Effect of Simultaneous Immediate Implant Placement and Guided Bone Reconstruction with Ultra-Fine Titanium Mesh Membranes on Radiographic and Clinical Parameters after 18 Months of Loading
Abstract
1. Introduction
2. Materials and Methods
3. Outcomes Measures Included:
- Implant survival rate: an implant was considered a failure if it presented any mobility, implant fracture or an infection that mandated implant removal.
- A restoration was considered failed if it needed to be replaced by an alternative restoration.
- Presence of biological (pain, swelling, suppuration, etc.) or mechanical (screw loosening or fracture of the framework and/or the veneering material, etc.) complications.
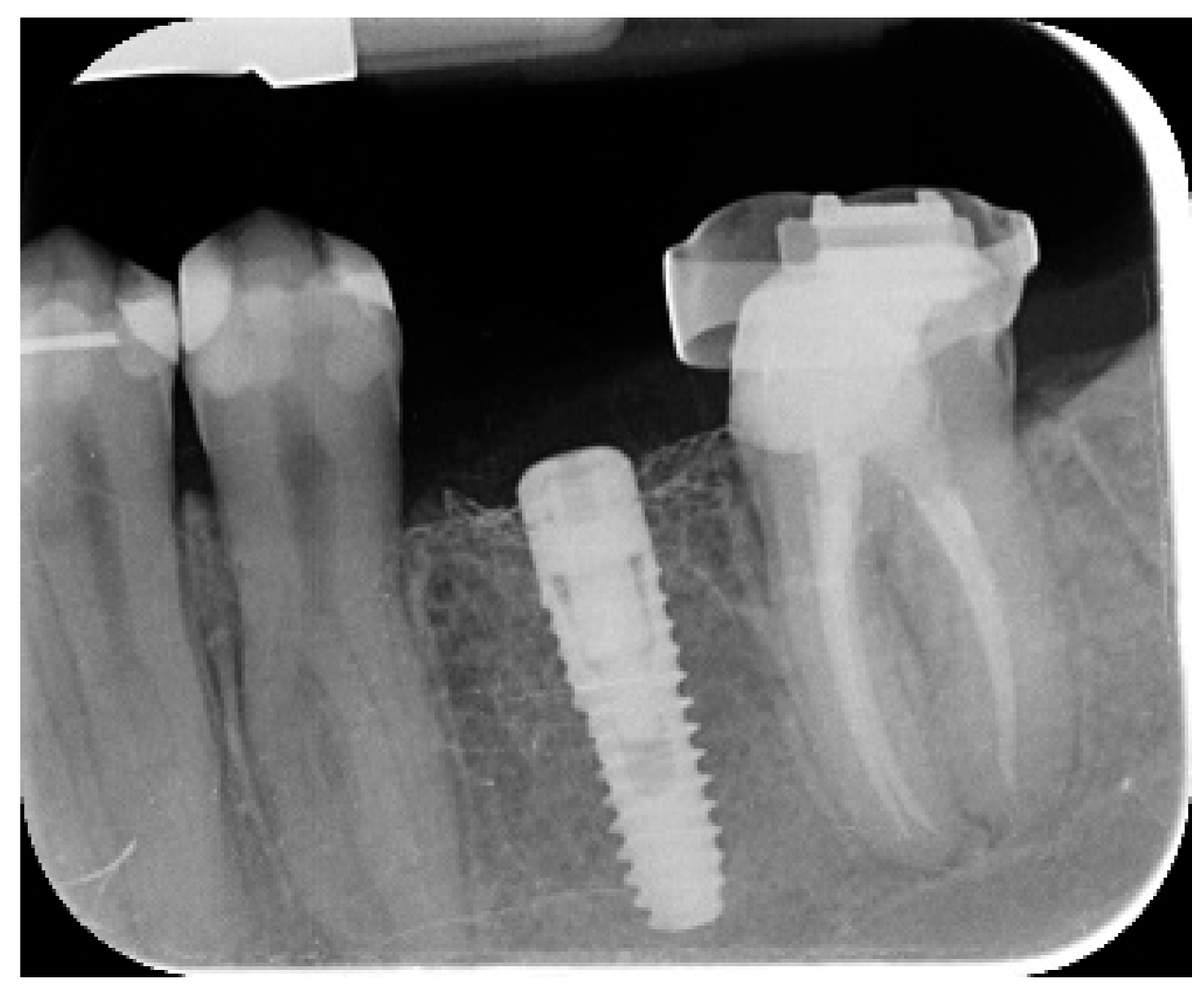
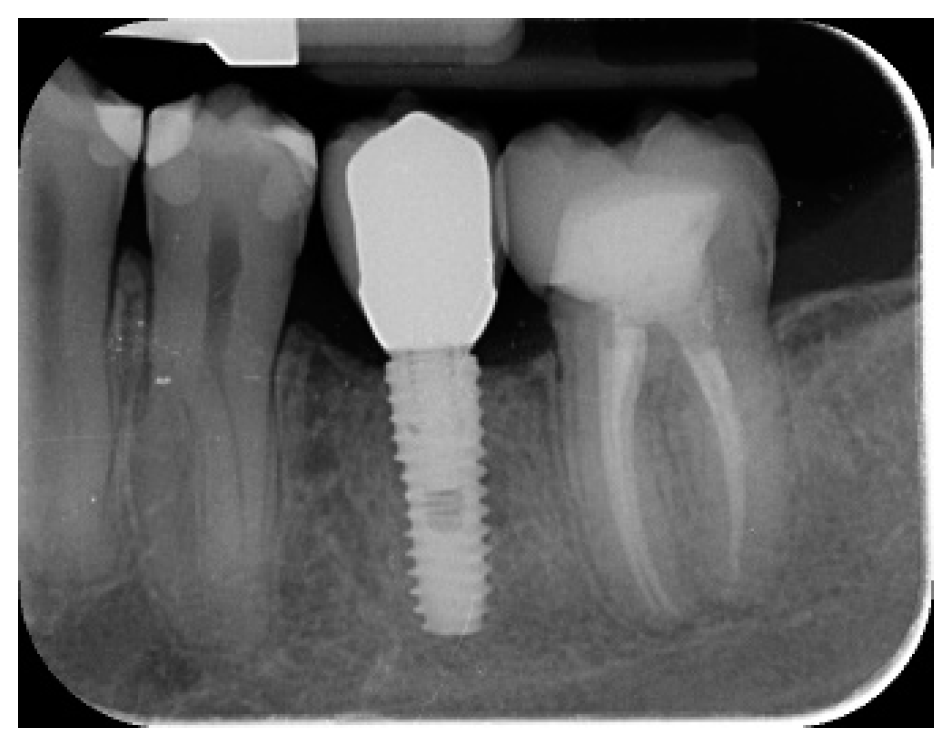
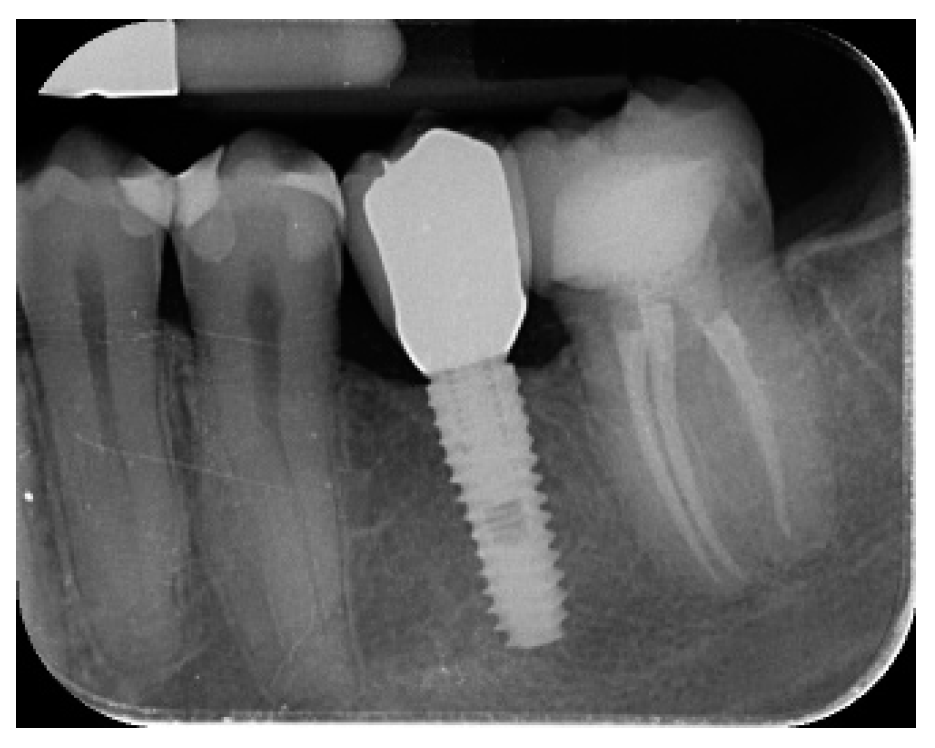
- Marginal bone level changes were assessed by digital periapical radiographs (Digora Optime; Soredex, Tuusula, Finland) using the parallel technique and commercially available film holders. Three time points were evaluated, at implant placement (baseline), immediately after the insertion of the restoration, and one year after loading. The averaged mesial and distal distances from the most coronal margin of the implant and the first bone-to-implant contact was measured to the nearest 0.01 mm and taken as the marginal bone level. The difference in levels between time points was taken as marginal bone loss (MBL).
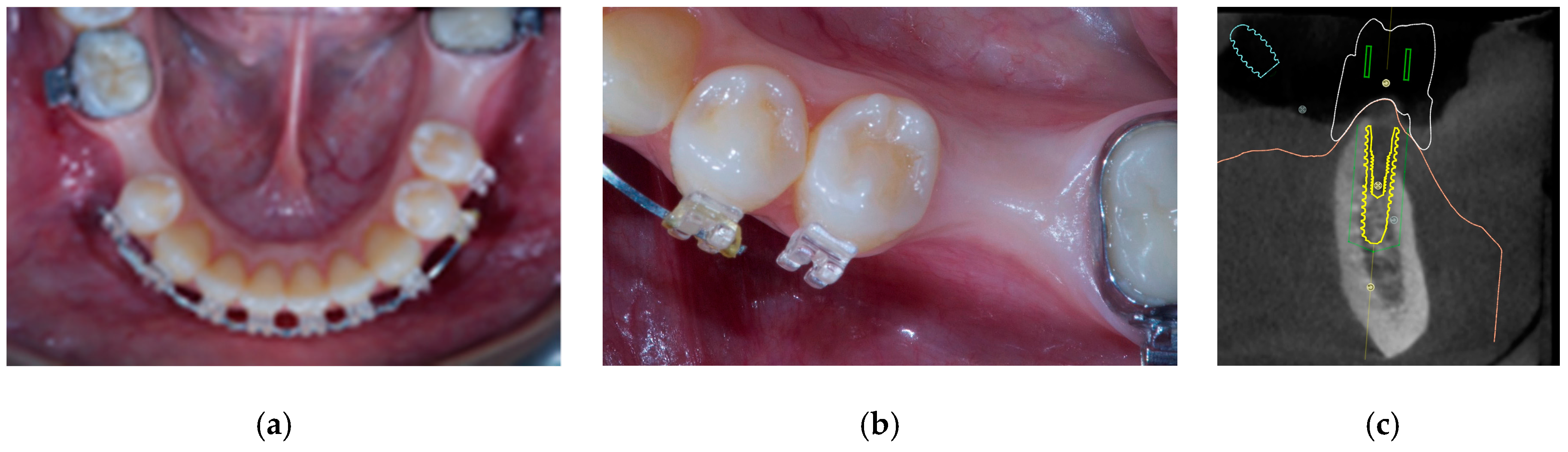
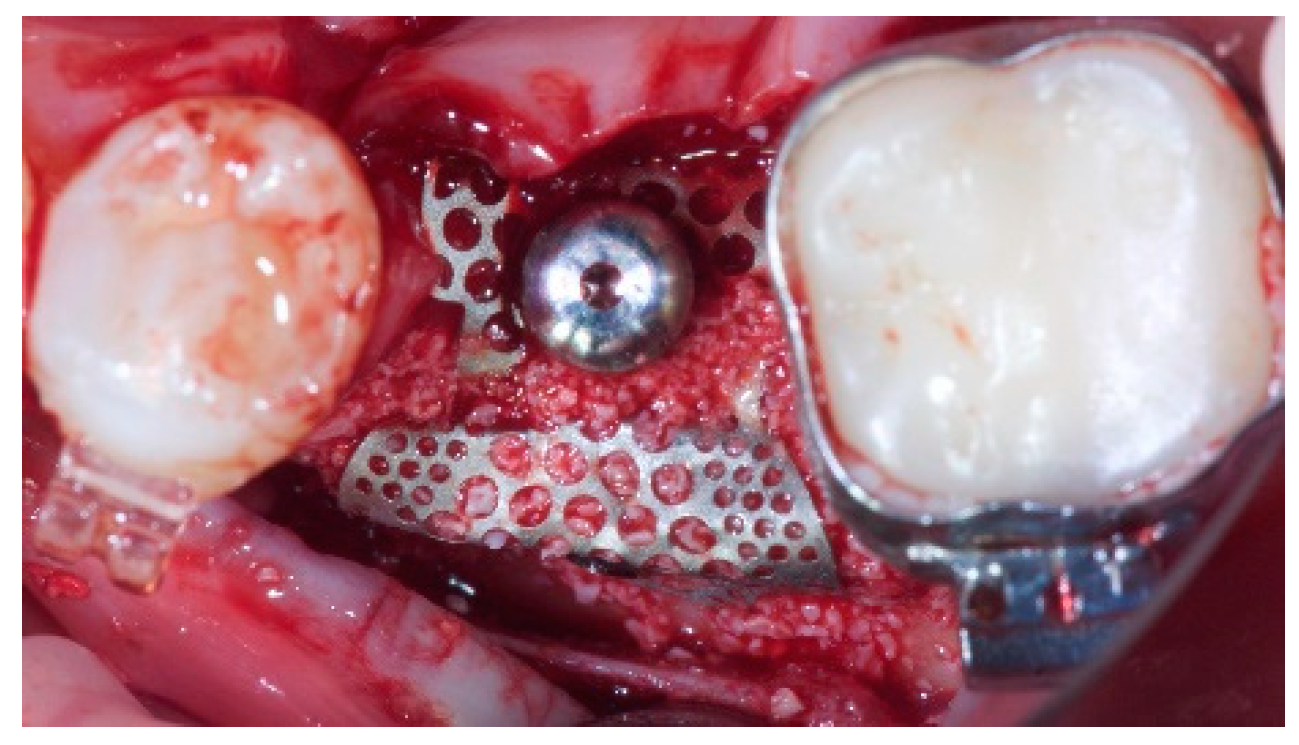
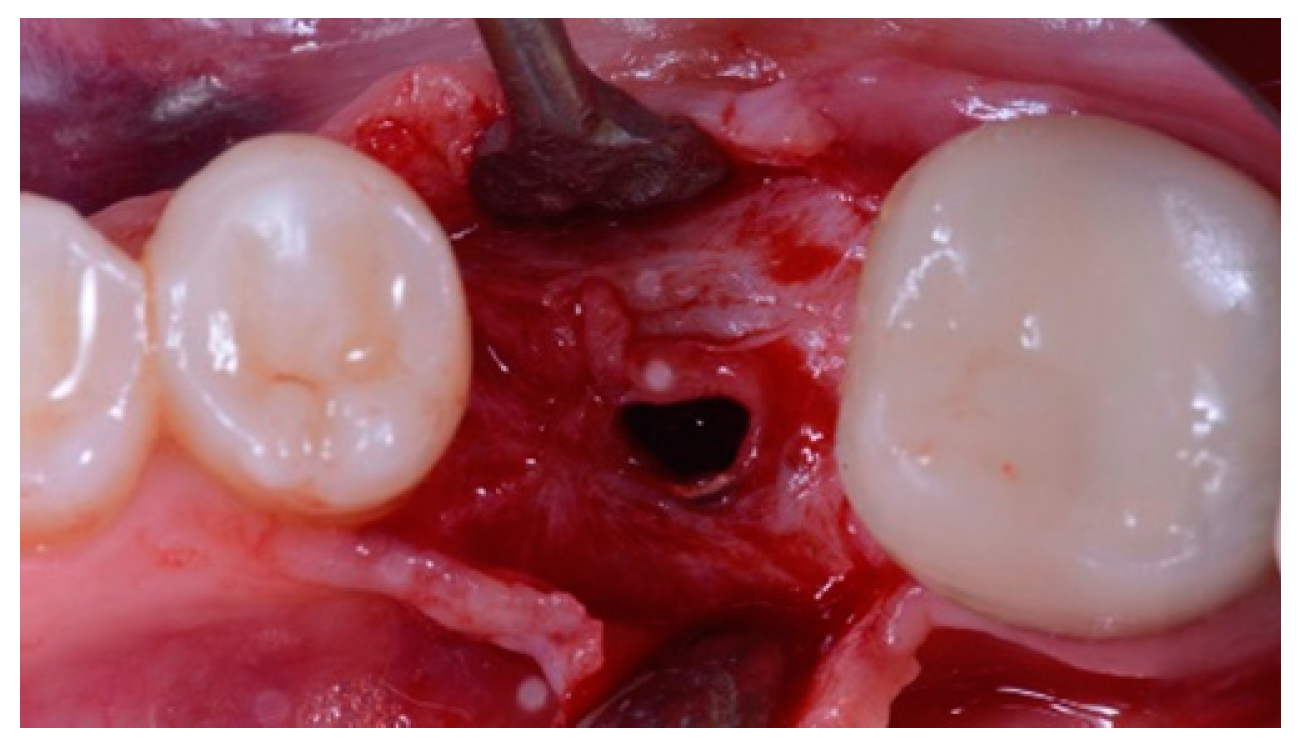
- Horizontal bone augmentation was evaluated at the CBCT scans, 1 mm below the original bone crest. The volumetric data were superimposed using the adjacent teeth as reference points, and a new generated set of DICOM data was stored as a separately files. Measures were taken before and after the treatment, and the difference of these two measurements was taken as horizontal bone augmentation (Figure 9).
- Volumetric measurements of the reconstructed bone was performed automatically on the merged CBCT set of volumetric data, using the Fusion module of the OnDemand 3D software (Cybermed Inc., Seoul, Korea), according a previously published protocol [8].
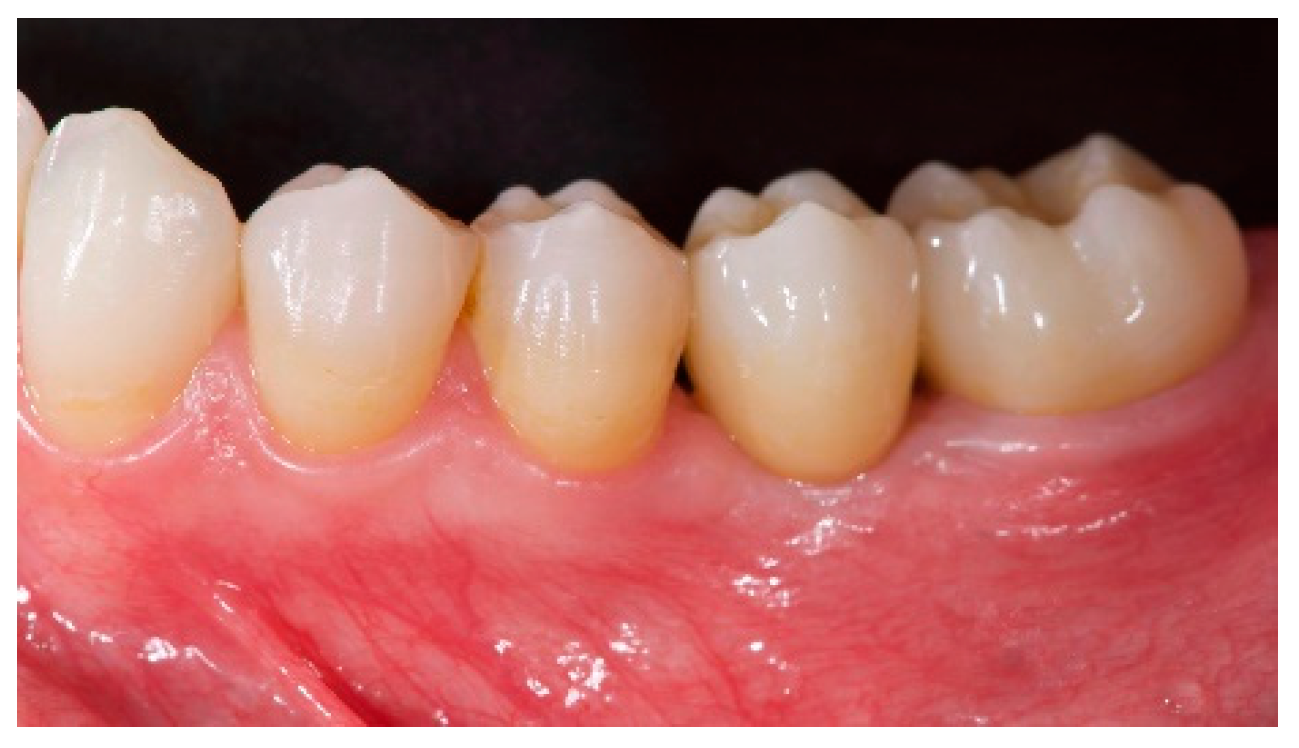
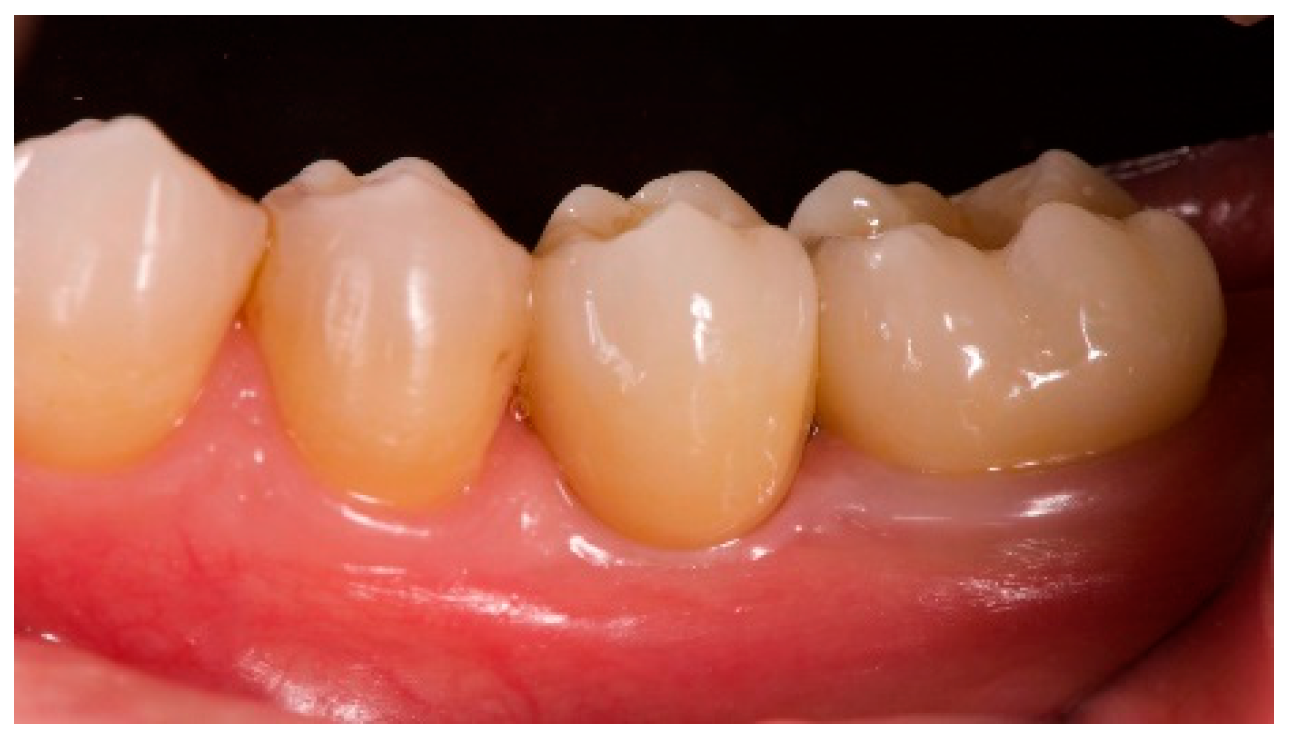
- The aesthetic evaluation was performed according to the pink aesthetic score (PES) on the vestibular and occlusal pictures taken including at least one adjacent tooth per side. The values were assessed at 6 and 12-months after loading follow-up examinations (18-months follow-up) [14] Seven variables (mesial papilla, distal papilla, soft-tissue level, soft-tissue contour, alveolar process deficiency, soft-tissue color and texture) were assessed with a 2-1-0 score (2 being best and 0 being poorest) by the same blinded dentist.
4. Results
5. Discussion
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Atwood, D.A. Reduction of residual ridges: a major oral disease entita. J. Prosthet. Dent. 1971, 26, 266–279. [Google Scholar] [CrossRef]
- Chiapasco, M.; Casentini, P.; Zaniboni, M. Bone augmentation procedures in implant dentistry. Int. J. Oral Implant. 2009, 24, 237–259. [Google Scholar]
- Rakhmatia, Y.D.; Jinno, Y.; Furuhashi, A.; Koyano, K.; Ayukawa, Y. Micro-computed tomography analysis of early stage bone healing using micro-porous titanium mesh for guided bone regeneration: preliminary experiment in a canine model. Odontology 2017, 8, 25–417. [Google Scholar] [CrossRef]
- Meloni, S.M.; Jovanovic, S.A.; Pisano, M.; De Riu, G.; Baldoni, E.; Tallarico, M. One-stage horizontal guided bone regeneration with autologous bone, anorganic bovine bone and collagen membranes: Follow-up of a prospective study 30 months after loading. Eur. J. Oral Implantol. 2018, 11, 89–95. [Google Scholar] [PubMed]
- Becker, W.; Becker, B.; Mellonig, J. A prospective multicenter study evaluating periodontal regeneration for class II furcation invasions and infrabony defects after treatment with a bioabsorbable barrier membrane: 1-year results. J. Periodontol. 1996, 67, 641–649. [Google Scholar] [CrossRef]
- Zitzmann, N.U.; Naef, R.; Scharer, P. Resorbable versus nonresorbable membranes in combination with Bio-Oss for guided bone regeneration. Int. J. Oral Maxillofac. Implants 1997, 12, 844–852. [Google Scholar] [PubMed]
- Merli, M.; Moscatelli, M.; Mariotti, G.; Rotundo, R.; Bernardelli, F.; Nieri, M. Bone level variation after vertical ridge augmentation: resorbable barriers versus titanium-reinforced barriers. A 6-year double-blind randomized clinical trial. Int. J. Oral Maxillofac. Implants 2014, 29, 905–913. [Google Scholar] [CrossRef] [PubMed]
- Meloni, S.M.; Jovanovic, S.A.; Urban, I.; Canullo, L.; Pisano, M.; Tallarico, M. Horizontal Ridge Augmentation using GBR with a Native Collagen Membrane and 1:1 Ratio of Particulated Xenograft and Autologous Bone: A 1-Year Prospective Clinical Study. Clin. Implant Dent. Relat. Res. 2017, 19, 38–45. [Google Scholar] [CrossRef]
- Sottosanti, J.S. Calcium sulfate: a valuable addition to the implant/bone regeneration complex. Dent. Implant. Updat. 1997, 8, 25–29. [Google Scholar]
- Roccuzzo, M.; Ramieri, G.; Spada, M.C.; Bianchi, S.D.; Berrone, S. Vertical alveolar ridge augmentation by means of a titanium mesh and autogenous bone grafts. Clin. Oral Implant. 2004, 15, 73–81. [Google Scholar] [CrossRef]
- Rakhmatia, Y.D.; Ayukawa, Y.; Furuhashi, A.; Koyano, K. Current barrier membranes: Titanium mesh and other membranes for guided bone regeneration in dental applications. J. Prosthodont. 2013, 57, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Tallarico, M.; Martinolli, M.; Kim, Y.; Cocchi, F.; Meloni, S.M.; Alushi, A.; Xhanari, E. Accuracy of Computer-Assisted Template-Based Implant Placement Using Two Different Surgical Templates Designed with or without Metallic Sleeves: A Randomized Controlled Trial. Dent. J. 2019, 7, 41. [Google Scholar] [CrossRef]
- Tallarico, M.; Kim, Y.J.; Cocchi, F.; Martinolli, M.; Meloni, S.M. Accuracy of newly developed sleeve-designed templates for insertion of dental implants: A prospective multicenters clinical trial. Clin. Implant Dent. Relat. Res. 2019, 21, 108–113. [Google Scholar] [CrossRef]
- Fürhauser, R.; Florescu, D.; Benesch, T.; Haas, R.; Mailath, G.; Watzek, G. Evaluation of soft tissue around single-tooth implant crowns: the pink esthetic score. Clin. Oral Implant. 2005, 16, 639–644. [Google Scholar] [CrossRef] [PubMed]
- Ciocca, L.; Lizio, G.; Baldissara, P.; Sambuco, A.; Scotti, R.; Corinadesi, G. Prosthetically CAD-CAM–Guided Bone Augmentation of Atrophic Jaws Using Customized Titanium Mesh: Preliminary Results of an Open Prospective Study. J. Oral Implant. 2018, 44, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Schopper, C.H.; Goriwoda, W.; Moser, D. Long-term results after guided bone regeneration with resorbable and microporous titanium membranes. J. Oral Maxillofac. Surg. Clin. North Am. 2001, 13, 449–458. [Google Scholar]
- Scantlebury, T.V. 1982-1992: a decade of technology development for guided tissue regeneration. J. Periodontol. 1993, 64, 1129–1137. [Google Scholar] [CrossRef]
- A Fugazzotto, P. Success and failure rates of osseointegrated implants in function in regenerated bone for 72 to 133 months. Int. J. Oral Implant. 2005, 20, 77–83. [Google Scholar]
- Kim, Y.-K.; Yun, P.-Y.; Kim, S.-G.; Oh, D.S. In vitro scanning electron microscopic comparison of inner surface of exposed and unexposed nonresorbable membranes. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontology 2009, 107, e5–e11. [Google Scholar] [CrossRef] [PubMed]
- Watzinger, F.; Luksch, J.; Millesi, W.; Schopper, C.; Neugebauer, J.; Moser, D.; Ewers, R. Guided bone regeneration with titanium membranes: a clinical study. Br. J. Oral Surg. 2000, 38, 312–315. [Google Scholar] [CrossRef]
- Selvig, K.A.; Kersten, B.G.; Chamberlain, A.D.H.; Wikesjö, U.M.E.; Nilvúus, R.E. Regenerative Surgery of Intrabony Periodontal Defects Using ePTFE Barrier Membranes: Scanning Electron Microscopic Evaluation of Retrieved Membranes Versus Clinical Healing. J. Periodontol. 1992, 63, 974–978. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, H.; Masui, S.; Ishihata, H.; Kaneko, T.; Ishida, D.; Endo, M.; Kanno, C.; Yamazaki, M.; Kitabatake, T.; Utsunomiya, S.; et al. Evaluation of a Newly Designed Microperforated Pure Titanium Membrane for Guided Bone Regeneration. Int. J. Oral Maxillofac. Implants 2019, 34, 411–422. [Google Scholar] [CrossRef] [PubMed]
- Roberts, E.W.; E Simmons, K.; Garetto, L.P.; A DeCastro, R. Bone physiology and metabolism in dental implantology: risk factors for osteoporosis and other metabolic bone diseases. Implant. Dent. 1992, 1, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Wessing, B.; Urban, I.; Montero, E.; Zechner, W.; Hof, M.; Chamorro, J.A.; Martin, N.A.; Polizzi, G.; Meloni, S.; Sanz, M. A multicenter randomized controlled clinical trial using a new resorbable non-cross-linked collagen membrane for guided bone regeneration at dehisced single implant sites: interim results of a bone augmentation procedure. Clin. Oral Implants Res. 2017, 28, e218–e226. [Google Scholar] [CrossRef] [PubMed]
- Urban, I.; Wessing, B.; Alández, N.; Meloni, S.; González-Martin, O.; Polizzi, G.; Sanz-Sanchez, I.; Montero, E.; Zechner, W. A multicenter randomized controlled trial using a novel collagen membrane for guided bone regeneration at dehisced single implant sites: Outcome at prosthetic delivery and at 1-year follow-up. Clin. Oral Implants Res. 2019. [Google Scholar] [CrossRef] [PubMed]

| Age (years) | 52.7 ± 20.3 |
| Mean Follow-up (months) | 20.85 ± 42.84 |
| Female patients | 5 |
| Male patients | 2 |
| Implant placed | 10 |
| Complications | 1 |
| Implant failure | 0 |
| Prosthesis failure | 0 |
| Insertion torque | 40 ± 5 |
| Implant Loading | 18-Month Follow-Up | |
|---|---|---|
| Mean marginal bone loss (mm) | 0.13 ± 0.09 (95% CI 0.08–0.19) | 0.28 ± 0.33 (95% CI 0.07–0.50) |
| Mean horizontal alveolar ridge (mm) | 2.92 ± 0.48 (95% CI 2.68–3.16) | 8.29 ± 2.14 (95% CI 7.59–8.99) |
| PES (mm) | 8.2 ± 0.8 (95% CI 7.7–8.7) | 12.0 ± 0.7 (95% CI 11.5–12.5) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tallarico, M.; Ceruso, F.M.; Muzzi, L.; Meloni, S.M.; Kim, Y.-J.; Gargari, M.; Martinolli, M. Effect of Simultaneous Immediate Implant Placement and Guided Bone Reconstruction with Ultra-Fine Titanium Mesh Membranes on Radiographic and Clinical Parameters after 18 Months of Loading. Materials 2019, 12, 1710. https://doi.org/10.3390/ma12101710
Tallarico M, Ceruso FM, Muzzi L, Meloni SM, Kim Y-J, Gargari M, Martinolli M. Effect of Simultaneous Immediate Implant Placement and Guided Bone Reconstruction with Ultra-Fine Titanium Mesh Membranes on Radiographic and Clinical Parameters after 18 Months of Loading. Materials. 2019; 12(10):1710. https://doi.org/10.3390/ma12101710
Chicago/Turabian StyleTallarico, Marco, Francesco Mattia Ceruso, Leonardo Muzzi, Silvio Mario Meloni, Yong-Jin Kim, Marco Gargari, and Matteo Martinolli. 2019. "Effect of Simultaneous Immediate Implant Placement and Guided Bone Reconstruction with Ultra-Fine Titanium Mesh Membranes on Radiographic and Clinical Parameters after 18 Months of Loading" Materials 12, no. 10: 1710. https://doi.org/10.3390/ma12101710
APA StyleTallarico, M., Ceruso, F. M., Muzzi, L., Meloni, S. M., Kim, Y.-J., Gargari, M., & Martinolli, M. (2019). Effect of Simultaneous Immediate Implant Placement and Guided Bone Reconstruction with Ultra-Fine Titanium Mesh Membranes on Radiographic and Clinical Parameters after 18 Months of Loading. Materials, 12(10), 1710. https://doi.org/10.3390/ma12101710







