Effect of Air Oxidation on Texture, Surface Properties and Dye Adsorption of Wood-Derived Porous Carbon Materials
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
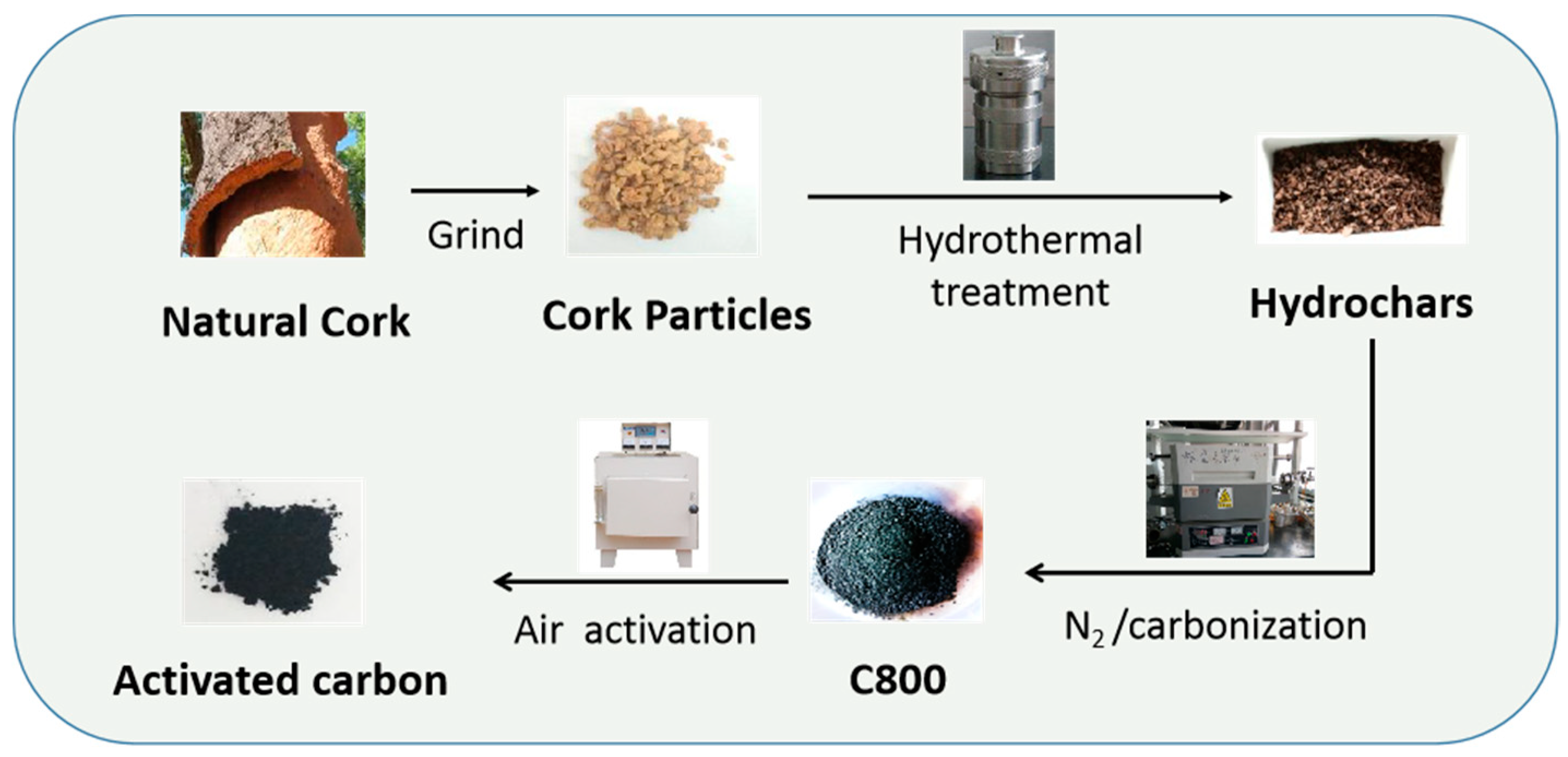
2.2. Preparation of Functional Porous Carbon Materials
2.3. Characterization
2.4. Adsorption Testing
3. Results and Discussion
3.1. Fabrication of the Functional Carbons
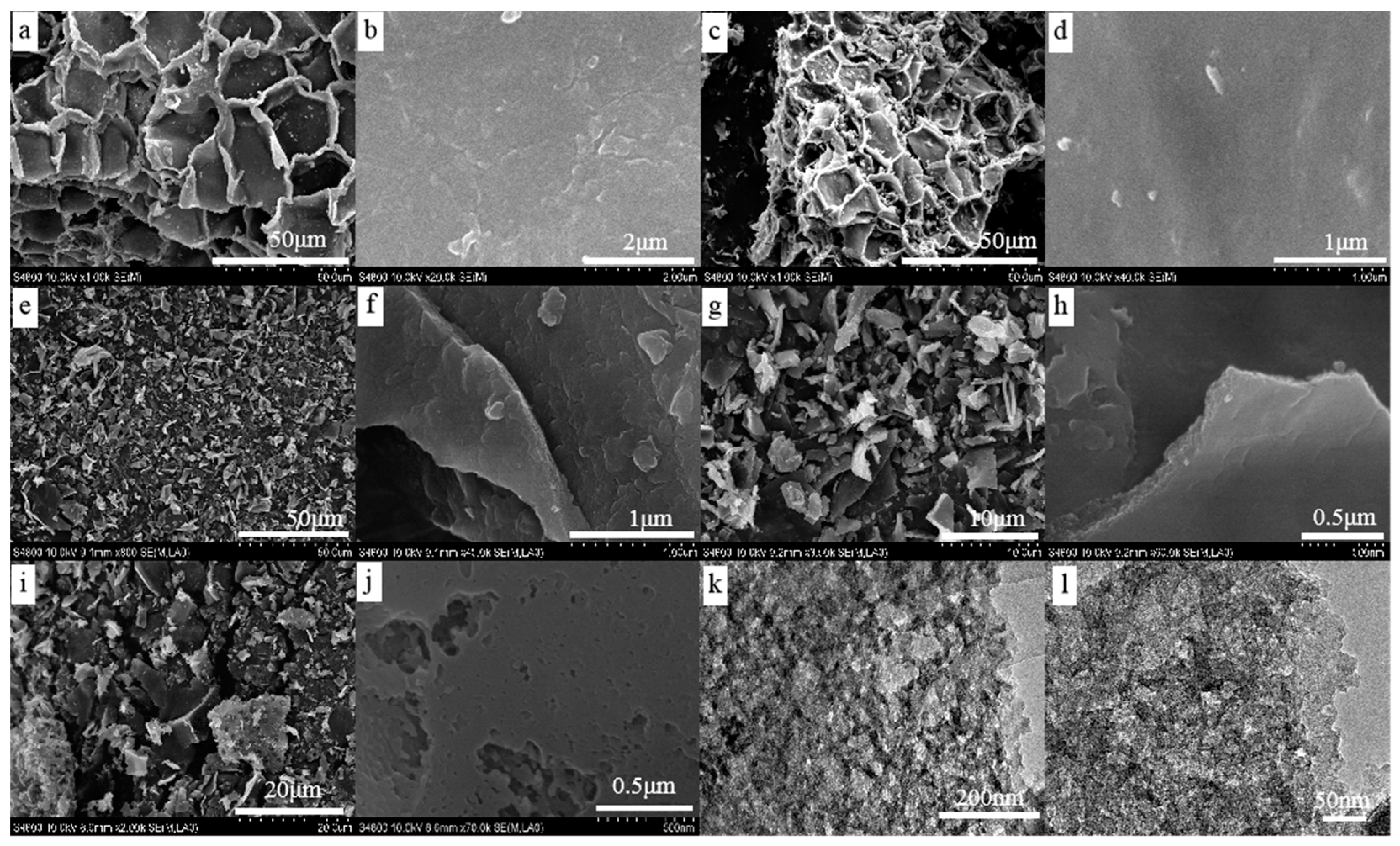
3.2. Surface Morphology of The Functional Carbons
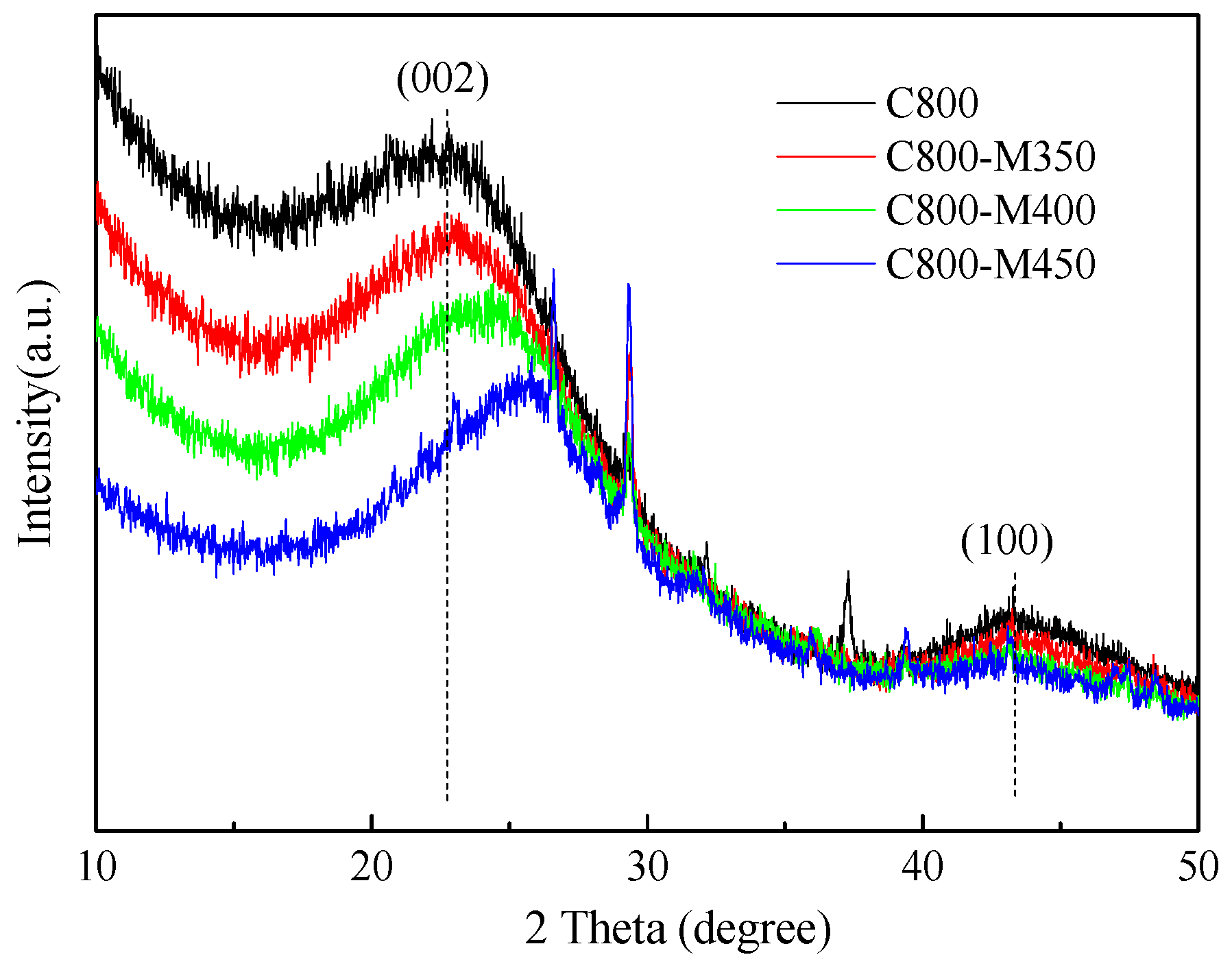
3.3. X-ray Diffraction of the Functional Carbons
3.4. Surface Areas and Pore Size Distribution of the Functional Carbons
3.5. FTIR Analysis of the Functional Carbons
3.6. XPS Analysis of the Functional Carbons
3.7. Adsorbent Analysis of the Functional Carbons for Methylene Blue/Methylene Orange
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Gupta, V.K.; Suhas. Application of low-cost adsorbents for dye removal—A review. J. Environ. Manag. 2009, 90, 2313–2342. [Google Scholar] [CrossRef]
- Brillasa, E.; Martínez-Huitleb, C.A. Decontamination of wastewaters containing synthetic organic dyes by electrochemical methods. An updated review. Appl. Catal. B Environ. 2015, 166, 603–643. [Google Scholar] [CrossRef]
- Verma, A.K.; Dash, R.R.; Bhunia, P. A review on chemical coagulation/flocculation technologies for removal of colour from textile wastewaters. J. Environ. Manag. 2011, 93, 154–168. [Google Scholar] [CrossRef]
- Ritika; Kaur, M.; Umar, A.; Mehta, S.K.; Singh, S.; Kansal, S.K.; Fouad, H.; Alothman, O.Y. Rapid Solar-Light Driven Superior Photocatalytic Degradation of methylene blue using MoS2-ZnO heterostructure nanorods photocatalyst. Materials 2018, 11, 2254. [Google Scholar] [CrossRef]
- Yin, A.S.; Xu, F.; Zhang, X.M. Fabrication of biomass-derived carbon aerogels with high adsorption of oils and organic solvents: Effect of hydrothermal and post-pyrolysis processes. Materials 2016, 9, 758. [Google Scholar] [CrossRef]
- Sachdeva, S.; Kumar, A. Preparation of nanoporous composite carbon membrane for separation of rhodamine B dye. J. Membr. Sci. 2009, 329, 2–10. [Google Scholar] [CrossRef]
- Ahmad, A.; Mohd-Setapar, S.H.; Chuong, C.S.; Khatoon, A.; Wani, W.A.; Kumar, R.; Rafatullah, M. Recent advances in new generation dye removal technologies: Novel search for approaches to reprocess wastewater. RSC Adv. 2015, 5, 30801–30818. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Zhang, B.; Chen, X.F.; Mi, B.B.; Wei, P.L.; Fei, B.H.; Mu, X.D. Antimicrobial bamboo materials functionalized with ZnO and graphene oxide nanocomposites. Materials 2017, 10, 239. [Google Scholar] [CrossRef]
- Gogate, P.R.; Pandit, A.B. A review of imperative technologies for wastewater treatment I: Oxidation technologies at ambient conditions. Adv. Environ. Res. 2004, 8, 501–551. [Google Scholar] [CrossRef]
- Lin, S.H.; Peng, C.F. Continuous treatment of textile wastewater by combined coagulation, electrochemical oxidation and activated sludge. Water Res. 1996, 30, 587–592. [Google Scholar] [CrossRef]
- Lv, L.J.; Huang, Y.; Cao, D.P. Nitrogen-doped porous carbons with ultrahigh specific surface area as bifunctional materials for dye removal of wastewater and supercapacitors. Appl. Surf. Sci. 2018, 456, 184–194. [Google Scholar] [CrossRef]
- Wang, R.; Wang, P.; Yan, X.; Lang, J.; Peng, C.; Xue, Q. Promising porous carbon derived from celtuce leaves with outstanding supercapacitance and CO2 capture performance. ACS Appl. Mater. Inter. 2012, 4, 5800–5806. [Google Scholar] [CrossRef]
- Sun, L.; Tian, C.; Li, M.; Meng, X.; Wang, L.; Wang, R.; Yin, J.; Fu, H. From coconut shell to porous graphene-like nanosheets for high-power supercapacitors. J. Mater. Chem. A 2013, 1, 6462–6470. [Google Scholar] [CrossRef]
- Kang, S.M.; Pan, J.M.; Gu, G.T.; Wang, C.; Wang, Z.P.; Tan, J.H.; Liu, G.H. Sequential production of levulinic acid and porous carbon material from cellulose. Materials 2018, 11, 1408. [Google Scholar] [CrossRef]
- Chen, Y.; Zhai, S.R.; Liu, N.; Song, Y.; An, Q.D.; Song, X.W. Dye removal of activated carbons prepared from NaOH-pretreated rice husks by low-temperature solution processed carbonization and H3PO4 activation. Bioresour. Technol. 2013, 144, 401–409. [Google Scholar] [CrossRef]
- Shopsowitz, K.E.; Hamad, W.Y.; MacLachlan, M.J. Chiral nematic mesoporous carbon derived from nanocrystalline cellulose. Angew. Chem. Int. Ed. 2011, 50, 10991–10995. [Google Scholar] [CrossRef]
- Liu, X.Y.; Zhang, B.; Fei, B.H.; Chen, X.F.; Zhang, J.Y.; Mu, X.D. Tunable and selective hydrogenation of furfural to furfuryl alcohol and cyclopentanone over Pt supported on biomass-derived porous heteroatom doped carbon. Faraday Discuss. 2017, 202, 79–98. [Google Scholar] [CrossRef]
- Schlienger, S.; Graff, A.L.; Celzard, A.; Parmentier, J. Direct synthesis of ordered mesoporous polymer and carbon materials by a biosourced precursor. Green Chem. 2012, 14, 313–316. [Google Scholar] [CrossRef]
- Ozdemir, I.; Sahin, M.; Orhan, R.; Erdem, M. Preparation and characterization of activated carbon from grape stalk by zinc chloride activation. Fuel Process. Technol. 2014, 125, 200–206. [Google Scholar] [CrossRef]
- Sun, H.; He, W.; Zong, C.; Lu, L. Template-free synthesis of renewable macroporous carbon via yeast cells for high-performance supercapacitor electrode materials. ACS Appl. Mater. Interfaces 2013, 5, 2261–2268. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Y.; Peng, B.; Yu, W.; Wang, H.; Wang, T.; Deng, B.; Chai, L.; Zhang, K.; Wang, J. Preparation of a macroscopic, robust carbon-fiber monolith from filamentous fungi and its application in Li-S batteries. Green Chem. 2014, 16, 3926–3934. [Google Scholar] [CrossRef]
- Xu, C.; Strømme, M. Sustainable porous carbon materials derived from wood-based biopolymers for CO2 capture. Nanomaterials 2019, 9, 103. [Google Scholar] [CrossRef]
- Xia, L.Y.; Li, X.J.; Wu, Y.Q.; Rong, M.Z. Wood-derived carbons with hierarchical porous structures and monolithic shapes prepared by biological-template and self-assembly strategies. ACS Sustain. Chem. Eng. 2015, 3, 1724–1731. [Google Scholar] [CrossRef]
- Chen, X.F.; Zhang, J.Y.; Zhang, B.; Dong, S.M.; Guo, X.C.; Mu, X.D.; Fei, B.H. A novel hierarchical porous nitrogen-doped carbon derived from bamboo shoot for high performance supercapacitor. Sci. Rep. 2017, 7, 7362. [Google Scholar] [CrossRef]
- Sayğılı, H.; Güzel, F. High surface area mesoporous activated carbon from tomato processing solid waste by zinc chloride activation: Process optimization, characterization and dyes adsorption. J. Clean. Prod. 2016, 113, 995–1004. [Google Scholar] [CrossRef]
- Baysal, M.; Bilge, K.; Yılmaz, B.; Papila, M.; Yürüm, Y. Preparation of high surface area activated carbon from waste-biomass of sunflower piths: Kinetics and equilibrium studies on the dye removal. J. Environ. Chem. Eng. 2018, 6, 1702–1713. [Google Scholar] [CrossRef]
- Wu, Z.B.; Zhong, H.; Yuan, X.Z.; Wang, H.; Wang, L.L.; Chen, X.H.; Zeng, G.M.; Wu, Y. Adsorptive removal of methylene blue by rhamnolipid-functionalized graphene oxide from wastewater. Water Res. 2014, 67, 330–344. [Google Scholar] [CrossRef]
- Alatalo, S.M.; Mäkilä, E.; Repo, E.; Heinonen, M.; Salonen, J.; Kukk, E.; Sillanpää, M.; Titirici, M.M. Meso-and microporous soft templated hydrothermal carbons for dye removal from water. Green Chem. 2016, 18, 1137–1146. [Google Scholar] [CrossRef]
- Liu, D.; Chen, X.C.; Xu, G.Q.; Guan, J.; Cao, Q.; Dong, B.; Qi, Y.F.; Li, C.H.; Mu, X.D. Iridium nanoparticles supported on hierarchical porous N-doped carbon: An efficient water-tolerant catalyst for bio-alcohol condensation in water. Sci. Rep. 2016, 6, 21365. [Google Scholar] [CrossRef]
- Yorgun, S.; Vural, N.; Demiral, H. Preparation of high-surface area activated carbons from Paulownia wood by ZnCl2 activation. Micropor. Mesopor. Mat. 2009, 122, 189–194. [Google Scholar] [CrossRef]
- Sing, K.S.W. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity. Pure Appl. Chem. 1982, 54, 2201–2218. [Google Scholar] [CrossRef]
- Sabio, E.; Gonzalez, E.; Gonzalez, J.F.; Gonzalez-García, C.M.; Ramiro, A.; Ganan, J. Thermal regeneration of activated carbon saturated with p-nitrophenol. Carbon 2004, 42, 2285–2293. [Google Scholar] [CrossRef]
- Shen, B.X.; Liu, Z.; Xu, H.; Wang, F.M. Enhancing the absorption of elemental mercury using hydrogen peroxide modified bamboo carbons. Fuel 2019, 235, 878–885. [Google Scholar] [CrossRef]
- Farghali, A.A.; Bahgat, M.; El Rouby, W.M.A.; Khedr, M.H. Preparation, decoration and characterization of grapheme sheets for methyl green adsorption. J. Alloy. Compd. 2013, 555, 193–200. [Google Scholar] [CrossRef]
- Chen, H.; Wang, X.X.; Li, J.X.; Wang, X.K. Cotton derived carbonaceous aerogels for the efficient removal of organic pollutants and heavy metal ions. J. Mater. Chem. A 2015, 3, 6073–6081. [Google Scholar] [CrossRef]
- Islam, M.A.; Benhouria, A.; Asif, M.; Hameed, B.H. Methylene blue adsorption on factory-rejected tea activated carbon prepared by conjunction of hydrothermal carbonization and sodium hydroxide activation processes. J. Taiwan Inst. Chem. Eng. 2015, 52, 57–64. [Google Scholar] [CrossRef]
- Guo, H.Q.; Bi, C.Y.; Zeng, C.C.; Ma, W.T.; Yan, L.S.; Li, K.X.; Wei, K. Camellia oleifera seed shell carbon as an efficient renewable bio-adsorbent for the adsorption removal of hexavalent chromium and methylene blue from aqueous solution. J. Mol. Liq. 2018, 249, 629–636. [Google Scholar] [CrossRef]
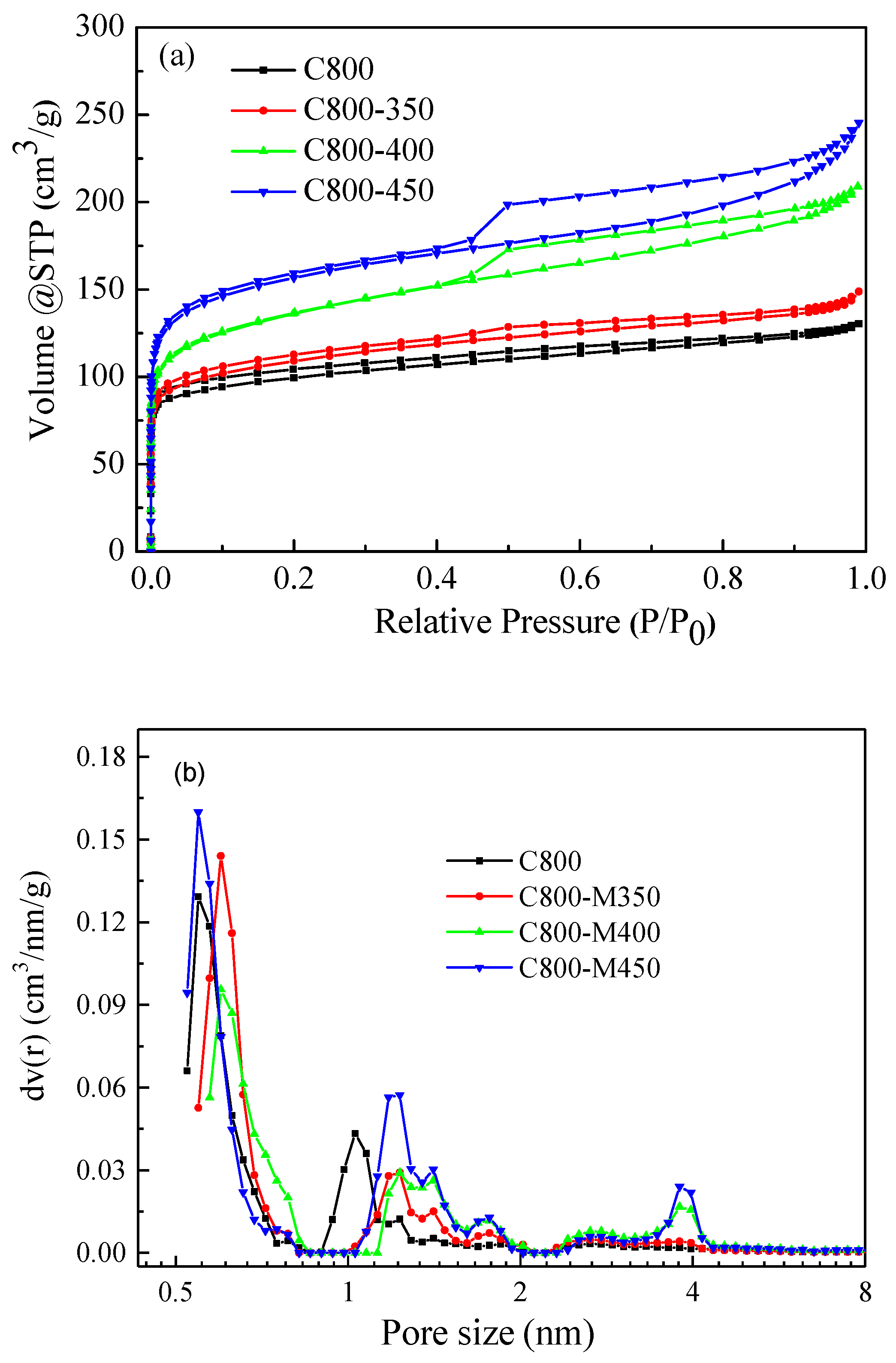
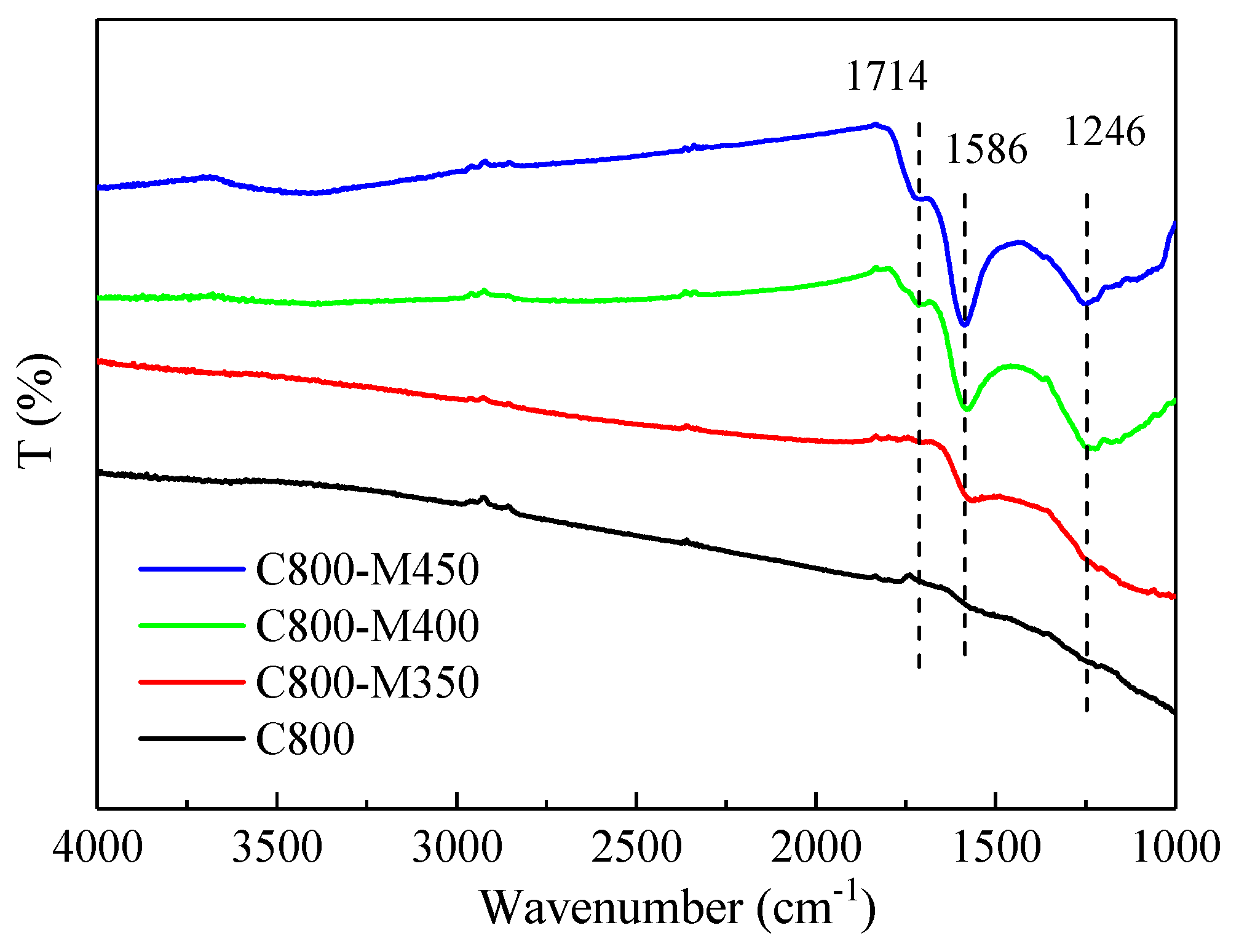
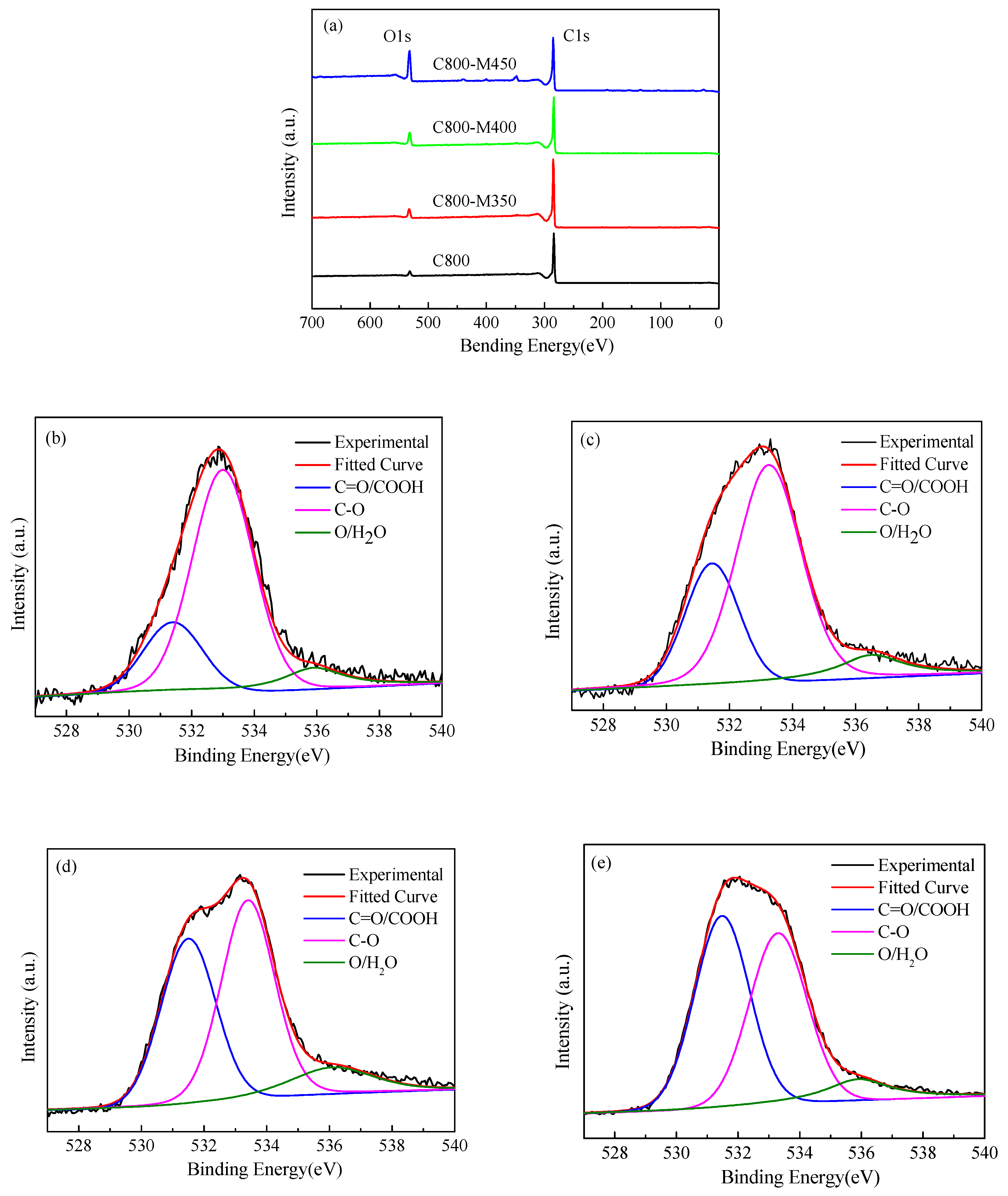
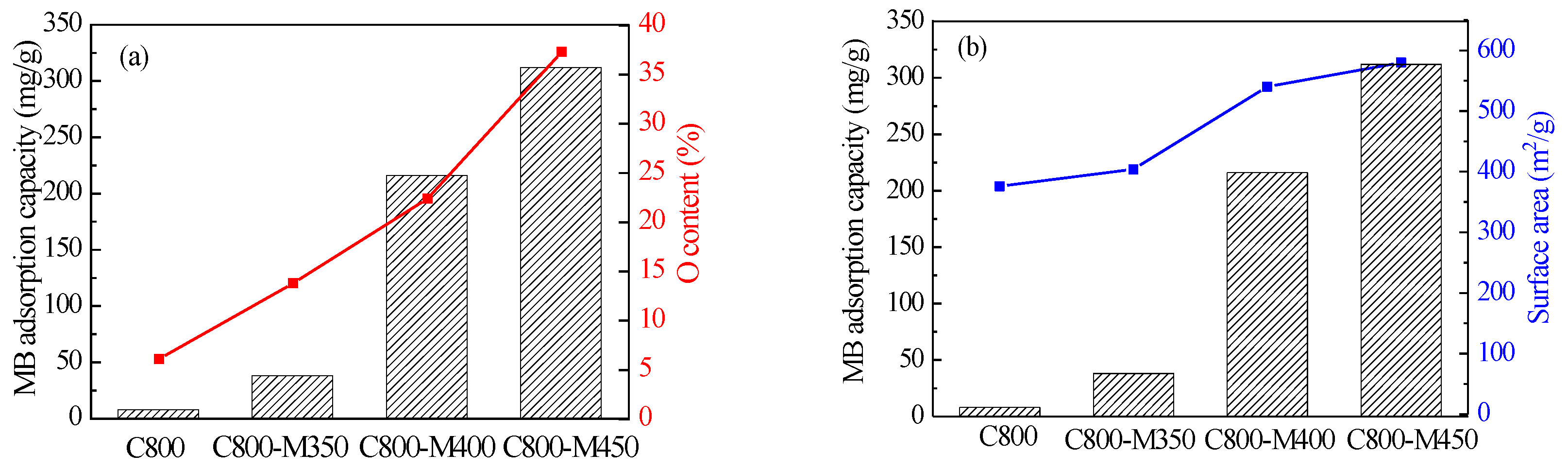
| Sample | SBET (m2/g) | Vtotal (cm3/g) | Vmeso (cm3/g) | Pore Size (nm) |
|---|---|---|---|---|
| C800 | 376 | 0.201 | 0.043 | 0.55/1.03 |
| C800-M350 | 404 | 0.232 | 0.059 | 0.60/1.22/3.80 |
| C800-M400 | 540 | 0.325 | 0.136 | 0.60/1.23/3.84 |
| C800-M450 | 580 | 0.379 | 0.167 | 0.55/1.23/3.86 |
| Sample | C 1s (%) | O 1s (%) | O 1s C=O/COOH (%) | O 1s C–O (%) | O 1s –O/H2O (%) |
|---|---|---|---|---|---|
| C800 | 94.8 | 4.4 | 0.9 | 3.1 | 0.4 |
| C800-M350 | 92.7 | 6.6 | 1.8 | 4.2 | 0.6 |
| C800-M400 | 88.5 | 10.8 | 4.3 | 5.2 | 1.3 |
| C800-M450 | 77.2 | 21.4 | 10.1 | 9.3 | 1.9 |
| Sample | Dye | KL (L/mg) | |
|---|---|---|---|
| C800 | MB | 0.2089 | 7.7 |
| C800-M350 | MB | 0.1139 | 38.6 |
| C800-M400 | MB | 1.2105 | 217.4 |
| C800-M450 | MB | 0.2353 | 312.5 |
| C800 | MO | 1.1422 | 6.4 |
| C800-M450 | MO | 0.4813 | 97.1 |
| Samples | Dye | R2 | ||
|---|---|---|---|---|
| C800 | MB | 6.9 | 0.0141 | 0.9060 |
| C800-M350 | MB | 14.1 | 0.1555 | 0.9157 |
| C800-M400 | MB | 162.9 | 0.1426 | 0.9515 |
| C800-M450 | MB | 220.2 | 0.0482 | 0.9091 |
| C800 | MO | 5.0 | 0.1211 | 0.9449 |
| C800-M450 | MO | 77.9 | 0.0943 | 0.8467 |
| Sample | Dye | kad (g/mg/min) | R2 | |
|---|---|---|---|---|
| C800 | MB | 8.1 | 0.2287 | 0.9992 |
| C800-M350 | MB | 39.1 | 0.0168 | 0.9949 |
| C800-M400 | MB | 189.1 | 0.0010 | 0.9912 |
| C800-M450 | MB | 200.0 | 0.0019 | 0.9964 |
| C800 | MO | 6.5 | 0.0250 | 0.9995 |
| C800-M450 | MO | 90.9 | 0.0010 | 0.9964 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, S.; Deng, L.; Zhang, B.; Lei, Y.; Ren, H.; Lv, J.; Zhao, R.; Chen, X. Effect of Air Oxidation on Texture, Surface Properties and Dye Adsorption of Wood-Derived Porous Carbon Materials. Materials 2019, 12, 1675. https://doi.org/10.3390/ma12101675
Ren S, Deng L, Zhang B, Lei Y, Ren H, Lv J, Zhao R, Chen X. Effect of Air Oxidation on Texture, Surface Properties and Dye Adsorption of Wood-Derived Porous Carbon Materials. Materials. 2019; 12(10):1675. https://doi.org/10.3390/ma12101675
Chicago/Turabian StyleRen, Suhong, Liping Deng, Bo Zhang, Yafang Lei, Haiqing Ren, Jianxiong Lv, Rongjun Zhao, and Xiufang Chen. 2019. "Effect of Air Oxidation on Texture, Surface Properties and Dye Adsorption of Wood-Derived Porous Carbon Materials" Materials 12, no. 10: 1675. https://doi.org/10.3390/ma12101675
APA StyleRen, S., Deng, L., Zhang, B., Lei, Y., Ren, H., Lv, J., Zhao, R., & Chen, X. (2019). Effect of Air Oxidation on Texture, Surface Properties and Dye Adsorption of Wood-Derived Porous Carbon Materials. Materials, 12(10), 1675. https://doi.org/10.3390/ma12101675