Chloride Induced Reinforcement Corrosion in Mortars Containing Coal Bottom Ash and Coal Fly Ash
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials, Mix Proportions and Specimen Details
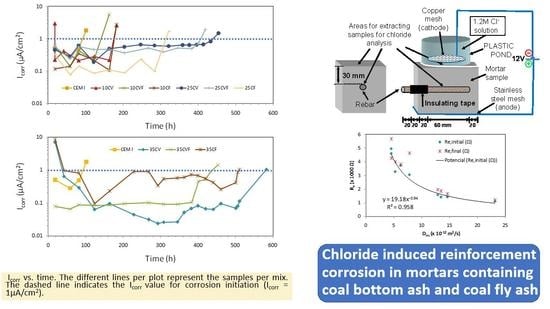
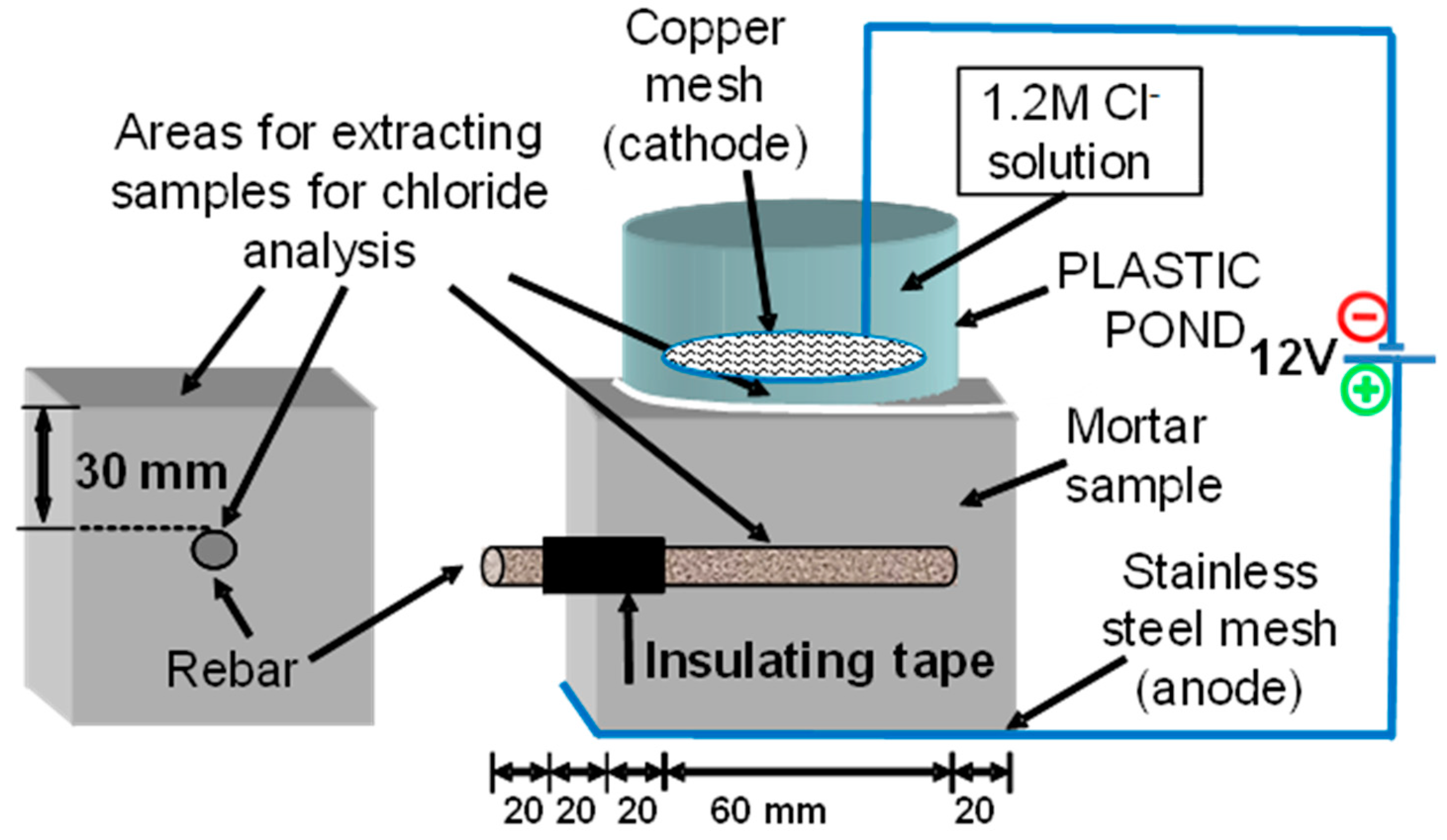
2.2. Testing Procedure
2.3. Calculation of the Non-Steady State (Apparent) Diffusion Coefficient
3. Results and Discussion
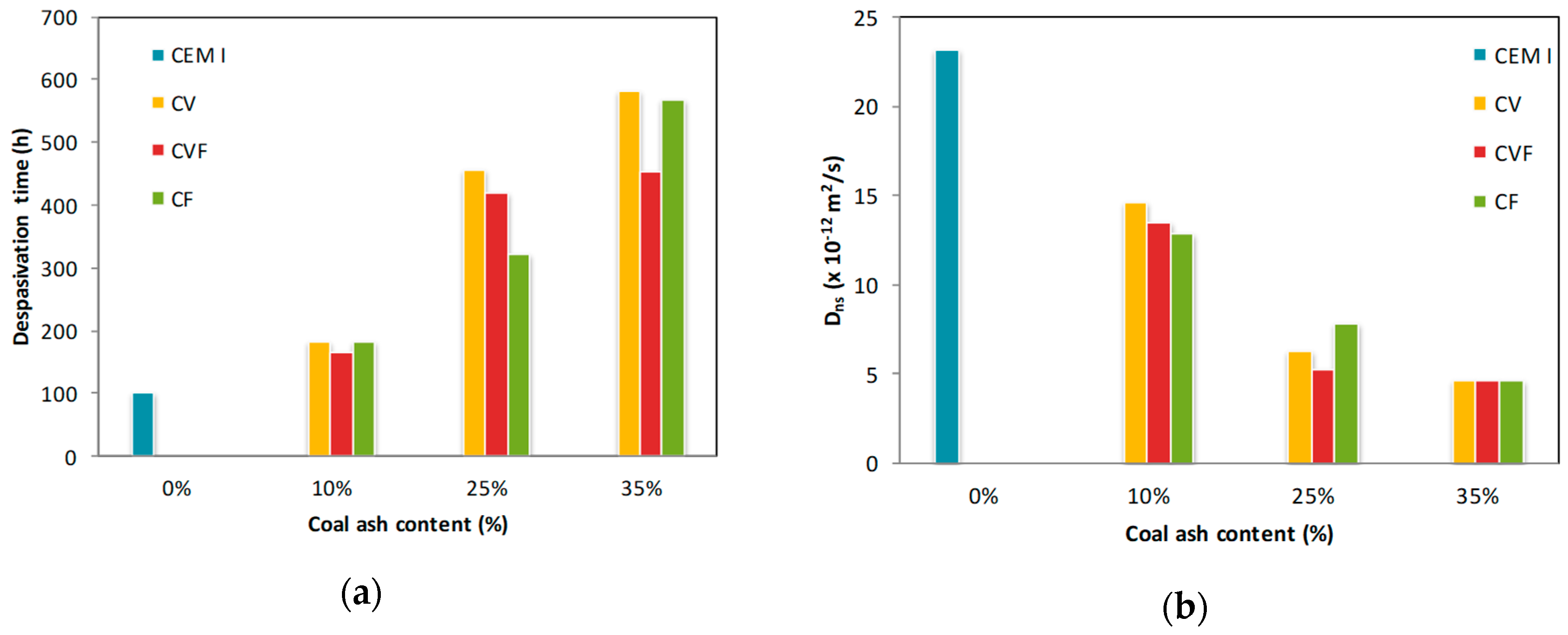
3.1. Depassivation Time and Non-Steady State Diffusion Coefficient
3.2. Critical and Surface Chloride Content
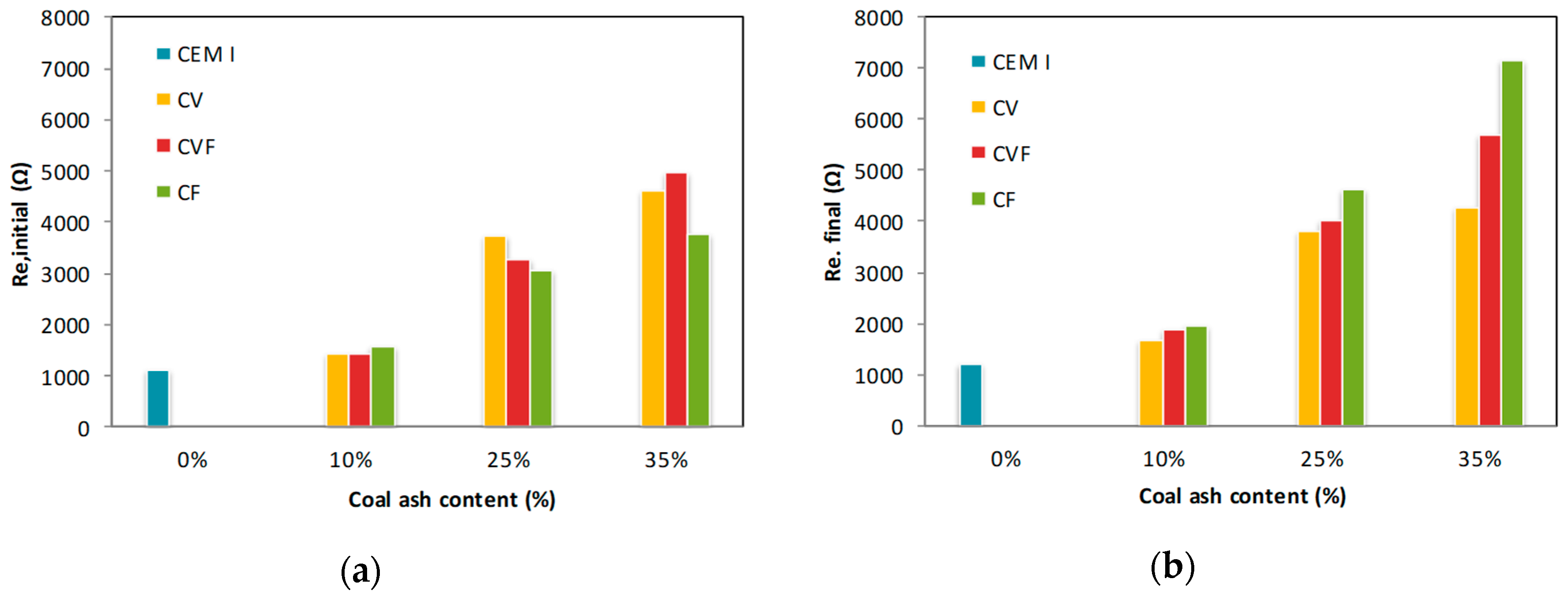
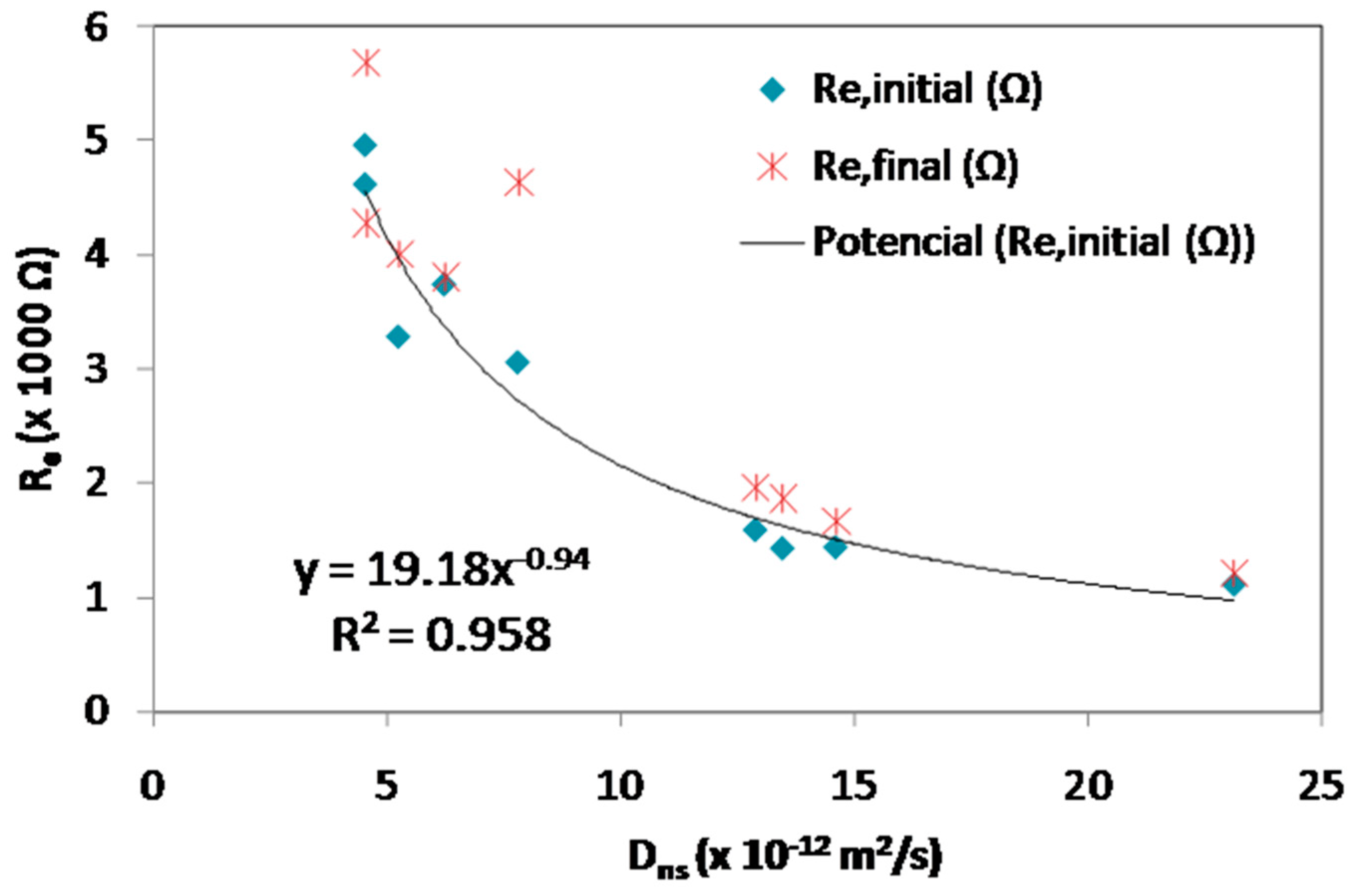
3.3. Initial and Final Resistance
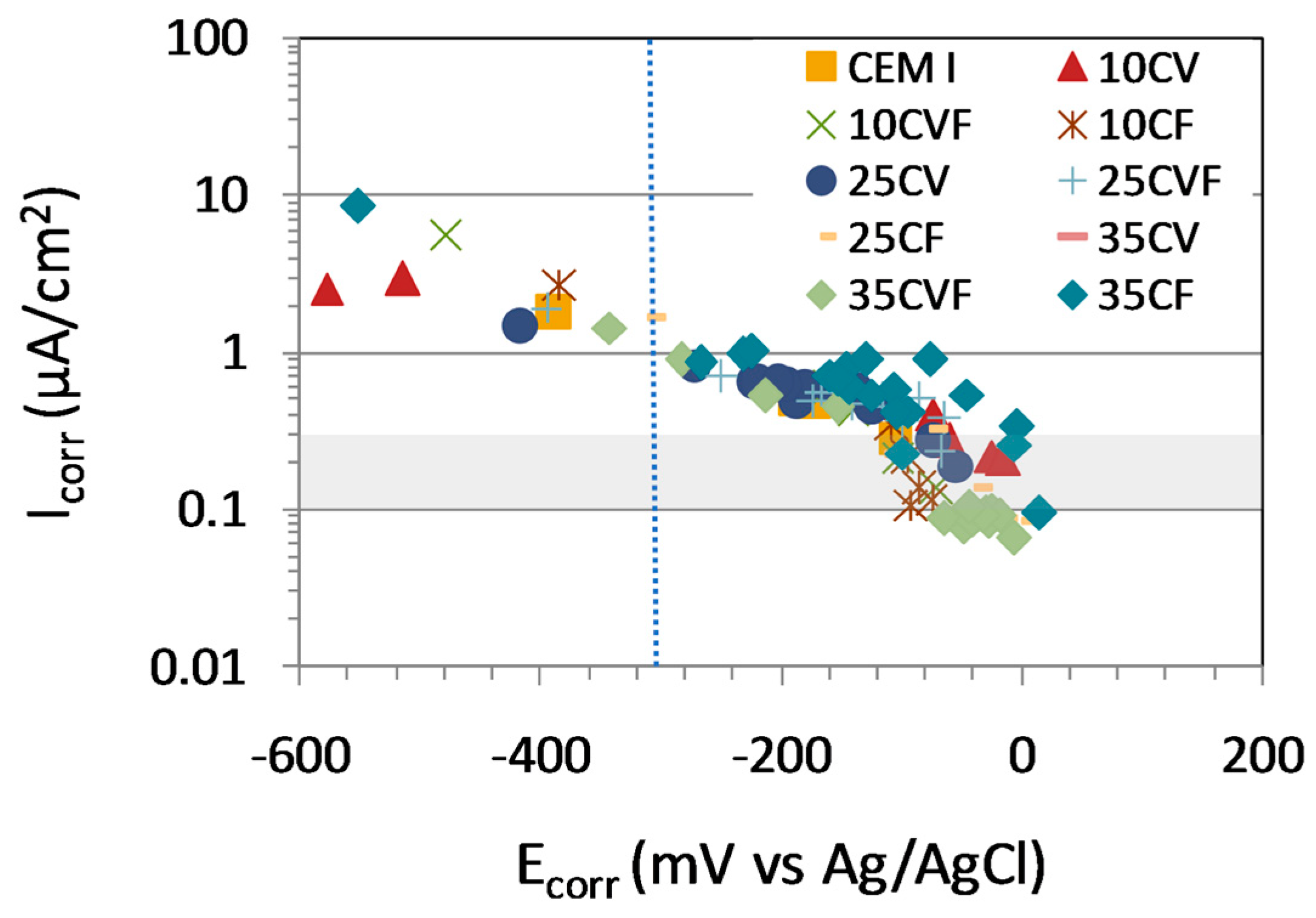
3.4. Potential Monitoring
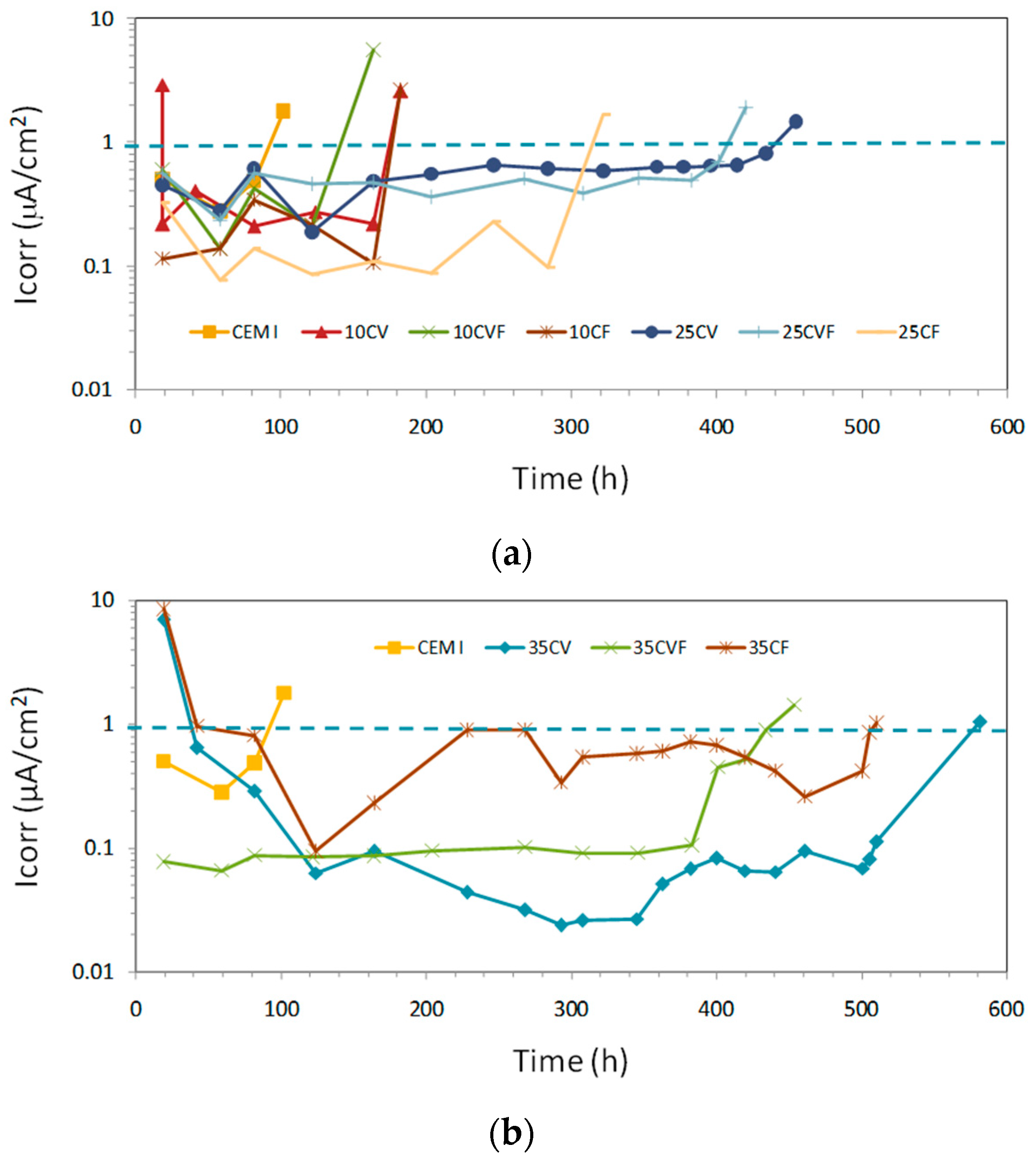
3.5. Corrosion Rate Monitoring
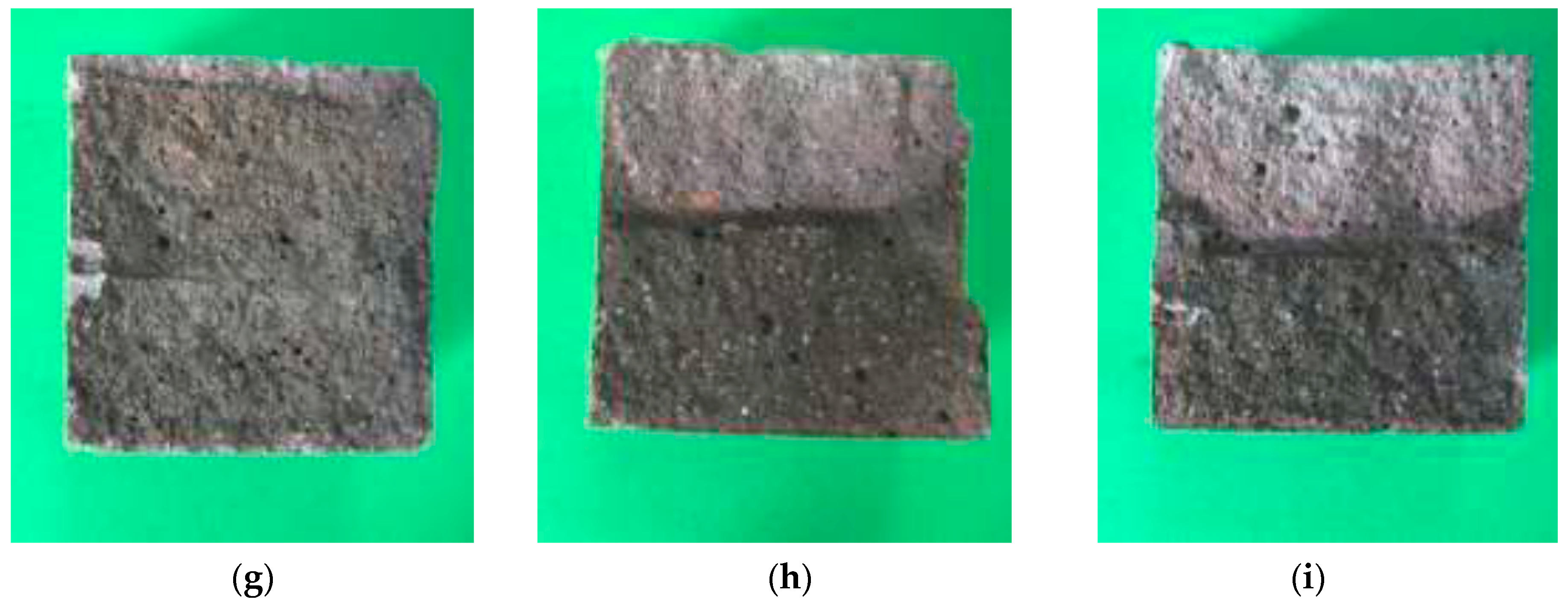
3.6. Visual Examination
4. Conclusions
- Chloride diffusion coefficient in natural test conditions decreased from 23 × 10−12 m2/s in cements without coal ashes to 4.5 × 10−12 m2/s in cements with 35% by weight of coal ashes. Moreover, the time to steel corrosion initiation went from 102 h to about 500 h, respectively.
- Coal bottom ash and coal fly ash showed a similar corrosion performance in reinforced mortars. Both of them have a positive effect on the chloride resistance of the reinforced mortars. However, the higher coal ash proportion in the mortar, the lower critical chloride content, Ccritical, was found. This is explained by the lower hydroxyl concentration in blended mortars and, therefore, the lower Cl−/OH− threshold value than in plain mortars.
- The most important parameter influencing the corrosion onset is the amount of coal ash independent of the type of ash.
- The results reveal that the experimental procedure used being accelerated appears a promising method to arrive at the chloride apparent diffusion coefficient, Dap, in mortars and concretes. It provided reliable information about the quality of the coal bottom ash investigated in this research program with regard to its durability.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tripathi, S.R.; Ogura, H.; Kawagoe, H.; Inoue, H.; Hasegawa, T.; Takeya, K.; Kawase, K. Measurement of chloride ion concentration in concrete structures using terahertz time domain spectroscopy (THz-TDS). Corros. Sci. 2012, 62, 5–10. [Google Scholar] [CrossRef]
- Song, H.-W.; Lee, C.-H.; Ann, K.Y. Factors influencing chloride transport in concrete structures exposed to marine environments. Cem. Concr. Compos. 2008, 30, 113–121. [Google Scholar] [CrossRef]
- Ann, K.Y.; Song, H.-W. Chloride threshold level for corrosion of steel in concrete. Corros. Sci. 2007, 49, 4113–4133. [Google Scholar] [CrossRef]
- Tuutti, K. Corrosion of Steel in Concrete; Swedish Cement and Concrete Research Institute: Stockholm, Sweeden, 1982. [Google Scholar]
- Muthulingam, S.; Rao, B.N. Non-uniform corrosion states of rebar in concrete under chloride environment. Corros. Sci. 2015, 93, 267–282. [Google Scholar] [CrossRef]
- Alonso, C.; Castellote, M.; Andrade, C. Chloride threshold dependence of pitting potential of reinforcements. Electrochim. Acta 2002, 47, 3469–3481. [Google Scholar] [CrossRef]
- Argiz, C.; Menéndez, E.; Moragues, A.; Sanjuán, M.A. Fly ash characteristics of Spanish coal-fired power plants. Afinidad 2015, 72, 269–277. [Google Scholar]
- Jorat, M.E.; Aziz, M.A.; Marto, A.; Zaini, N.; Jusoh, S.N.; Manning, D.A. Sequestering Atmospheric CO2 Inorganically: A Solution for Malaysia’s CO2 Emission. Geosciences 2018, 8, 483. [Google Scholar] [CrossRef]
- Ali, H.L.; Yusuf, B.; Mohammed, T.A.; Shimizu, Y.; Ab Razak, M.S.; Rehan, B.M. Improving the Hydro-Morpho Dynamics of A River Confluence by Using Vanes. Resources 2019, 8, 9. [Google Scholar] [CrossRef]
- Segismundo, E.Q.; Kim, L.-H.; Jeong, S.-M.; Lee, B.-S. A Laboratory Study on the Filtration and Clogging of the Sand-Bottom Ash Mixture for Stormwater Infiltration Filter Media. Water 2017, 9, 32. [Google Scholar] [CrossRef]
- Kim, J.-H.; Sung, J.-H.; Jeon, C.-S.; Lee, S.-H.; Kim, H.-S. A Study on the Properties of Recycled Aggregate Concrete and Its Production Facilities. Appl. Sci. 2019, 9, 1935. [Google Scholar] [CrossRef]
- Chung, S.-Y.; Abd Elrahman, M.; Stephan, D. Effect of Different Gradings of Lightweight Aggregates on the Properties of Concrete. Appl. Sci. 2017, 7, 585. [Google Scholar] [CrossRef]
- Fan, C.-C.; Huang, R.; Hwang, H.; Chao, S.-J. The Effects of Different Fine Recycled Concrete Aggregates on the Properties of Mortar. Materials 2015, 8, 2658–2672. [Google Scholar] [CrossRef] [Green Version]
- Valentim, B.; Białecka, B.; Gonçalves, P.A.; Guedes, A.; Guimarães, R.; Cruceru, M.; Całus-Moszko, J.; Popescu, L.G.; Predeanu, G.; Santos, A.C. Undifferentiated Inorganics in Coal Fly Ash and Bottom Ash: Calcispheres, Magnesiacalcispheres, and Magnesiaspheres. Minerals 2018, 8, 140. [Google Scholar] [CrossRef]
- Kuo, W.-T.; Gao, Z.-C. Engineering Properties of Controlled Low-Strength Materials Containing Bottom Ash of Municipal Solid Waste Incinerator and Water Filter Silt. Appl. Sci. 2018, 8, 1377. [Google Scholar] [CrossRef]
- Tuan, L.Q.; Thenepalli, T.; Chilakala, R.; Vu, H.H.T.; Ahn, J.W.; Kim, J. Leaching Characteristics of Low Concentration Rare Earth Elements in Korean (Samcheok) CFBC Bottom Ash Samples. Sustainability 2019, 11, 2562. [Google Scholar] [CrossRef]
- Argiz, C.; Menéndez, E.; Sanjuán, M.A. Effect of mixes made of coal bottom ash and fly ash on the mechanical strength and porosity of Portland cement. Mater. Construcc. 2013, 309, 49–64. [Google Scholar]
- Choia, Y.-S.; Kima, J.-G.; Lee, K.-M. Corrosion behavior of steel bar embedded in fly ash concrete. Corros. Sci. 2006, 48, 1733–1745. [Google Scholar] [CrossRef]
- Andrade, C.; Buják, R. Effects of some mineral additions to Portland cement on reinforcement corrosion. Cem. Concr. Res. 2013, 53, 59–67. [Google Scholar] [CrossRef]
- Faustino, P.; Chastre, C.; Nunes, Â.; Brás, A. Lifetime modelling of chloride-induced corrosion in concrete structures with Portland and blended cements. Struct. Infrastruct. Eng. 2016, 12, 1013–1023. [Google Scholar] [CrossRef]
- European Committee for Standardization. EN 197-1:2011. Cement—Part. 1: Composition, Specifications and Conformity Criteria for Common Cements; European Committee for Standardization (CEN) Standards: Brussels, Belgium, 2011. [Google Scholar]
- ASTM C618-15. Standard Specification for Coal Fly Ash and Raw or Calcined Natural Pozzolan for Use as a Mineral Admixture in Concrete; ASTM Standards: West Conshohocken, PA, USA, 2001. [Google Scholar]
- European Committee for Standardization. EN 196-2:2013. Method of Testing Cement. Chemical Analysis of Cement; European Committee for Standardization (CEN) Standards: Brussels, Belgium, 2013. [Google Scholar]
- UNE—Asociación Española de Normalización. UNE 83992-2:2012 EX. Durability of Concrete. Test Methods. Chloride Penetration Tests on Concrete. Part 2: Integral Accelerated Method; UNE: Madrid, Spain, 2012. [Google Scholar]
- Paul, S.C.; van Zijl, G.P. Crack Formation and Chloride Induced Corrosion in Reinforced Strain Hardening Cement-Based Composite (R/SHCC). J. Adv. Concr. Technol. 2014, 12, 340–351. [Google Scholar] [CrossRef] [Green Version]
- Paul, S.C.; van Zijl, G.P.; Babafemi, A.J.; Tan, M.J. Chloride ingress in cracked and uncracked SHCC under cyclic wetting-drying exposure. Constr. Build. Mater. 2016, 114, 232–240. [Google Scholar] [CrossRef]
- King, F. Corrosion of Copper in Alkaline Chloride Environments; Technical Report TR-02-25; Swedish Nuclear Fuel and Waste Management Co.: Stockholm, Sweden, 2002; p. 71. [Google Scholar]
- Liu, J.; Qiu, Q.; Chen, X.; Wang, X.; Xing, F.; Han, N.; He, Y. Degradation of fly ash concrete under the coupled effect of carbonation and chloride aerosol ingress. Corros. Sci. 2016, 112, 364–372. [Google Scholar] [CrossRef]
- Hussain, S.E.; Al-Musallam, A.; Al-Gahtani, A.S. Factors affecting threshold chloride for reinforcement corrosion in concrete. Cem. Concr. Res. 1995, 25, 1543–1555. [Google Scholar] [CrossRef]
- Jiang, L.; Liu, R.; Mo, L.; Xu, J.; Yang, H. Influence of chloride salt type on critical chloride content of reinforcement corrosion in concrete. Mag. Concr. Res. 2013, 65, 319–331. [Google Scholar] [CrossRef]
- Oh, B.H.; Jang, S.Y.; Shin, Y.S. Experimental investigation of the threshold chloride concentration for corrosion initiation in reinforced concrete structures. Mag. Concr. Res. 2003, 55, 117–124. [Google Scholar] [CrossRef]
- Xu, J.; Jiang, L.; Wang, W.; Jiang, Y. Influence of CaCl2 and NaCl from different sources on chloride threshold value for the corrosion of steel reinforcement in concrete. Constr. Build. Mater. 2011, 25, 663–669. [Google Scholar] [CrossRef]
- Liu, R.; Jiang, L.; Huang, G.; Zhu, Y.; Liu, X.; Chu, H.; Xiong, C. The effect of carbonate and sulfate ions on chloride threshold level of reinforcement corrosion in mortar with/without fly ash. Constr. Build. Mater. 2016, 113, 90–95. [Google Scholar] [CrossRef]
- Härdtl, R.; Schiessl, P.; Wiens, U. Limits of pozzolanic additions with respect to alkalinity and corrosion protection of reinforcement. In Durability of High Performance Concrete; Sommer, H., Ed.; RILEM International Workshop: Vienna, Austria, 1994; pp. 189–193. [Google Scholar]
- Thomas, M. Chloride thresholds in marine concrete. Cem. Concr. Res. 1996, 26, 513–519. [Google Scholar] [CrossRef]
- Hausmann, D.A. Electrochemical behaviour of steel in concrete. ACI J. Proc. 1964, 61, 171–188. [Google Scholar]
- Gouda, V.K. Corrosion and corrosion inhibition of reinforcing steel I. Immersed in alkaline solutions. Brit. Corros. 1970, 5, 198–203. [Google Scholar] [CrossRef]
- Andrade, C.; Alonso, C. Corrosion rate monitoring in the laboratory and on-site. Constr. Build. Mater. 1996, 10, 315–328. [Google Scholar] [CrossRef]
- Hornbostel, K.; Angst, U.M.; Elsener, B.; Larsen, C.K.; Geiker, M.R. Influence of mortar resistivity on the rate-limiting step of chloride-induced macro-cell corrosion of reinforcing steel. Corros. Sci. 2016, 110, 46–56. [Google Scholar] [CrossRef] [Green Version]
- Paul, S.C.; van Zijl, G.P. Corrosion Deterioration of Steel in Cracked SHCC. Int. J. Concr. Struct. Mater. 2017, 11, 557–572. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, K.; Iizuka, T.; Kurachi, H.; Rokugo, K. Corrosion protection performance of High Performance Fiber Reinforced Cement Composites as a repair material. Cem. Concr. Compos. 2010, 32, 411–420. [Google Scholar] [CrossRef]
- Alonso, C.; Andrade, C.; Castellote, M.; Castro, P. Chloride threshold values to depassivate reinforcing bars embedded in a standardized OPC mortar. Cem. Concr. Res. 2000, 30, 1047–1055. [Google Scholar] [CrossRef]


| Compositions | SiO2 | Al2O3 | Fe2O3 | CaO | MgO | SO3 | TiO2 | P2O5 | Soluble Residue 1 | Loss on Ignition |
|---|---|---|---|---|---|---|---|---|---|---|
| Cement | 19.04 | 3.85 | 3.43 | 57.16 | 1.54 | 3.14 | 0.17 | 0.07 | 2.15 | 3.93 |
| Bottom ash | 48.12 | 25.55 | 5.86 | 7.07 | 1.28 | 0.15 | 1.5 | 0.96 | 81.24 | 1.85 |
| Fly ash | 46.84 | 26.66 | 4.72 | 5.55 | 1.33 | 0.37 | 1.5 | 1.03 | 76.00 | 3.63 |
| Composition 1 | CEM I | 10CV | 10CVF | 10CF | 25CV | 25CVF | 25CF | 35CV | 35CVF | 35CF |
|---|---|---|---|---|---|---|---|---|---|---|
| Cement | 100 | 90 | 90 | 90 | 75 | 75 | 75 | 65 | 65 | 65 |
| Fly ash | 0 | 10 | 8 | 0 | 25 | 20 | 0 | 35 | 28 | 0 |
| Bottom ash | 0 | 0 | 2 | 10 | 0 | 5 | 25 | 0 | 7 | 35 |
| Sand | 300 | 300 | 300 | 300 | 300 | 300 | 300 | 300 | 300 | 300 |
| Water | 50 | 50 | 50 | 50 | 50 | 50 | 50 | 50 | 50 | 50 |
| Code | tlag (h) | Dns (× 10−12 m2/s) | Icorr at tlag (µA/cm2) | Ecorr (mV) | Ccritical (% wt Dry Sample) | Cs (wt % Dry Sample) | Re,initial (Ω) | Re,final (Ω) | Cover Thickness (cm) |
|---|---|---|---|---|---|---|---|---|---|
| CEM I | 102 | 23.13 | 1.78 | −389 | 0.19 | 0.59 | 1105 | 1212 | 3.10 |
| 10CV | 183 | 14.61 | 1.18 | −578 | 0.18 | 0.97 | 1432 | 1660 | 3.30 |
| 10CVF | 164 | 13.47 | 5.51 | −478 | 0.14 | 0.53 | 1423 | 1863 | 3.00 |
| 10CF | 183 | 12.89 | 2.65 | −384 | 0.09 | 0.49 | 1583 | 1960 | 3.10 |
| 25CV | 455 | 6.24 | 1.46 | −416 | 0.03 | 1.13 | 3738 | 3800 | 3.40 |
| 25CVF | 420 | 5.26 | 1.91 | −394 | 0.09 | 1.04 | 3279 | 4000 | 3.00 |
| 25CF | 322 | 7.81 | 1.68 | −310 | 0.05 | 0.77 | 3053 | 4633 | 3.20 |
| 35CV | 582 | 4.55 | 6.94 | −326 | 0.03 | 0.53 | 4615 | 4270 | 3.60 |
| 35CVF | 454 | 4.55 | 1.43 | −342 | 0.06 | 1.17 | 4959 | 5673 | 2.90 |
| 35CF | 567 | 4.63 | 1.06 | −331 | 0.03 | 1.32 | 3750 | 7143 | 3.27 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Menéndez, E.; Argiz, C.; Sanjuán, M.Á. Chloride Induced Reinforcement Corrosion in Mortars Containing Coal Bottom Ash and Coal Fly Ash. Materials 2019, 12, 1933. https://doi.org/10.3390/ma12121933
Menéndez E, Argiz C, Sanjuán MÁ. Chloride Induced Reinforcement Corrosion in Mortars Containing Coal Bottom Ash and Coal Fly Ash. Materials. 2019; 12(12):1933. https://doi.org/10.3390/ma12121933
Chicago/Turabian StyleMenéndez, Esperanza, Cristina Argiz, and Miguel Ángel Sanjuán. 2019. "Chloride Induced Reinforcement Corrosion in Mortars Containing Coal Bottom Ash and Coal Fly Ash" Materials 12, no. 12: 1933. https://doi.org/10.3390/ma12121933