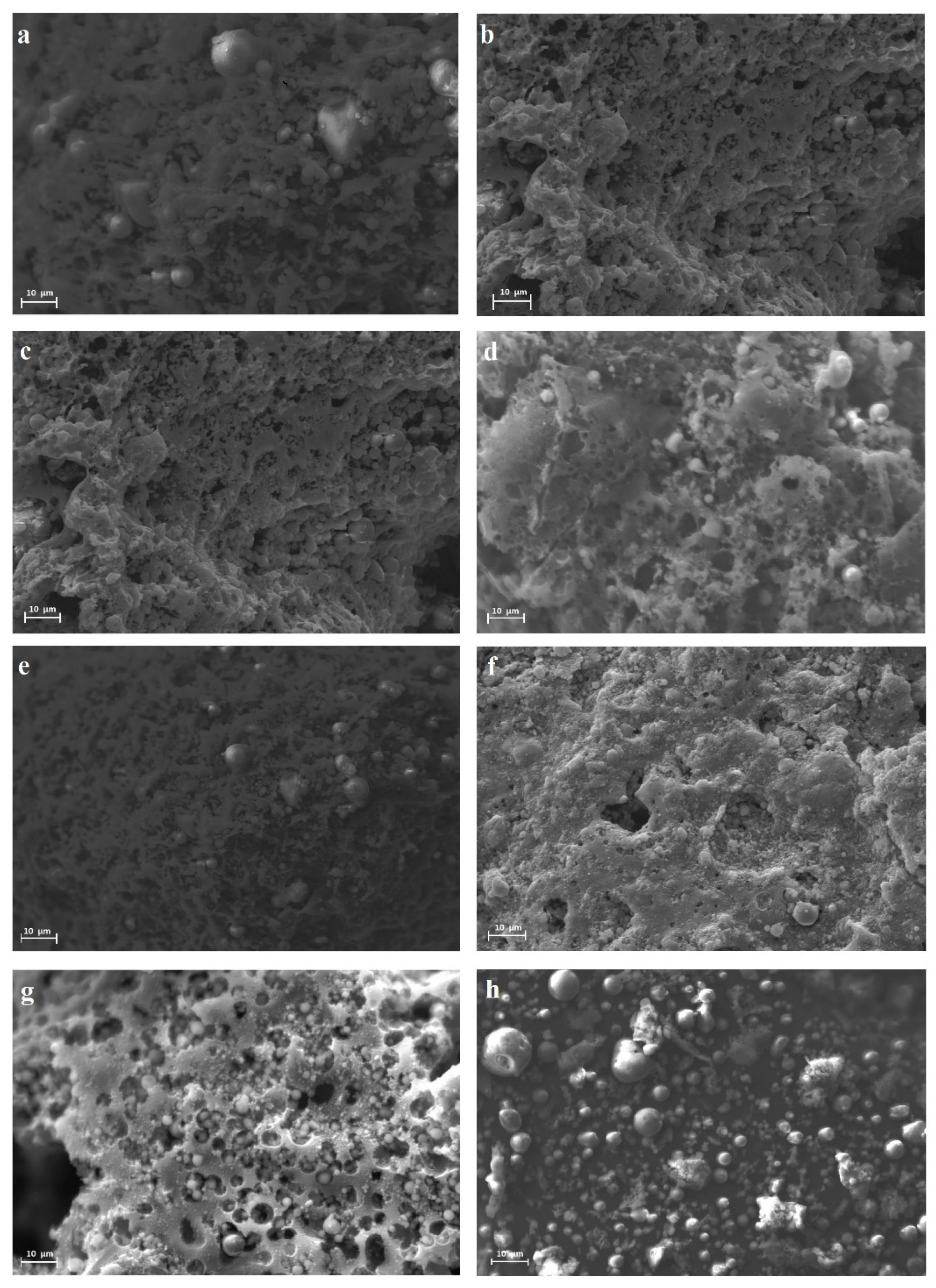
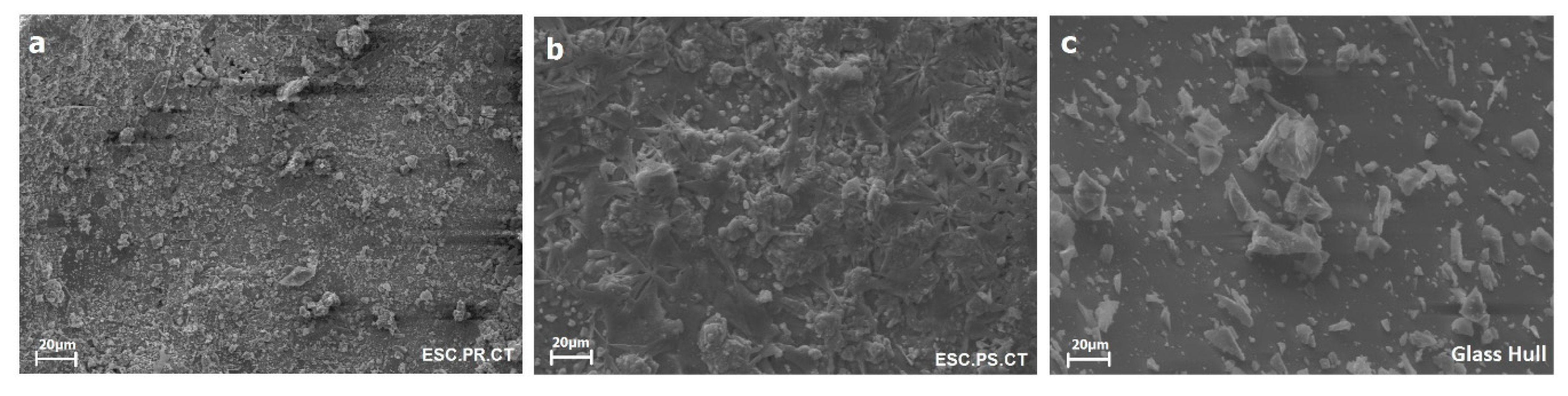
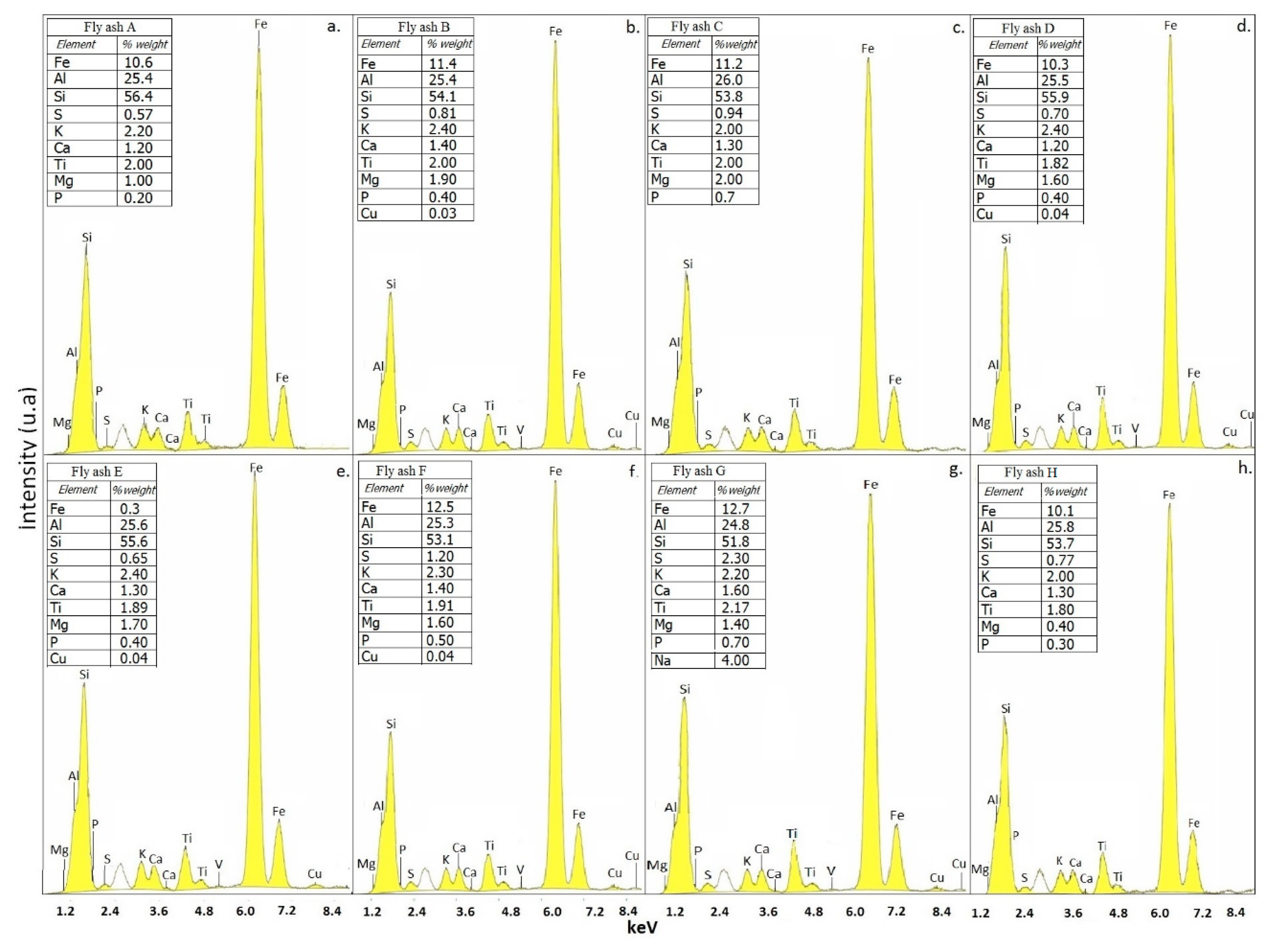
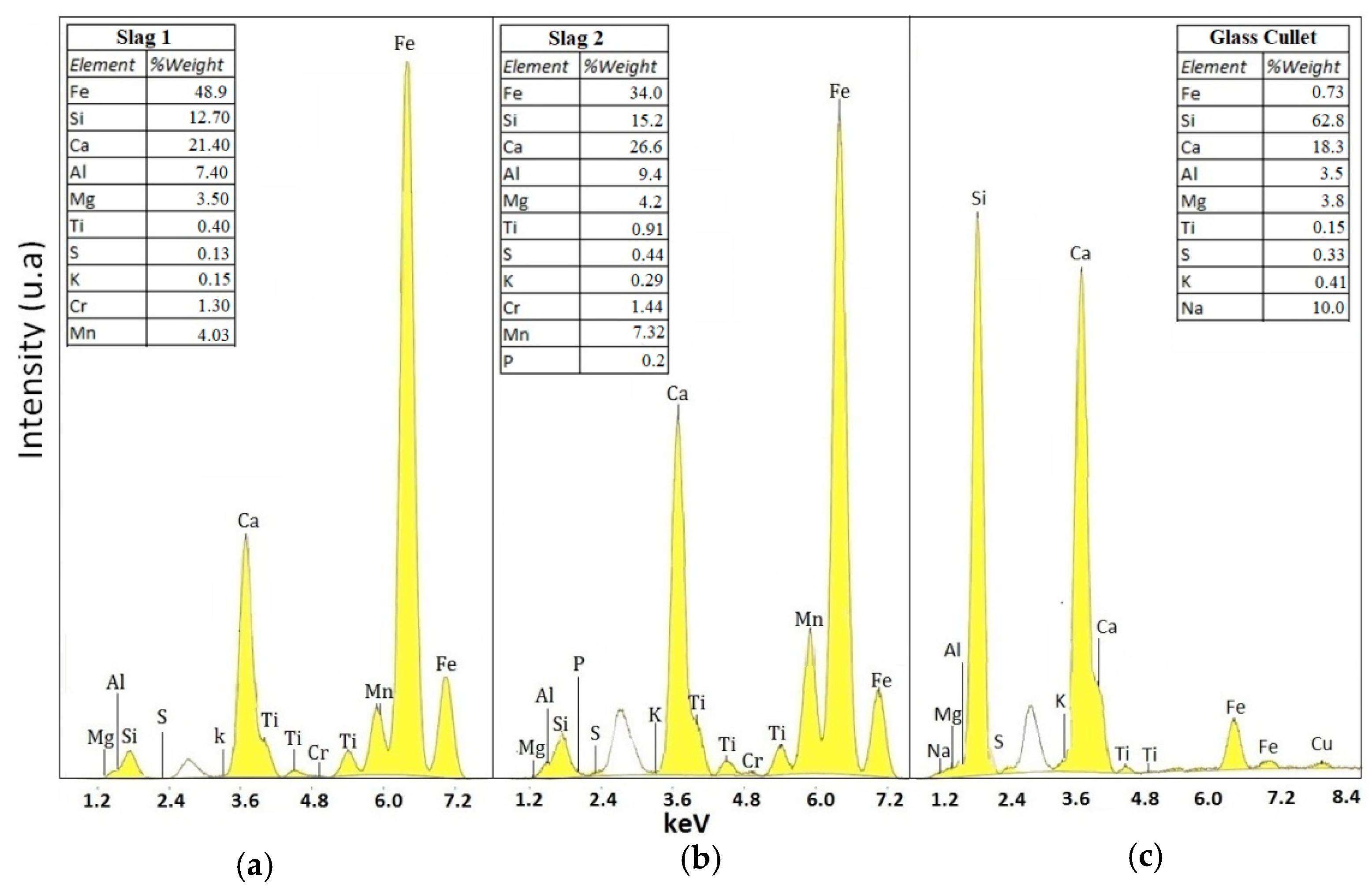
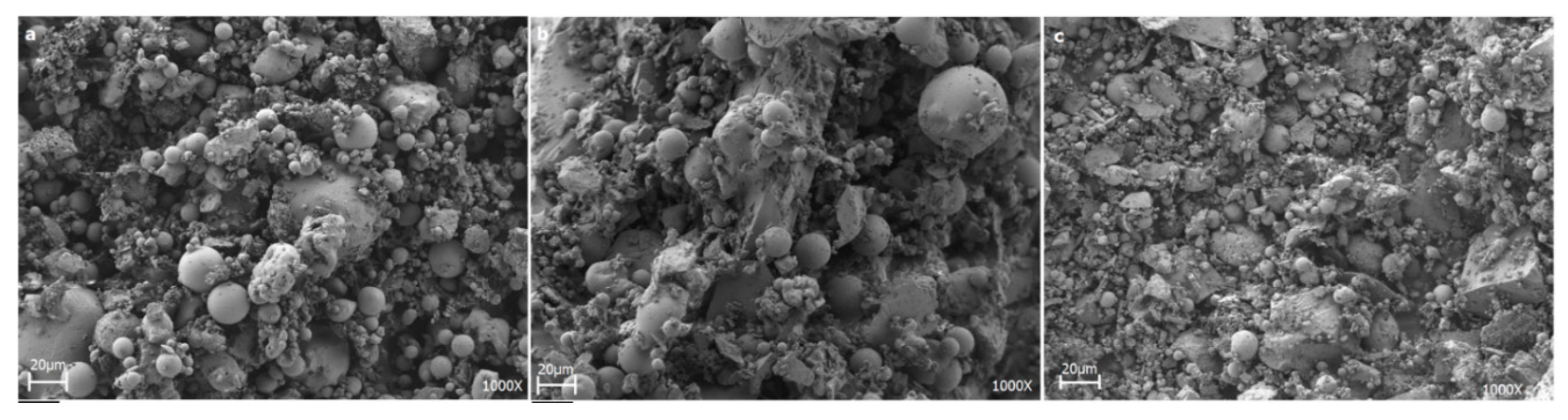
2.1. Characterization of Raw Materials
2.2. Preparation, Analysis, and Design of Mixtures
2.3. Glass-Ceramic Synthesis
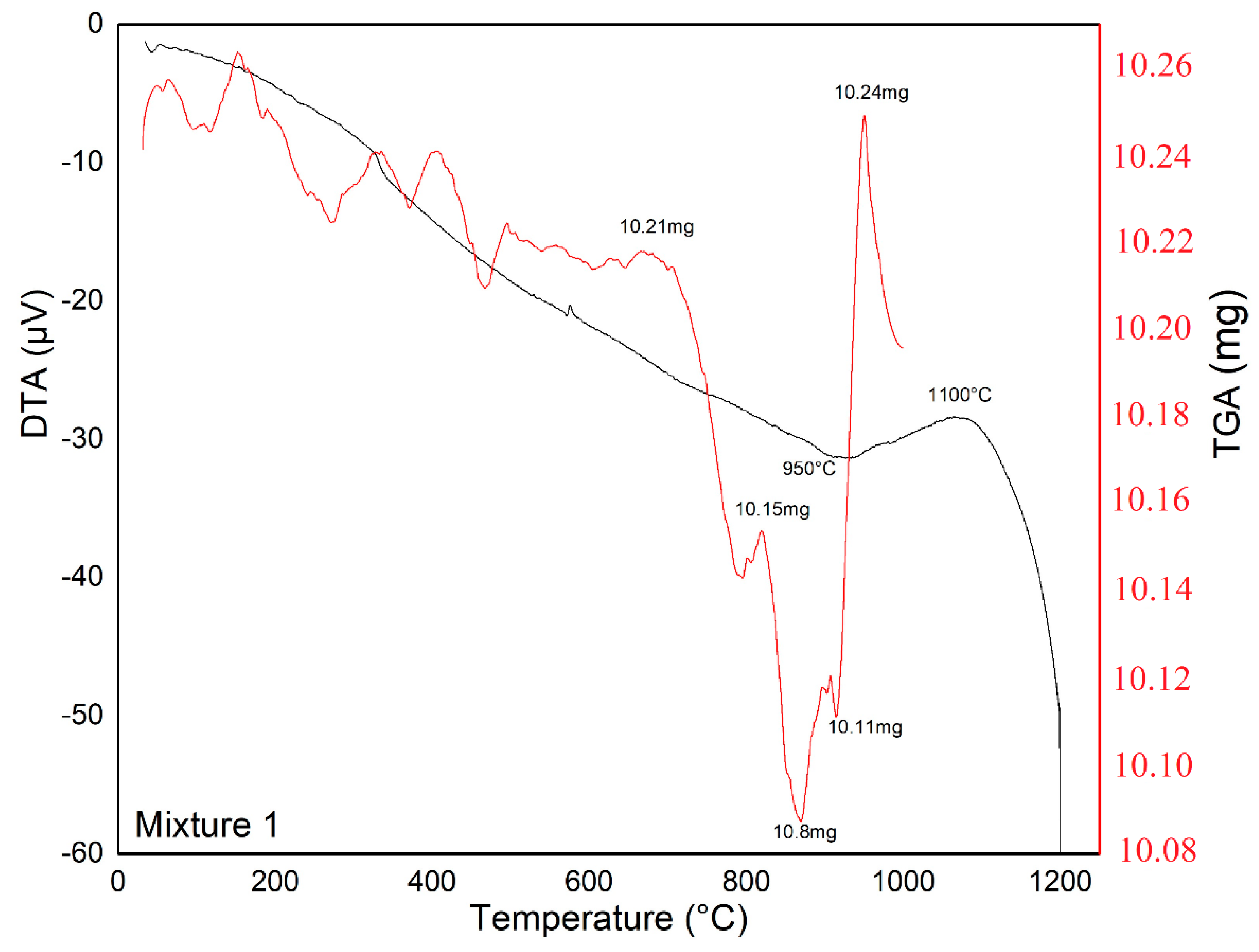
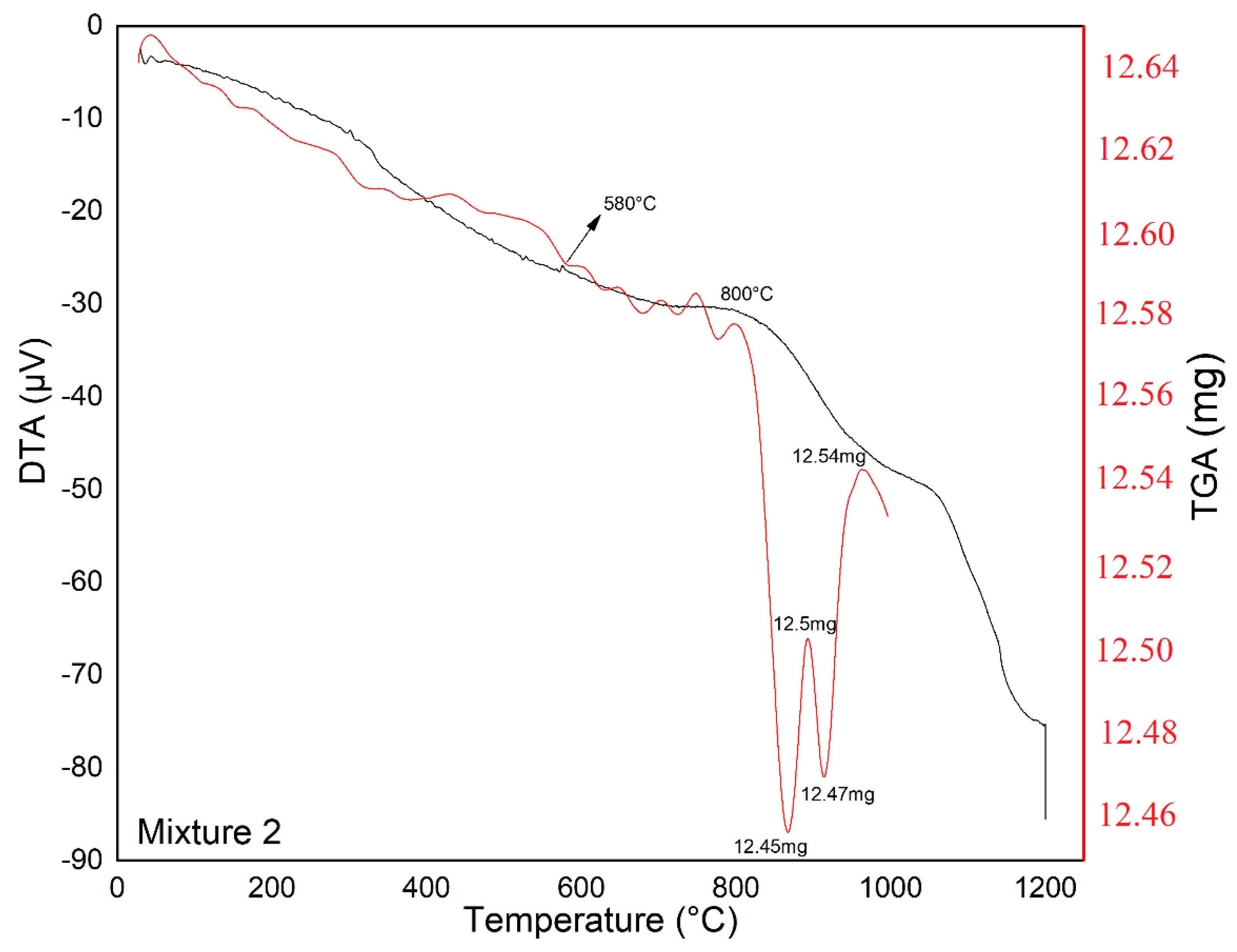
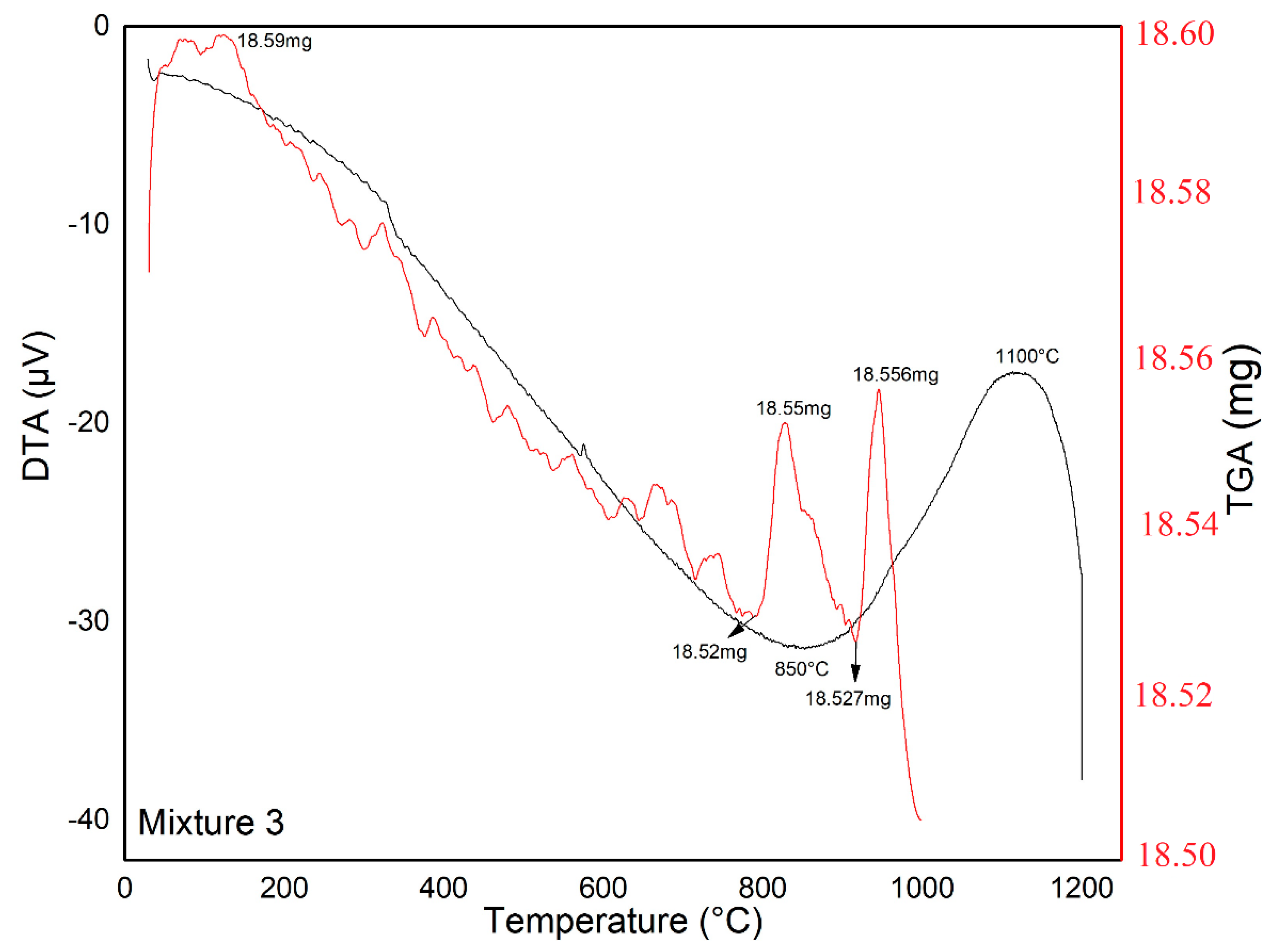
2.3.1. Thermal Analysis (DTA-TGA)
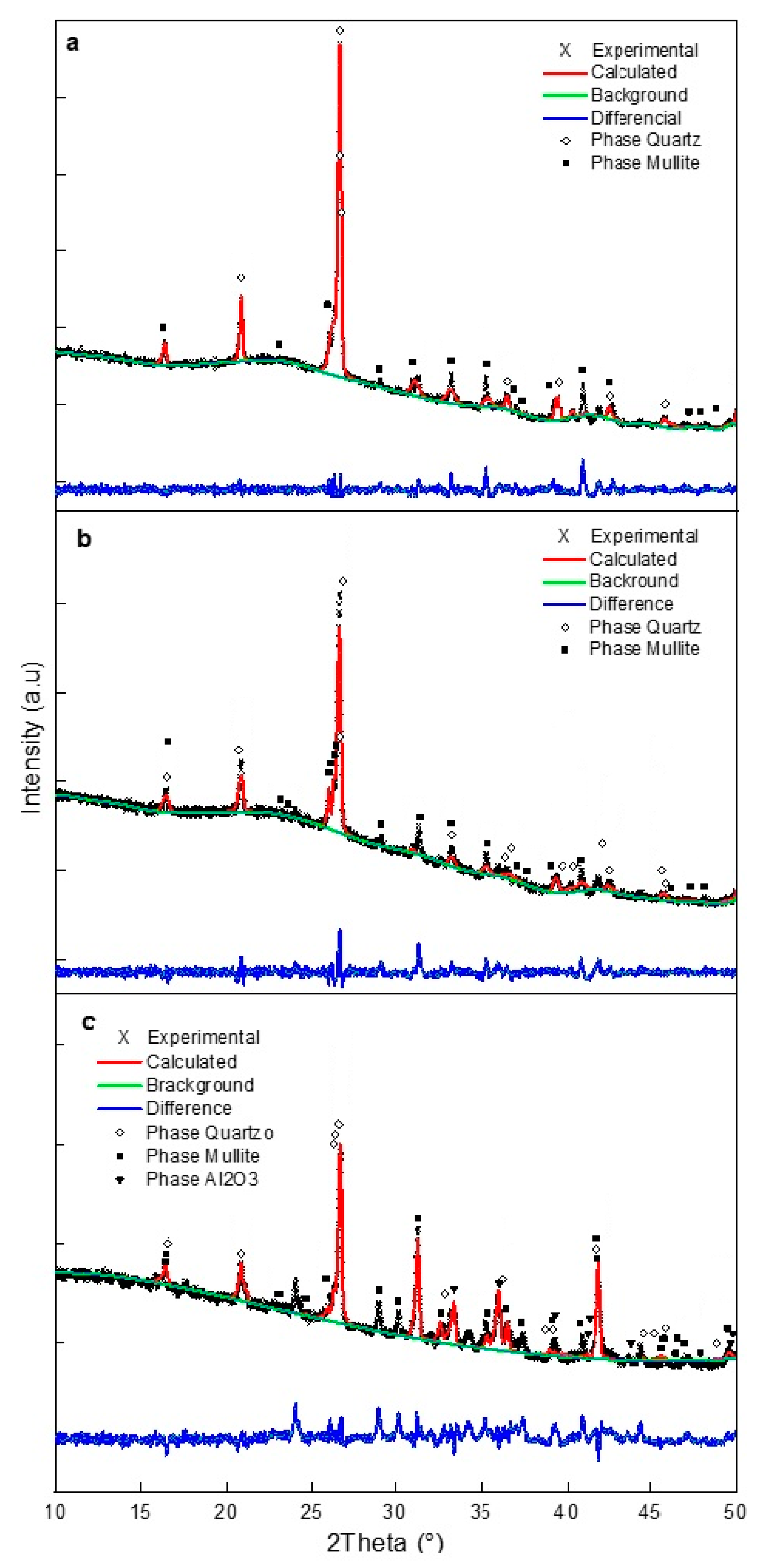
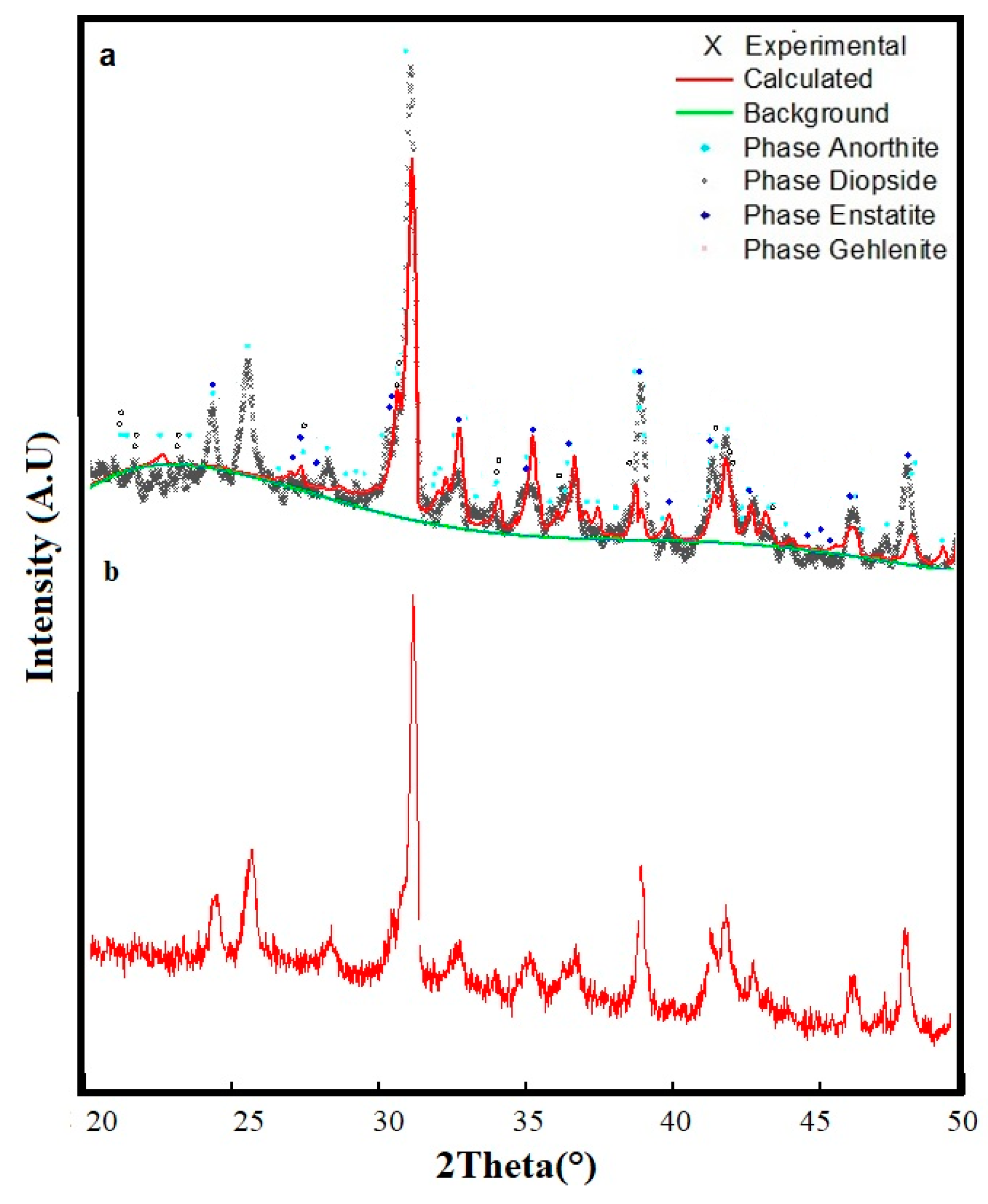
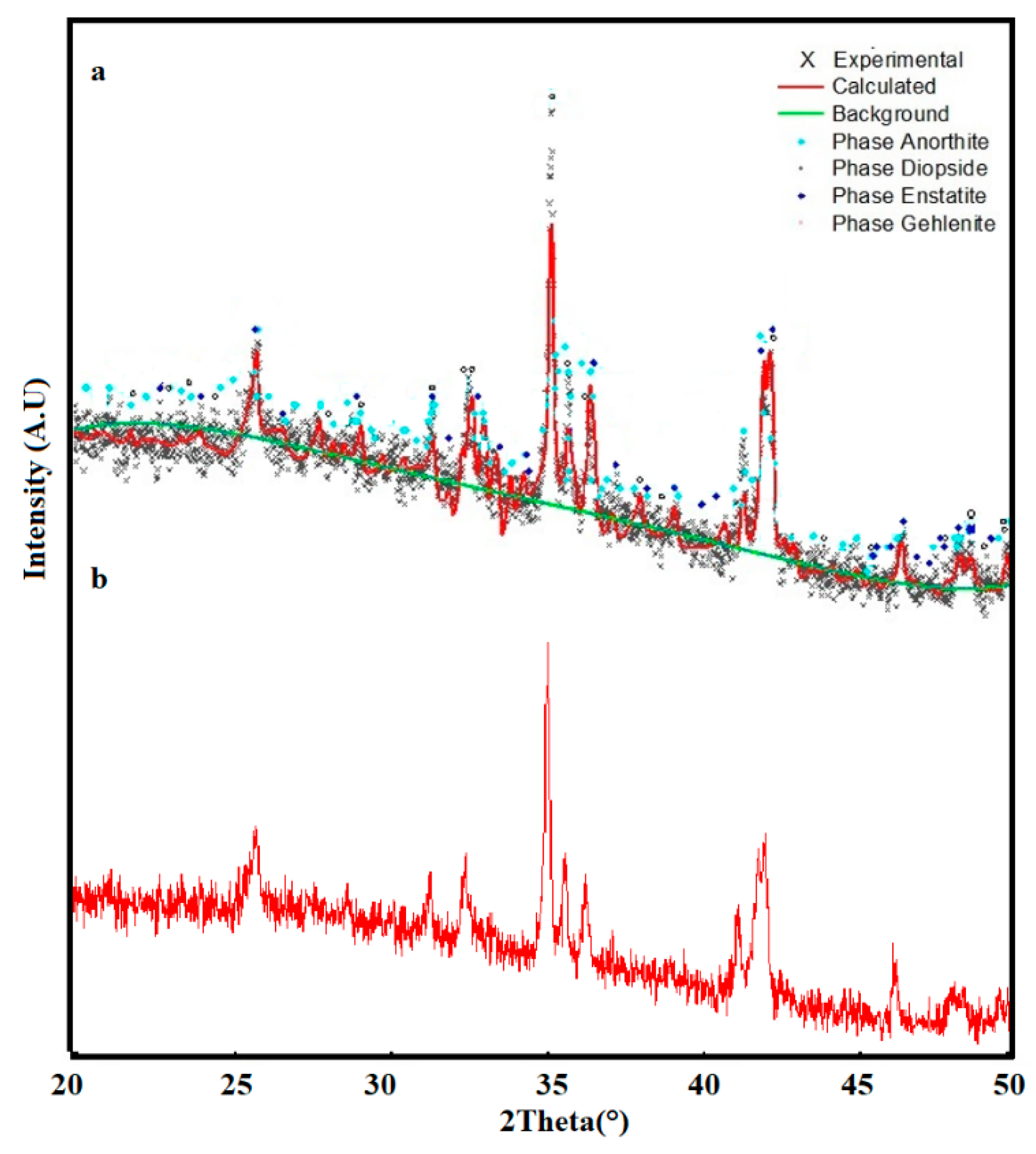
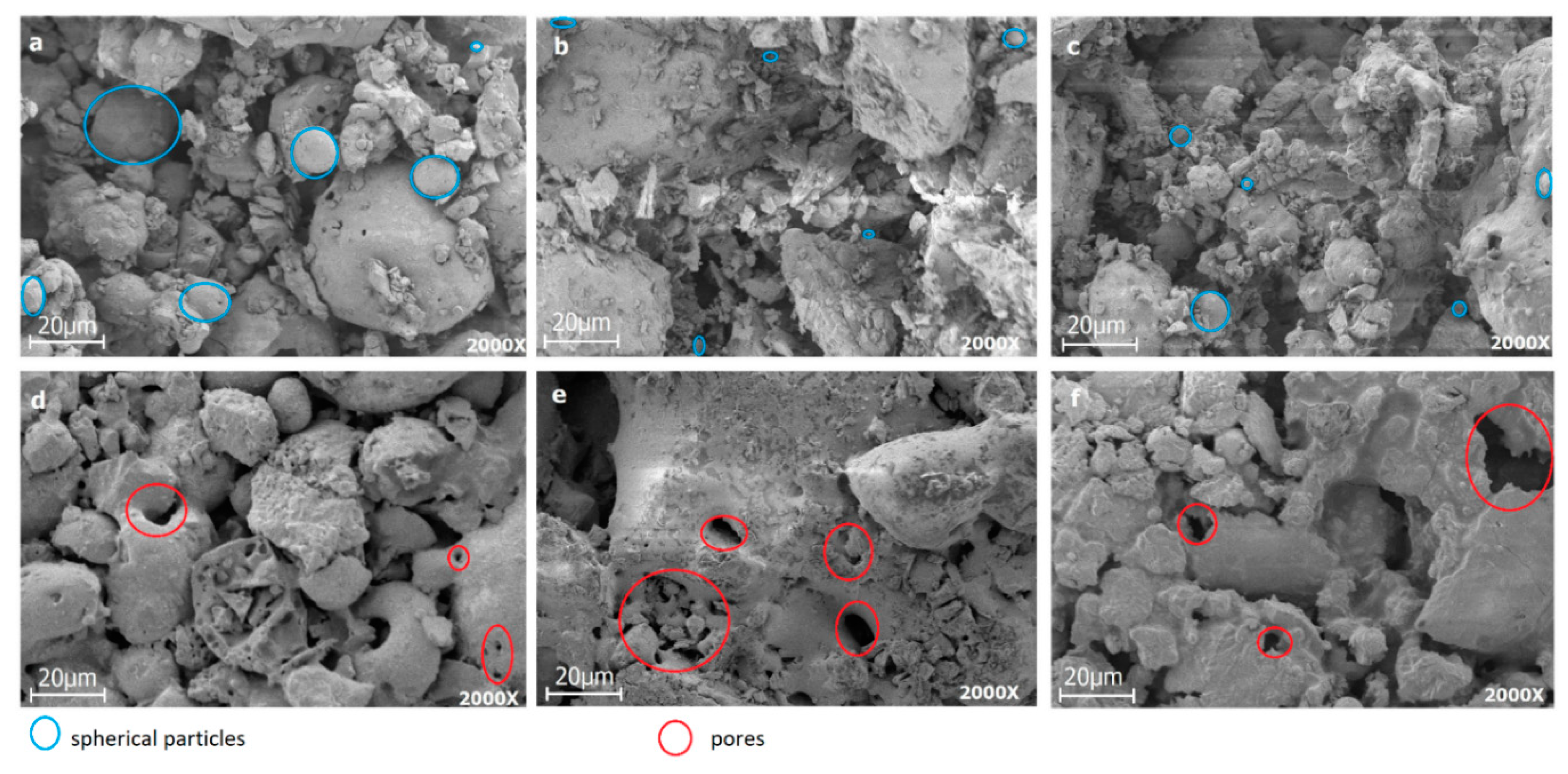
2.3.2. Sintering
3. Discussion
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sanchez, A.; Mauricio, H. Estado del arte sobre las escorias negras de horno de arco electrico y sus aplicaciones en pavimentos. L’esprit Ingéniux 2016, 7, 63–72. Available online: http://revistas.ustatunja.edu.co/index.php/lingenieux/article/view/1367/1266. (accessed on 15 June 2019).
- Jarrige, A. Empleo de las cenizas volantes en la construcción. Mater. Constr. 1959, 95, 521. [Google Scholar] [CrossRef]
- Ossa, M.; Jorquera, H. Cementos con cenizas volantes. Mater. Constr. 1984, 193, 1–17. [Google Scholar] [CrossRef]
- Valderrama; Patricia, C.; Agredo, J.T.; de Gutiérrez, R.M. Características de desempeño de un concreto adicionado con cenizas volantes de alto nivel de inquemados. Ingeniería e Investigación 2011, 31, 39–46. [Google Scholar]
- Sánchez-Vázquez, A.I.; Ruiz-Valdés, J.J.; Ramírez-Blanco, E.; Álvarez-Méndez, A.; Arciniega, S.M.D. Synthesis, characterisation and kinetic study of a glassy material in the BaO-TiO2-Ta2O5-B2O3-Al2O3 system obtained by a traditional glass fusion-casting method. J. Non-Cryst. Solids 2013, 380, 65–70. [Google Scholar] [CrossRef]
- Ahmaruzzaman, M. A review on the utilization of fly ash. Prog. Energy Combust. Sci. 2010, 36, 327–363. [Google Scholar] [CrossRef]
- Ortiz, O.J.R.; Tauta, J.F.C. Efecto del desperdicio de una siderurgica en bases y subbases granulares. Ciencia e Ingeniería Neogranadina 2003, 13, 25–29. [Google Scholar] [CrossRef]
- Rawlings, R.D.; Wu, J.P.; Boccaccini, A.R. Glass-ceramics: Their production from wastes-A Review. J. Mater. Sci. 2006, 41, 733–761. [Google Scholar] [CrossRef]
- Santaella, L.E.; Correa, R.S. Comportamiento de concreto con bajos porcentajes de ceniza volante (termo paipa IV) y agua constante. Ciencia e Ingeniería Neogranadina 2004, 14, 2–7. [Google Scholar] [CrossRef]
- Vianchá, G.; Roldan, P.R. Propuesta para la utilización de cenizas volantes como adición en la fabricación de cemento tipo I en la planta cementera de Holcim Colombia S.A. Universidad de la Sabana: Chía, Colombia, 2007. Available online: https://studylib.es/doc/5118574/propuesta-para-la-utilizaci%C3%B3n-de-cenizas-volantes (accessed on 15 June 2019).
- Karamanov, A.; Pelino, M. Crystallization phenomena in iron-rich glasses. J. Non-Cryst. Solids 2001, 281, 139–151. [Google Scholar] [CrossRef]
- Ojovan, W.; Juoi, J.M.; Boccaccini, A.R. Glass Composite Materials for Nuclear and Hazardous Waste Immobilisation. MRS Online Proc. Libr. Arch. 2008, 245, 1107. [Google Scholar] [CrossRef]
- Bai, H.; Zhang, X.; Cang, D.; Zhao, L.; Wei, W. Synthesis of steel slag ceramics: Chemical composition and crystalline phases of raw materials. Int. J. Miner. Metall. Mater. 2015, 22, 325–333. [Google Scholar] [CrossRef]
- Erol, M.; Küçükbayrak, S.; Ersoy-Meriçboyu, A. Production of glass-ceramics obtained from industrial wastes by means of controlled nucleation and crystallization. Chem. Eng. J. 2007, 132, 335–343. [Google Scholar] [CrossRef]
- Rezvani, M.; Eftekhari-Yekta, B.; Solati-Hashjin, M.; Marghussian, V.K. Effect of Cr2O3, Fe2O3 and TiO2 nucleants on the crystallization behaviour of SiO2-Al2O3-CaO-MgO(R2O) glass-ceramics. Ceram. Int. 2005, 13, 75–80. [Google Scholar] [CrossRef]
- Axinte, E. Glasses as engineering materials: A review. Mater. Des. 2011, 32, 1717–1732. [Google Scholar] [CrossRef]
- Asquini, L.; Furlani, E.; Bruckner, S.; Maschio, S. Production and characterization of sintered ceramics from paper mill sludge and glass cullet. Chemosphere 2008, 17, 83–89. [Google Scholar] [CrossRef]
- Höland, W.; Beall, G.H. Glass Ceramics Technology; American Ceramic Socienty: Westerville, OH, USA, 2012. [Google Scholar]
- Chinnam, R.K.; Francis, A.A.; Will, J.; Bernardo, E.; Boccaccini, A.R. Review. Functional glasses and glass-ceramics derived from iron rich waste and combination of industrial residues. J. Non-Cryst. Solids 2013, 365, 63–74. [Google Scholar] [CrossRef]
- Li, D.; Wong, L.N.Y. The brazilian disc test for rock mechanics applications: Review and new insights. Rock Mech. Rock Eng. 2013, 46, 269–287. [Google Scholar] [CrossRef]
- Karmakar, B. Functional Glasses and Glass-Ceramics from Solid Waste Materias. In Functional Glasses and Glass-Ceramic; Butterworth-Heinemann: Oxford, UK, 2017; pp. 295–315. [Google Scholar]
- Hanif, A.; Lu, Z.; Li, Z. Utilization of fly ash cenosphere as lightweight filler in cement-based composites—A review. Constr. Build. Mater. 2017, 144, 373–384. [Google Scholar] [CrossRef]
- Valderrama, D.A.; Cuaspud, J.G. Characterization of fly ash, slag and glass hull for the obtaining of vitreous materials. J. Phys. 2017, 935, 012040. [Google Scholar] [CrossRef]
- Quaranta, N.; Benavidey, E.; Grasselli, C.; Aguilar, I. Densificación de cenizas de una central térmica. Mem. Jorn. SAM-CONAMET-AAS 2001, 883–890. [Google Scholar]
- Ghosal, S.; Self, S.A. Particle size-density relation and cenosphere content of coal fly ash. Fuel 1995, 74, 522–529. [Google Scholar] [CrossRef]
- Gao, H.T.; Liu, X.H.; Chen, J.Q.; Qi, J.L.; Wang, Y.B.; Ai, Z.R. Preparation of glass-ceramics with low density and high strength using blast furnace slag, glass fiber and water glass. Ceram. Int. 2018, 44, 6044–6053. [Google Scholar] [CrossRef]
- Puertas, F. Escorias de alto horno: Composición y comportamiento hidráulico. Mater. Constr. 1993, 43, 37–48. [Google Scholar] [CrossRef]
- Javier, G.-F.d.A. Caracterización de Cenizas de Centrales térmicas del Norte de España para su Aplicación en usos Alternativos. Master’s Thesis, Universidad de Oviedo, Oviedo, Spain, 2015; pp. 296–304. [Google Scholar]
- Wang, S.; Baxter, L.; Fonseca, F. Biomass fly ash in concrete: SEM, EDX and ESEM analysis. Fuel 2008, 87, 372–379. [Google Scholar] [CrossRef]
- Folguer, M.V.; de Oliveira, P.N.; Alarcon, O.E. Glass-Ceramics obtained from processed slag and fly ash. Am. Ceram. Soc. Bull. 2005, 84, 9201–9205. [Google Scholar]
- Zyrkowski, M.; Neto, R.C.; Santos, L.F.; Witkowski, K. Characterization of fly-ash cenospheres from coal-fired power plant unit. Fuel 2016, 174, 49–53. [Google Scholar] [CrossRef]
- Liu, H.; Wei, G.; Liang, Y.; Dong, F. Glass-ceramics made from arc-melting slag of waste incineration fly ash. J. Cent. South Univ. Technol. 2011, 18, 1945–1952. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, C.; Chen, J. Utilization of coal fly ash for the production of glass-ceramics with unique performances: A brief review. J. Mater. Sci. Technol. 2014, 30, 1208–1212. [Google Scholar] [CrossRef]
- Liu, Y.; Zheng, L.; Li, X.; Xie, S. SEM/EDS and XRD characterization of raw and washed MSWI fly ash sintered at different temperatures. J. Hazard. Mater. 2009, 162, 161–173. [Google Scholar] [CrossRef]
- Rincón, J.M.; Hernández-Crespo, M.; Romero, M. Vitrificación de residuos industriales inorgánicos para la producción de nuevas fritas, así como de plaquetas porcelanitas y vitroceramicas. I Simposio Iberoamericano Ingeniería de Residuo 2008, 23–24. [Google Scholar]
- Liu, H.; Lu, H.; Chen, D.; Wang, H.; Xu, H.; Zhang, R. Preparation and properties of glass-ceramics derived from blast-furnace slag by a ceramic-sintering process. Ceram. Int. 2009, 35, 3181–3184. [Google Scholar] [CrossRef]
- Harabi, A.; Zaiou, S.; Guechi, A.; Foughali, L.; Harabi, E.; Zouai, S.; Guerfa, F. Mechanical properties of anorthite based ceramics prepared from kaolin DD2 and calcite. Cerâmica 2017, 63, 311–317. [Google Scholar] [CrossRef]
- Han, W. Glass ceramic of high hardness and fracture toughness developed from iron-rich wastes. Acta Metall. Sin. Eng. Lett. 2009, 22, 181–190. [Google Scholar] [CrossRef]
- Boccaccini, A.R.; Han, W.X.; Dimech, C.; Rawlings, R.D. Glass ceramics of high hardness and fracture toughness developed from steel fly ash. Mater. Sci. Technol. 2006, 22, 1148–1154. [Google Scholar] [CrossRef]
- Mirza, A.; Riaz, M.; Zia, R.; Hussain, T.; Bashir, F. Effect of temperature on mechanical and bioactive properties of glass-ceramics. J. Alloys Compd. 2017, 726, 348–351. [Google Scholar] [CrossRef]
- Park, Y.J.; Heo, J. Conversion to glass-ceramics from glasses made by MSW incinerator fly ash for recycling. Ceram. Int. 2002, 28, 689–694. [Google Scholar] [CrossRef]
- Hameed, S.A.M.A.; Elkheshen, A.A. Thermal and chemical properties of diopside-wollastonite glass-ceramics in the SiO2-CaO-MgO system from raw materials. Ceram. Int. 2003, 29, 265–269. [Google Scholar] [CrossRef]
- Beall, G.H. Design and Properties of Glass-Ceramics. Annu. Rev. Mater. Sci. 1992, 22, 91–119. [Google Scholar] [CrossRef]
- Karayannis, V.; Moutsatsou, A.; Domopoulou, A.; Katsika, E.; Drossou, C.; Baklavaridis, A. Fired ceramics 100% from lignite fly ash and waste glass cullet mixtures. J. Build. Eng. 2017, 14, 1–6. [Google Scholar] [CrossRef]
- Ptáček, P.; Opravil, T.; Šoukal, F.; Havlica, J.; Holešinský, R. Kinetics and mechanism of formation of gehlenite, Al-Si spinel and anorthite from the mixture of kaolinite and calcite. Solid State Sci. 2013, 26, 53–58. [Google Scholar] [CrossRef]
- Ercenk, E.; Sen, U.; Bayrak, G.; Yilmaz, S. Glass and glass-ceramics produced from fly ash and boron waste. Acta Phys. Pol. A 2014, 125, 626–628. [Google Scholar] [CrossRef]
- Zhang, W.Y.; Gao, H.; Xu, Y. Sintering and reactive crystal growth of diopside-albite glass-ceramics from waste glass. J. Eur. Ceram. Soc. 2011, 31, 1669–1675. [Google Scholar] [CrossRef]
- Romero, M.; Rincón, J.M.; Rawlings, R.D.; Boccaccini, A.R. Use of vitrified urban incinerator waste as raw material for production of sintered glass-ceramics. Mater. Res. Bull. 2001, 36, 383–395. [Google Scholar] [CrossRef]
- Goeuriot, D.; Dubois, J.C.; Merle, D.; Thevenot, F.; Exbrayat, P. Enstatite Based Ceramics for Machinable Prosthesis Applications. J. Eur. Ceram. Soc. 1998, 18, 2045–2056. [Google Scholar] [CrossRef]
- Snellings, R.; Mertens, G.; Elsen, J. Supplementary Cementitious Materials. Rev. Miner. Geochem. 2014, 74, 211–278. [Google Scholar] [CrossRef]
- Toya, T.; Kameshima, Y.; Yasumori, A.; Okada, K. Preparation and properties of glass-ceramics from wastes (Kira) of silica sand and kaolin clay refining. J. Eur. Ceram. Soc. 2004, 24, 2367–2372. [Google Scholar] [CrossRef]
- Park, Y.J.; Moon, S.O.; Heo, J. Crystalline phase control of glass ceramics obtained from sewage sludge fly ash. Ceram. Int. 2003, 29, 223–227. [Google Scholar] [CrossRef]
- Bernardo, E.; Castellan, R.; Hreglich, S. Sintered glass-ceramics from mixtures of wastes. Ceram. Int. 2007, 33, 27–33. [Google Scholar] [CrossRef]
- Baowei, L.; Leibo, D.; Xuefeng, Z.; Xiaolin, J. Structure and performance of glass–ceramics obtained by Bayan Obo tailing and fly ash. J. Non-Cryst. Solids 2013, 380, 103–108. [Google Scholar] [CrossRef]
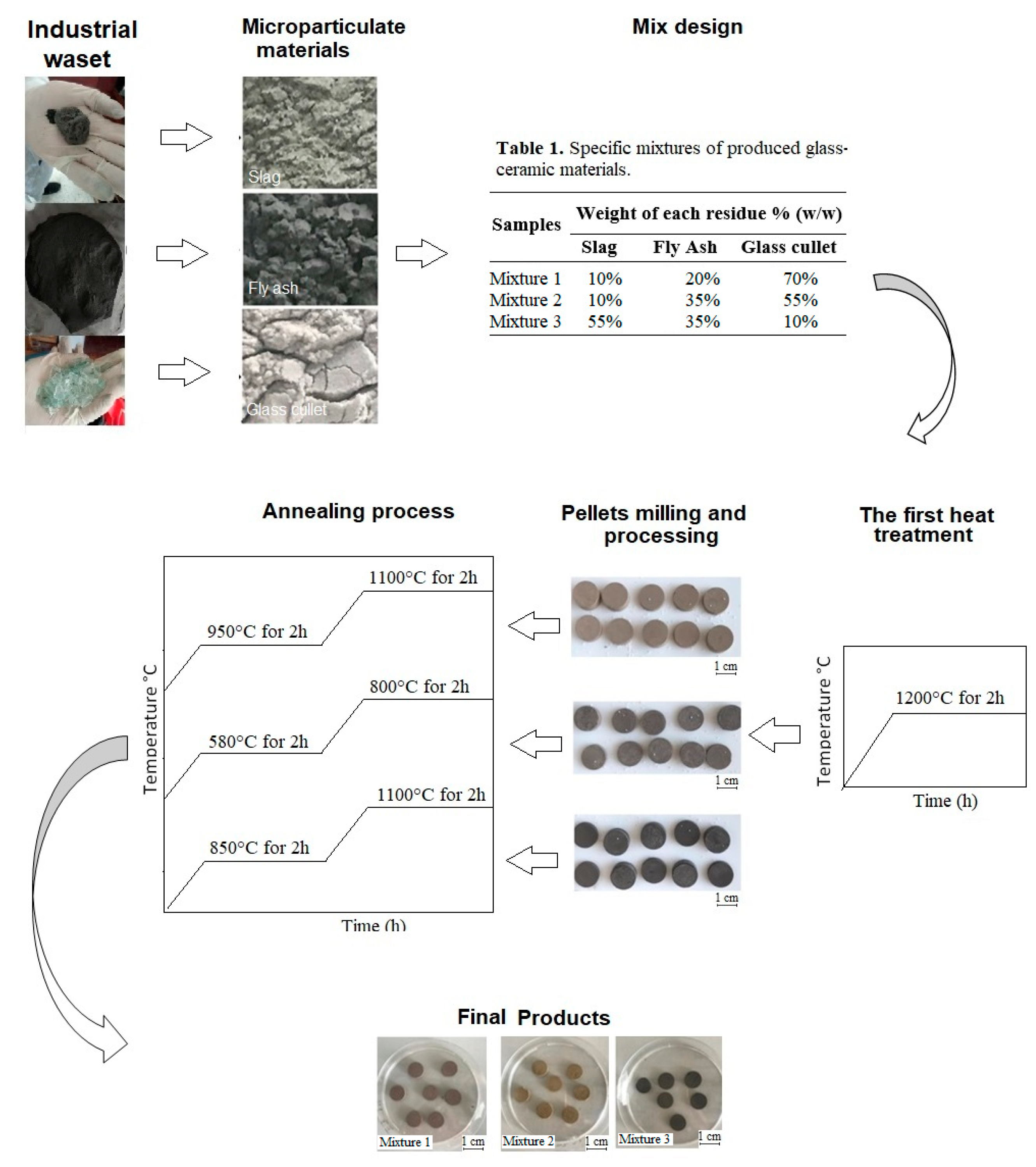

| Samples | Weight of Each Residue % (w/w) | ||
|---|---|---|---|
| Slag | Fly Ash | Glass Cullet | |
| Mixture 1 | 10% | 20% | 70% |
| Mixture 2 | 10% | 35% | 55% |
| Mixture 3 | 55% | 35% | 10% |
| Samples | Na2O | MgO | Al2O3 | SiO2 | SO3 | K2O | CaO | TiO2 | Fe2O3 | Mn | Other |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mixture 1 | 3.627 | 1.85 | 13.64 | 66.3 | 0.8 | 0.7 | 7.1 | 1.09 | 3.924 | 0.7 | 0.285 |
| Mixture 2 | 4.914 | 3.38 | 8.161 | 66.4 | 0.6 | 0.4 | 12 | 0.64 | 2.828 | 0.7 | 0.227 |
| Mixture 3 | 1.813 | 3.12 | 11.24 | 46.9 | 0.7 | 0.5 | 20 | 1.03 | 9.686 | 3.7 | 0.927 |
| Samples | Melting Temperature (°C) | Nucleation Temperature (°C) | Crystallization Temperature (°C) | Time (h) |
|---|---|---|---|---|
| Mixture 1 | 1200 | 930 | 1100 | 2 |
| Mixture 2 | 1200 | 580 | 800 | 2 |
| Mixture 3 | 1200 | 850 | 1100 | 2 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ayala Valderrama, D.M.; Gómez Cuaspud, J.A.; Roether, J.A.; Boccaccini, A.R. Development and Characterization of Glass-Ceramics from Combinations of Slag, Fly Ash, and Glass Cullet without Adding Nucleating Agents. Materials 2019, 12, 2032. https://doi.org/10.3390/ma12122032
Ayala Valderrama DM, Gómez Cuaspud JA, Roether JA, Boccaccini AR. Development and Characterization of Glass-Ceramics from Combinations of Slag, Fly Ash, and Glass Cullet without Adding Nucleating Agents. Materials. 2019; 12(12):2032. https://doi.org/10.3390/ma12122032
Chicago/Turabian StyleAyala Valderrama, Diana M., Jairo A. Gómez Cuaspud, Judith A. Roether, and Aldo R. Boccaccini. 2019. "Development and Characterization of Glass-Ceramics from Combinations of Slag, Fly Ash, and Glass Cullet without Adding Nucleating Agents" Materials 12, no. 12: 2032. https://doi.org/10.3390/ma12122032
APA StyleAyala Valderrama, D. M., Gómez Cuaspud, J. A., Roether, J. A., & Boccaccini, A. R. (2019). Development and Characterization of Glass-Ceramics from Combinations of Slag, Fly Ash, and Glass Cullet without Adding Nucleating Agents. Materials, 12(12), 2032. https://doi.org/10.3390/ma12122032






