Effects of Lignin-Based Hollow Nanoparticle Structure on the Loading and Release Behavior of Doxorubicin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
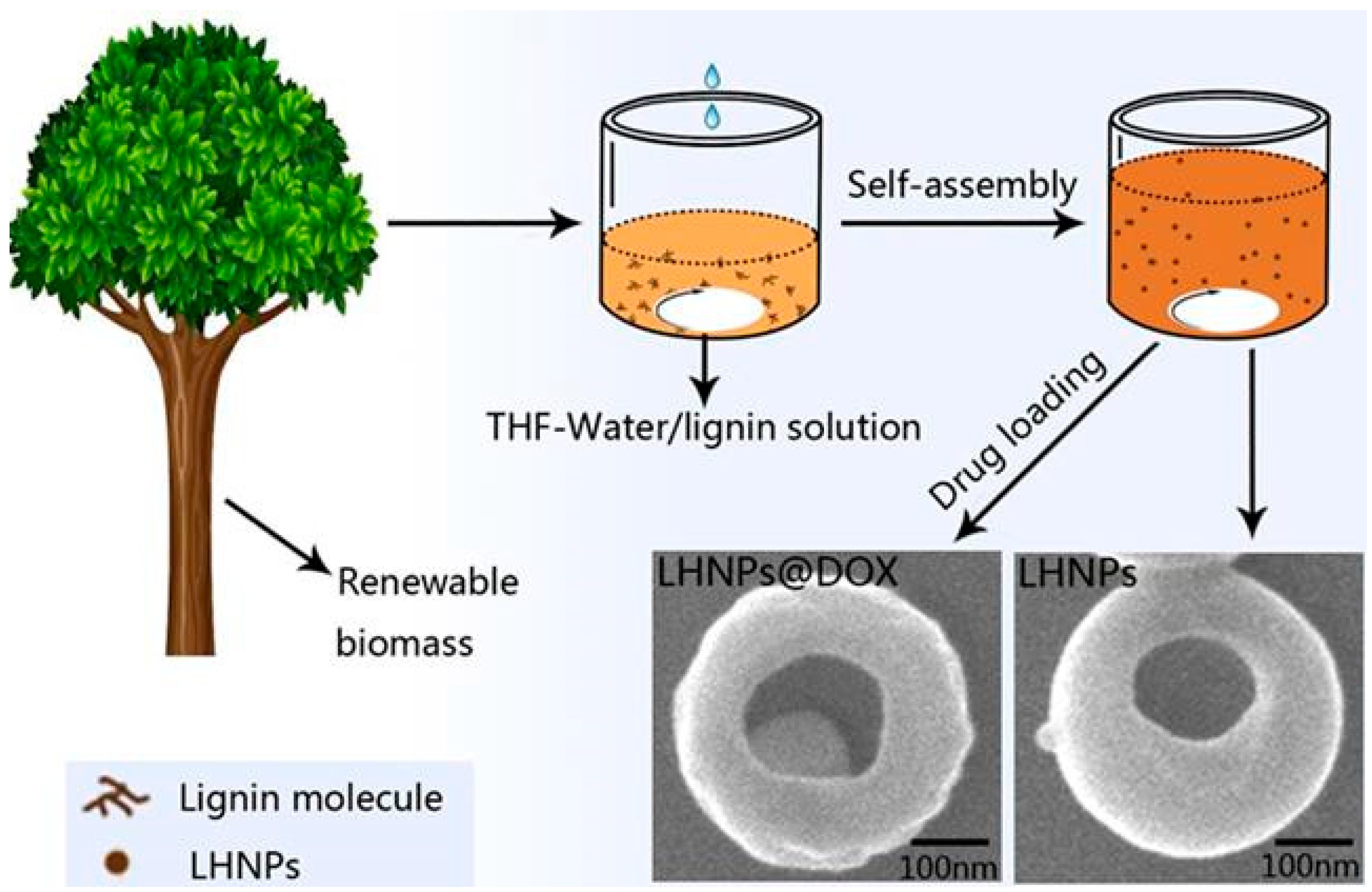
2.2. Synthesis of Lignin Hollow Nanoparticles
2.3. Drug Loading
2.4. In Vitro Release Studies
2.5. Cellular Uptake of Modified LHNPs Assays
3. Results and Discussion
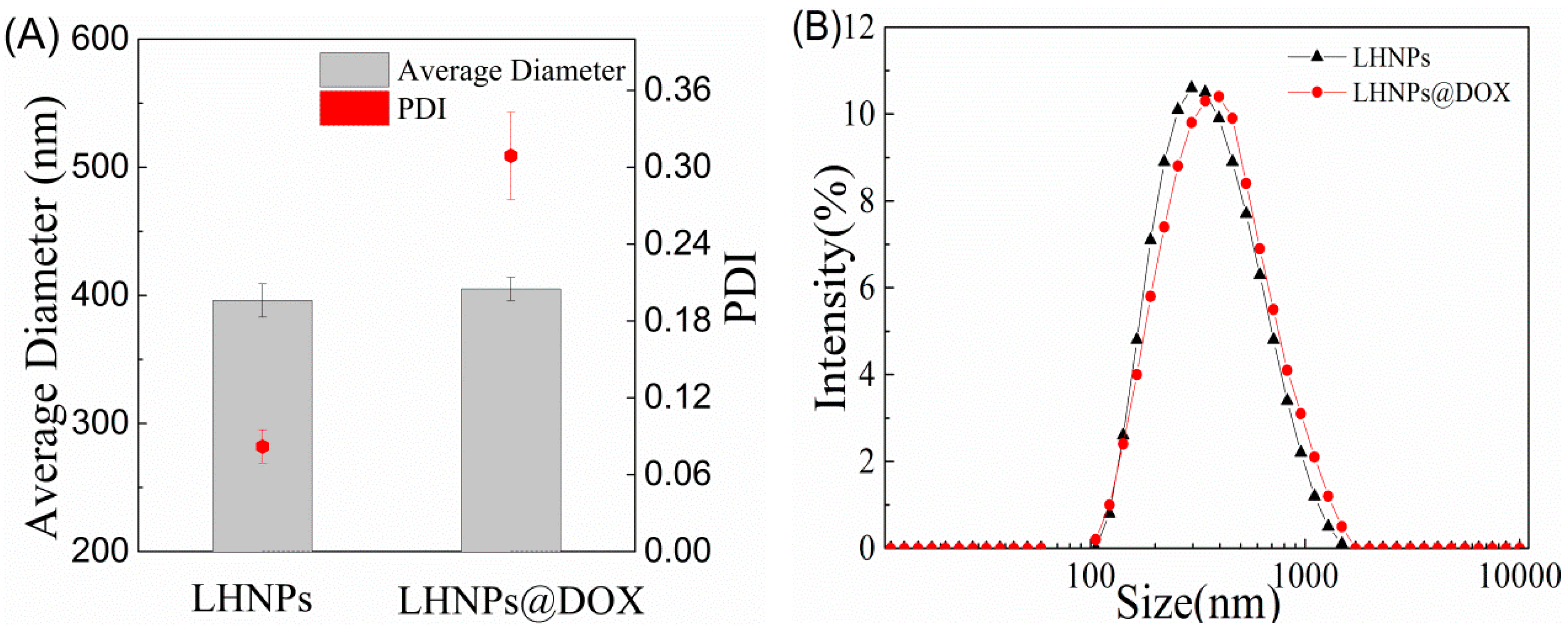
3.1. Morphology of EHL, Lignin Hollow Nanoparticles, and Drug-Loaded Lignin Nanoparticles
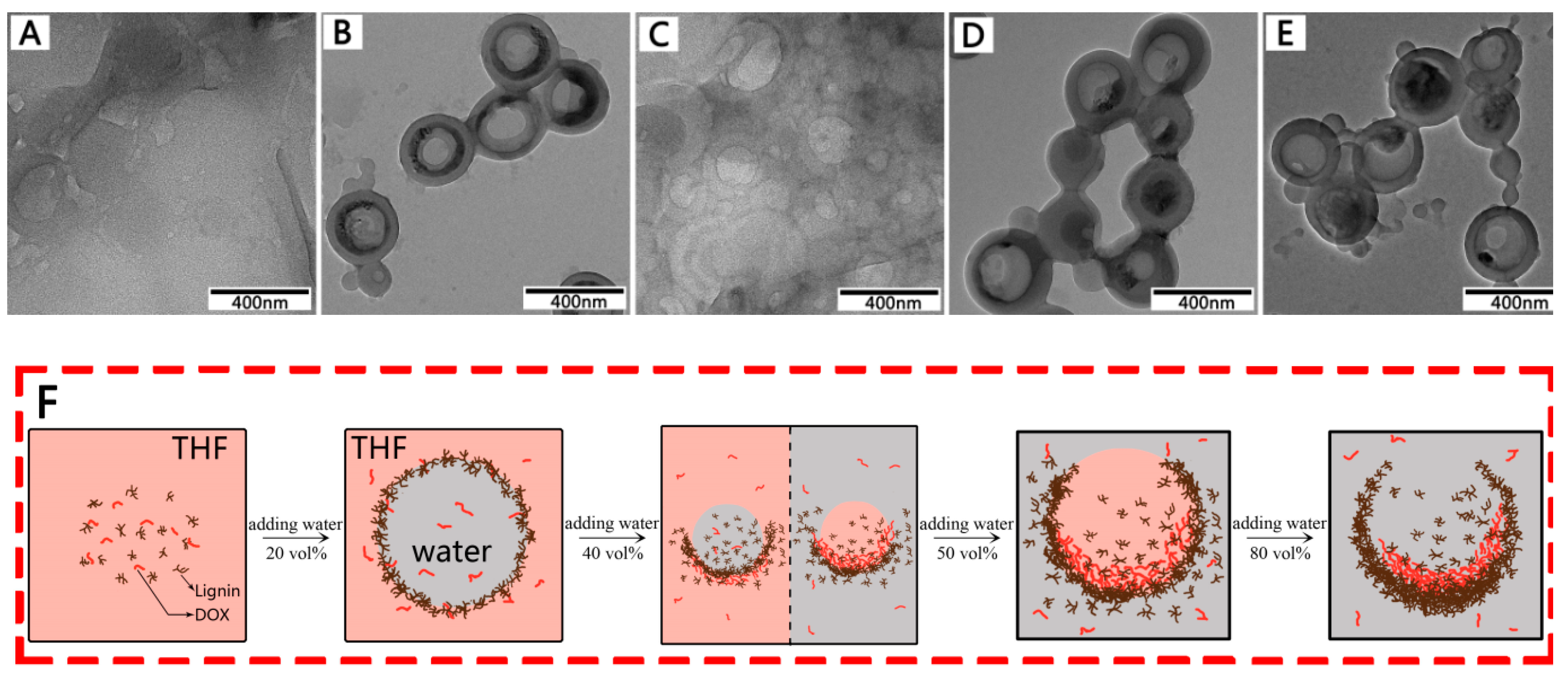
3.2. Morphological Structure Regulation of Lignin Hollow Nanoparticles
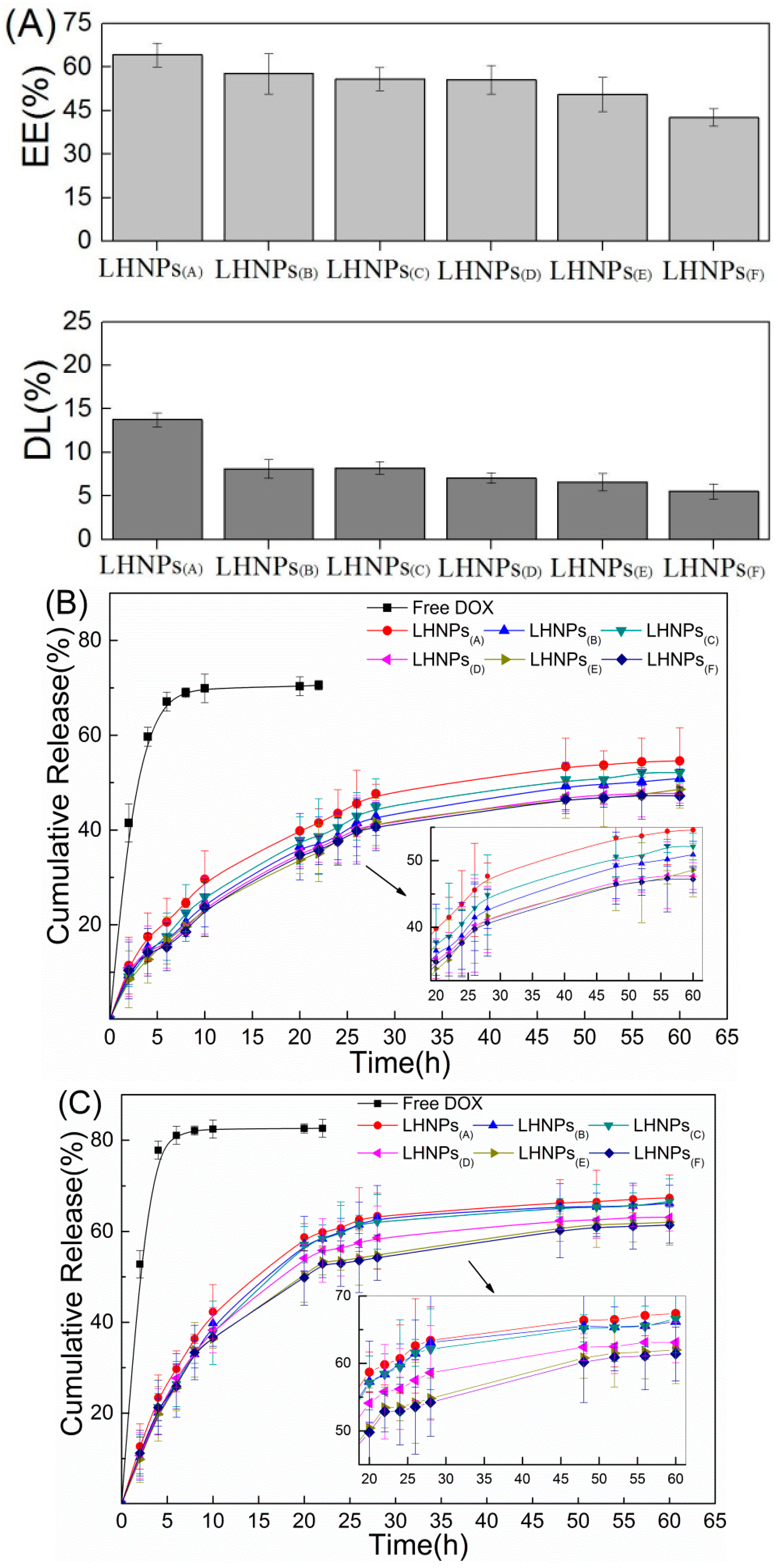
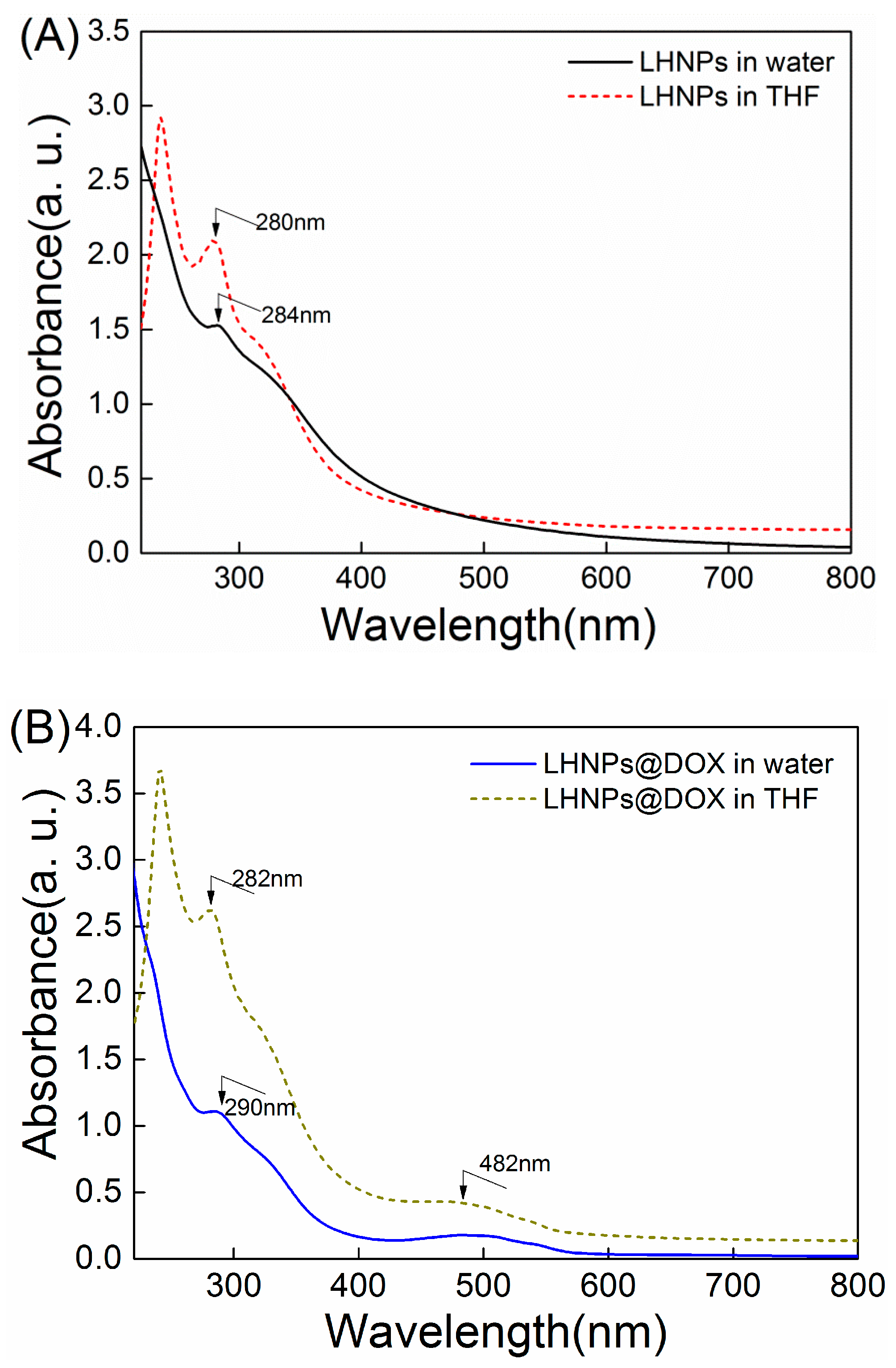
3.3. Drug Loading and In Vitro Release Studies
3.4. Formation Mechanism of DOX-Loaded Lignin Nanoparticles
3.5. LHNPs and DOX-Loading LHNPs Cellular Uptake
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Duval, A.; Lawoko, M. A review on lignin-based polymeric, micro- and nano-structured materials. React. Funct. Polym. 2014, 85, 78–96. [Google Scholar] [CrossRef]
- Laurichesse, S.; Avérous, L. Chemical modification of lignins: Towards biobased polymers. Prog. Poly. Sci. 2014, 39, 1266–1290. [Google Scholar] [CrossRef]
- Figueiredo, P.; Lintinen, K.; Kiriazis, A.; Hynninen, V.; Liu, Z.; Baulethramos, T.; Rahikkala, A.; Correia, A.; Kohout, T.; Sarmento, B. In vitro evaluation of biodegradable lignin-based nanoparticles for drug delivery and enhanced antiproliferation effect in cancer cells. Biomaterials 2017, 121, 97. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Deng, Y.; Liu, B.; Ren, Y.; Liang, J.; Qian, Y.; Qiu, X.; Li, C.; Zheng, D. Preparation of nanocapsules via the self-assembly of kraft lignin: A totally green process with renewable resources. ACS Sustain. Chem. Eng. 2016, 4, 1946–1953. [Google Scholar] [CrossRef]
- Hong, N.; Li, Y.; Qiu, X. A highly efficient dispersant from black liquor for carbendazim suspension concentrate: Preparation, self-assembly behavior and investigation of dispersion mechanism. J. Appl. Polym. Sci. 2016, 133. [Google Scholar] [CrossRef]
- Flavahan, W.A.; Gaskell, E.; Bernstein, B.E. Epigenetic plasticity and the hallmarks of cancer. Science 2017, 357, eaal2380. [Google Scholar] [CrossRef]
- Blanco, E.; Shen, H.; Ferrari, M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol. 2015, 33, 941–951. [Google Scholar] [CrossRef]
- Mou, X.; Ali, Z.; Li, S.; He, N. Applications of magnetic nanoparticles in targeted drug delivery system. J. Nanosci. Nanotechnol. 2015, 15, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Chidambaram, M.; Manavalan, R.; Kathiresan, K. Nanotherapeutics to overcome conventional cancer chemotherapy limitations. J. Pharm. Pharm. Sci. 2011, 14, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Qiu, M.; Sun, H.; Meng, F.; Cheng, R.; Zhang, J.; Deng, C.; Zhong, Z. Lipopepsomes: A novel and robust family of nano-vesicles capable of highly efficient encapsulation and tumor-targeted delivery of doxorubicin hydrochloride in vivo. J. Control. Release 2018, 272, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Si, Y.; Chen, M.; Wu, L. Syntheses and biomedical applications of hollow micro-/nano-spheres with large-through-holes. Chem. Soc. Rev. 2016, 45, 690–714. [Google Scholar] [CrossRef]
- Xu, J.; Ma, A.; Xu, Z.; Liu, X.; Chu, D.; Xu, H. Synthesis of au and pt hollow capsules with single holes via pickering emulsion strategy. J. Phys. Chem. C 2016, 119, 28055–28060. [Google Scholar] [CrossRef]
- Carvalho, C.; Santos, R.X.; Cardoso, S.; Correia, S.; Oliveira, P.J.; Santos, M.S.; Moreira, P.I. Doxorubicin: The good, the bad and the ugly effect. Curr. Med. Chem. 2009, 16, 3267–3285. [Google Scholar] [CrossRef]
- Minotti, G.; Menna, P.; Salvatorelli, E.; Cairo, G.; Gianni, L. Anthracyclines: Molecular advances and pharmacologic developments in antitumor activity and cardiotoxicity. Pharmacol. Rev. 2004, 56, 185–229. [Google Scholar] [CrossRef] [PubMed]
- Mcrae, P.S.; Henchey, E.; Chen, X.; Schneider, S.; Emrick, T. Efficacy of polympc-dox prodrugs in 4t1 tumor-bearing mice. Mol. Pharm. 2014, 11, 1715–1720. [Google Scholar] [CrossRef]
- Xiong, F.; Han, Y.; Wang, S.; Li, G.; Qin, T.; Chen, Y.; Chu, F. Preparation and formation mechanism of renewable lignin hollow nanospheres with a single hole by self-assembly. ACS Sustain. Chem. Eng. 2017, 5, 2273–2281. [Google Scholar] [CrossRef]
- Chen, N.; Dempere, L.A.; Tong, Z. Synthesis of pH-responsive lignin based nanocapsules for controlled release of hydrophobic molecules. ACS Sustain. Chem. Eng. 2016, 4, 5204–5211. [Google Scholar] [CrossRef]
- Dai, L.; Liu, R.; Hu, L.; Zou, Z.; Si, C. Lignin nanoparticle as a novel green carrier for the efficient delivery of resveratrol. ACS Sustain. Chem. Eng. 2017, 5, 8241–8249. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, M.; Pang, Y.; Qiu, X. Lignin-based microsphere: Preparation and performance on encapsulating the pesticide avermectin. ACS Sustain. Chem. Eng. 2017, 5, 3321–3328. [Google Scholar] [CrossRef]
- Lievonen, M.; Valle-Delgado, J.J.; Mattinen, M.L.; Hult, E.L.; Lintinen, K.; Kostiainen, M.A.; Paananen, A.; Szilvay, G.R.; Setälä, H.; Österberg, M. Simple process for lignin nanoparticle preparation. Green Chem. 2016, 18, 1416–1422. [Google Scholar] [CrossRef]
- Mateovic, T.; Kriznar, B.; Bogataj, M.; Mrhar, A. The influence of stirring rate on biopharmaceutical properties of eudragit rs microspheres. J. Microencapsul. 2002, 19, 29–36. [Google Scholar] [CrossRef]
- Liu, Z.; Qie, R.; Li, W.; Hong, N.; Li, Y.; Li, C.; Wang, R.; Shi, Y.; Guo, X.; Jia, X. Preparation of avermectin microcapsules with anti-photodegradation and slow-release by the assembly of lignin derivatives. New J. Chem. 2017, 41, 3190–3195. [Google Scholar] [CrossRef]
- Liu, J.; Pang, Y.; Huang, W.; Zhu, Z.; Zhu, X.; Zhou, Y.; Yan, D. Redox-responsive polyphosphate nanosized assemblies: A smart drug delivery platform for cancer therapy. Biomacromolecules 2011, 12, 2407–2415. [Google Scholar] [CrossRef] [PubMed]
- Sheng, W.B.; Li, W.; Zhang, G.X.; Tong, Y.B.; Liu, Z.Y.; Jia, X. Study on the uv-shielding and controlled-release properties of a polydopamine coating for avermectin. New J. Chem. 2015, 39, 2752–2757. [Google Scholar] [CrossRef]
- Antipov, A.A.; Sukhorukov, G.B.; Edwin Donath, A.; Möhwald, H. Sustained release properties of polyelectrolyte multilayer capsules. J. Phys. Chem. B 2001, 105, 2281–2284. [Google Scholar] [CrossRef]
- Yu, P.; Xia, X.M.; Wu, M.; Cui, C.; Zhang, Y.; Liu, L.; Wu, B.; Wang, C.X.; Zhang, L.J.; Zhou, X. Folic acid-conjugated iron oxide porous nanorods loaded with doxorubicin for targeted drug delivery. Colloids Surf. B Biointerfaces 2014, 120, 142–151. [Google Scholar] [CrossRef]
- Xiong, F.; Chu, F.; Li, G.; Wang, S.; Qin, T.; Han, Y.; Chen, Y. Preparation and formation mechanism of size-controlled lignin nanospheres by self-assembly. Ind. Crop. Prod. 2017, 100, 146–152. [Google Scholar] [CrossRef]
- Norato, M.A.; Tavlarides, L.L.; Tsouris, C. Phase inversion studies in liquid-liquid dispersions. Can. J. Chem. Eng. 2010, 76, 486–494. [Google Scholar] [CrossRef]
- Deng, Y.; Zhao, H.; Qian, Y.; Lei, L.; Wang, B.; Qiu, X. Hollow lignin azo colloids encapsulated avermectin with high anti-photolysis and controlled release performance. Ind. Crop. Prod. 2016, 87, 191–197. [Google Scholar] [CrossRef]
- Lv, H.; Lin, Q.; Zhang, K.; Yu, K.; Yao, T.; Zhang, X.; Zhang, J.; Yang, B. Facile fabrication of monodisperse polymer hollow spheres. Langmuir ACS J. Surf. Colloids 2008, 24, 13736–13741. [Google Scholar] [CrossRef]
- Ding, J.; Liang, T.; Zhou, Y.; He, Z.; Min, Q.; Jiang, L.; Zhu, J. Hyaluronidase-triggered anticancer drug and sirna delivery from cascaded targeting nanoparticles for drug-resistant breast cancer therapy. Nano Res. 2017, 10, 690–703. [Google Scholar] [CrossRef]
- Wang, H.; He, J.; Zhang, M.; Tao, Y.; Li, F.; Tam, K.C.; Ni, P. Biocompatible and acid-cleavable poly(ε-caprolactone)-acetal-poly(ethylene glycol)-acetal-poly(ε-caprolactone) triblock copolymers: Synthesis, characterization and ph-triggered doxorubicin delivery. J. Mater. Chem. B 2013, 1, 6596–6607. [Google Scholar] [CrossRef]
- Dai, L.; Zhu, W.; Liu, R.; Si, C. Lignin-containing self-nanoemulsifying drug delivery system for enhance stability and oral absorption of trans-resveratrol. Part. Part. Syst. Charact. 2018, 35, 1700447. [Google Scholar] [CrossRef]




© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Y.; Han, Y.; Li, G.; Chu, F. Effects of Lignin-Based Hollow Nanoparticle Structure on the Loading and Release Behavior of Doxorubicin. Materials 2019, 12, 1694. https://doi.org/10.3390/ma12101694
Zhou Y, Han Y, Li G, Chu F. Effects of Lignin-Based Hollow Nanoparticle Structure on the Loading and Release Behavior of Doxorubicin. Materials. 2019; 12(10):1694. https://doi.org/10.3390/ma12101694
Chicago/Turabian StyleZhou, Yu, Yanming Han, Gaiyun Li, and Fuxiang Chu. 2019. "Effects of Lignin-Based Hollow Nanoparticle Structure on the Loading and Release Behavior of Doxorubicin" Materials 12, no. 10: 1694. https://doi.org/10.3390/ma12101694
APA StyleZhou, Y., Han, Y., Li, G., & Chu, F. (2019). Effects of Lignin-Based Hollow Nanoparticle Structure on the Loading and Release Behavior of Doxorubicin. Materials, 12(10), 1694. https://doi.org/10.3390/ma12101694




