Evaluating the Effects of KCl on Thermal Behavior and Reaction Kinetics of Medium Density Fiberboard Pyrolysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Material Samples
2.2. TG-FTIR
2.3. Kinetic Reaction Scheme
2.4. Optimization Technique—Differential Evolution Algorithm
3. Results and Discussion
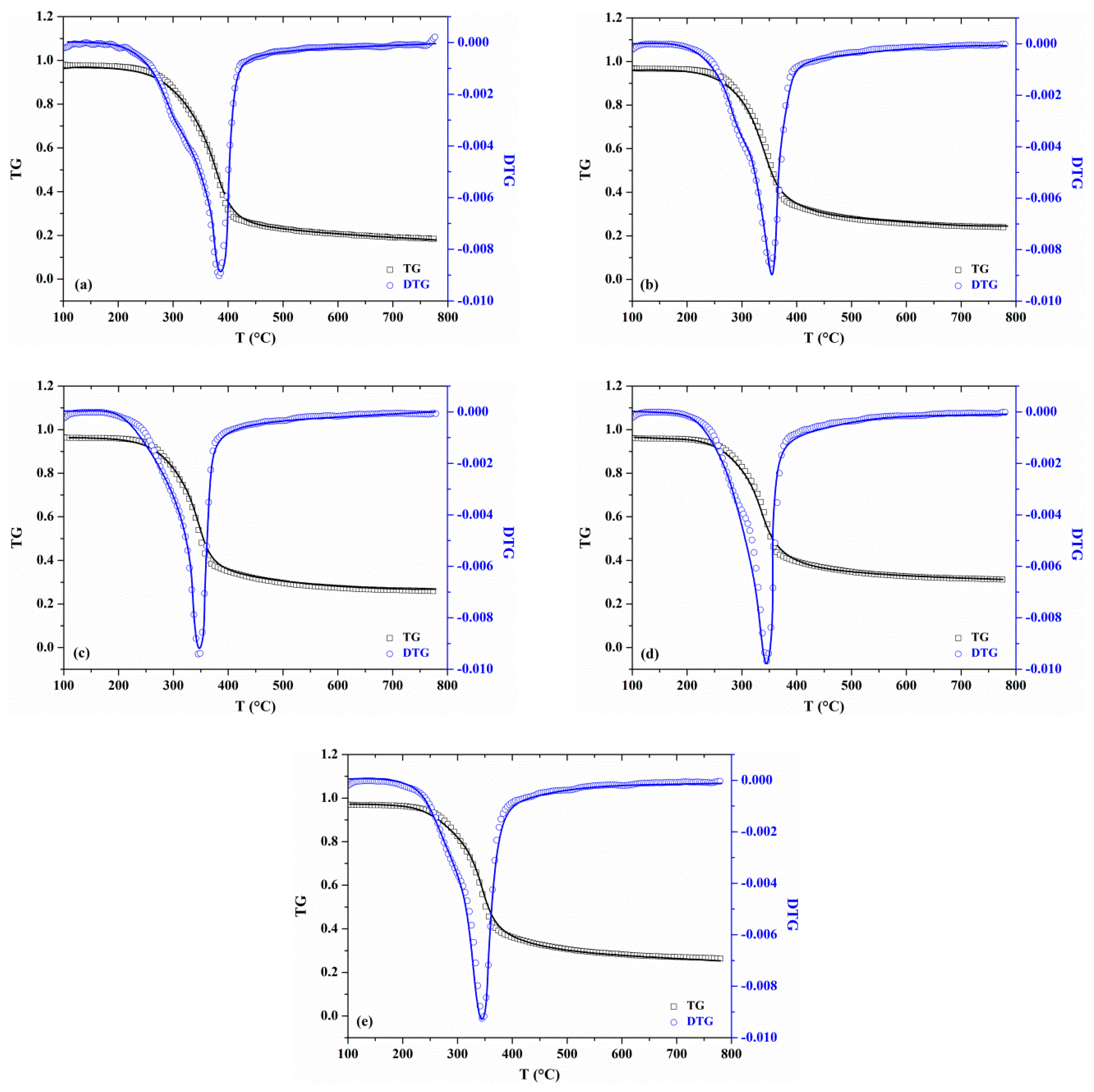
3.1. Thermogravimetric Analysis
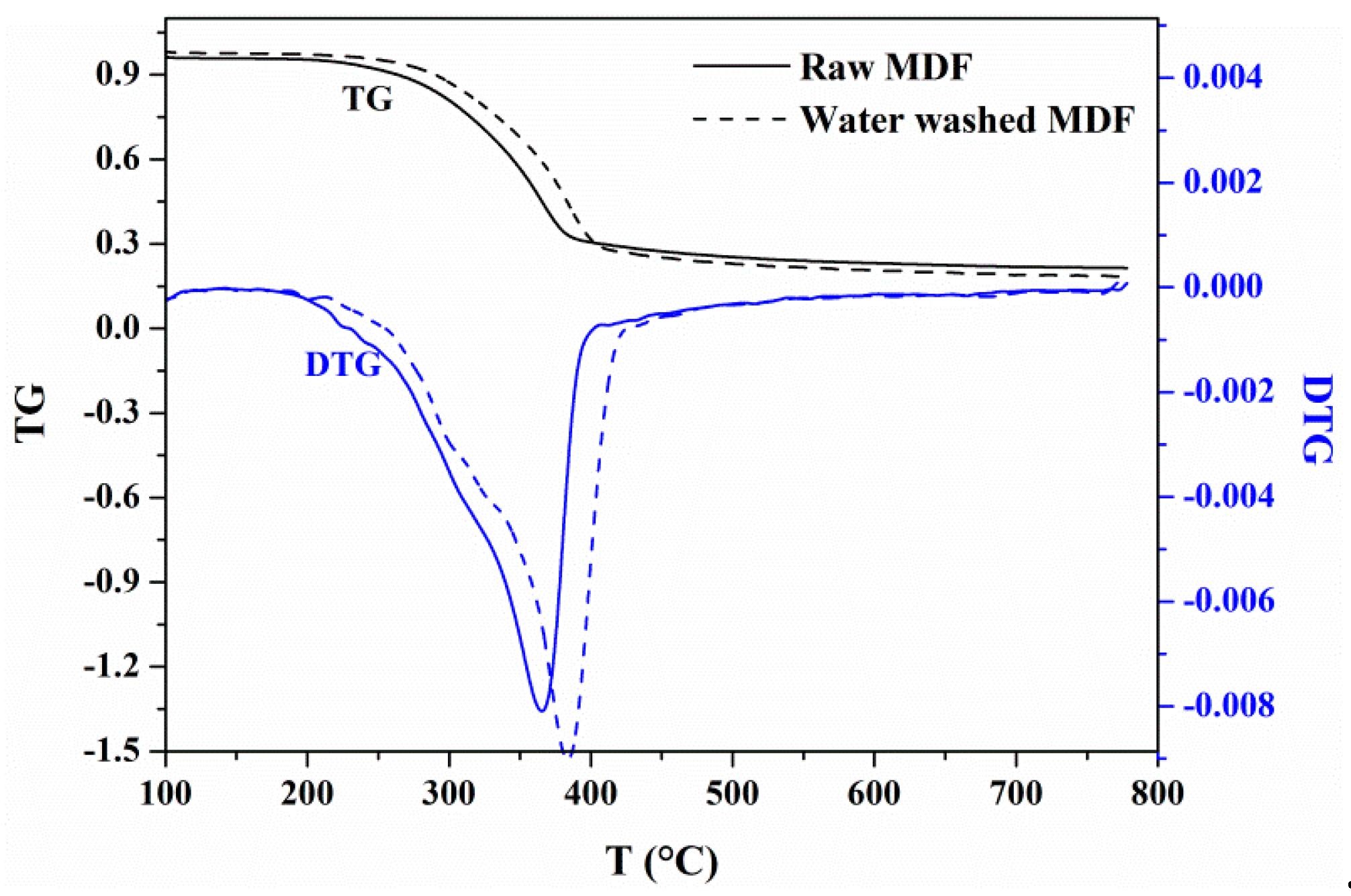
3.1.1. Effects of Water Washing on Weight Loss
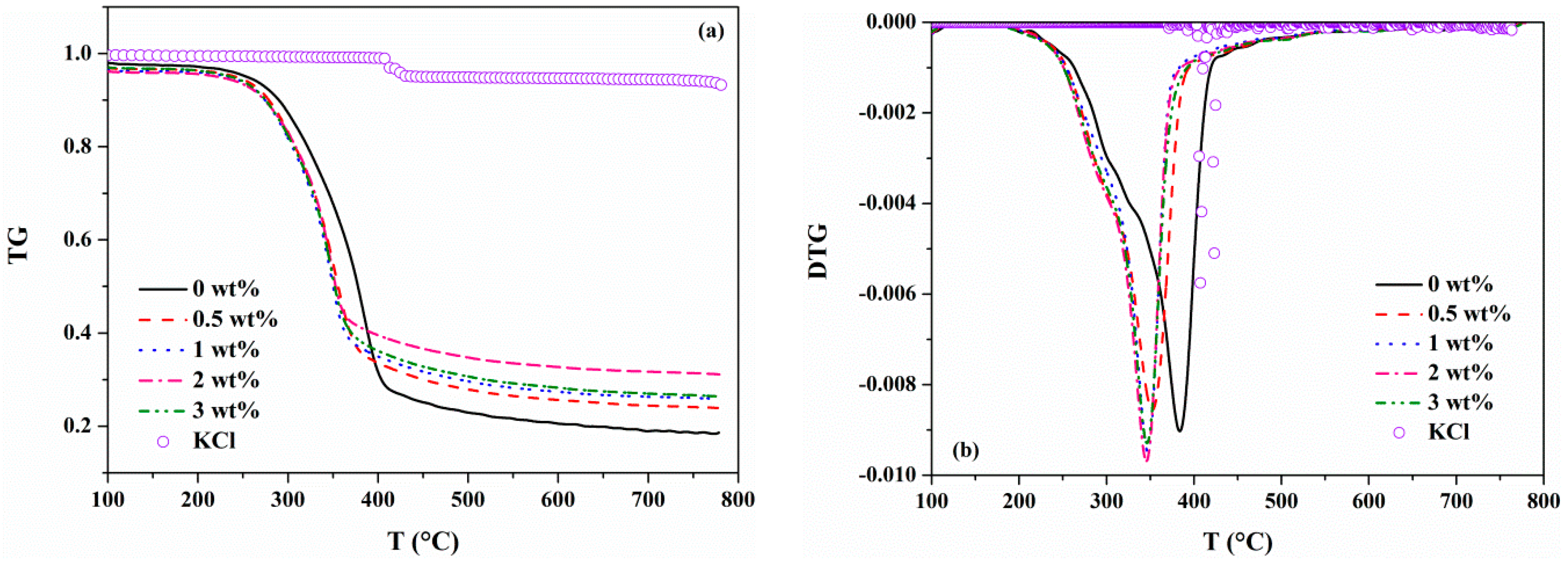
3.1.2. Effects of KCl on the Weight Loss Profile
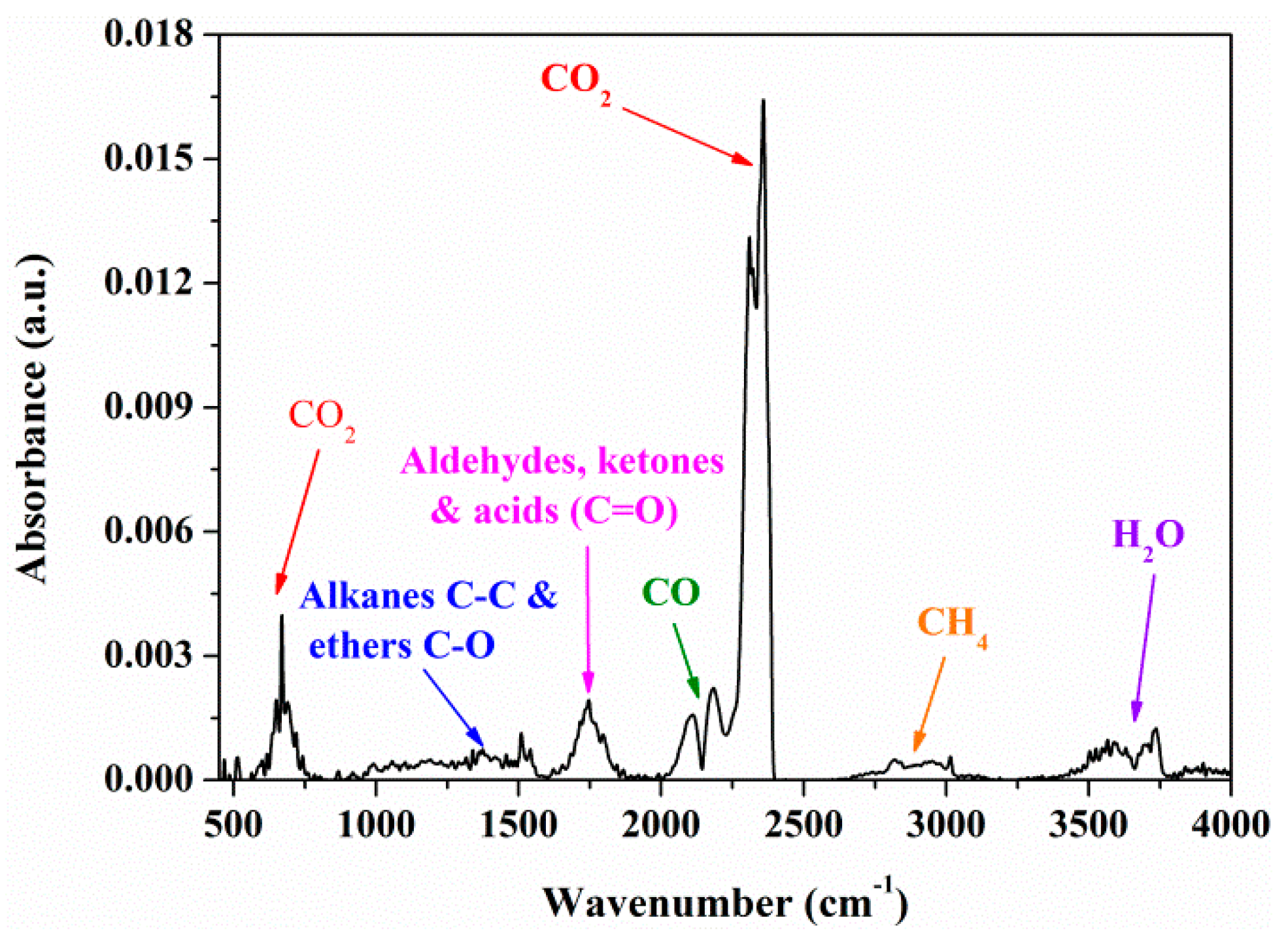
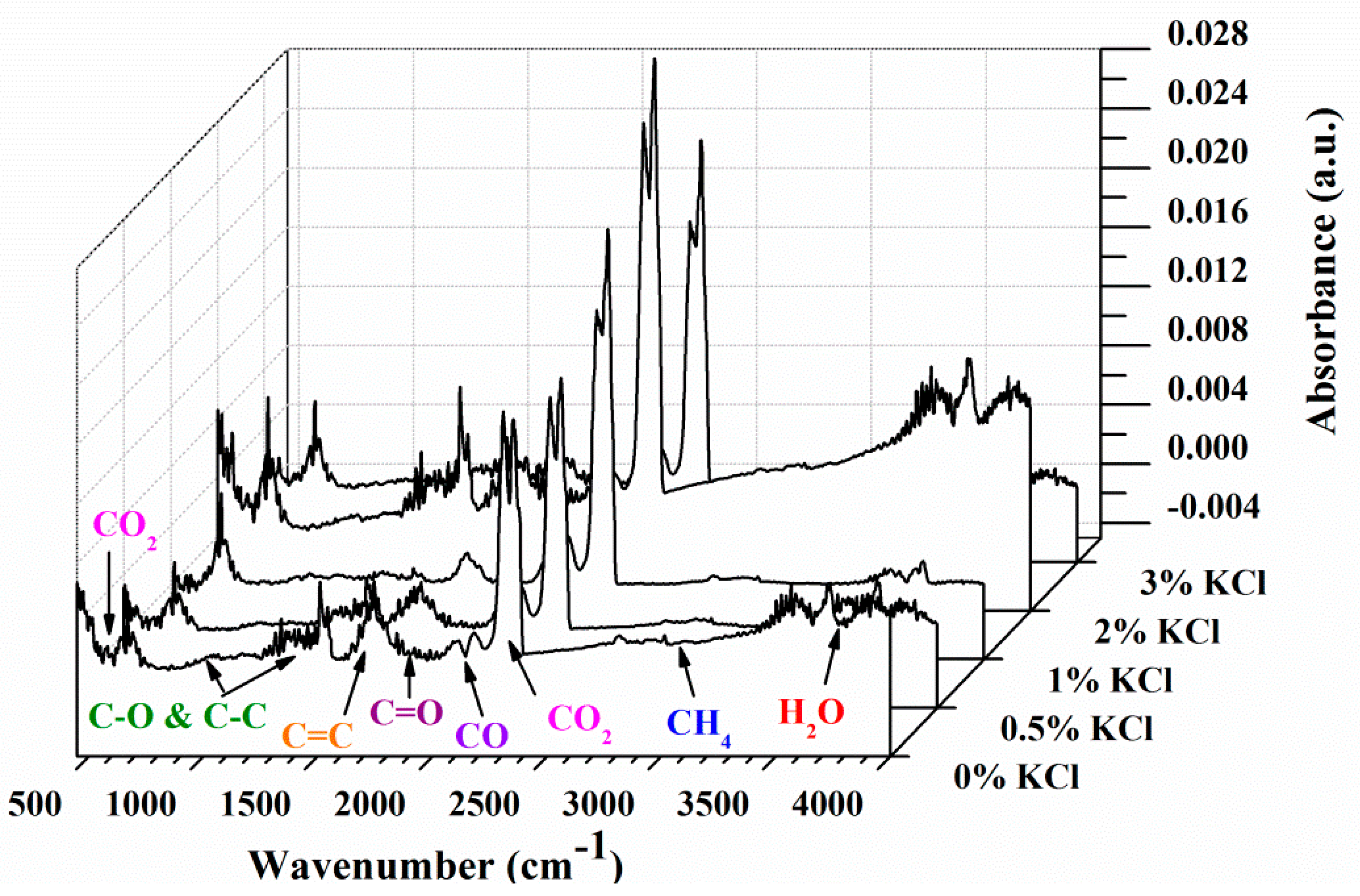
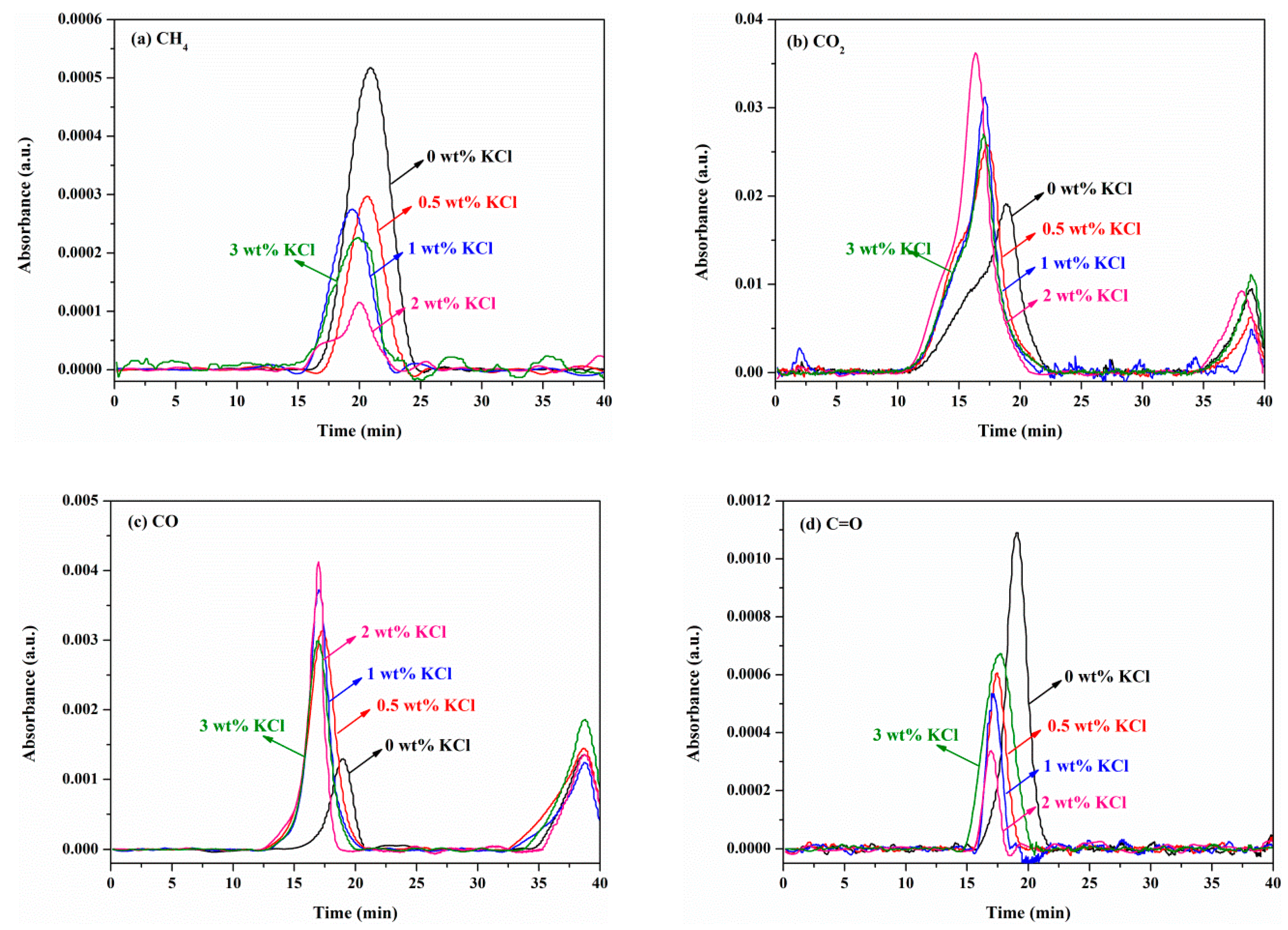
3.2. FTIR Analysis
3.3. Kinetic Analysis
3.3.1. Model Parameters Optimization
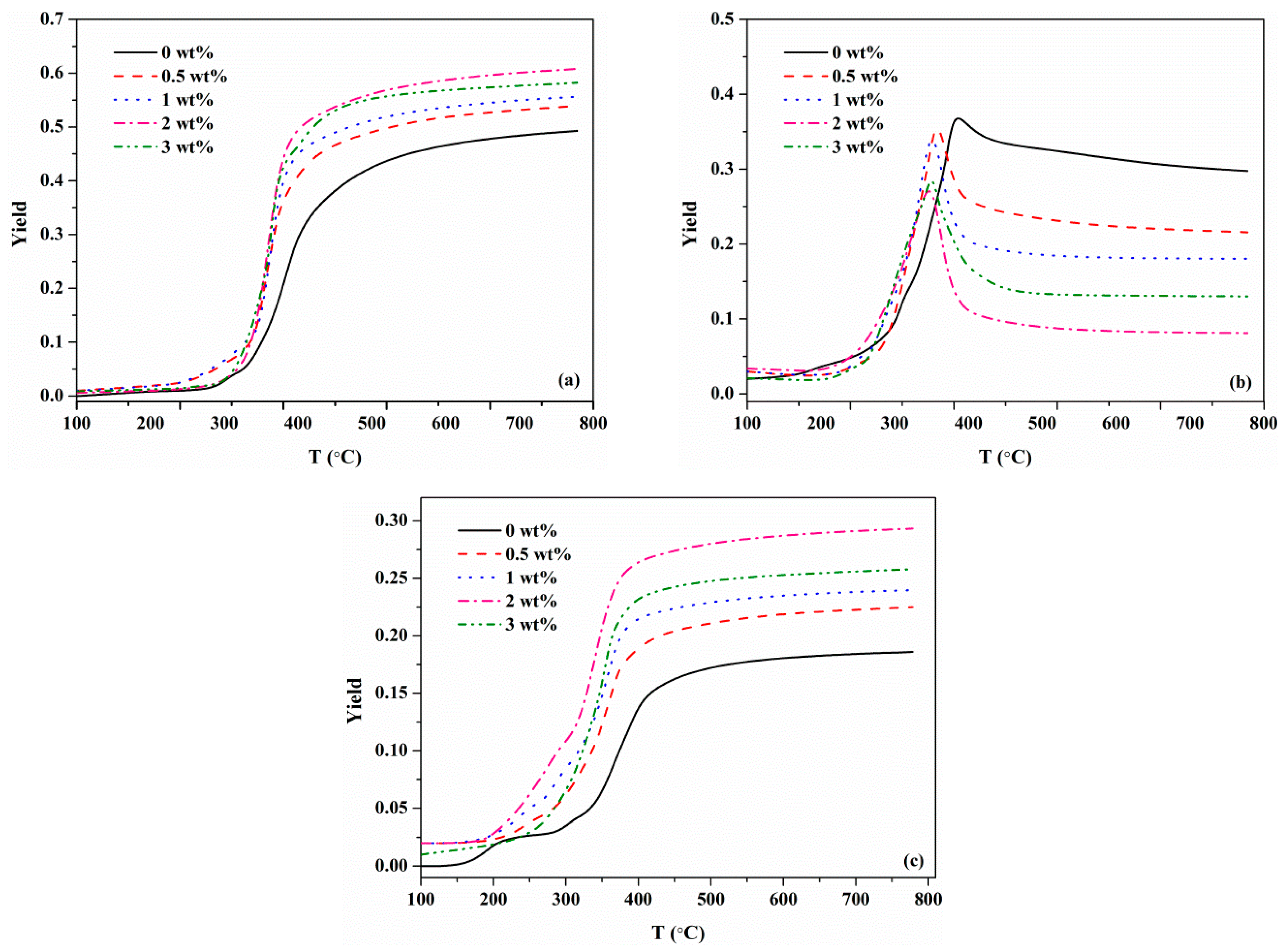
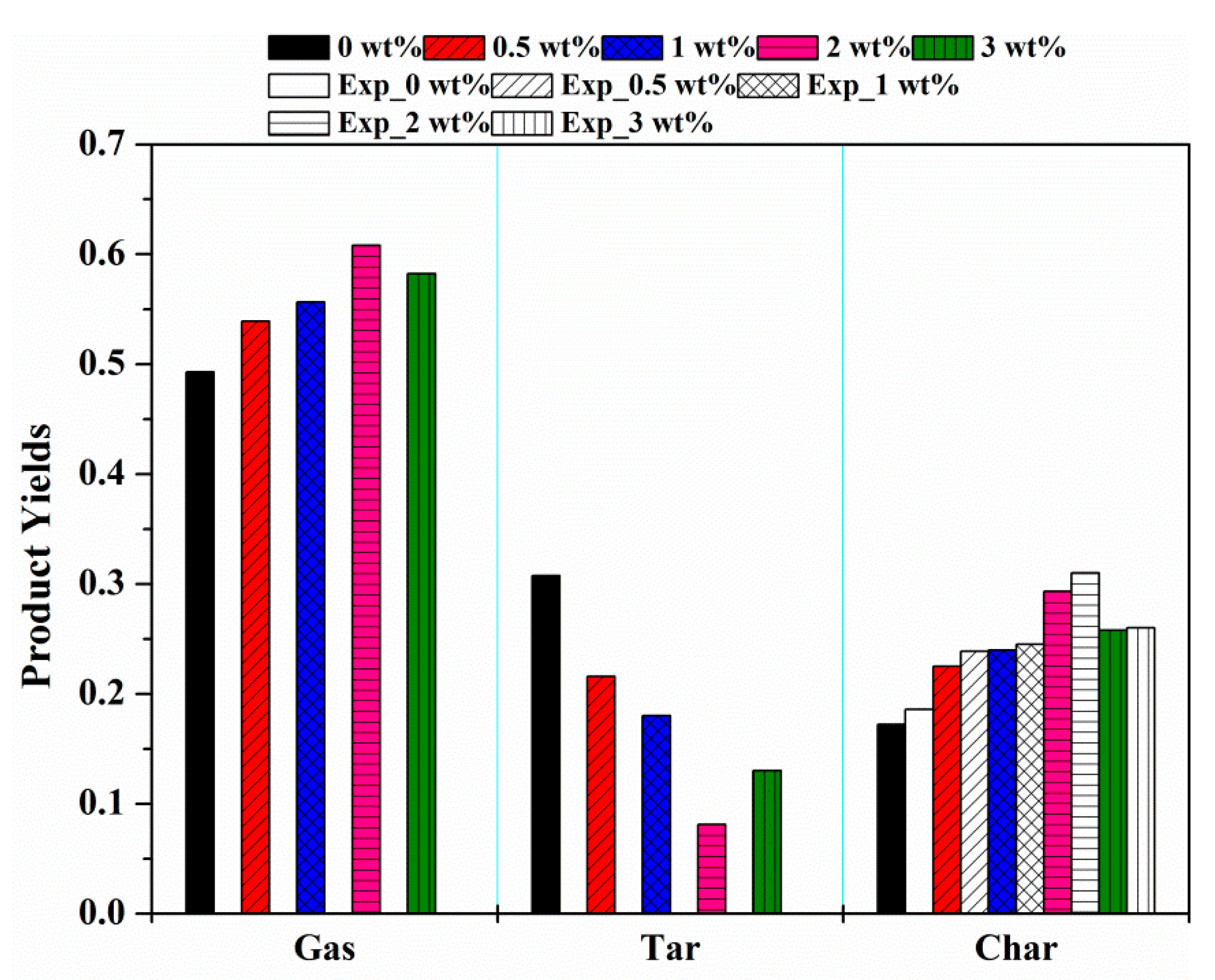
3.3.2. Effects of KCl on MDF Components and Pyrolysis Products
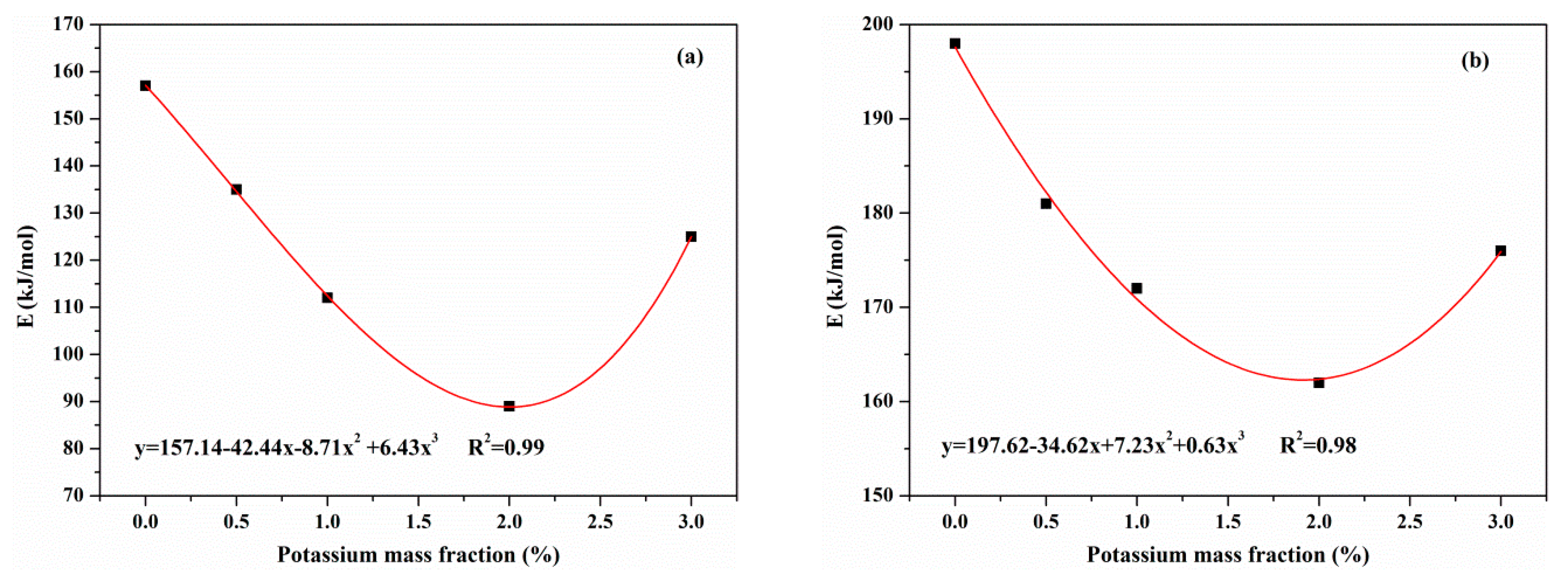
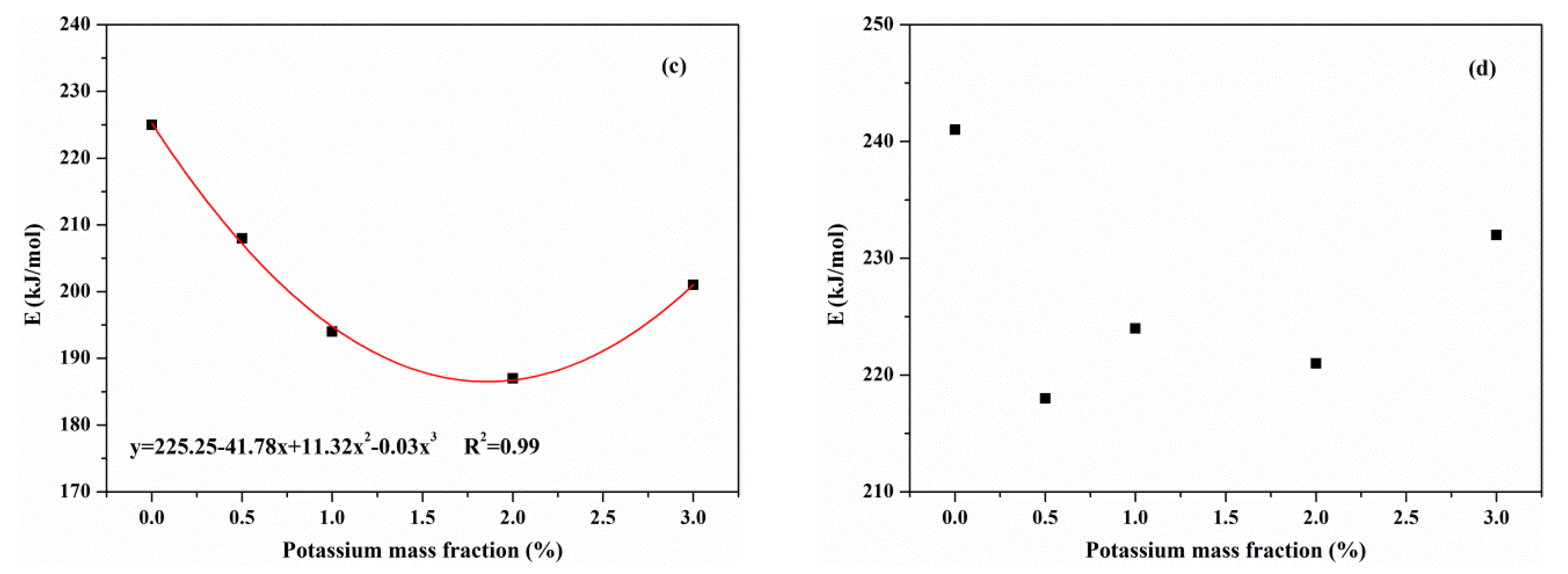
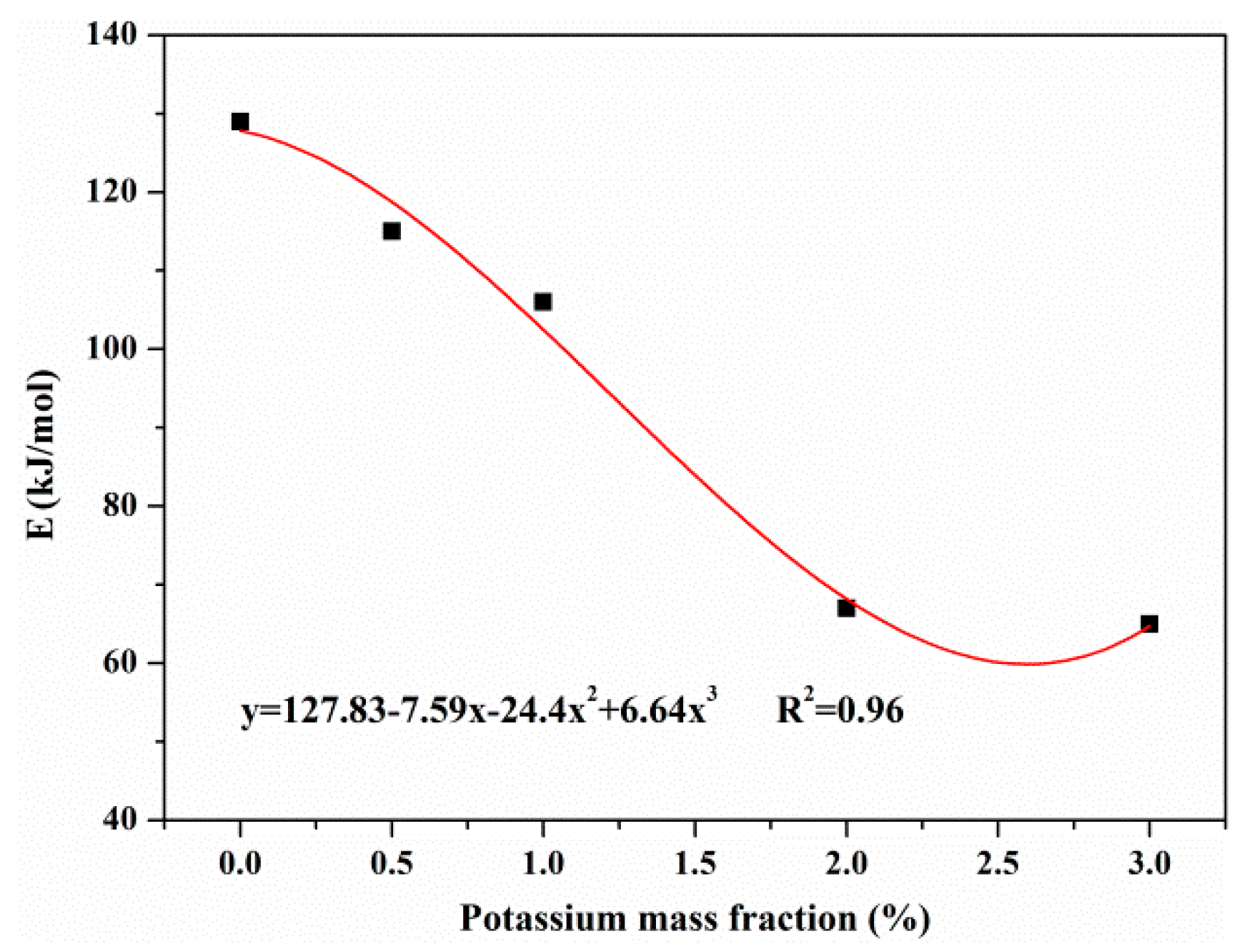
3.3.3. Effects of KCl on Activation Energies
4. Conclusions
- (1)
- The presence of KCl shifted the primary pyrolysis stage towards lower temperatures. As the addition of KCl increased, the characteristic initial temperature showed little change while the final temperature decreased. The temperature range of the primary pyrolysis process was narrowed by nearly 24−36 °C. The mass loss rate of the whole pyrolysis process reached its peak in advance with the concentration of KCl.
- (2)
- KCl affected the release of pyrolysis gaseous products in terms of amounts instead of composition. KCl inhibited the formation of CH4 and C=O functional groups while promoting the formation of CO2 and CO.
- (3)
- KCl changed the initial degradation temperature for each component of MDF. With the increasing concentration of KCl, the initial degradation temperature of resin, hemicellulose and cellulose dropped. For lignin, the effect of KCl on the initial degradation temperature was relatively weak.
- (4)
- Char and gas yields increased with the increasing concentration of KCl, whereas the tar yield was reduced.
- (5)
- KCl had an effect on the activation energies of reactions during the pyrolysis process. In the primary pyrolysis stage, the activation energies of resin, hemicellulose and cellulose decreased with the concentration of KCl. Aside from this, KCl had little effect on the activation energy of lignin. In the secondary charring reaction, KCl reduced the activation energy of tar. The catalytic effect of KCl on MDF pyrolysis manifested in the combination of the primary pyrolysis and secondary charring reaction.
- (6)
- It is reasonable to take 2 wt% and 2.5 wt% as the optimal concentrations of KCl for the catalytic effects on MDF pyrolysis in primary pyrolysis and the secondary charring reaction, respectively.
Author Contributions
Funding
Conflicts of Interest
References
- FAO. Food and Agriculture Organization of the United Nations. Forestry Production and Trade. Available online: http://www.fao.org/faostat/en/#data/FO (accessed on 16 September 2018).
- Jahromi, H.; Agblevor, F.A. Hydrodeoxygenation of aqueous-phase catalytic pyrolysis oil to liquid hydrocarbons using multifunctional nickel catalyst. Ind. Eng. Chem. Res. 2018, 57, 13257–13268. [Google Scholar] [CrossRef]
- Ozsin, G.; Putun, A.E. TGA/MS/FT-IR study for kinetic evaluation and evolved gas analysis of a biomass/PVC co-pyrolysis process. Energy Convers. Manag. 2019, 182, 143–153. [Google Scholar] [CrossRef]
- Sipra, A.T.; Gao, N.; Sarwar, H. Municipal solid waste (MSW) pyrolysis for bio-fuel production: A review of effects of MSW components and catalysts. Fuel Process. Technol. 2018, 175, 131–147. [Google Scholar] [CrossRef]
- Niu, B.; Chen, Z.; Xu, Z. Application of pyrolysis to recycling organics from waste tantalum capacitors. J. Hazard. Mater. 2017, 335, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Jiang, Y.; Wang, L. Thermal decomposition of rape straw: Pyrolysis modeling and kinetic study via particle swarm optimization. Energy Convers. Manag. 2017, 146, 124–133. [Google Scholar] [CrossRef]
- Anca-Couce, A. Reaction mechanisms and multi-scale modelling of lignocellulosic biomass pyrolysis. Prog. Energy Combust. Sci. 2016, 53, 41–79. [Google Scholar] [CrossRef]
- Feng, D.; Zhang, Y.; Zhao, Y.; Sun, S. Catalytic effects of ion-exchangeable K+ and Ca2+ on rice husk pyrolysis behavior and its gas-liquid-solid product properties. Energy 2018, 152, 166–177. [Google Scholar] [CrossRef]
- Raveendran, K.; Ganesh, A.; Khilar, K.C. Pyrolysis characteristics of biomass and biomass components. Fuel 1996, 75, 987–998. [Google Scholar] [CrossRef]
- Perander, M.; DeMartini, N.; Brink, A.; Kramb, J.; Karlstrom, O.; Hemming, J.; Moilanen, A.; Konttinen, J.; Hupa, M. Catalytic effect of Ca and K on CO2 gasification of spruce wood char. Fuel 2015, 150, 464–472. [Google Scholar] [CrossRef]
- Scott, D.S.; Piskorz, J.; Radlein, D. Liquid products from the continuous flash pyrolysis of biomass. Ind. Eng. Chem. Proc. Des. Dev. 1985, 24, 581–588. [Google Scholar] [CrossRef]
- Nowakowski, D.J.; Jones, J.M.; Brydson, R.M.D.; Ross, A.B. Potassium catalysis in the pyrolysis behaviour of short rotation willow coppice. Fuel 2007, 86, 2389–2402. [Google Scholar] [CrossRef]
- Shimada, N.; Kawamoto, H.; Saka, S. Different action of alkali/alkaline earth metal chlorides on cellulose pyrolysis. J. Anal. Appl. Pyrolysis 2008, 81, 80–87. [Google Scholar] [CrossRef]
- Pan, W.P.; Richards, G.N. Influence of metal ions on volatile products of pyrolysis of wood. J. Anal. Appl. Pyrolysis 1989, 16, 117–126. [Google Scholar] [CrossRef]
- Patwardhan, P.R.; Satrio, J.; Brown, R.; Shanks, B. Influence of inorganic salts on the primary pyrolysis products of cellulose. Bioresour. Technol. 2010, 101, 4646–4655. [Google Scholar] [CrossRef] [PubMed]
- Trendewicz, A.; Evans, R.; Dutta, A.; Sykes, R.; Carpenter, D.; Braun, R. Evaluating the effect of potassium on cellulose pyrolysis reaction kinetics. Biomass Bioenergy 2015, 74, 15–25. [Google Scholar] [CrossRef]
- Olsson, J.G.; Jaglid, U.; Pettersson, J.B.C.; Hald, P. Alkali metal emission during pyrolysis of biomass. Energy Fuel 1997, 11, 779–784. [Google Scholar] [CrossRef]
- Shafizadeh, F.; Wolfrom, M.L.; Tipson, R.S. Pyrolysis and combustion of cellulosic materials. Adv. Carbohydr. Chem. 1968, 23, 419–474. [Google Scholar]
- Vassilev, S.V.; Baxter, D.; Andersen, L.K.; Vassileva, C.G. An overview of the composition and application of biomass ash. Part 1. Phase-mineral and chemical composition and classification. Fuel 2013, 105, 40–76. [Google Scholar] [CrossRef]
- Zhao, Y.; Cheng, G.; Long, F.; Liu, L.; Dong, C.; Wang, X.; Zhao, J. Analysis and prediction of corrosion of refractory materials by potassium during biomass combustion-thermodynamic study. Materials 2018, 11, 2584. [Google Scholar] [CrossRef]
- Pan, L.; Jiang, Y.; Wang, L.; Xu, W. Kinetic study on the pyrolysis of medium density fiberboard: Effects of secondary charring reactions. Energies 2018, 11, 2481. [Google Scholar] [CrossRef]
- Ferreiro, A.I.; Rabaçal, M.; Costa, M.; Giudicianni, P.; Grottola, C.M.; Ragucci, R. Modeling the impact of the presence of KCl on the slow pyrolysis of cellulose. Fuel 2018, 215, 57–65. [Google Scholar] [CrossRef]
- Tortosa Masia, A.A.; Buhre, B.J.P.; Gupta, R.P.; Wall, T.F. Characterising ash of biomass and waste. Fuel Process. Technol. 2007, 88, 1071–1081. [Google Scholar] [CrossRef]
- Storn, R. On the usage of differential evolution for function optimization. In Proceedings of the 1996 Biennial Conference of the North American Fuzzy Information Processing Society, Berkeley, CA, USA, 19–22 June 1996; IEEE Press: Piscataway, NJ, USA, 1996; pp. 519–523, ISBN 0780332261. [Google Scholar]
- Storn, R.; Price, K. Differential evolution—A Simple and efficient heuristic for global optimization over continuous spaces. J. Glob. Optim. 1997, 11, 341–359. [Google Scholar] [CrossRef]
- Biagini, E.; Barontini, F.; Tognotti, L. Devolatilization of biomass fuels and biomass components studied by TG/FTIR Technique. Ind. Eng. Chem. Res. 2006, 45, 4486–4493. [Google Scholar] [CrossRef]
- Leng, E.; Wang, Y.; Gong, X.; Zhang, B.; Zhang, Y.; Xu, M. Effect of KCl and CaCl2 loading on the formation of reaction intermediates during cellulose fast pyrolysis. Proc. Combust. Inst. 2017, 36, 2263–2270. [Google Scholar] [CrossRef]
- Gargiulo, V.; Giudicianni, P.; Alfe, M.; Ragucci, R. Influence of possible interactions between biomass organic components and alkali metal ions on steam assisted pyrolysis: A case study on Arundodonax. J. Anal. Appl. Pyrolysis 2015, 112, 244–252. [Google Scholar] [CrossRef]
- Jensen, A.; Dam-Johansen, K.; Wojtowicz, M.A.; Serio, M.A. TG-FTIR study of the influence of potassium chloride on wheat straw pyrolysis. Energy Fuels 1998, 12, 929–938. [Google Scholar] [CrossRef]
- Deng, S.; Wang, X.; Zhang, J.; Liu, Z.; Mikulcic, H.; Vujanovic, M.; Tan, H.; Duic, N. A kinetic study on the catalysis of KCl, K2SO4, and K2CO3 during oxy-biomass combustion. J. Environ. Manag. 2018, 218, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Hao, Q.L.; Li, B.L.; Liu, L.; Zhang, Z.B.; Dou, B.J.; Wang, C. Effect of potassium on pyrolysis of rice husk and its components. J. Fuel Chem. Technol. 2015, 43, 34–41. [Google Scholar] [CrossRef]
- Yang, H.; Rong, Y.; Chen, H.; Dong, H.L.; Zheng, C. Characteristics of hemicellulose, cellulose and lignin pyrolysis. Fuel 2007, 86, 1781–1788. [Google Scholar] [CrossRef]
- Quan, C.; Ma, Z.; Gao, N.; He, C. Pyrolysis and combustion characteristics of corncob hydrolysis residue. J. Anal. Appl. Pyrolysis 2018, 130, 72–78. [Google Scholar] [CrossRef]
- Ding, W.; Zhang, X.; Zhao, B.; Zhou, W.; Xu, A.; Chen, L.; Sun, L.; Yang, S.; Guan, H.; Xie, X.; et al. TG-FTIR and thermodynamic analysis of the herb residue pyrolysis with in-situ CO2 capture using CaO catalyst. J. Anal. Appl. Pyrolysis 2018, 134, 389–394. [Google Scholar] [CrossRef]
- Liang, F.; Wang, R.; Hongzhong, X.; Yang, X.; Zhang, T.; Hu, W.; Mi, B.; Liu, Z. Investigating pyrolysis characteristics of moso bamboo through TG-FTIR and Py-GC/MS. Bioresour. Technol. 2018, 256, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yang, L.; Ma, C.; Zhang, Y. Thermal behavior of sweet potato starch by non-isothermal thermogravimetric analysis. Materials 2019, 12, 699. [Google Scholar] [CrossRef] [PubMed]
- Vecchio Ciprioti, S.; Tuffi, R.; Dell’Era, A.; Dal Poggetto, F.; Bollino, F. Thermal behavior and structural study of SiO(2)/Poly(epsilon-caprolactone) hybrids synthesized via sol-gel method. Materials 2018, 11, 275. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wei, R.; Wang, X.; He, J.; Wang, J. Pyrolysis and combustion of polyvinyl chloride (PVC) sheath for new and aged cables via thermogravimetric analysis-fourier transform infrared (TG-FTIR) and calorimeter. Materials 2018, 11, 1997. [Google Scholar] [CrossRef] [PubMed]
- Tao, L.; Zhao, G.-B.; Qian, J.; Qin, Y.-K. TG–FTIR characterization of pyrolysis of waste mixtures of paint and tar slag. J. Hazard. Mater. 2010, 175, 754–761. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Lu, S.; Zhang, Y.; Lo, S. Pyrolysis study of waste cable hose with thermogravimetry/Fourier transform infrared/mass spectrometry analysis. Energy Convers. Manag. 2017, 153, 83–92. [Google Scholar] [CrossRef]

| Proximate Analysis (wt%) | Ultimate Analysis (wt%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Moisture | Ash | Volatiles | Fixed Carbon | error | C | H | Oa | N | S | error |
| 8.28 | 4.69 | 81.2 | 5.83 | 0.98% | 44.96 | 6.26 | 44.87 | 3.35 | 0.56 | 1.48% |
| Samples | Ash Chemical Compositions (wt%) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ca | K | S | Si | Mg | P | Al | Fe | Na | Cl | Mean Error | |
| Raw MDF | 22.87 | 10.66 | 3.45 | 3.11 | 2.52 | 1.53 | 1.09 | 0.91 | 0.62 | 0.53 | 1.82% |
| Water washed MDF | 18.01 | 1.25 | 1.64 | 6.41 | 1.73 | 0.48 | 2.62 | 1.29 | 1.19 | 0.20 | 1.46% |
| Sample | Ti (°C) | Tmax (°C) | Tf (°C) | tmax (min) |
|---|---|---|---|---|
| raw MDF | 182.6 | 365.3 | 395.1 | 18.6 |
| 0 wt% KCl (water washed MDF) | 192.5 | 383.5 | 416.5 | 19.5 |
| 0.5 wt% KCl | 192.4 | 352.0 | 391.5 | 17.9 |
| 1 wt% KCl | 191.4 | 347.1 | 383.7 | 17.7 |
| 2 wt% KCl | 191.3 | 345.3 | 379.9 | 17.6 |
| 3 wt% KCl | 188.9 | 345.7 | 387.1 | 17.6 |
| Wavenumbers, cm−1 | Chemical Bond | Vibrations | Compounds |
|---|---|---|---|
| 4000−3400 | O−H | stretching | H2O |
| 3050−2650 | C−H | stretching | CH4 |
| 2400−2240 | C=O | stretching | CO2 |
| 2230−2000 | C−O | stretching | CO |
| 1880−1620 | C=O | stretching | aldehydes, ketones, acids |
| 1600−1420 | C=C | stretching | aromatics |
| 1300−950 | C−O, C−C | stretching | alkanes & ethers |
| 750−560 | C=O | bending | CO2 |
| Component | Parameter | Range | 0 wt% KCl | 0.5 wt% KCl | 1 wt% KCl | 2 wt% KCl | 3 wt% KCl |
|---|---|---|---|---|---|---|---|
| Resin | lnAr (ln s−1) | (2.4, 47.6) | 21.82 | 24.34 | 20.9 | 24.12 | 22 |
| Er (kJ/mol) | (6.9, 200) | 157.5 | 136.56 | 112 | 89 | 125.3 | |
| nr | (0.1, 8) | 2.05 | 2.07 | 2.2 | 2 | 2.5 | |
| Wr,o (%) | (1, 20) | 9.80 | 9.75 | 9.7 | 9.6 | 9.51 | |
| φr/σr (%) | (5, 95) | 31.8 | 42 | 23.65 | 58.32 | 53.21 | |
| γr (%) | (5, 95) | 7.0 | 8.78 | 21.36 | 16.85 | 8.7 | |
| Hemicellulose | lnAh (ln s−1) | (3.1, 62.4) | 32 | 33.04 | 39.95 | 29.96 | 33.9 |
| Eh (kJ/mol) | (11.3, 225.3) | 198 | 181 | 172.35 | 162.56 | 176 | |
| nh | (0.1, 8) | 1.9 | 1.7 | 1.89 | 1.78 | 1.49 | |
| Wh,o (%) | (1.8, 36) | 20.23 | 20.22 | 20.03 | 19.83 | 19.64 | |
| φh/σh (%) | (5, 95) | 10.4 | 16.7 | 21.46 | 11.36 | 17.58 | |
| γh (%) | (5, 95) | 49.5 | 48.3 | 61.1 | 50.32 | 30.36 | |
| Cellulose | lnAc (ln s−1) | (3.9, 78.2) | 35 | 40.4 | 38.06 | 35.69 | 39.4 |
| Ec (kJ/mol) | (16, 319.4) | 225 | 208.65 | 194.63 | 187.53 | 201.64 | |
| nc | (0.1, 8) | 2.5 | 1.9 | 2.26 | 2.41 | 2.42 | |
| Wc,o (%) | (4.8, 96) | 42.9 | 42.68 | 42.48 | 42.06 | 41.65 | |
| φc/σc (%) | (5, 95) | 12.3 | 13.4 | 12.94 | 30.58 | 25.64 | |
| γc (%) | (5, 95) | 42.9 | 27.2 | 43.85 | 52.25 | 28.33 | |
| Lignin | lnAl (ln s−1) | (5, 100.7) | 39 | 35 | 39.01 | 42.24 | 38.83 |
| El (kJ/mol) | (22.6, 451.8) | 241 | 218 | 224.36 | 221.43 | 232.96 | |
| nl | (0.1, 8) | 1.4 | 2 | 2.24 | 1.82 | 2.27 | |
| Wl,o (%) | 27 | 26.89 | 26.73 | 26.47 | 26.21 | ||
| φl /σl (%) | (5, 95) | 3.4 | 8.1 | 7.32 | 12.25 | 5.39 | |
| γl (%) | (5, 95) | 69.2 | 78 | 73.25 | 63.02 | 38.65 | |
| Tar | lnAt (ln s−1) | (1.8, 35.8) | 33.4 | 33.85 | 33.8 | 33.93 | 33.5 |
| Et (kJ/mol) | (12.4, 248) | 129.3 | 115.85 | 106.65 | 67.74 | 65.69 | |
| nt | (0.1, 8) | 2.87 | 2.29 | 1.44 | 1.57 | 1.46 | |
| θ (%) | (5, 95) | 14.1 | 18.2 | 15.20 | 5.65 | 23.02 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, L.; Jiang, Y. Evaluating the Effects of KCl on Thermal Behavior and Reaction Kinetics of Medium Density Fiberboard Pyrolysis. Materials 2019, 12, 1826. https://doi.org/10.3390/ma12111826
Pan L, Jiang Y. Evaluating the Effects of KCl on Thermal Behavior and Reaction Kinetics of Medium Density Fiberboard Pyrolysis. Materials. 2019; 12(11):1826. https://doi.org/10.3390/ma12111826
Chicago/Turabian StylePan, Longwei, and Yong Jiang. 2019. "Evaluating the Effects of KCl on Thermal Behavior and Reaction Kinetics of Medium Density Fiberboard Pyrolysis" Materials 12, no. 11: 1826. https://doi.org/10.3390/ma12111826
APA StylePan, L., & Jiang, Y. (2019). Evaluating the Effects of KCl on Thermal Behavior and Reaction Kinetics of Medium Density Fiberboard Pyrolysis. Materials, 12(11), 1826. https://doi.org/10.3390/ma12111826




