1. Introduction
Complete oxidation of carbon-based fossil fuel in industrial chemistry and the automotive industry based on internal combustion engines is one of the biggest scientific challenges, aiming to reduce polluting gases being released into the atmosphere [
1] with short-term toxic effects on people’s health and long-term effects on the environment in terms of global warming [
2], as well as efficient energy generation. Historically, in this direction of minimizing the toxic emissions to the atmosphere, a major breakthrough for automotive and petrochemical industry was the invention of the catalytic converter, which further oxidized fossil fuel combustion products on the surface of high specific area metal oxides modified with platinum group metals [
3]. However, the efficiency of these converters is low during the cold-start regime of the engine, when the temperature of the exhaust gases from the vehicle is below 200 °C and thus unable to heat the catalyst enough to trigger the catalytic oxidation of those gases [
1]. During this critical operation time, the diesel oxidation catalysts can emit up to 80% of CO gas, for example [
4].
Under these considerations, where the aerobic oxidation of these fuels requires high activation energy for the reactions of the C(sp
3)–H bonds with the atmosphere air [
5], incomplete combustion of the fuel will release into the atmosphere not only CO
2 and water but also CO, hydrocarbon (HCs) (like soot or particle matter with a size below 2.5 µm) and NO
x, as it is in the case of diesel fuel oxidation [
6]. From this point of view, the utilization of natural gas as a fuel was considered a better and apparently cleaner alternative to both gasoline and diesel fuel, due to the increased efficiency of the natural gas motors by about 10–20% and an apparently softer impact on the environment [
7,
8].
These relatively improved properties of natural gas fuel come from the fact that it is mainly composed of methane and its combustion generates less NO
x. On the other hand, the CH
4 molecule is chemically more stable than the CO molecule and therefore higher temperatures are required for complete catalytic oxidation in the catalytic converter [
9]. The complete combustion of methane will provide CO
2 and H
2O as reaction products. However, in the case of the incomplete oxidation of natural gas fuel, the release into the atmosphere of the remaining unoxidized CH
4 will counterbalance the above benefits, due to the high global warming potential (it is a measure of how much energy the emissions of 1 ton of a gas will absorb over a given period of time, relative to the emissions of 1 ton of carbon dioxide) of methane, which is about 21 to 36 higher (over 100 years) than that of CO
2 considered as reference and this result is explained by the higher amount of energy absorbed by CH
4 molecules with respect to the CO
2 molecule [
10,
11].
The present catalysts used for the oxidation of the mentioned fossil fuel consist of palladium as an active phase supported on a high specific area metal oxide [
12]. In such catalytic converters, the key metal oxide supporting the noble metal catalyst is CeO
2 due to its oxygen store and release capabilities, multiple oxidation states of the cerium cations and their easy redox reactions (Ce
4+/Ce
3+), which promote oxygen vacancy formation [
13]. Such Pd–CeO
2 catalysts take advantage of the low cost Pd catalyst, high oxidation capability and better sintering resistance with respect to platinum and rhodium-based catalysts [
4]. However, despite the improvements brought to these Pd-ceria based catalysts, there are still issues with complete oxidation of fossil fuel at low temperatures, for both diesel and natural gas, where CO and/or soot and/or CH
4 are released into the atmosphere not only from the vehicles but also from coal mines (up to 1% CH
4 in air) [
14] and from different chemical processes in the petrochemical industry.
In the last two decades, intensive research has been pursued in the field of development of novel catalysts able to improve the efficiency and the reliability of the existing noble metal-based catalysts [
15], as well as to create novel generations of noble metal-free catalysts, starting from the “traditional” ceria as a host, to which a great number of oxides of transition metals were added as possible dopants, as one of the starting approaches [
16,
17]. However, using pure bulk ceria as a catalyst was not a solution due to sintering processes, which occurred at higher temperatures, which further triggered the increase of the crystallite size and the reduction of the specific surface area, of oxygen vacancies number, as well as of catalytic properties [
18]. Therefore, ceria-based composites, obtained by mixing with different metal oxides, became a clear option for the further improvement of these materials.
As a result of an extensive comparative study of the physical, morphological, structural and catalytical properties of noble metal-free ceria doped with cations of transition metals like manganese (Mn), zirconium (Zr), lanthanum (La), praseodymium (Pr), hafnium (Hf) and iron (Fe), very useful results were obtained, which allowed performance of a ranking of the catalytic properties of the ceria based composites with respect to low temperature combustion of carbon monoxide (CO) and soot. It was thus demonstrated that CeO
2–MnO
x composites have shown the best catalytic behaviour; by showing that the temperature at which 50% of the CO or soot concentration in air was oxidized (T
50) was lowest for CeO
2–MnO
x in comparison with all the other mixed oxides. (T
50 for CO was equal to 117 °C and T
50 for soot was equal to 396 °C) [
16].
These results for manganese-doped ceria were explained in terms of the lowest binding energy of lattice oxygen due to the major differences in the electronegativity of the Ce host and doping Mn cations, a maximum lattice strain enhancing the oxygen diffusion from bulk to the surface of the catalyst, as well as the multiple oxidation states of Mn in ceria.
An important experimental study by Venkataswamy et al. showed the catalytic activity during CO oxidation of the CeO
2–MnO
x composite supported on pure alumina [
19]. It was emphasized that for CO oxidation on CeO
2–MnO
x composite calcinated at 500 °C and deposited on alumina substrate, the T
50 was about 67 °C, which was much lower with respect to the T
50 of pure ceria deposited on alumina or pure manganese oxide deposited on alumina, which were 304 °C and 374 °C, respectively. This result was excellent proof of the synergy of the two oxides in the composite formed between them. This study has also shown that the increase of calcination temperature of the alumina supported CeO
2–MnO
x composite from 500 °C up to 800 °C has increased the T
50 value up to 177 °C and such a result could be correlated to the structural changes in both the catalyst and pure alumina substrate.
Recently, an improved catalytic property of the mesoporous CeO
2–MnO
x composite on low temperature oxidation of hydrocarbons has been demonstrated by Zhang et al. [
5]. It was shown by these authors that dissolving and gelling of the two metal oxides precursors in the presence of a ionic liquid template followed by its extraction at 200 °C and thermal treatment at 500 °C has created a solid solution of CeO
2–MnO
x with a high concentration of active oxygen, a lower energy of vacancy formation and high oxygen vacancy migration as an explanation of low temperature and selective oxidation of hydrocarbons (from cyclohexane to cyclohexanone), with T
50 in the range of 100–120 °C and carbon monoxide with T
50 at about 50 °C. In the same direction, Wang et al. have shown the role of the hydrothermal synthesis and optimization of the molar ratio of the Ce and Mn cations on the low temperature oxidation of benzene by the solid solution of CeO
2–MnO
x, with T
50 at about 260 °C [
20].
As can be inferred from the above studies, despite the high interest in the use of natural gas fuel instead of gasoline and Diesel fuel, due to its fewer toxic oxidation by-products, there are quite a few papers on the catalytic properties of the CeO
2–MnO
x composite in the catalytic combustion of methane, as a main component of natural gas [
21,
22]. Thus, Shi et al. have shown the sensibility of the catalytic properties of CeO
2–MnO
x composite to the effect of supporting substrate and the precursors used for the synthesis.
It is the purpose of our work to perform extended research on the catalytic performances of CeO2–MnOx composite supported on lanthanum-modified alumina substrate as a function of three syntheses methods like coprecipitation, incipient impregnation and citrate based-sol gel, for methane combustion. For this study, for the incipient impregnation method we have used, for the first time, a commercial, thermally stable 4 wt.% La2O3–Al2O3 support for the distribution of the CeO2–MnOx catalyst on the outer surfaces of support particles, while for the rest of the experiments, the precursors of the ceria and manganese oxide were mixed and precipitated simultaneously together with the precursors of the lanthanum oxide and alumina. Therefore, the role of bulk versus surface modification of alumina with Mn and Ce will be discussed.
Finally, for the evaluation of the catalytic properties of the above catalysts as a function of these process parameters, we have devised a self-heated catalytic micro-converter, consisting of an alumina strand above which a resistive platinum meander was deposited by thick film technology. Above this future heater, we have deposited the CeO2–MnOx catalyst supported on 4 wt.% La2O3–Al2O3. This way, we were able to mimic the catalytic combustion process of methane in a real catalytic converter. By monitoring the voltage and current through the platinum heater in the absence and presence of methane, the thermal effects of catalytic combustion were indicated in the electric current variation, thanks to the positive temperature coefficient (TCR) of platinum. Thus, we were able to evaluate and finally discriminate between different synthetized catalysts.
2. Materials and Methods
2.1. Materials Preparation
All chemicals used in the preparation of catalytic materials come from commercial sources and have been used without the need for an additional purification step. The following chemical substances were used: cerium (III) nitrate hexahydrate 99.999%, manganese (II) nitrate hexahydrate 99.99%, lanthanum (III) nitrate hexahydrate 99.999%, aluminium nitrate nonahydrate 99.997%, citric acid ≥99.5% and a 25% solution of ammonia (to adjust the pH), all of which being purchased from Merck. As a support, commercially available Sasol Puralox TH100, an alumina containing 4 wt.% La2O3 with a specific surface area of 150 m2/g, was also used. In order to ensure the highest possible purity of the final materials, MiliQ deionized water was used in all the preparation steps.
The coprecipitation, incipient impregnation and citrate-based sol-gel were the three types of preparation methods employed in this study to obtain final catalysts with the CeO2:MnOx:(4 wt.% La2O3–Al2O3) molar ratio based on oxides of 7:3:10. The obtained materials were denoted as 7Ce3Mn/10LaAl_pp, 7Ce3Mn/10LaAl_imp and 7Ce3Mn/10LaAl_cit, with the front number corresponding to the molar ratio, while the termination representing the preparation procedures employed in these cases: coprecipitation, incipient impregnation and citrate-based sol-gel method, respectively.
2.1.1. Coprecipitation Method
For the case of the coprecipitation method, in a first step, individual solutions were prepared containing the calculated amounts of metal precursors according to the molar ratio of 7:3:10. The required solutions were mixed and the pH of the resulting mixtures was adjusted to 9 by the addition of ammonia solution (percent concentration of 25%). The mixture was stirred overnight to ensure maturation of the material. The resulting solid material was filtered on a Buchner funnel and washed with deionized water. Drying of the materials was done in an oven at 60 °C for 1h (under vacuum), then the oven temperature was raised to 160 °C and left overnight (under vacuum). Finally, the materials were calcined at 500 °C for 6 h in air with a heating rate of 5 °C/min A 4 wt.% La2O3–Al2O3 support was also prepared by coprecipitation method as well, denoted further by LaAl_pp.
2.1.2. Impregnation Method
For the case of incipient impregnation, in a first step the calculated amounts of metal precursors were dissolved in a required volume equal to the porous volume of deionized water necessary to completely wet the support. After dissolution of the precursors, the Puralox TH100 is added and homogenized well with a glass rod. The support was thus completely wetted without leaving solution outside the pores. The drying process plays an important role in the final properties of the catalytic material, special attention has been paid to the next stage. Therefore, the materials were slowly dried in the oven at 60 °C (under vacuum) for 1 h. The oven temperature is then raised to 80 °C (under vacuum) for another hour and was finally left at 160 °C (under vacuum) overnight. In all cases the heating rate was kept at 1 °C/min. Finally, the materials were calcined at 500 °C for 6 h in air with a heating rate of 5 °C/min.
2.1.3. Citrate-Based Sol-Gel Method
Lastly, for the synthesis of the catalysts with the CeO2:MnOx:(4 wt.% La2O3–Al2O3) molar ratio based on oxides of 7:3:10, the citrate-based sol-gel method was used. Thus, in the first step, individual solutions containing the calculated amounts of metal precursors were prepared by dissolving the corresponding inorganic salts in deionized water and stirring at room temperature until homogeneous solutions were obtained. Later on, the citric acid complexing agent was added to each solution in calculated amounts to obtain a 1:2 weight ratio toward metal ions to citric acid but also an excess of 10%. The citrate mixtures were stirred at 50 °C for 2h. The water was evaporated using a rotavapor until gelling of the materials occurred. The obtained materials were dried under vacuum at 60 °C for 1 h and then the temperature was increased to 160 °C and kept overnight. The thermal stabilization process was performed at 500 °C for 6 h in air with a heating ramp of 5 °C/min. A 4 wt.% La2O3–Al2O3 support was also prepared by citrate method as well, denoted further as LaAl_cit.
2.2. Materials Characterization
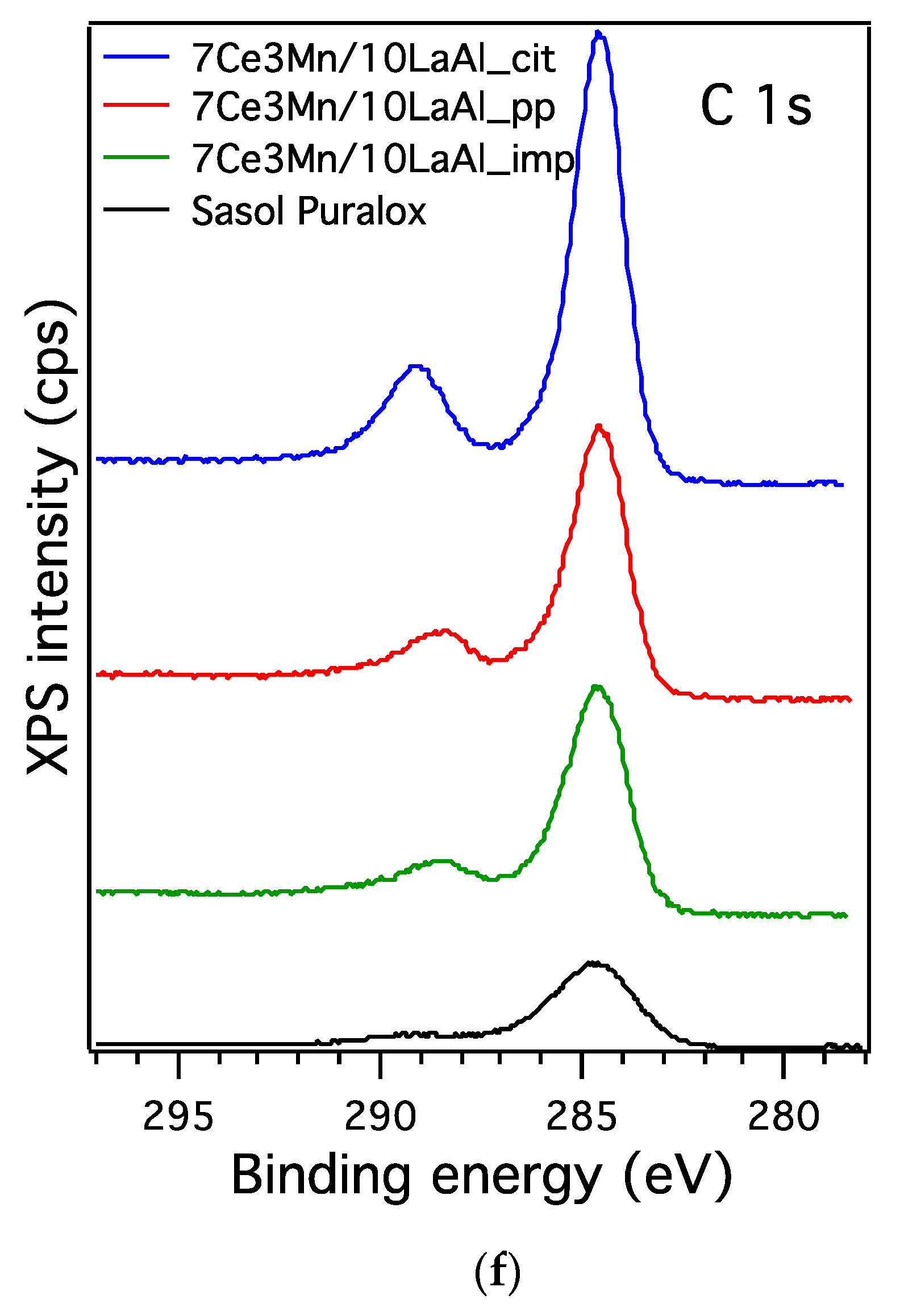
Detailed features of all the samples were observed using a scanning electron microscope type GeminiSEM 500 (Carl Zeiss AG, Oberkochen, Germania) equipped with a Quantax XFlash 6/10 energy-dispersive spectrometry detector from Bruker (Billerica, MA, USA) for elemental analysis. Images of all samples were taken at the same magnification of 10,000 × and acceleration voltage of 1 kV using the InLens detector.
XPS measurements were performed using a Kratos Ultra DLD Setup spectrometer ( Kratos Analytical Ltd., Manchester, UK) using the Al–Kα (1486.74 eV) radiation produced by an X-ray source operating at a total power of 300 W (12.0 kV × 25 mA) and an approx. 1 × 10−7 Pa.
The temperature programmed reduction experiments in hydrogen (H2–TPR) were performed using a Porotec TPDRO 1100 device (Thermo Fisher Scientific Inc., Waltham, MA, USA). Prior to the reduction step, approximately 50 mg of sample was pre-treated for 1 h at 200 °C in a helium gas flow to ensure the surface cleaning, after which it was cooled down to room temperature also in a helium gas flow. Then a 5 vol. % H2–He mixture was passed over the sample with a flow rate of 50 mL/min and the temperature was linearly increased by 10 °C/min to 800 °C. The quantification of the hydrogen consumption during the reduction process was carried out by using the equipped thermal conductivity detector of the TPDRO device.
The X-ray diffraction measurements were performed using a Bruker-AXS D8 Advance diffractometer (Bruker Corporation, Billerica, MA, USA.) equipped with a LynxEye 1D detector and Cu-Kα (0.1541 nm) radiation source and a scintillation counter detector. The diffraction patterns were recorded over a 2θ range of 10–80° with a 0.01° step size and using a counting time of 1s per point. For the identification of the XRD phases present in the samples, the Powder Diffraction File from the International Centre for Diffraction Data (PDF-ICDD) was used.
The surface areas and pore size distribution of the as-prepared materials were determined by N
2 adsorption–desorption isotherms at liquid N
2 temperature (77 K) on a Micromeritics (ASAP 2020) analyser (Micromeritics Instrument Corporation, Norcross, GA, USA). Specific surface area and pore size distribution were calculated by Brunauer–Emmett–Teller (BET) formalism [
23] and Barrett-Joyner-Halenda (BJH) method [
24], respectively, while Langmuir surface area was determined by Langmuir formalism [
25]. In order to efficiently remove the surface adsorbed residues, a degassing step at 150 °C for 4 h was employed.
2.2.1. Preparation of the Self-Heated Micro-Converters
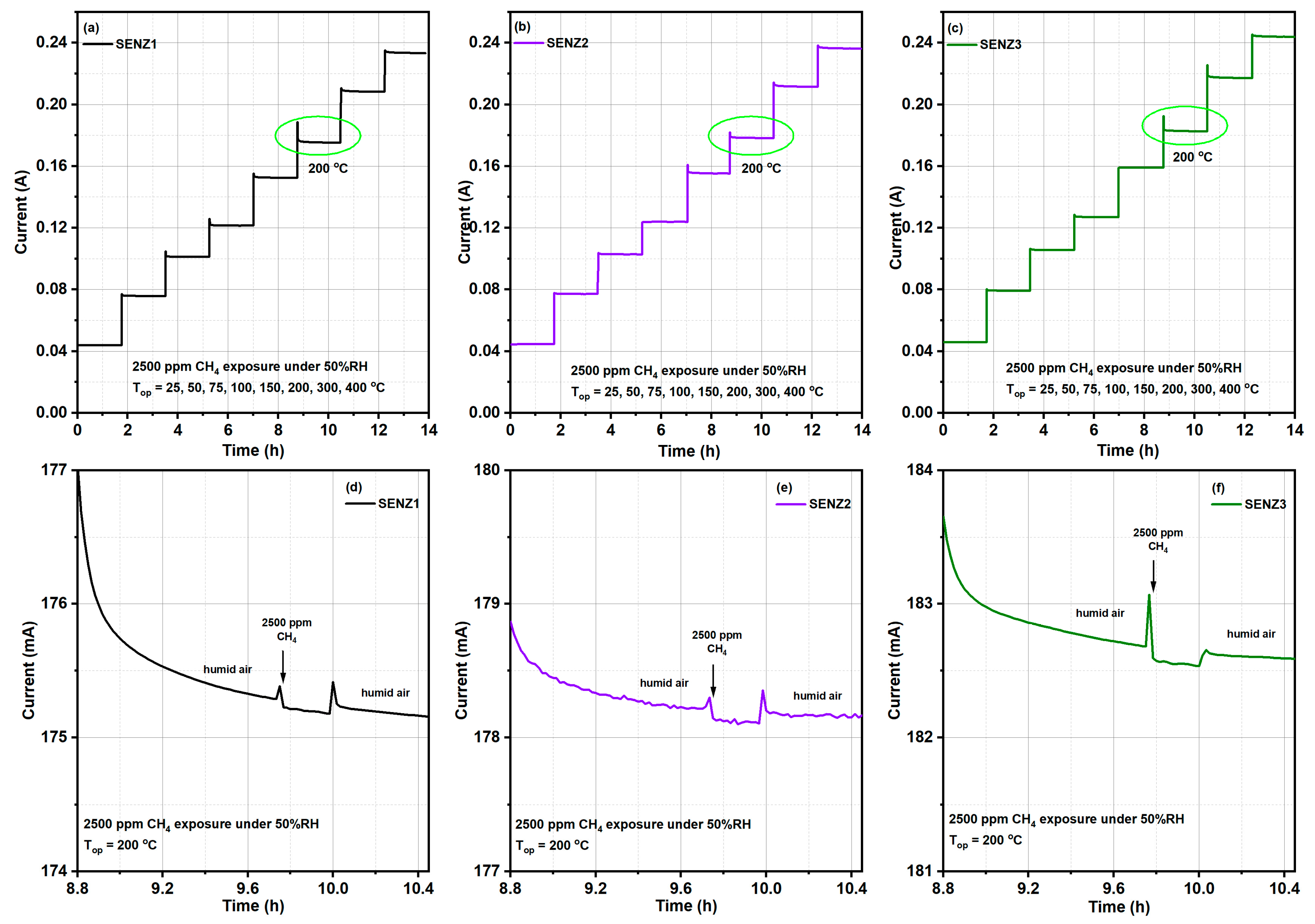
In order to proceed with the catalytic combustion investigations, catalytic micro-converters have been made on alumina substrate above which the catalyst was deposited by a drop coating method. More specific, the as received calcined powders based on CeMn/LaAl have been mixed with 1,2–propanediol as an organic binder and deposited over the platinum heater meander. The obtained catalytic layers were subjected to two heating stages. Within the first stage, the layers were kept at 60 °C for 18 h in order to settle the paste and to ensure optimum adhesion. As for the second heating stage, a programmed oven was used, applying the following protocol: 200–400 °C with 30 min at 50 °C step and 60 min at 450 °C. Consequently, the organic binder is removed leading to a homogeneous and porous catalytic layer formation. The as obtained catalytic micro-converters were labelled as follow: SENZ1 (7Ce3Mn/10LaAl_pp), SENZ2 (7Ce3Mn/10LaAl_cit) and SENZ3 (7Ce3Mn/10LaAl_imp).
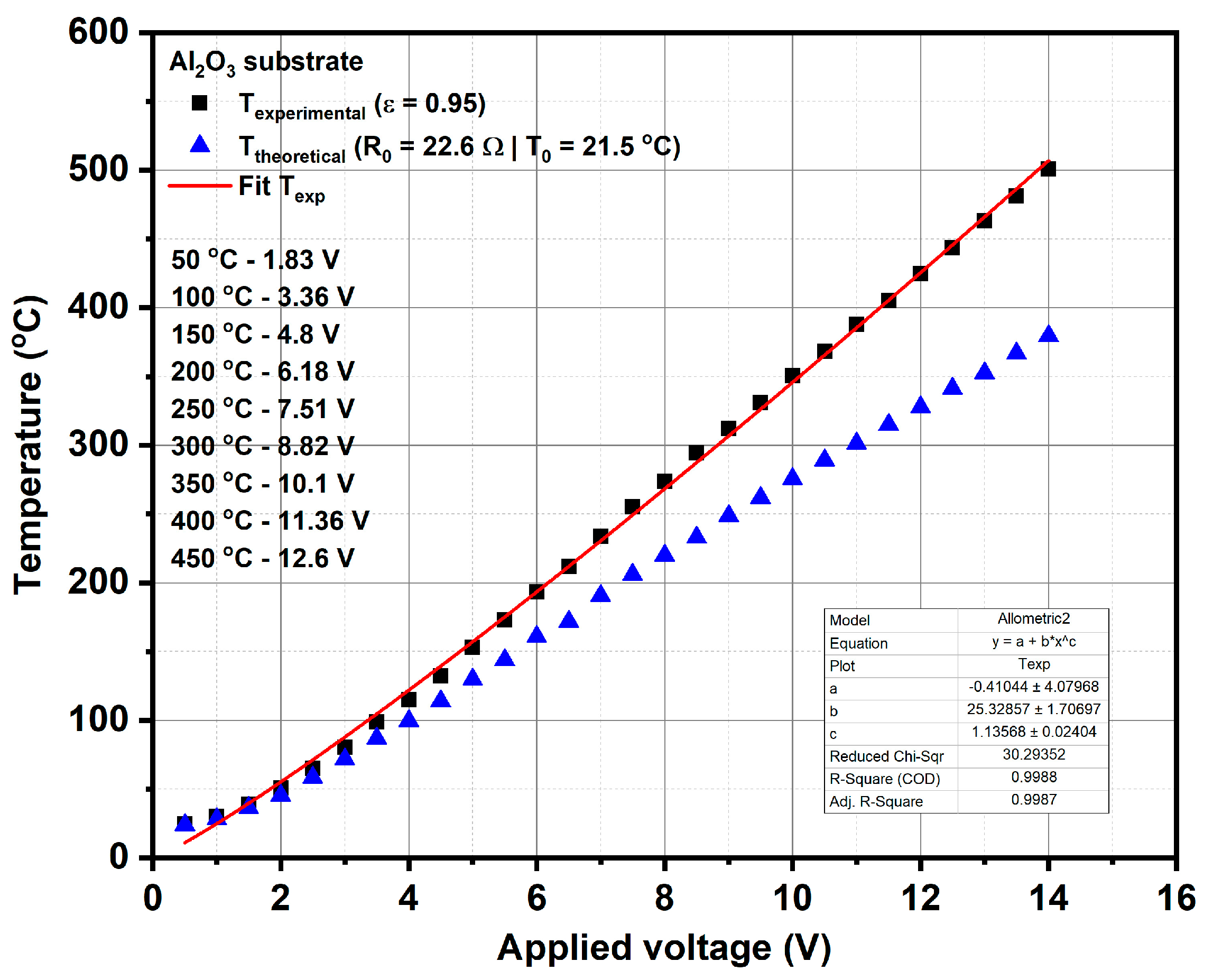
The calibration curve of the temperature of heater meander versus applied voltage was obtained using LumaSense IN-5L Plus pyrometer (LumaSense Technologies GmbH, Frankfurt, Germany) (see
Figure 1), which provided the experimental temperature on the surface of micro-converter by considering a value of emissivity equal to 0.95 for the surface of the platinum meander. The calibration curve drawn with a continuous line, as shown in
Figure 1, was obtained based on the linear fitting of the experimental results obtained by the pyrometer, at different applied voltages.
This calibration procedure is very important for subsequent catalytic combustion evaluations on our micro-converters in the presence of methane, because the operation temperature of these catalytic devices is controlled via the applied voltage on the heater side. This means that when the platinum heater is biased to a certain constant voltage, an electric current will flow through the platinum resistance and a temperature known via a previously-made calibration process will be obtained. In the presence of methane, due to the catalytic combustion of methane on the surface of the catalyst (which is an exothermic reaction), an increase in temperature will occur. A portion of this generated heat will be transferred to the platinum heater, which will increase its electrical resistance due to its +TCR and thus a decrease in the current flowing through the heater will be recorded. Such an electric current decrease (ΔI) through the platinum in the presence of methane in air, for the same applied voltage as in the case of humid air (without any methane added) will provide information about the presence of the catalytic combustion. Obviously, when the applied voltage is low and the temperature of the catalyst is too low, there is no catalytic process and the electric current through the platinum will not decrease even if methane is present at the surface of the catalyst.
2.3. CH4 Combustion Evaluation on the Self-Heated Catalytic Micro-Converter
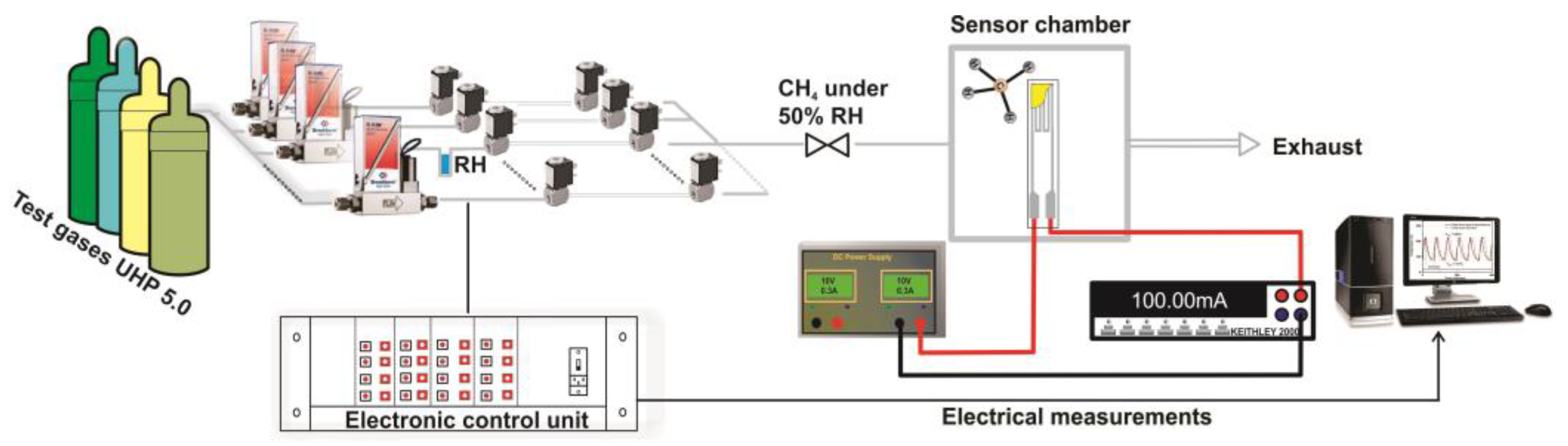
The catalytic properties of different catalytic micro-converters built with different CeMn/LaAl layers have been evaluated using a computer-controlled Gas Mixing System (GMS) consisting of a test chamber with 4 sensor sockets, a Keithley 2000-multimeter, DC regulated power supply source, mass flow controllers, vaporizers, valves and data acquisition cards. A dedicated software was used to control the gas mixture protocol and to record the electrical current changes passing through the heater (see
Figure 2).
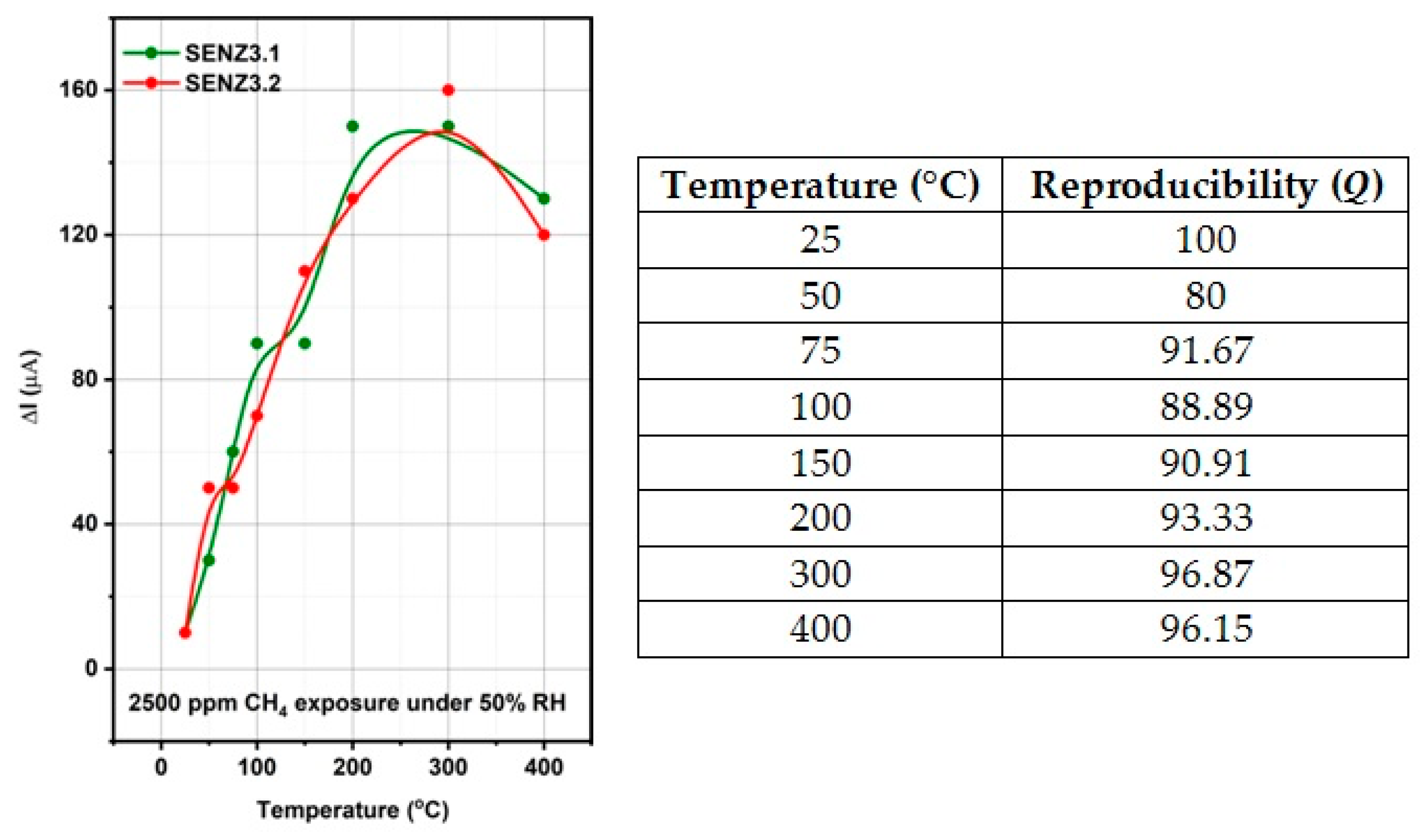
The catalytic micro-converters were exposed to 2500 ppm CH
4 in synthetic air with 50% relative humidity (RH) specific for infield conditions and the flow rate was maintained constant at 200 cm
3/min. The gas sensing performances have been acquired over a wide temperature range from 25 to 400 °C, obtained by applying a sequence of voltages from 1 V up to 11.36 V, on the heater as shown in the inset of
Figure 1, for a period of 14 h. Each applied voltage was maintained for 1.75 h. During each time interval of 1.75 h, the catalytic micro-converter was first exposed to synthetic air with 50% RH for 1 h, followed by applying 2500 ppm of CH
4 in synthetic air with 50% RH for 0.25 h and followed by exposure to the same synthetic air for 0.5 h, while the electric current was monitored and recorded continuously. In case of no catalytic combustion at a certain temperature, no current variation appears during the interval of time when CH
4 is present in the synthetic air.
For the detection of light-off temperature of CH4 combustion, detectable by our specific approach, the experiment started at low applied voltages on the heater of catalytic micro-converter.
4. General Assessment
The catalytic properties of a material, as shown also by our own experimental results, are affected by the preparation step and due to its complexity is probably the most important step in the catalyst manufacture [
37]. There are several factors, like geometry and the redox state of the metal cations that might alter the strength of the oxygen chemical bondings near the catalytic sites, thus affecting the catalytic activity. Also, to increase the rate of a catalytic reaction the catalyst must possess as many catalytic sites exposed to reactants and in this direction a special attention is dedicated to catalyst bulk versus surface properties modifications. Therefore, it is very important to find new ways to improve the preparation strategy to obtain more active catalysts, very well dispersed on a large surface area with enhanced stability.
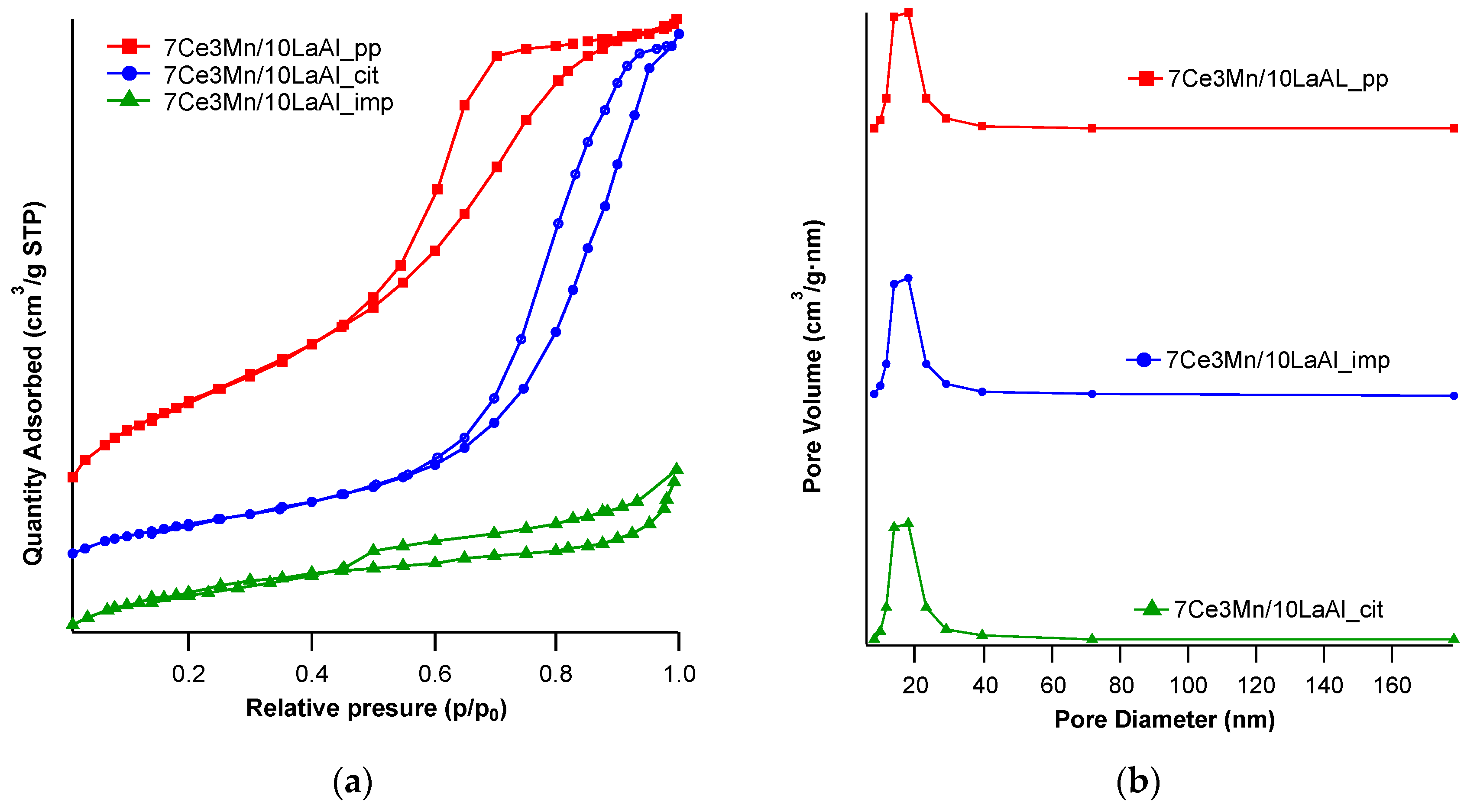
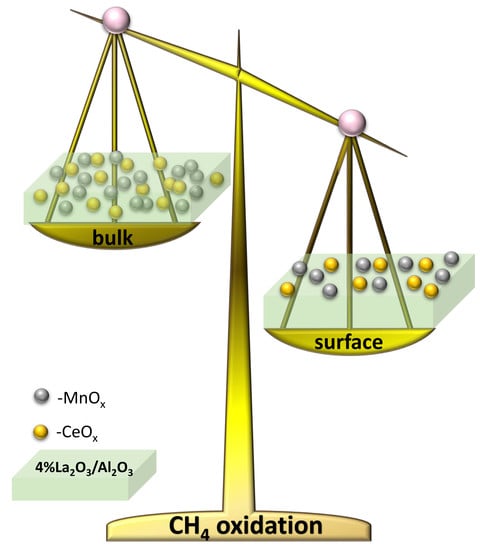
Our different synthesis methods allowed us to investigate the effect of accessible active centre distribution on the surface of the support or intimately mixing of the catalyst at the bulk level with the support, as follows. In case of 7Ce3Mn/10LaAl_pp and 7Ce3Mn/10LaAl_cit catalysts, the precursors of the support were mixed with the precursors of the active species and therefore a bulk mixing between the active species and the support was obtained, while in the case of 7Ce3Mn/10LaAl_imp catalyst the active species were impregnated only on the surface of the commercial support, Puralox and therefore the active species are well dispersed on the surface, preserving an enough surface area (118 m
2/g) with respect to initial value of the support (150 m
2/g), as shown by our BET analysis (see
Table 1).
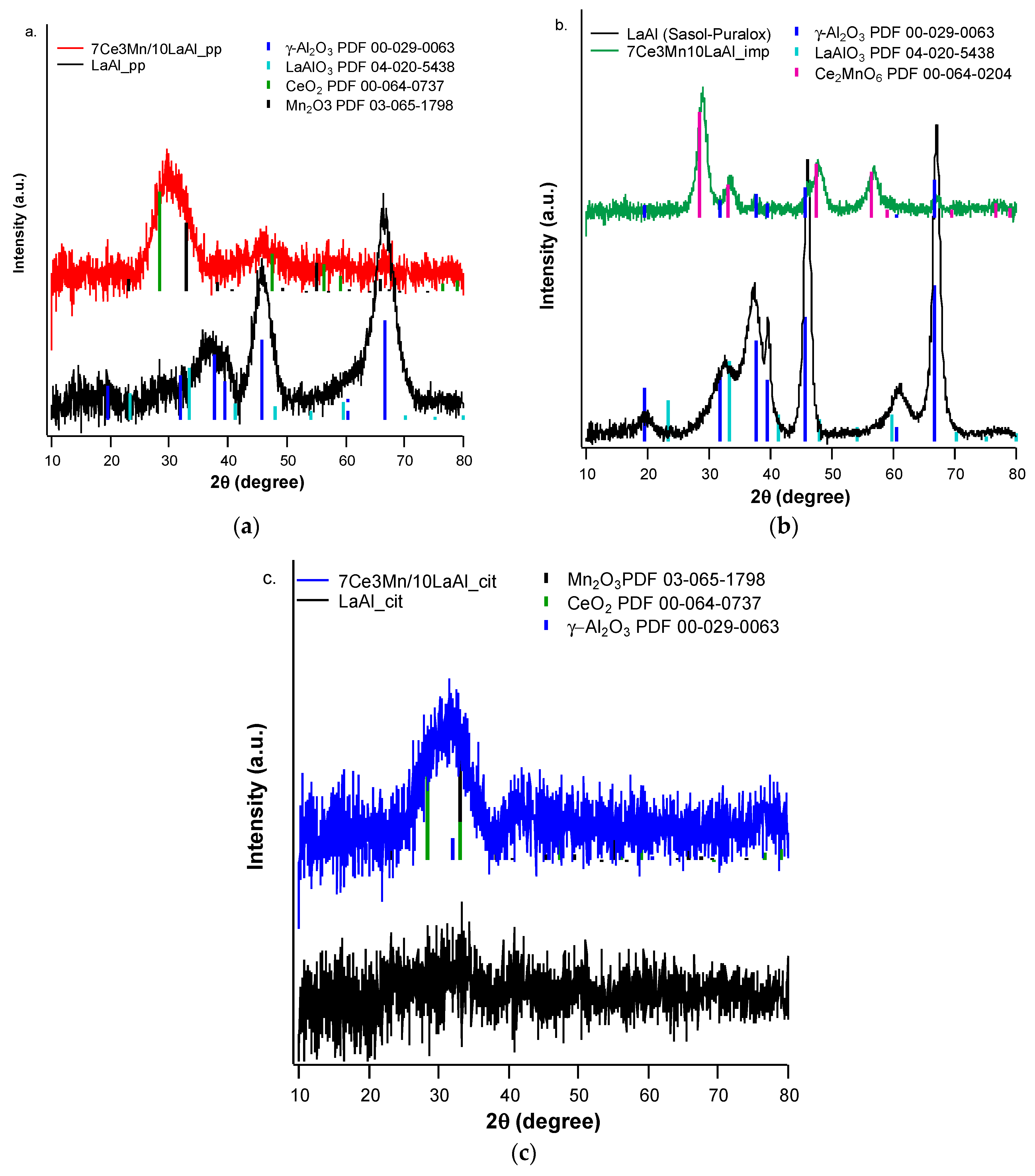
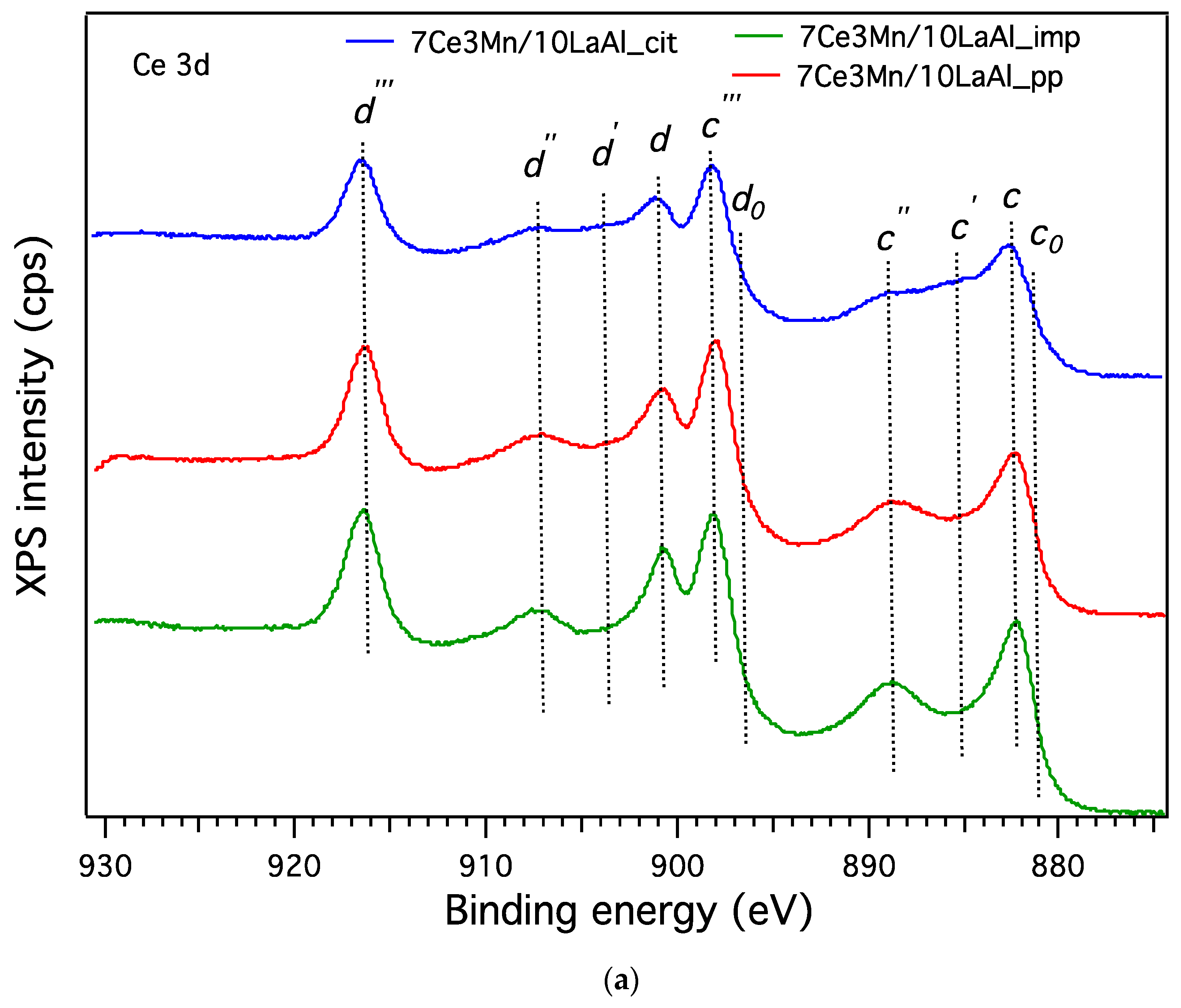
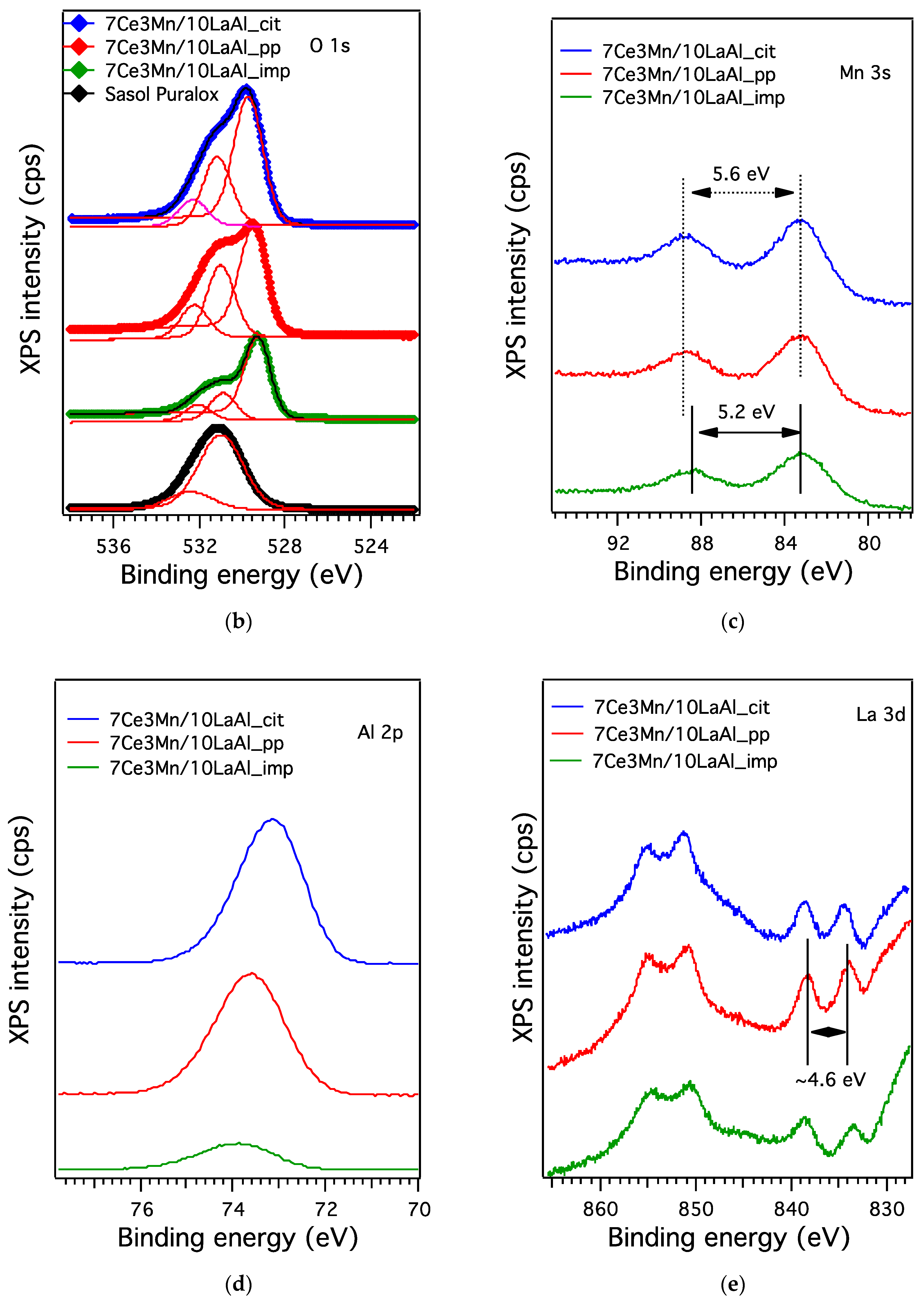
In order to compare the behaviour of a bulk versus surface modification of alumina with Mn and Ce based oxides, a higher consideration was given to the interaction between Mn and Ce oxides and support. In the case of CeO2–MnOx impregnated on commercial Puralox La-modified alumina support, our XRD results have indicated the presence of a mixed phase of Ce2MnO6 on the surface. Such a mixed phase may contribute locally to increased defects and further distortions of the atomic architecture of the catalytic sites and thus may further increase the catalytic activity of this type of supported catalyst.
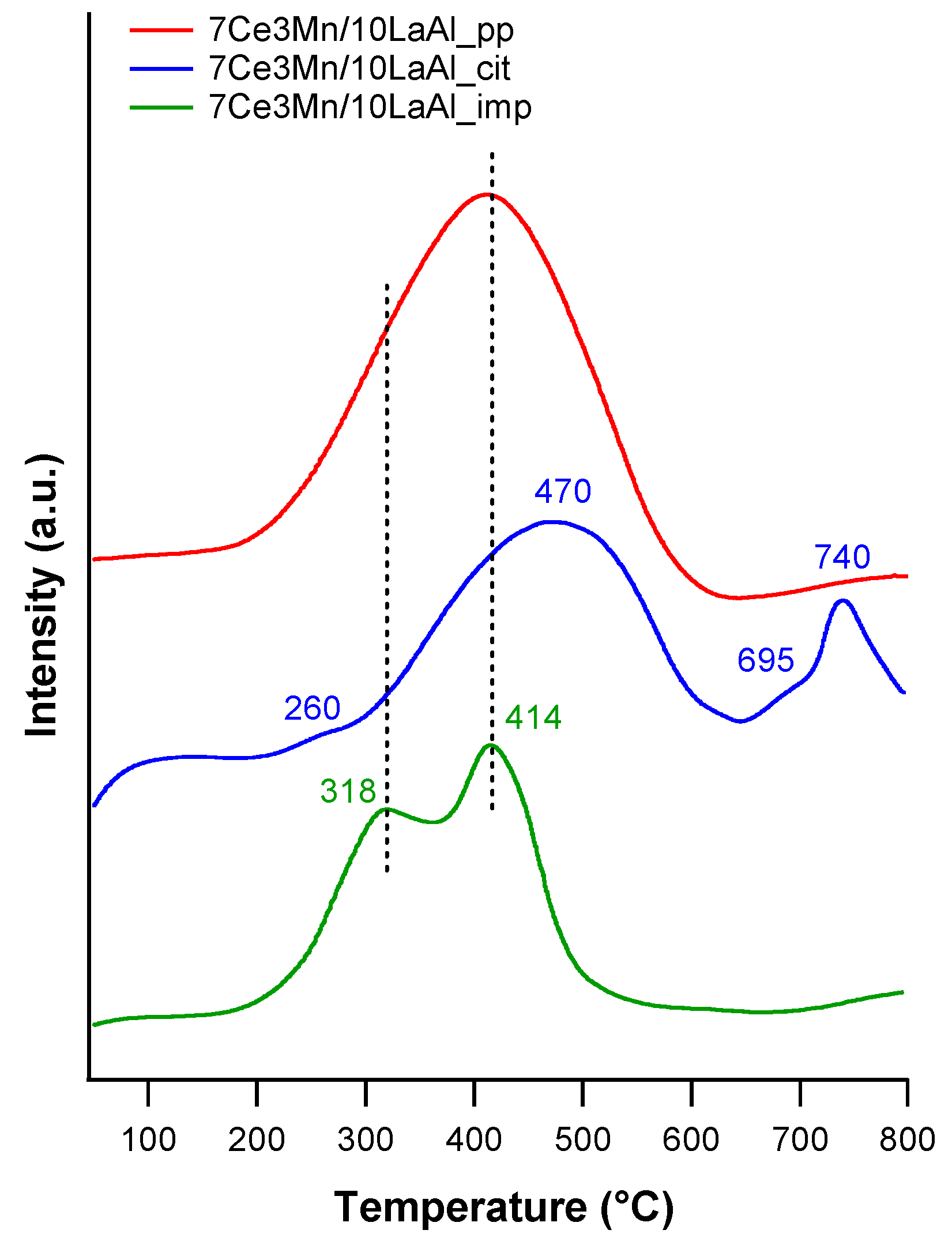
The interaction of Mn and Ce oxides with alumina, on the surface or in bulk, is clearly demonstrated by the H
2–TPR and XPS data. TPR-H
2 analyses (
Figure 5) evidenced the presence of the same type of reducible species for all samples. However, taking into account the shift in the reduction temperature and the lowest onset, it can be inferred that 7Ce3Mn/10LaAl_imp catalyst can be more active than the bulk ones, due to the higher mobility of the oxygen species and lower interaction of Mn and Ce oxides with alumina support. Moreover, more reducible sites on the CeMn/LaAl_imp catalyst surface facilitate the adsorption and activation of CH
4 during the catalytic reaction and thus enhance its catalytic activity, in agreement with the theoretical predictions obtained by density functional theory (DFT) which showed a direct correlation between reaction energy for catalytic methane oxidation and surface reducibility of doped CeO
2. [
38]. From XPS data, higher ratio between O bonded to Mn and Ce and the O bonded to Al for the impregnated sample indicates that the oxygen species related to Mn and Ce are more active for CH
4 combustion and have moderate strength (from H
2–TPR data) with the support during the reaction. Thus, surface deposition of CeO
2–MnO
x is much more desirable than the bulk one. Moreover, the data obtained from H
2–TPR and XPS indicate the presence in higher amount of Mn
4+ and Ce
4+ for the impregnated sample, that promote the catalytic activity, as also indicated by other studies [
39].
The presence of reducible Ce4+ and Mn4+ at the surface of impregnated the catalyst is an indication of Mars Van Krevelen mechanism, in which the oxidation of CH4 takes place using the oxygen from the lattice, with its rather labile chemical bonds to manganese and cerium cations, consecutively with the reduction of Ce4+ and Mn4+ to Ce3+ and Mn3+, respectively. Furthermore, the oxygen from the gaseous phase is used to reoxidize the surface to assure another catalytic cycle.
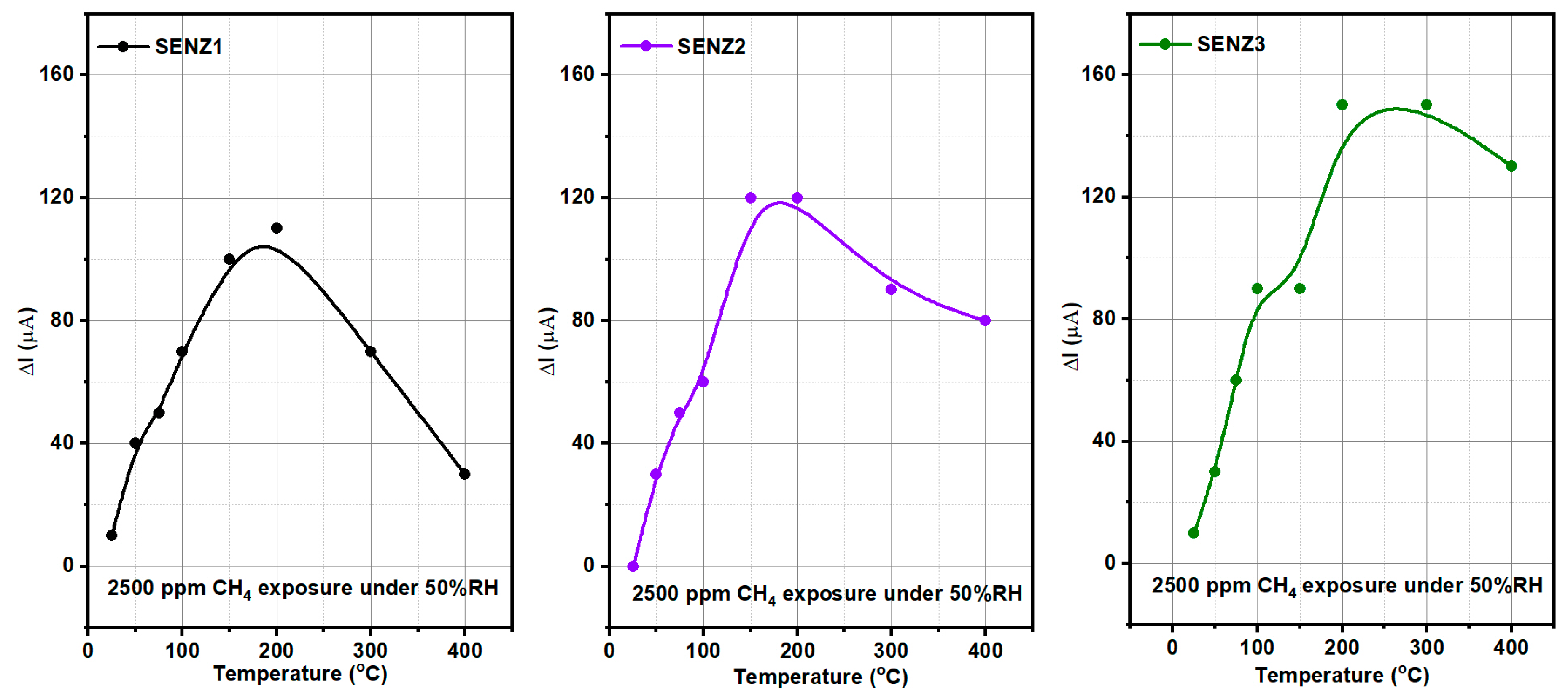
In agreement with the above material and catalytic properties of the developed catalysts, as depicted in
Figure 9, the highest conversion rate was attained by the self-heated catalytic micro-converter denoted by SENZ3, obtained by impregnation of the CeO
2–MnO
x catalyst on the commercial lanthanum-modified alumina support, Puralox. An additional explanation for this increased catalytic conversion of methane might be related to the increased diffusion of oxygen and CH
4 toward catalytic sites due to higher pore sizes found for this impregnated catalyst, too (see
Table 1) [
40].
5. Conclusions
In this paper we studied noble metal-free catalysts consisting of Mn and Ce based oxides and lanthanum-modified alumina by three methods (coprecipitation, citrate-based sol-gel method and impregnation on a commercial 4 wt.% La2O3–Al2O3 support) for CH4 catalytic combustion. The experimental study aimed the elucidation of the role of the surface versus bulk incorporation of the CeO2–MnOx catalyst on/in the support, respectively.
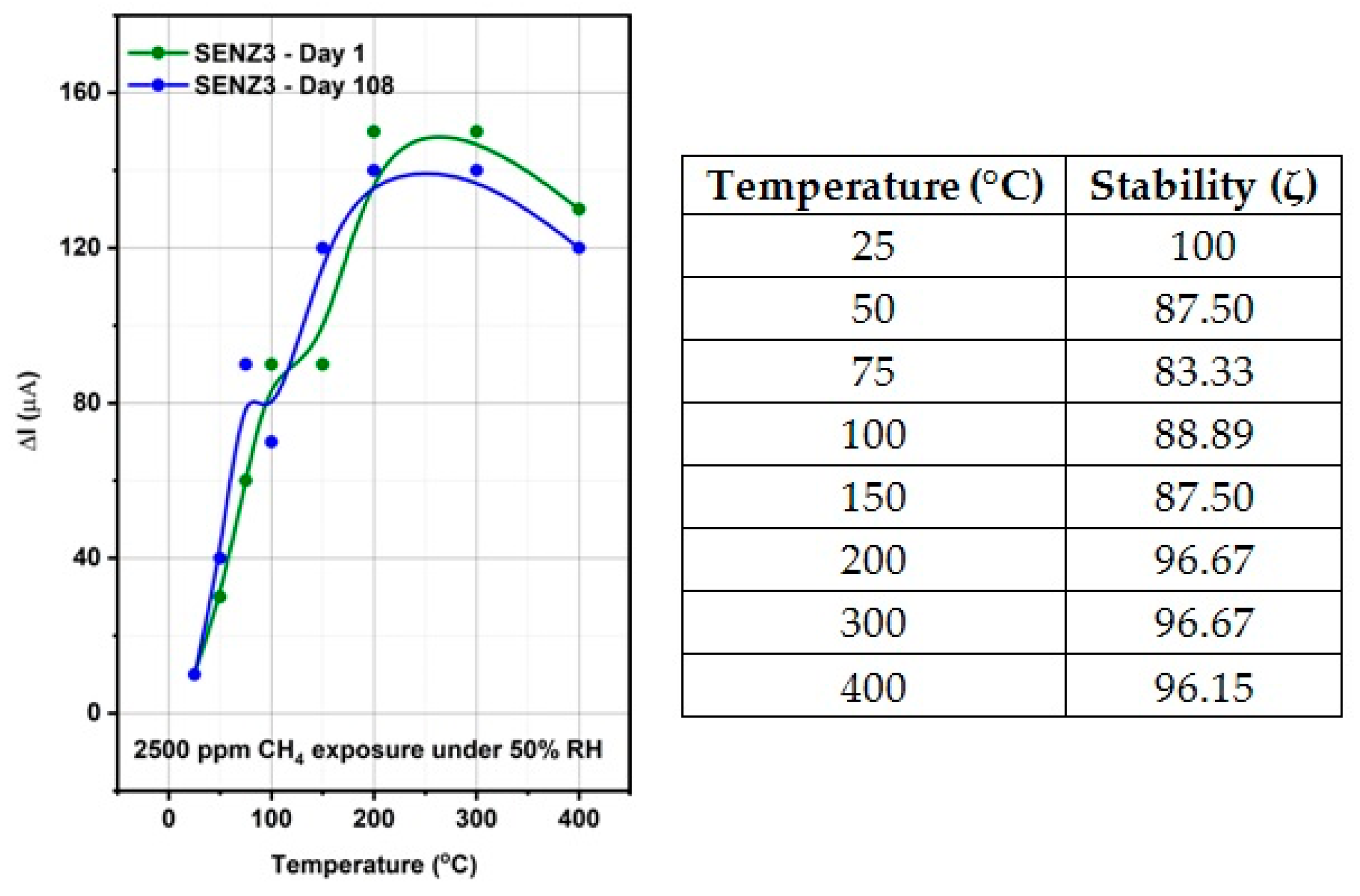
The self-heated catalytic micro-converters, which have been built for the functional testing of the catalysts, proved to be a sensitive test structure that identified very weak methane oxidation at temperatures below 200 °C.
The highest catalytic conversion of CH4 was obtained on micro-converts (SENZ3) where CeO2–MnOx catalyst was impregnated on a commercial 4 wt.% La2O3–Al2O3 support (7Ce3Mn/10LaAl_imp). The heterogeneous combustion results with the maximum CH4 conversion for the CeO2–MnOx impregnated catalyst were correlated with the collective features such as: the highest pore sizes dimensions according with the BET investigations, the highest amount of Mn4+ or/and Ce4+ on the surface, presence of a mixed phase (Ce2MnO6) on the surface and a high reducibility of Mn4+ or/and Ce4+ species. The ranking of the catalytic activity of the three CeO2–MnOx catalysts evaluated by means of self-heated micro-converters was in direct correlation with the experimental surface reducibility results in hydrogen, as it was theoretically demonstrated in the prior art by DFT simulations.
This study has shown the superior catalytic activity of the CeO2–MnOx catalyst impregnated on the surface of the support with respect to incorporation of the same catalyst in bulk.
By means of an extensive material characterization comprising XRD, BET, XPS and H2–TPR it was shown that surface modification of a commercial 4 wt.% La2O3–Al2O3 support with more accessible CeO2–MnOx catalyst may be a promising direction for pushing further the research on complete oxidation of methane at lower temperatures, a major challenge of today’s research.