The Evolution of Complex Carbide Precipitates in a Low Alloy Cr–Mo–V Steel after Long-Term Aging Treatment
Abstract
1. Introduction
2. Experimental Procedure
3. Results
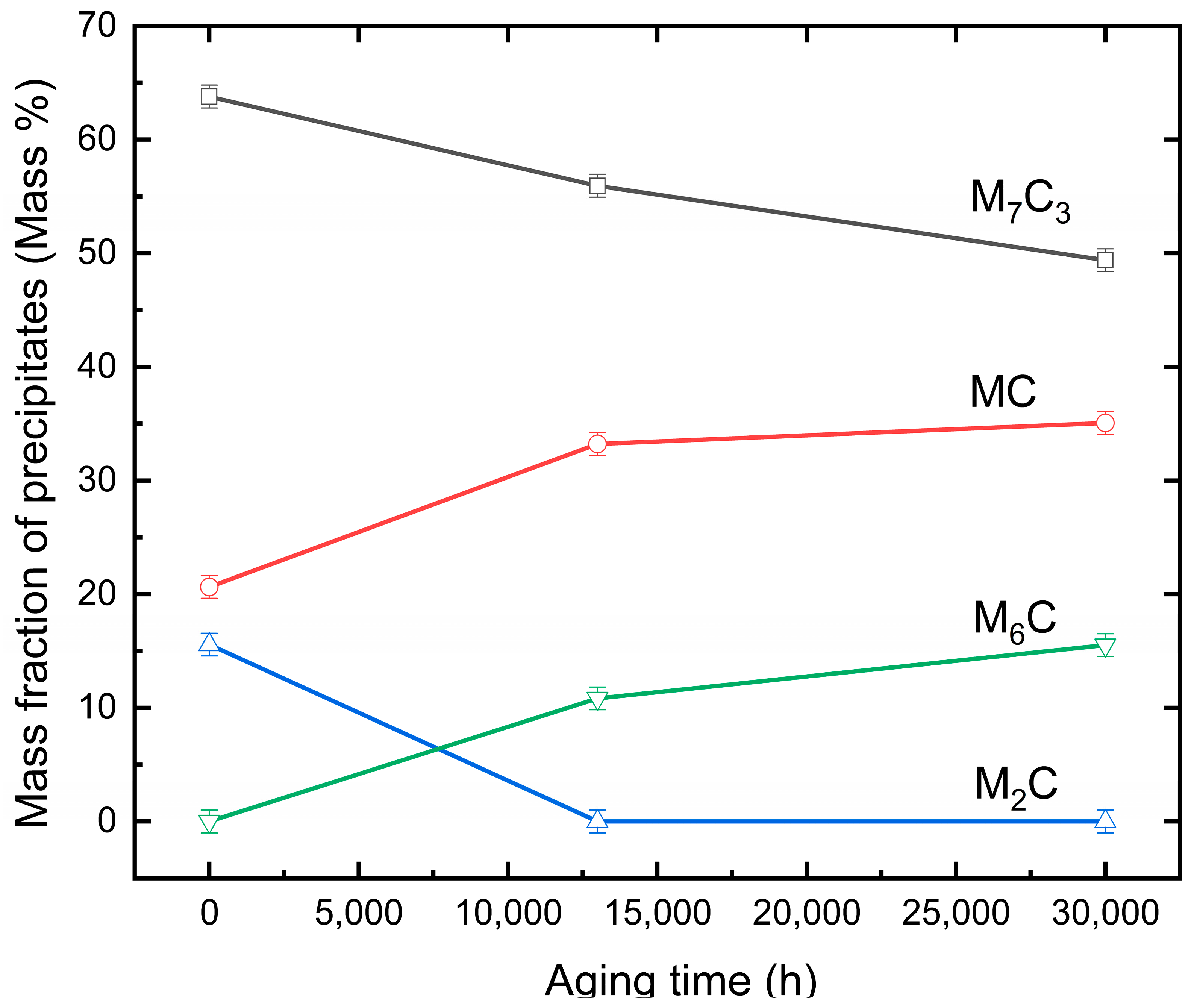
3.1. Mass Fraction of Precipitates in Different Aging Conditions
3.2. Particle Size Distribution
3.3. X-ray Diffraction Analysis of the Extracted Precipitates
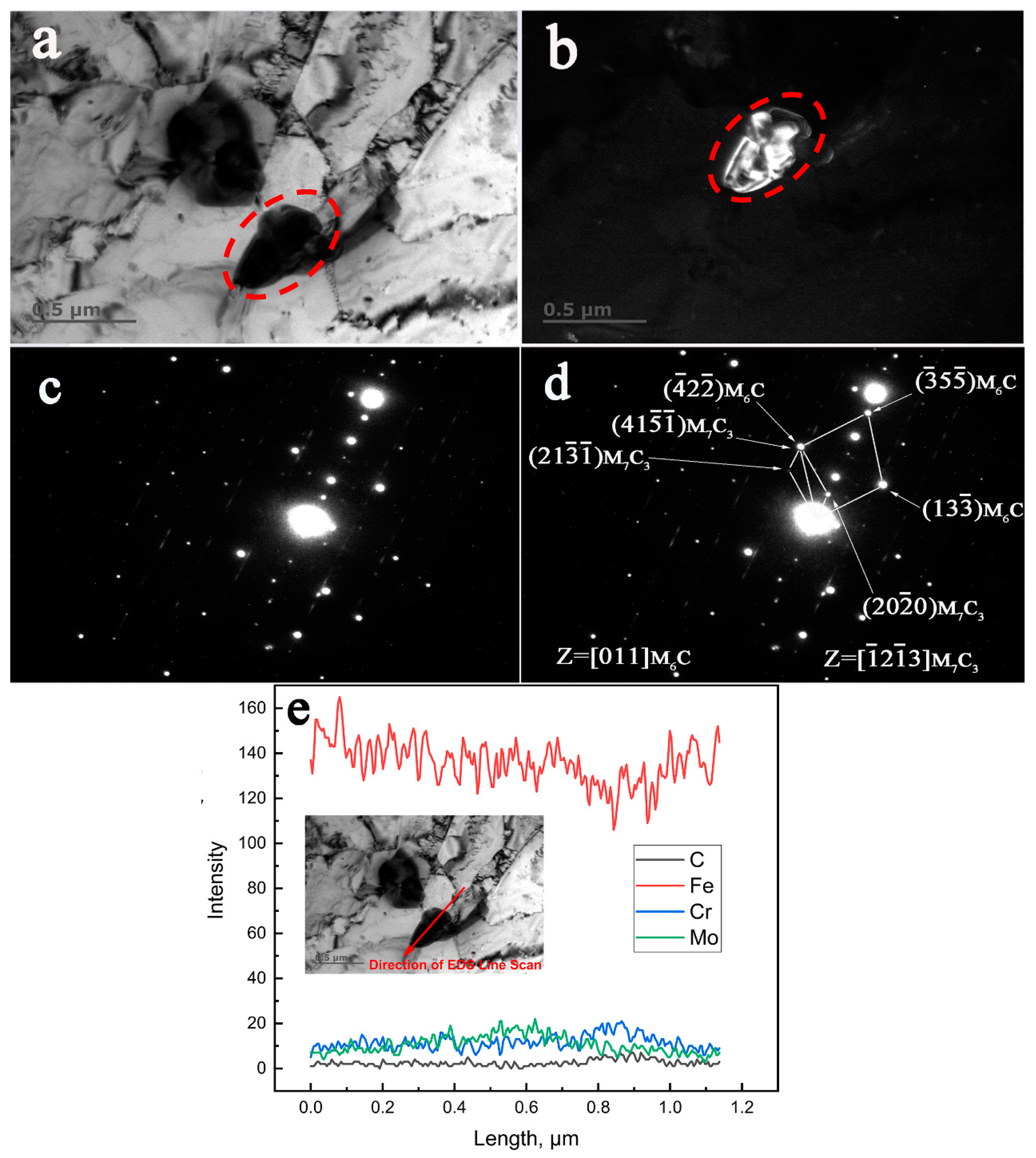
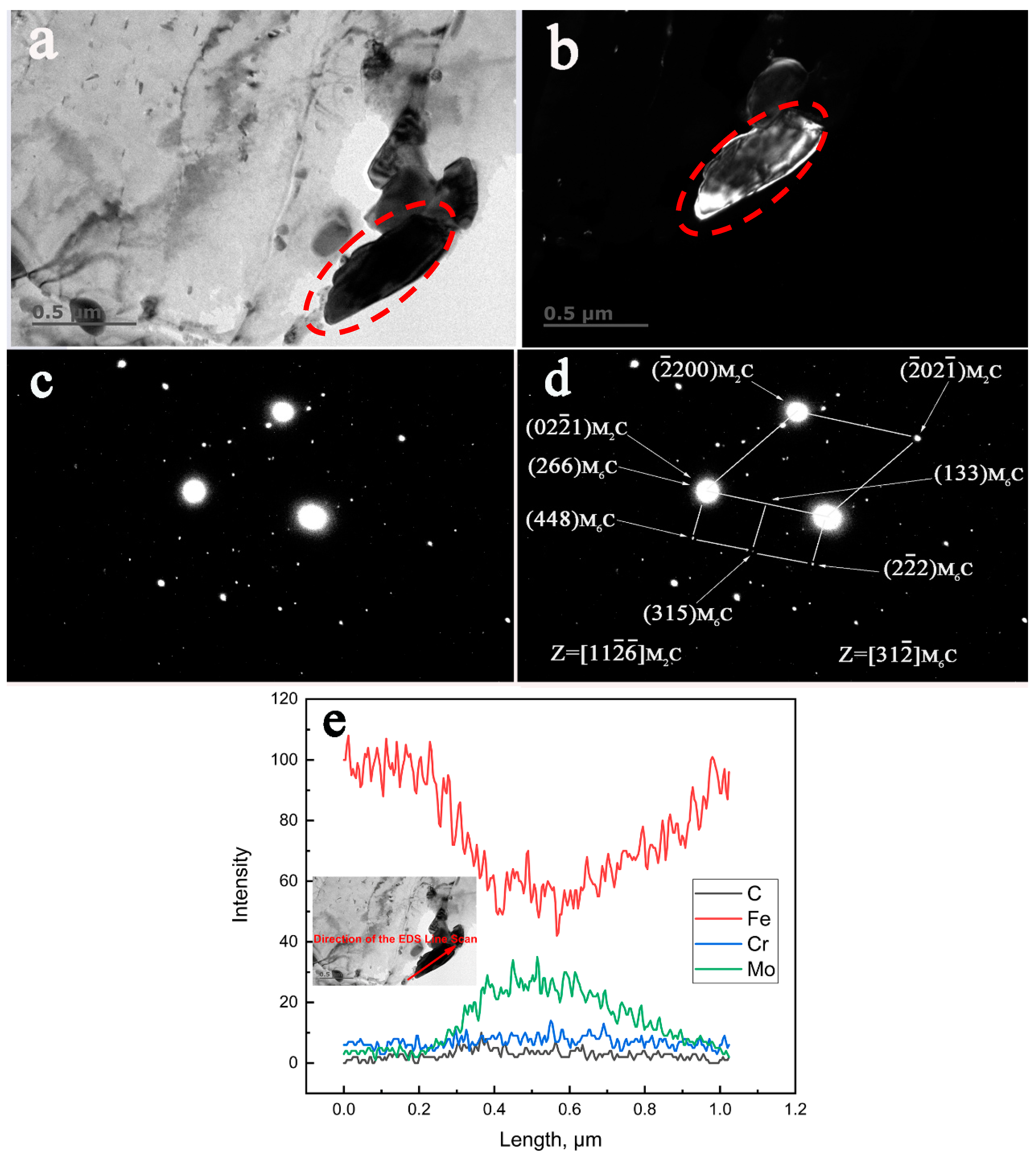
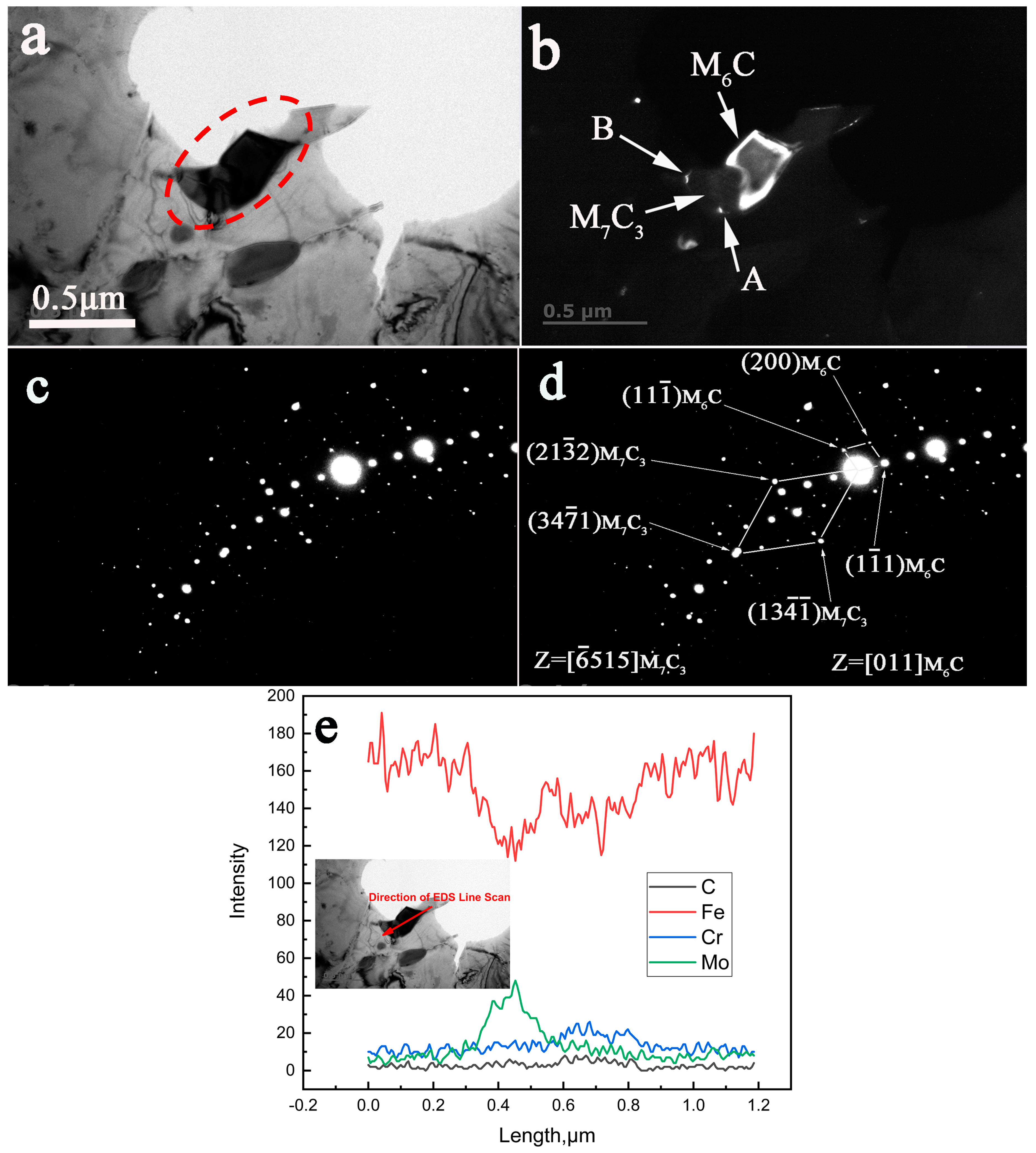
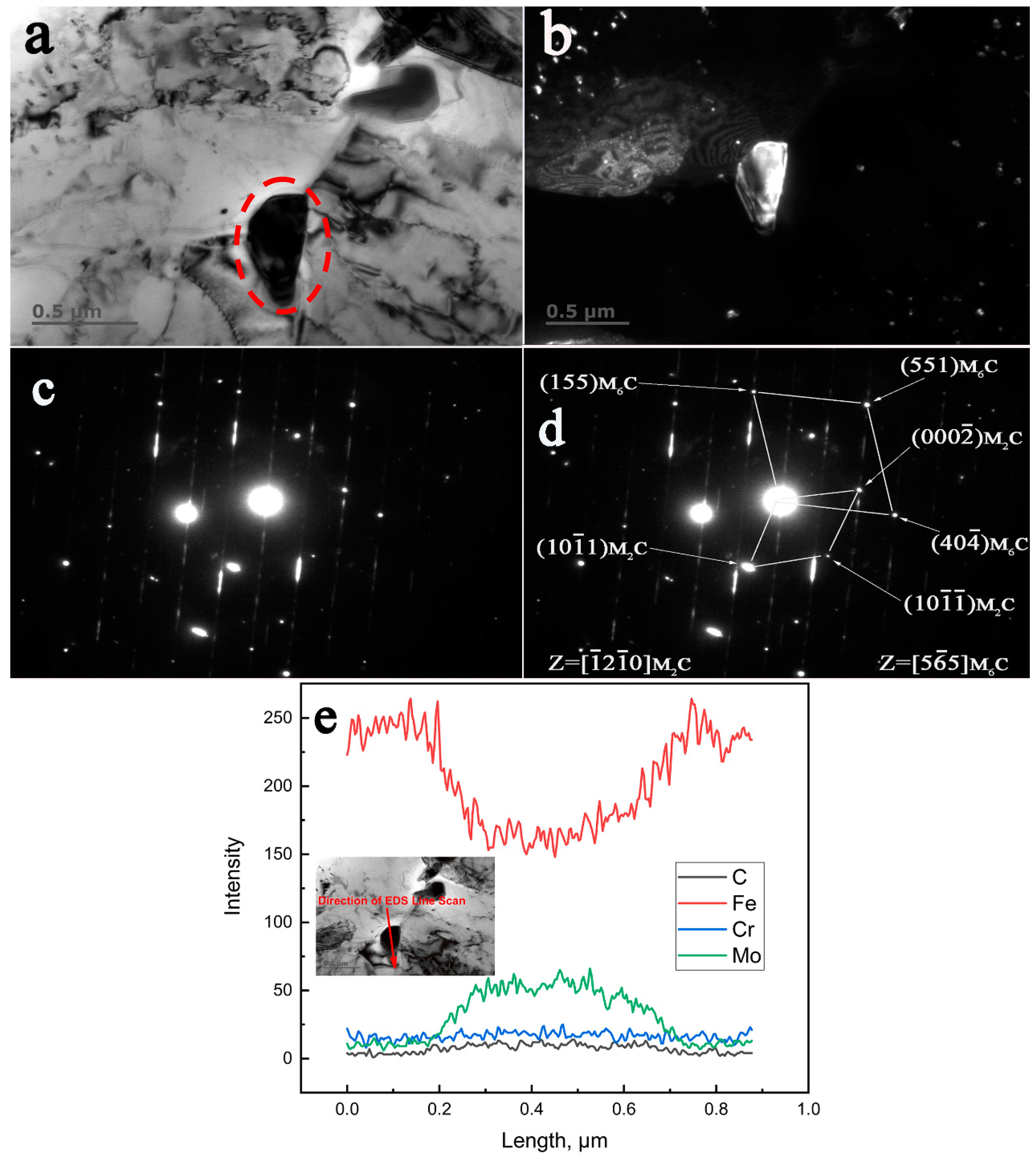
3.4. TEM, SAED, and EDS Characterization of the Crystallographic Orientation Relationship of the In-Situ Transformed Carbides
4. Discussion
4.1. The Identification of Complex Carbide Precipitates in Long-Term Aged Cr–Mo–V Steel
4.2. The Sequence of Carbide Precipitation
4.3. The Nucleation of the M6C Carbides
4.4. The Planar Mismatch between Different Phases in the Complex Precipitates
5. Conclusions
- A near-equilibrium state of the low alloy Cr–Mo–V steel was achieved by aging at 650 °C for up to 30,000 h. The transformation of M2C to M6C and M7C3 to M6C were observed and confirmed by means of SAED and EDS line scan. Four types of precipitates, including MC, M7C3, M6C, and M2C, were found in the specimens in the near-equilibrium state.
- The crystallographic orientation relationships between the in-situ transformed phases of the specimen aged for 13,000 h at 650 °C were established as follows:
- The crystallographic orientation relationships between the in-situ transformed phases of the specimen aged for 30,000 h at 650 °C were identified as:
- The carbide precipitate sequence in the investigated Cr–Mo–V steel aged at 650 °C for 30,000 h was found to be: MC + M7C3 + M2C → MC + M2C + M7C3 + M6C → MC + M7C3 + M6C, which is consistent with the equilibrium calculations.
- The direct evidence from TEM characterization revealed that the M6C nucleated at the interfaces of the existing M7C3 carbides.
- The coherent interfaces between the in-situ transformed complex carbide precipitates became incoherent at prolonged aging time of 30,000 h.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bhadeshia, H.K.D.H. Design of Ferritic Creep-resistant Steels. ISIJ Int. 2001, 41, 626–640. [Google Scholar] [CrossRef]
- Mitchell, D.R.G.; Ball, C.J. A quantitative X-ray diffraction and analytical electron microscopy study of service-exposed 2.25Cr-1Mo steels. Mater. Charact. 2001, 47, 17–26. [Google Scholar] [CrossRef]
- Wang, Y.; Cheng, G.; Qin, M.; Li, Q.; Zhang, Z.; Chen, K.; Li, Y.; Hu, H.; Wu, W.; Zhang, J. Effect of high temperature deformation on the microstructure, mechanical properties and hydrogen embrittlement of 2.25Cr–1Mo-0.25 V steel. Int. J. Hydrogen Energy 2017, 42, 24549–24559. [Google Scholar] [CrossRef]
- Pereira, P.A.S.; Franco, C.S.G.; Guerra Filho, J.L.M.; Dos Santos, D.S. Hydrogen effects on the microstructure of a 2.25Cr-1Mo-0.25 V steel welded joint. Int. J. Hydrogen Energy 2015, 40, 17136–17143. [Google Scholar] [CrossRef]
- Chen, J.B.; Liu, H.B.; Pan, Z.Y.; Shi, K.; Zhang, H.Q.; Li, J.F. Carbide evolution and service life of simulated post weld heat treated 2.25Cr-1Mo steel. Mater. Sci. Eng. A 2015, 622, 153–159. [Google Scholar] [CrossRef]
- Taneike, M.; Abe, F.; Sawada, K. Creep-strengthening of steel at high temperatures using nano-sized carbonitride dispersions. Nature 2003, 424, 294–296. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Babu, S.S.; Terasaki, H.; Komizo, Y.; Yamamoto, Y.; Santella, M.L. Correlation of precipitate stability to increased creep resistance of Cr-Mo steel welds. Acta Mater. 2013, 61, 2194–2206. [Google Scholar] [CrossRef]
- Nguyen, T.D.; Sawada, K.; Kushima, H.; Tabuchi, M.; Kimura, K. Change of precipitate free zone during long-term creep in 2.25Cr-1Mo steel. Mater. Sci. Eng. A 2014, 591, 130–135. [Google Scholar] [CrossRef]
- Dépinoy, S.; Toffolon- Masclet, C.; Urvoy, S.; Roubaud, J.; Marini, B.; Roch, F.; Kozeschnick, E.; Gourgues, A.F. Carbide precipitation in 2.25 Cr-1 Mo bainitic steel: Effect of heating and isothermal tempering conditions. Metall. Mater. Trans. A 2017, 48, 2164–2178. [Google Scholar] [CrossRef]
- Zhang, Y.; Luo, P.; Yan, H.; Zhang, H.; Li, J. The Effect of Bainite Type on the Evolution of Carbide Constituent During an Accelerated Aging in Cr-Mo-V Steel. J. Mater. Eng. Perform 2018, 28, 578–585. [Google Scholar] [CrossRef]
- Wang, X.; Li, Y.; Li, H.; Lin, S.; Ren, Y. Effect of long-term aging on the microstructure and mechanical properties of T23 steel weld metal without post-weld heat treatment. J. Mater. Process Tech. 2018, 252, 618–627. [Google Scholar] [CrossRef]
- Jiang, Z.; Wang, P.; Li, D.; Li, Y. Influence of the decomposition behavior of retained austenite during tempering on the mechanical properties of 2.25Cr-1Mo-0.25V steel. Mater. Sci. Eng. A 2019, 742, 540–552. [Google Scholar] [CrossRef]
- Zieliński, A.; Golański, G.; Sroka, M. Influence of long-term ageing on the microstructure and mechanical properties of T24 steel. Mater. Sci. Eng. A 2017, 682, 664–672. [Google Scholar] [CrossRef]
- Baker, R.G.; Nutting, J. The tempering of a Cr-Mo-V-W and a Mo-V steel. Iron and Steel Inst. Spec. Rep. 1959, 64, 1–22. [Google Scholar]
- Janovec, J.; Vyrostkova, A. Effect of tempering on development of carbide particles in 2.7Cr-0.6Mo-0.3V steel. J. Mater. Sci. 1992, 27, 6564–6572. [Google Scholar] [CrossRef]
- Ishiguro, T.; Ohnishi, K.; Watanabe, J. Effects of chromium and vanadium on the hydrogen attack susceptibility of boron added Cr-Mo steels. Tetsu-to-Hagane 1986, 72, 70–77. [Google Scholar] [CrossRef][Green Version]
- Vodarek, V.; Strang, A. Effect of nickel on the precipitation processes in 12CrMoV steels during creep at 550 °C. Scripta Mater. 1997, 38, 101–106. [Google Scholar] [CrossRef]
- Jiang, Z.H.; Wang, P.; Li, D.Z.; Li, Y.Y. The evolution of microstructure and mechanical properties of 2.25Cr-1Mo-0.25V steel with different initial microstructures during tempering. Mater. Sci. Eng. A 2017, 699, 165–175. [Google Scholar] [CrossRef]
- Zhang, Y.; Miao, L.; Wang, X.; Zhang, H.; Li, J. Evolution behavior of carbides in 2.25Cr-1Mo-0.25V steel. Mater. Trans. 2009, 50, 2507–2511. [Google Scholar]
- Wen, T.; Hu, X.F.; Song, Y.Y.; Yan, D.S.; Rong, L.J. Carbides and mechanical properties in a Fe-Cr-Ni-Mo high strength steel with different V contents. Mater. Sci. Eng. A 2013, 588, 201–207. [Google Scholar] [CrossRef]
- Seung, P.H.; Seong, I.K.; Tae, Y.A.; Soon, T.H.; Young, W.K. Effects of extended heat treatment on carbide evolution in Cr-Mo steels. Mater. Charact. 2016, 115, 8–13. [Google Scholar]
- Vyrostkova, A.; Kroupa, A.; Janovec, J.; Svoboda, M. Carbide reactions and phase equilibria in low alloy Cr-Mo-V steels tempered at 773-993 K. Part I: Experimental measurements. Acta Mater. 1998, 46, 31–38. [Google Scholar] [CrossRef]
- Yu, J. Carbide stability diagrams in 2.25Cr-1Mo Steels. Metall. Trans. A 1989, 20, 1561–1564. [Google Scholar] [CrossRef]
- Senior, B.A. A critical review of precipitation behaviour in 1Cr-Mo-V rotor steels. Mater. Sci. Eng. A 1988, 103, 263–271. [Google Scholar] [CrossRef]
- Pitsch, W. The orientation relationship between cementite and ferrite in pearlite. Acta Metall. 1962, 10, 79–80. [Google Scholar] [CrossRef]
- Heikkinen, V.K.; Hakkarainen, T.J. Precipitation associated with the climb of a <100> dislocations in a low-carbon iron-vanadium alloy. Philos. Mag. 1973, 28, 237–280. [Google Scholar] [CrossRef]
- Rong, W.; Dunlop, G.L. The crystallography of secondary carbide precipitation in high speed steel. Acta Metall. 1984, 32, 1591–1599. [Google Scholar] [CrossRef]
- Lee, T.; Oh, C.; Ryu, S.; Kim, J. Crystallography and morphology of carbides in a low-cycle fatigued 1Cr-1Mo-0.25V steel. Metall. Mater. Trans. A 2011, 42, 147–157. [Google Scholar] [CrossRef]
- Dyson, D.J.; Andrews, K.W. Carbide M7C3 and its formation in alloy steels. J. Iron Steel Inst. 1969, 207, 208–219. [Google Scholar]
- Kuo, K.H.; Jia, C.L. Crystallography of M23C6 and M6C precipitated in a low alloy steel. Acta Mater. 1985, 33, 991–996. [Google Scholar] [CrossRef]
- Baker, R.G.; Nutting, J. The tempering of 2.25Cr-1Mo steel after quenching and normalizing. J. Iron Steel Inst. 1959, 192, 257–268. [Google Scholar]
- Woodhead, J.H.; Quarrell, A.G. Role of carbides in low-alloy creep resisting steels. J. Iron Steel Inst. 1965, 203, 605–620. [Google Scholar]
- Kroupa; Havránková, J.; Svoboda, M.; Coufalová, M.; Vřešt’Ál, J. Phase diagram in the iron-rich corner of the Fe-Cr-Mo-V-C system below 1000 K. J. Phase Equ. 2001, 22, 312–323. [Google Scholar] [CrossRef]
- Miyata, K.; Sawaragi, Y. Effect of Mo and W on the phase stability of precipitates in low Cr heat resistant steels. ISIJ Int. 2001, 41, 281–289. [Google Scholar] [CrossRef]
- Bhadeshia, H.K.D.H.; Christian, J.W. Bainite in steels. Metall. Trans. A 1990, 21, 767–797. [Google Scholar] [CrossRef]
- Rempel, A.A. Atomic and vacancy ordering in nonstoichiometric carbides. Physics-Uspekhi 1996, 39, 31–56. [Google Scholar] [CrossRef]
- Andrews, K.W.; Dyson, D.J.; Keown, S.R. Interpretation of Electron Diffraction Pattern, 1st ed.; Hilger & Watts Ltd.: London, UK, 1967; p. 173. [Google Scholar]
- Morniroli, J.P.; Bauer-Grosse, E.; Gantois, M. Crystalline defect in M7C3 carbide. Phil. Mag. A 1983, 48, 311–327. [Google Scholar] [CrossRef]
- Zhang, Y.T.; Han, H.B.; Miao, L.D.; Zhang, H.Q.; Li, J.F. Quantitative carbide analysis using the Rietveld method for 2.25Cr-1Mo-0.25V steel. Mater. Charact. 2009, 60, 953–956. [Google Scholar]
- Kroupa, A.; Vyrostkova, A.; Svoboda, M.; Janovec, J. Carbide reactions and phase equilibria in low alloy Cr-Mo-V steels tempered at 773-993 K. Part II: Theoretical calculations. Acta Mater. 1998, 46, 39–49. [Google Scholar] [CrossRef]
- Fu, R.D.; Wang, T.S.; Zhou, W.H. Characterization of precipitates in a 2.25Cr-1Mo-0.25V steel for large-scale cast-forged products. Mater. Charact. 2007, 58, 968–973. [Google Scholar] [CrossRef]
- Andrews, K.W.; Hughes, H.; Dyson, D.J. Constitution diagrams for Cr-Mo-V steels. J. Iron Steel Inst. 1972, 210, 337–350. [Google Scholar]
- Inoue, A.; Masumoto, T. Carbide reactions (M3C→M7C3→M23C6→M6C) during tempering of rapidly solidified high carbon Cr-W and Cr-Mo steels. Metall. Trans. A 1980, 11, 739–747. [Google Scholar] [CrossRef]


| Carbide | Orientation Relationships with Matrix | Reference |
|---|---|---|
| M3C | [25] | |
| MC (M4C3) | [26] | |
| M2C | [27] [28] | |
| M7C3 | [29] | |
| M6C | [30] | |
| M23C6 | [30] |
| Carbide | Lattice Parameters (nm) | Crystal System | Space Group | Reference |
|---|---|---|---|---|
| M3C | a = 0.4525 b = 0.5087 c = 0.6743 | orthorhombic | Pnma | [35] |
| M6C | a = 1.1082 | cubic | Fd mm | [35] |
| M23C6 | a = 1.0621 | cubic | Fm m | [35] |
| MC | a = 0.8333 | cubic | P4332 | [36] |
| M2C | a = 0.3002 c = 0.4724 | hexagonal | P63/mmc | [37] |
| M7C3 | a = 0.4526 b = 0.7010 c = 1.2142 | orthorhombic | Pnma | [38] |
| C | Mn | P | S | Cr | Mo | Nb | V | Al | N |
|---|---|---|---|---|---|---|---|---|---|
| 0.14 | 0.55 | 0.008 | 0.003 | 2.5 | 1.0 | 0.03 | 0.3 | 0.020 | 0.005 |
| Aging Temperature (°C) | Aging Time (h) | Types of Carbides |
|---|---|---|
| 650 | 0 | M7C3 + MC + M2C |
| 650 | 13,000 | M7C3 + MC + M6C |
| 650 | 30,000 | M7C3 + MC + M6C |
| Aging Time (h) | In-Situ Transformation | Crystallographic Relationships between the Precipitates |
|---|---|---|
| 13,000 | M7C3 → M6C | |
| M2C → M6C | ||
| 30,000 | M7C3 → M6C | |
| M2C → M6C |
| Face | (hkil)carbide | Lattice Spacing, d1, nm | (hkl)carbide | Lattice Spacing, d2, nm | Lattice Misfits, (d1 – d2)/d1 |
|---|---|---|---|---|---|
| 0.2281 | 0.2262 | 0.83% | |||
| 2 × 0.1253 | 0.2542 | −1.44% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Liu, C.; Miao, L.; Guo, X.; Ding, J.; Zhang, H. The Evolution of Complex Carbide Precipitates in a Low Alloy Cr–Mo–V Steel after Long-Term Aging Treatment. Materials 2019, 12, 1724. https://doi.org/10.3390/ma12101724
Liu Z, Liu C, Miao L, Guo X, Ding J, Zhang H. The Evolution of Complex Carbide Precipitates in a Low Alloy Cr–Mo–V Steel after Long-Term Aging Treatment. Materials. 2019; 12(10):1724. https://doi.org/10.3390/ma12101724
Chicago/Turabian StyleLiu, Zili, Chunming Liu, Lede Miao, Xiaofei Guo, Jianhua Ding, and Hanqian Zhang. 2019. "The Evolution of Complex Carbide Precipitates in a Low Alloy Cr–Mo–V Steel after Long-Term Aging Treatment" Materials 12, no. 10: 1724. https://doi.org/10.3390/ma12101724
APA StyleLiu, Z., Liu, C., Miao, L., Guo, X., Ding, J., & Zhang, H. (2019). The Evolution of Complex Carbide Precipitates in a Low Alloy Cr–Mo–V Steel after Long-Term Aging Treatment. Materials, 12(10), 1724. https://doi.org/10.3390/ma12101724





