The Phase Diagram and Exotic Magnetostrictive Behaviors in Spinel Oxide Co(Fe1−xAlx)2O4 System
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Composition Analysis and Structural Characterization
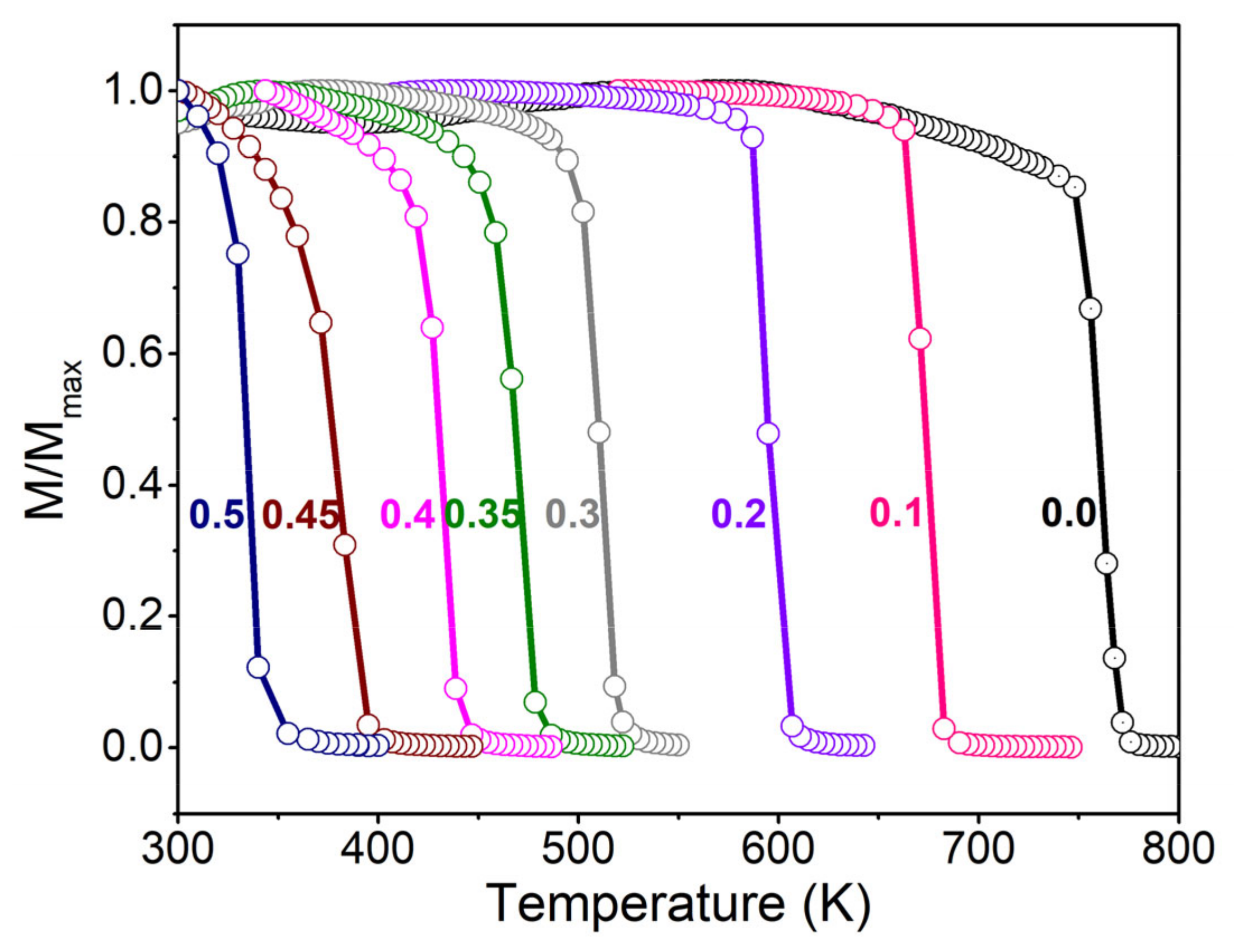
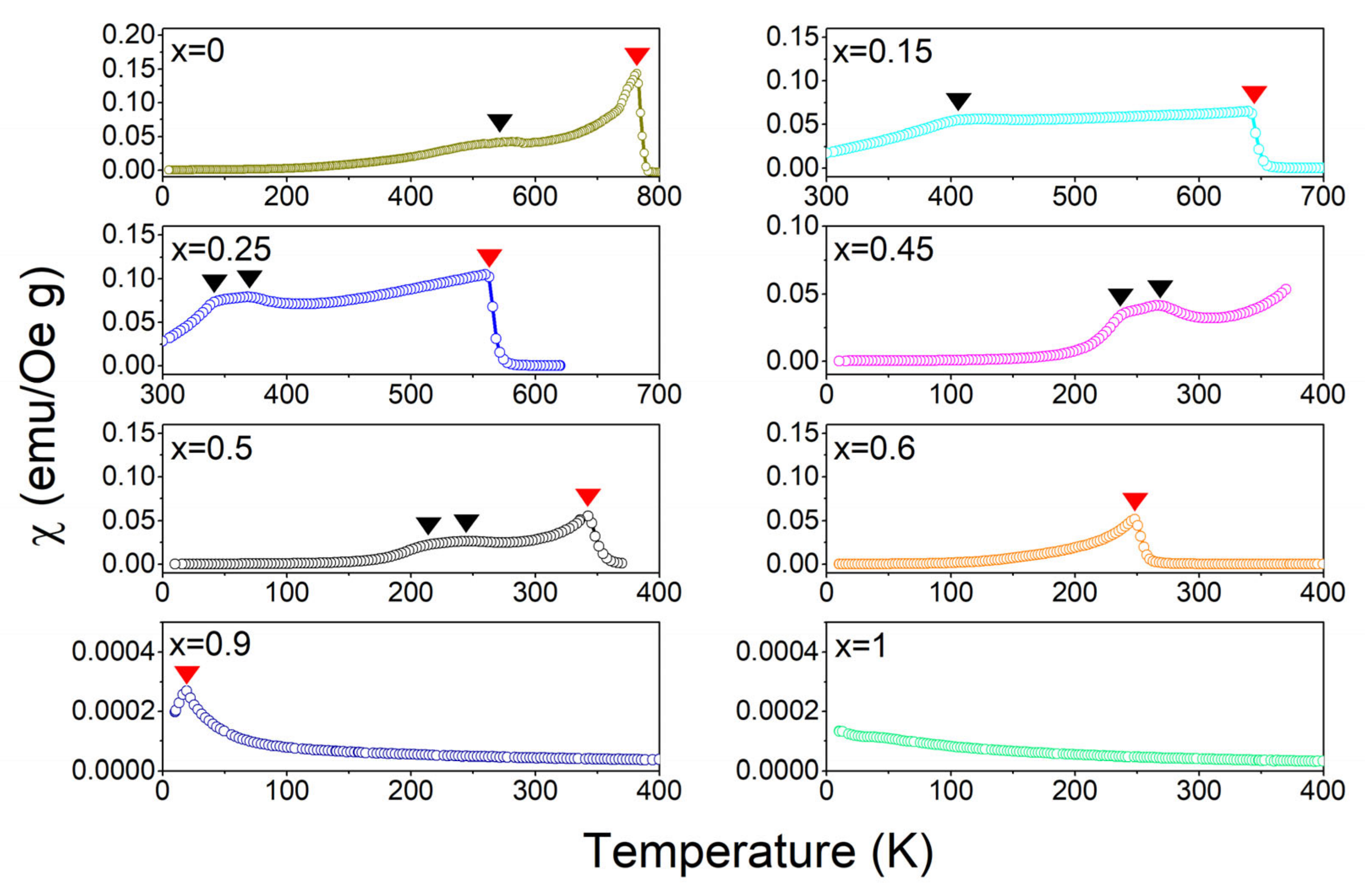
3.2. Temperature (T) Spectra of Magnetization (M) and Magnetic Susceptibility (χ)
3.3. Phase Diagram
3.4. M–H Hysteresis Loops
3.5. Magnetostriction as a Function of Composition and Temperature
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- O’Handley, R.C. Modern Magnetic Materials: Principles and Applications; John Wiley&Sons, Inc.: New York, NY, USA, 1999. [Google Scholar]
- Bozorth, R.M.; Tilden, E.F.; Williams, A.J. Anisotropy and Magnetostriction of Some Ferrites. Phys. Rev. 1955, 99, 1788–1798. [Google Scholar] [CrossRef]
- Nlebedim, I.; Snyder, J.; Moses, A.; Jiles, D. Dependence of the magnetic and magnetoelastic properties of cobalt ferrite on processing parameters. J. Magn. Magn. Mater. 2010, 322, 3938–3942. [Google Scholar] [CrossRef]
- Bhame, S.D.; Joy, P.A. Effect of Sintering Conditions and Microstructure on the Magnetostrictive Properties of Cobalt Ferrite. J. Am. Ceram. Soc. 2008, 91, 1976–1980. [Google Scholar] [CrossRef]
- Chae, K.P.; Lee, J.-G.; Kweon, H.S.; Lee, Y.B. The crystallographic, magnetic properties of Al, Ti doped CoFe2O4 powders grown by sol–gel method. J. Magn. Magn. Mater. 2004, 283, 103–108. [Google Scholar] [CrossRef]
- Atif, M.; Turtelli, R.S.; Grössinger, R.; Kubel, F. Influence of manganese substitution on the microstructure and magnetostrictive properties of Co1−xMnxFe2O4 (x = 0.0–0.4) ferrite. J. Appl. Phys. 2013, 113, 153902. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, W.; Liu, E.; Tang, X.; Li, G.; Zhang, H.; Wu, G. Compensation Effect and Magnetostriction in CoCr2−xFexO4. Phys. Status Solidi (b) 2013, 250, 1287–1292. [Google Scholar] [CrossRef]
- Ranvah, N.; Nlebedim, I.C.; Melikhov, Y.; Snyder, J.E.; Jiles, D.C.; Moses, A.J.; Williams, P.I.; Anayi, F.; Song, S. Temperature Dependence of Magnetostriction of Co1+xGexFe2−2xO4 for Magnetostrictive Sensor and Actuator Applications. IEEE Trans. Magn. 2008, 44, 3013–3016. [Google Scholar] [CrossRef]
- Song, S.H.; Lo, C.C.H.; Lee, S.J.; Aldini, S.T.; Snyder, J.E.; Jiles, D.C. Magnetic and magnetoelastic properties of Ga-substituted cobalt ferrite. J. Appl. Phys. 2007, 101, 9. [Google Scholar] [CrossRef]
- Rao, G.S.N.; Caltun, O.F.; Rao, K.H.; Rao, B.P.; Wamocha, H.L.; Hamdeh, H.H. Influence of silicon and cobalt substitutions on magnetostriction coefficient of cobalt ferrite. ICAME 2007 2008, 184, 593–598. [Google Scholar]
- Nlebedim, I.C.; Ranvah, N.; Melikhov, Y.; Williams, P.I.; Snyder, J.E.; Moses, A.J.; Jiles, D.C. Magnetic and Magnetomechanical Properties of CoAlxFe2−xO4 for Stress Sensor and Actuator Applications. IEEE Trans. Magn. 2009, 45, 4120–4123. [Google Scholar] [CrossRef]
- Nlebedim, I.C.; Ranvah, N.; Melikhov, Y.; Williams, P.I.; Snyder, J.E.; Moses, A.J.; Jiles, D.C. Paulsen. Effect of Temperature Variation on the Magnetostrictive Properties of CoAlxFe2−xO4. J. Appl. Phys. 2010, 107, 09A936. [Google Scholar] [CrossRef]
- Aghav, P.; Dhage, V.N.; Mane, M.L.; Shengule, D.; Dorik, R.; Jadhav, K.; Jadhav, K. Effect of aluminum substitution on the structural and magnetic properties of cobalt ferrite synthesized by sol–gel auto combustion process. B: Condens. Matter 2011, 406, 4350–4354. [Google Scholar] [CrossRef]
- Toksha, B.G.; Shirsath, S.E.; Mane, M.L.; Jadhav, K.M. Auto-Ignition Synthesis of CoFe2O4 with Al3+ Substitution for High Frequency Applications. Ceram. Int. 2017, 43, 14347–14353. [Google Scholar] [CrossRef]
- Anantharamaiah, P.N.; Joy, P.A. Effect of Size and Site Preference of Trivalent Non-Magnetic Metal Ions (Al3+, Ga3+, In3+) Substituted for Fe3+ on the Magnetostrictive Properties of Sintered CoFe2O4. J. Phys. D: Appl. Phys. 2017, 50, 435005. [Google Scholar] [CrossRef]
- Kim, Y.I.; Kim, D.; Lee, C.S. Synthesis and characterization of CoFe2O4 magnetic nanoparticles prepared by temperature-controlled coprecipitation method. B: Condens. Matter 2003, 337, 42–51. [Google Scholar] [CrossRef]
- Mikac, L.; Marić, I.; Štefanić, G.; Jurkin, T.; Ivanda, M.; Gotić, M. Radiolytic Synthesis of Manganese Oxides and Their Ability to Decolorize Methylene Blue in Aqueous Solutions. Appl. Surf. Sci. 2019, 476, 1086–1095. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. Sect. A: Cryst. Physics, Diffraction, Theor. Crystallogr. 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Anantharamaiah, P.N.; Joy, P. Magnetic and magnetostrictive properties of aluminium substituted cobalt ferrite synthesized by citrate-gel method. J. Mater. Sci. 2015, 50, 6510–6517. [Google Scholar] [CrossRef]
- Roy, B.; Pandey, A.; Zhang, Q.; Heitmann, T.W.; Vaknin, D.; Johnston, D.C.; Furukawa, Y. Experimental evidence of a collinear antiferromagnetic ordering in the frustrated CoAl 2 O 4 spinel. Phys. B 2013, 88, 174415. [Google Scholar]
- Supriya, S.; Kumar, S.; Pradhan, L.K.; Pandey, R.; Kar, M. Multiple electrical phase transitions in nanocrystalline aluminium-substituted cobalt ferrite. Int. J. Phys. B 2018, 32, 1850358. [Google Scholar] [CrossRef]
- Vaingankar, A.S.; Khasbardar, B.V.; Patil, R.N. X-ray spectroscopic study of cobalt ferrite. J. Phys. F: Phys. 1980, 10, 1615–1619. [Google Scholar] [CrossRef]
- Yousif, A.A.; Elzain, M.E.; Sutherland, H.H.; Salah, S.H. Mössbauer studies on Al–Co ferrites. Hyperfine Interactions 1992, 68, 323–327. [Google Scholar] [CrossRef]
- Ghatage, A.K.; Choudhari, S.C.; Patil, S.A.; Paranjpe, S.K. X-ray, infrared and magnetic studies of chromium substituted nickel ferrite. J. Mater. Sci. Lett. 1996, 15, 1548–1550. [Google Scholar] [CrossRef]
- Ranvah, N.; Nlebedim, I.C.; Melikhov, Y.; Snyder, J.E.; Williams, P.I.; Moses, A.J.; Jiles, D.C. Temperature Dependence of Magnetic Properties of CoAlxFe2−xO4. IEEE Trans. Magn. 2009, 45, 4261–4264. [Google Scholar] [CrossRef]
- Zhou, C.; Ren, S.; Bao, H.; Yang, S.; Yao, Y.; Ji, Y.; Ren, X.; Matsushita, Y.; Katsuya, Y.; Tanaka, M.; et al. Inverse Effect of Morphotropic Phase Boundary on the Magnetostriction of Ferromagnetic Tb1−xGdxCo2. Phys. Rev. B 2014, 89, 100101. [Google Scholar] [CrossRef]





| Nominal Composition | Composition by EDX Analysis | |||
|---|---|---|---|---|
| Co | Fe | Al | O | |
| CoFe2O4 | 14.2% | 27.7% | 0 | 58.1% |
| Co(Fe0.95Al0.05)2O4 | 14.3% | 26.9% | 1.3% | 57.6% |
| Co(Fe0.85Al0.15)2O4 | 14.5% | 24.5% | 4.1% | 56.9% |
| Co(Fe0.8Al0.2)2O4 | 13.4% | 21.3% | 5.7% | 59.6% |
| Co(Fe0.75Al0.25)2O4 | 13.4% | 19.7% | 7.1% | 59.8% |
| Co(Fe0.7Al0.3)2O4 | 13.7% | 18.0% | 7.7% | 60.6% |
| Co(Fe0.65Al0.35)2O4 | 13.4% | 17.2% | 9.8% | 59.6% |
| Co(Fe0.6Al0.4)2O4 | 12.5% | 16.0% | 10.7% | 60.8% |
| Co(Fe0.55Al0.45)2O4 | 13.5% | 15.8% | 12.6% | 58.0% |
| Co(Fe0.5Al0.5)2O4 | 14.5% | 12.5% | 13.2% | 59.8% |
| Co(Fe0.4Al0.6)2O4 | 14.2% | 10.3% | 17.1% | 58.5% |
| Co(Fe0.25Al0.75)2O4 | 14.7% | 6.7% | 19.3% | 59.4% |
| Co(Fe0.1Al0.9)2O4 | 15.0% | 2.2% | 23.4% | 59.4% |
| CoAl2O4 | 13.5% | 0 | 25.7% | 60.8% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, C.; Zhang, A.; Chang, T.; Chen, Y.; Zhang, Y.; Tian, F.; Zuo, W.; Ren, Y.; Song, X.; Yang, S. The Phase Diagram and Exotic Magnetostrictive Behaviors in Spinel Oxide Co(Fe1−xAlx)2O4 System. Materials 2019, 12, 1685. https://doi.org/10.3390/ma12101685
Zhou C, Zhang A, Chang T, Chen Y, Zhang Y, Tian F, Zuo W, Ren Y, Song X, Yang S. The Phase Diagram and Exotic Magnetostrictive Behaviors in Spinel Oxide Co(Fe1−xAlx)2O4 System. Materials. 2019; 12(10):1685. https://doi.org/10.3390/ma12101685
Chicago/Turabian StyleZhou, Chao, Azhen Zhang, Tieyan Chang, Yusheng Chen, Yin Zhang, Fanghua Tian, Wenliang Zuo, Yang Ren, Xiaoping Song, and Sen Yang. 2019. "The Phase Diagram and Exotic Magnetostrictive Behaviors in Spinel Oxide Co(Fe1−xAlx)2O4 System" Materials 12, no. 10: 1685. https://doi.org/10.3390/ma12101685
APA StyleZhou, C., Zhang, A., Chang, T., Chen, Y., Zhang, Y., Tian, F., Zuo, W., Ren, Y., Song, X., & Yang, S. (2019). The Phase Diagram and Exotic Magnetostrictive Behaviors in Spinel Oxide Co(Fe1−xAlx)2O4 System. Materials, 12(10), 1685. https://doi.org/10.3390/ma12101685





