Thermochemical Route for Extraction and Recycling of Critical, Strategic and High Value Elements from By-Products and End-of-Life Materials, Part I: Treatment of a Copper By-Product in Air Atmosphere
Abstract
1. Introduction
2. Materials and Methods
3. Results
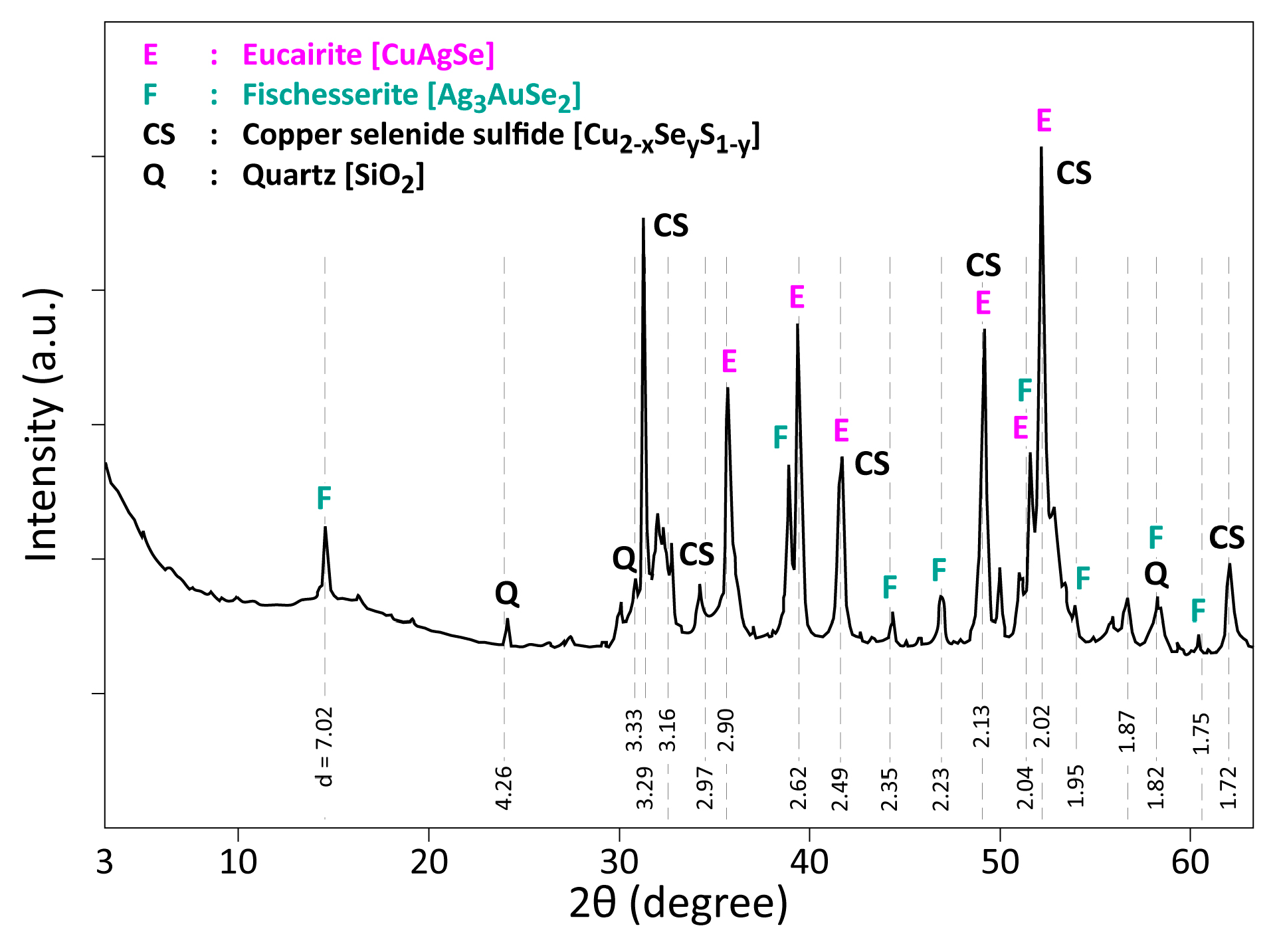
3.1. Elemental and Mineralogical Analysis of CAS Sample
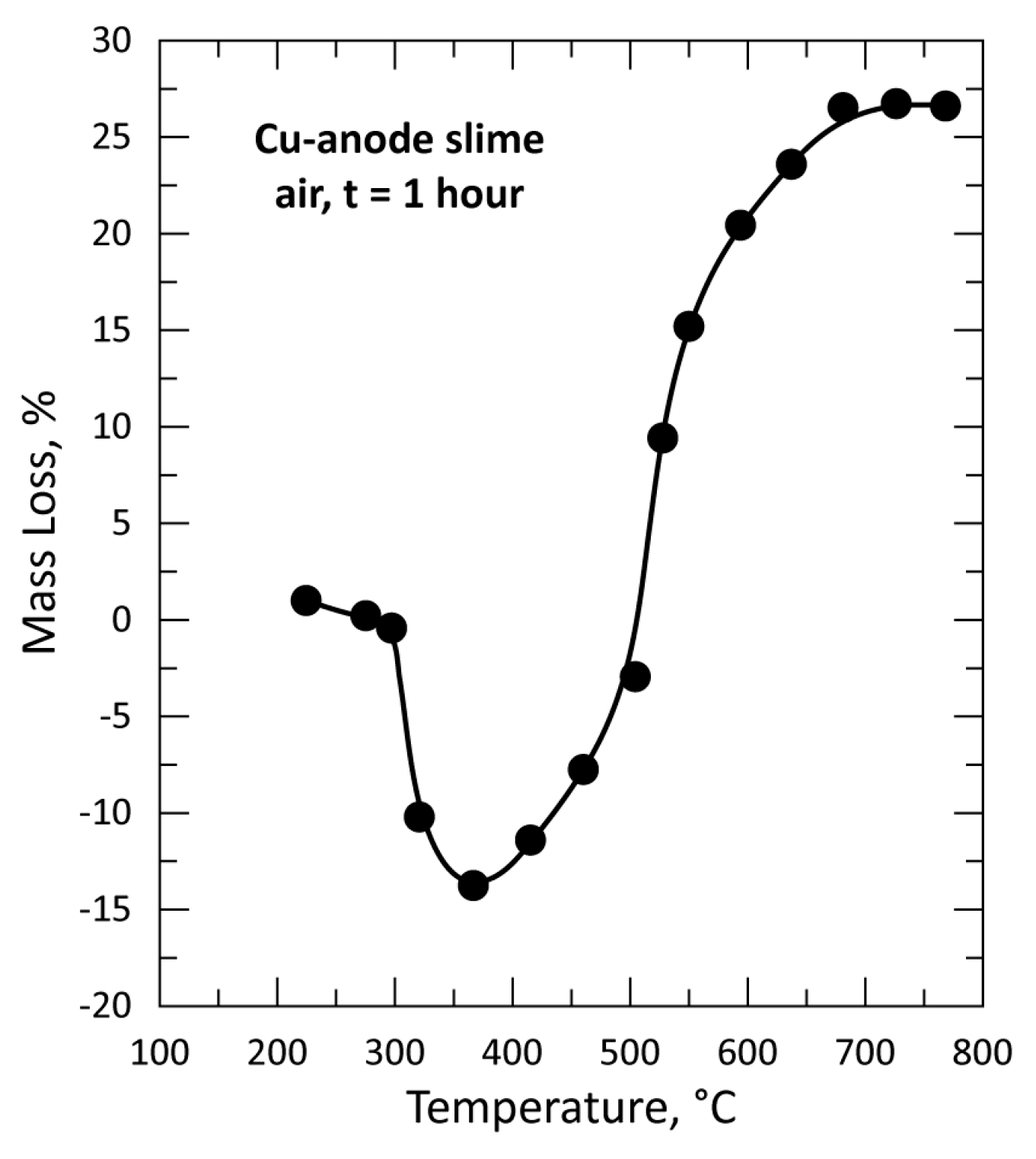
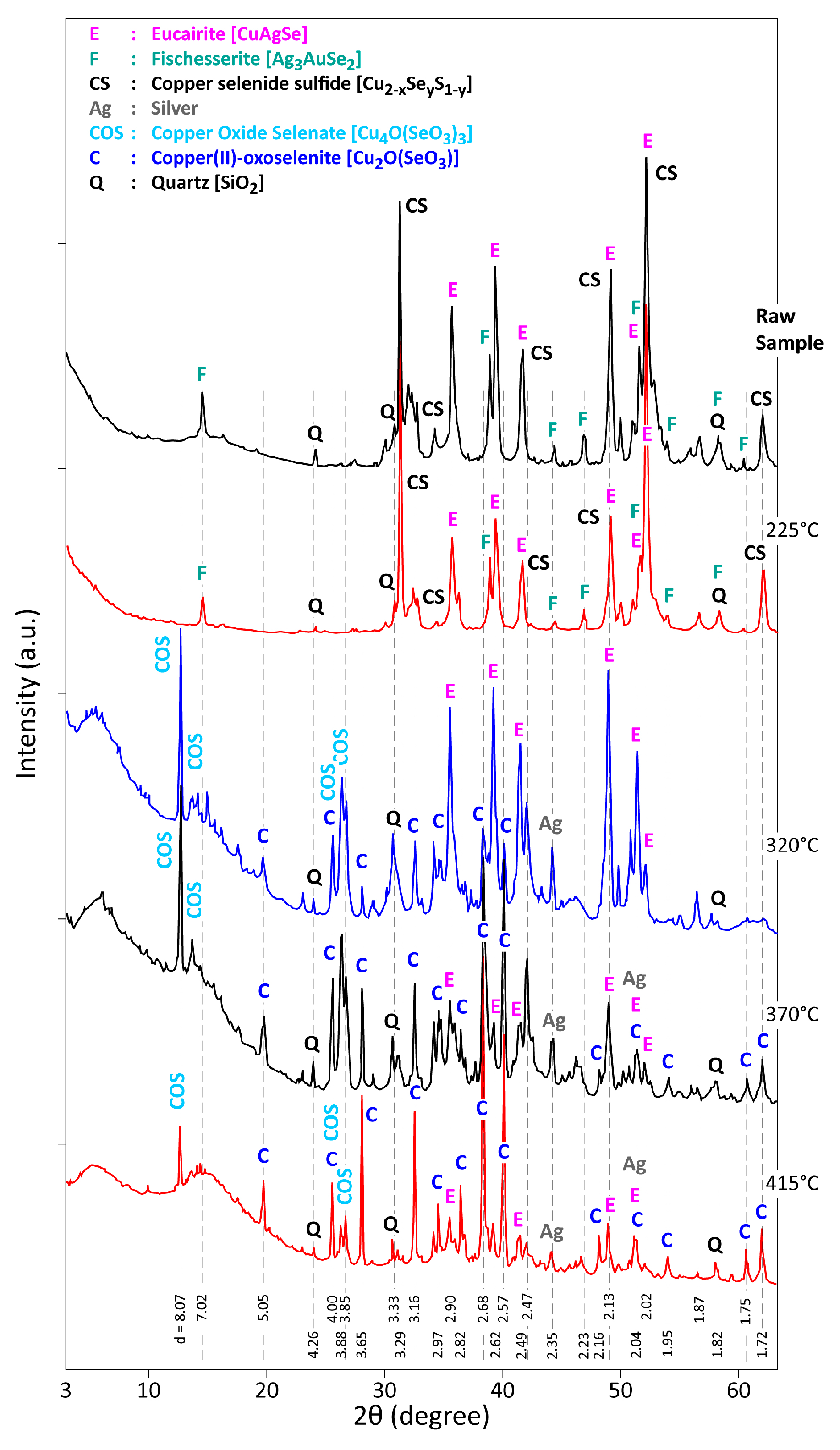
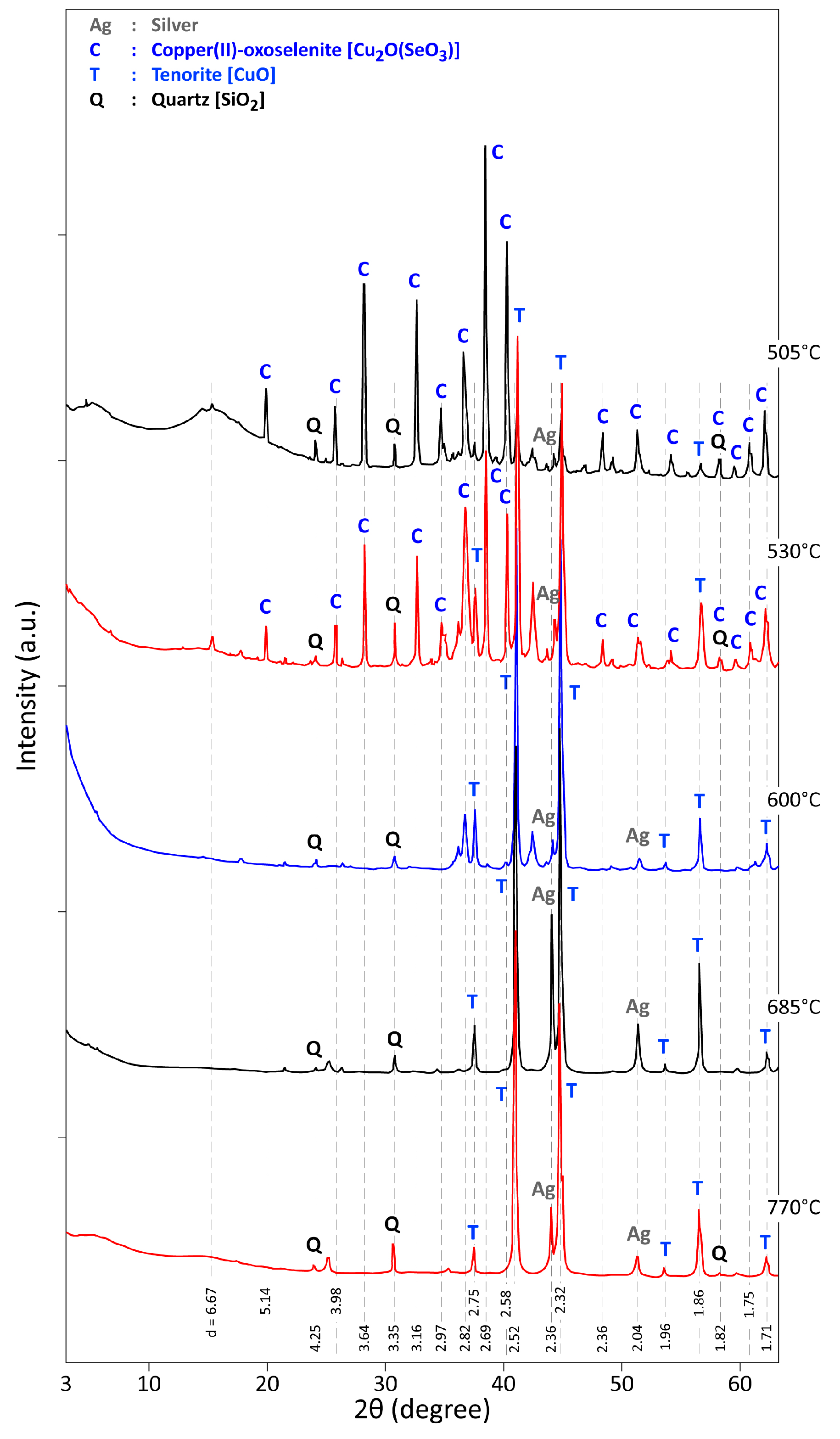
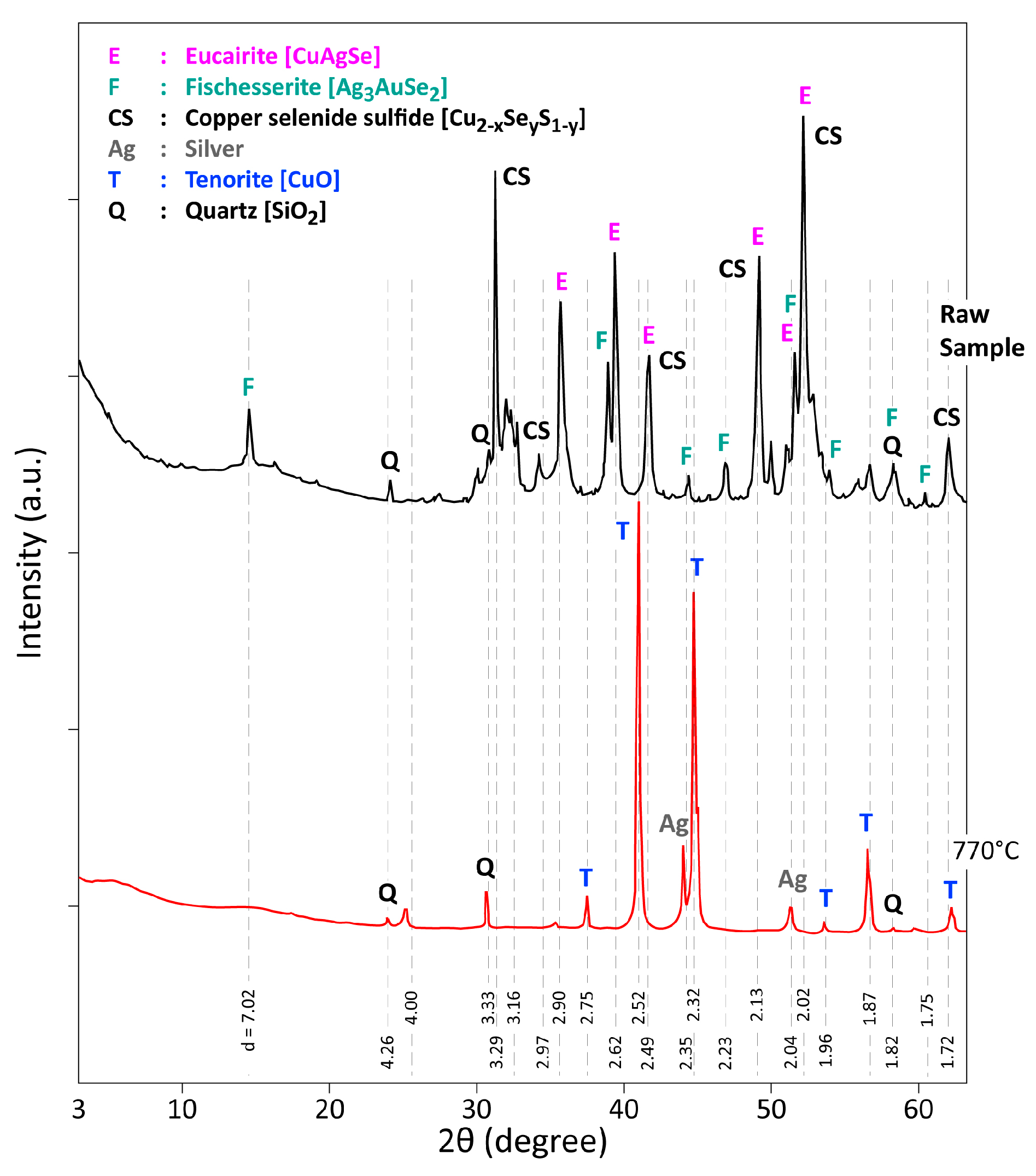
3.2. Thermal Treatment of CAS in Air for 1 h
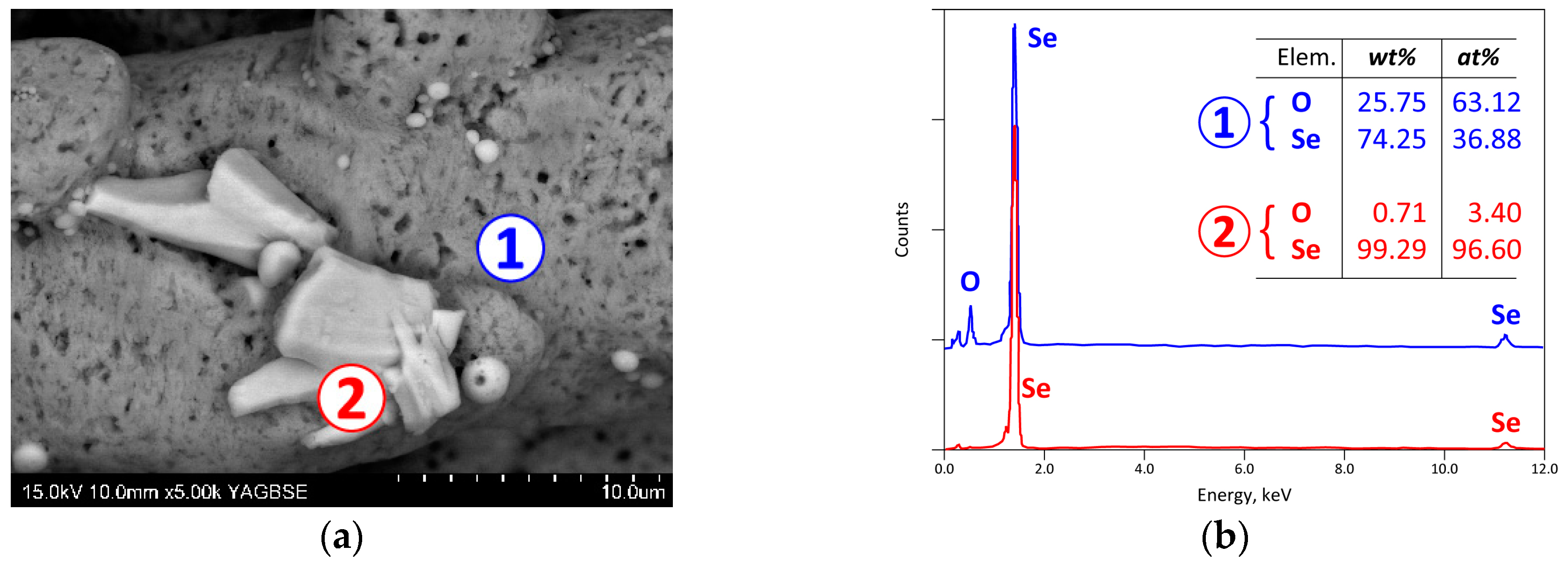
3.3. Analysis of the Reaction Products
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Available online: http://ec.europa.eu/growth/sectors/raw-materials/specific-interest/critical_en (accessed on 15 March 2019).
- Allain, E.; Kanari, N.; Diot, F.; Yvon, J. Development of a process for the concentration of the strategic tantalum and niobium oxides from tin slags. Miner. Eng. 2019, 134, 97–103. [Google Scholar] [CrossRef]
- Holgersson, S.; Steenari, B.-M.; Björkman, M.; Cullbrand, K. Analysis of the metal content of small-size Waste Electric and Electronic Equipment (WEEE) printed circuit boards—Part 1: Internet routers, mobile phones and smartphones. Resour. Conserv. Recycl. 2018, 133, 300–308. [Google Scholar] [CrossRef]
- Kumar, S.; Rawat, S. Future e-Waste: Standardisation for reliable assessment. Gov. Inf. Q. 2018, 35, S33–S42. [Google Scholar] [CrossRef]
- Otto, S.; Kibbe, A.; Henn, L.; Hentschke, L.; Kaiser, F.G. The economy of E-waste collection at the individual level: A practice oriented approach of categorizing determinants of E-waste collection into behavioral costs and motivation. J. Clean. Prod. 2018, 204, 33–40. [Google Scholar] [CrossRef]
- Jayaraman, K.; Vejayon, S.; Raman, S.; Mostafiz, I. The proposed e-waste management model from the conviction of individual laptop disposal practices-An empirical study in Malaysia. J. Clean. Prod. 2019, 208, 688–696. [Google Scholar] [CrossRef]
- Abbondanza, M.; Souza, R. Estimating the generation of household e-waste in municipalities using primary data from surveys: A case study of Sao Jose dos Campos, Brazil. Waste Manag. 2019, 85, 374–384. [Google Scholar] [CrossRef]
- Cho, B.-G.; Lee, J.-C.; Yoo, K. Valuable Metal Recycling. Metals 2018, 8, 345. [Google Scholar] [CrossRef]
- Ding, Y.; Zhang, S.; Liu, B.; Zheng, H.; Chang, C.-C.; Ekberg, C. Recovery of precious metals from electronic waste and spent catalysts: A review. Resour. Conserv. Recycl. 2019, 141, 284–298. [Google Scholar] [CrossRef]
- Avarmaa, K.; Klemettinen, L.; O’Brien, H.; Taskinen, P. Urban mining of precious metals via oxidizing copper smelting. Miner. Eng. 2019, 133, 95–102. [Google Scholar] [CrossRef]
- Amer, A. Processing of copper anodic-slimes for extraction of valuable metals. Waste Manag. 2003, 23, 763–770. [Google Scholar] [CrossRef]
- Khaleghi, A.; Ghader, S.; Afzali, D. Ag recovery from copper anode slime by acid leaching at atmospheric pressure to synthesize silver nanoparticles. Int. J. Min. Sci. Technol. 2014, 24, 251–257. [Google Scholar] [CrossRef]
- Li, X.J.; Yang, H.Y.; Jin, Z.N.; Chen, G.B.; Tong, L.L. Transformation of Selenium-Containing Phases in Copper Anode Slimes During Leaching. JOM 2017, 69, 1932–1938. [Google Scholar] [CrossRef]
- Xiao, L.; Wang, Y.; Yu, Y.; Fu, G.; Han, P.; Sun, Z.; Ye, S. An environmentally friendly process to selectively recover silver from copper anode slime. J. Clean. Prod. 2018, 187, 708–716. [Google Scholar] [CrossRef]
- Kilic, Y.; Kartal, G.; Timur, S. An investigation of copper and selenium recovery from copper anode slimes. Int. J. Miner. Process. 2013, 124, 75–82. [Google Scholar] [CrossRef]
- Gaballah, I.; Allain, E.; Djona, M. Extraction of tantalum and niobium from tin slags by chlorination and carbochlorination. Metall. Mater. Trans. B 1997, 28, 359–369. [Google Scholar] [CrossRef]
- Kanari, N.; Gaballah, I.; Allain, E. Kinetics of oxychlorination of chromite: Part I. Effect of temperature. Thermochim. Acta 2001, 371, 143–154. [Google Scholar] [CrossRef]
- Kanari, N.; Gaballah, I.; Allain, E. Kinetics of oxychlorination of chromite: Part II. Effect of reactive gases. Thermochim. Acta 2001, 371, 75–86. [Google Scholar] [CrossRef]
- Kanari, N.; Allain, E.; Joussemet, R.; Mochón, J.; Ruiz-Bustinza, I.; Gaballah, I. An overview study of chlorination reactions applied to the primary extraction and recycling of metals and to the synthesis of new reagents. Thermochim. Acta 2009, 495, 42–50. [Google Scholar] [CrossRef]
- Kanari, N.; Menad, N.-E.; Ostrosi, E.; Shallari, S.; Diot, F.; Allain, E.; Yvon, J. Thermal Behavior of Hydrated Iron Sulfate in Various Atmospheres. Metals 2018, 8, 1084. [Google Scholar] [CrossRef]
- Kanari, N.; Evrard, O.; Neveux, N.; Ninane, L. Recycling ferrous sulfate via super-oxidant synthesis. JOM 2001, 53, 32–33. [Google Scholar] [CrossRef]
- Kanari, N.; Ostrosi, E.; Ninane, L.; Neveux, N.; Evrard, O. Synthesizing alkali ferrates using a waste as a raw material. JOM 2005, 57, 39–42. [Google Scholar] [CrossRef]
- Kanari, N.; Filippov, L.; Diot, F.; Mochon, J.; Ruiz-Bustinza, I.; Allain, E.; Yvon, J. Synthesis of potassium ferrate using residual ferrous sulfate as iron bearing material. J. Phys. Conf. Ser. 2013, 416, 012013. [Google Scholar] [CrossRef]
- Kanari, N.; Filippova, I.; Diot, F.; Mochón, J.; Ruiz-Bustinza, I.; Allain, E.; Yvon, J. Utilization of a waste from titanium oxide industry for the synthesis of sodium ferrate by gas–solid reactions. Thermochim. Acta 2014, 575, 219–225. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, Z. A critical review of material flow, recycling technologies, challenges and future strategy for scattered metals from minerals to wastes. J. Clean. Prod. 2018, 202, 1001–1025. [Google Scholar] [CrossRef]
- Davidsson, S.; Höök, M. Material requirements and availability for multi-terawatt deployment of photovoltaics. Energy Policy 2017, 108, 574–582. [Google Scholar] [CrossRef]
- Padoan, F.C.; Altimari, P.; Pagnanelli, F. Recycling of end of life photovoltaic panels: A chemical prospective on process development. Sol. Energy 2019, 177, 746–761. [Google Scholar] [CrossRef]
- Domínguez, A.; Geyer, R. Photovoltaic waste assessment of major photovoltaic installations in the United States of America. Renew. Energy 2019, 133, 1188–1200. [Google Scholar] [CrossRef]
- Chen, T.T.; Dutrizac, J.E. A mineralogical study of the deportment and reaction of silver during copper electrorefining. Metall. Mater. Trans. B 1989, 20, 345–361. [Google Scholar] [CrossRef]
- Petkova, E. Microscopic examination of copper electrorefining slimes. Hydrometallurgy 1990, 24, 351–359. [Google Scholar] [CrossRef]
- Petkova, E. Hypothesis about the origin of copper electrorefining slime. Hydrometallurgy 1994, 34, 343–358. [Google Scholar] [CrossRef]
- Chen, T.T.; Dutrizac, J.E. Mineralogical characterization of a copper anode and the anode slimes from the la caridad copper refinery of mexicana de cobre. Metall. Mater. Trans. B 2005, 36, 229–240. [Google Scholar] [CrossRef]
- Available online: http://s1.iran-mavad.com/ASM%20hanbooks/Vol_3_ASM%20handbooks_iran-mavad.com.pdf (accessed on 29 March 2019).
- Roine, A. Outokumpu HSC Chemistry for Windows; Version 3.0; Outokumpu Research: Pori, Finland, 1997. [Google Scholar]
- Portnichenko, P.Y.; Romhanyi, J.; Onykiienko, Y.A.; Henschel, A.; Schmidt, M.; Cameron, A.S.; Surmach, M.A.; Lim, J.A.; Park, J.T.; Schneidewind, A.; et al. Magnon spectrum of the helimagnetic insulator Cu2OSeO3. Nat. Commun. 2016, 7, 10725. [Google Scholar] [CrossRef] [PubMed]
- Fokina, E.L.; Klimova, E.V.; Charykova, M.V.; Krivovichev, V.G.; Platonova, N.V.; Semenova, V.V.; Depmeier, W. The thermodynamics of arsenates, selenites, and sulfates in the oxidation zone of sulfide ores: VIII. Field of thermal stability of synthetic analog of chalcomenite, its dehydration and dissociation. Geol. Ore Deposits 2014, 56, 538–545. [Google Scholar] [CrossRef]
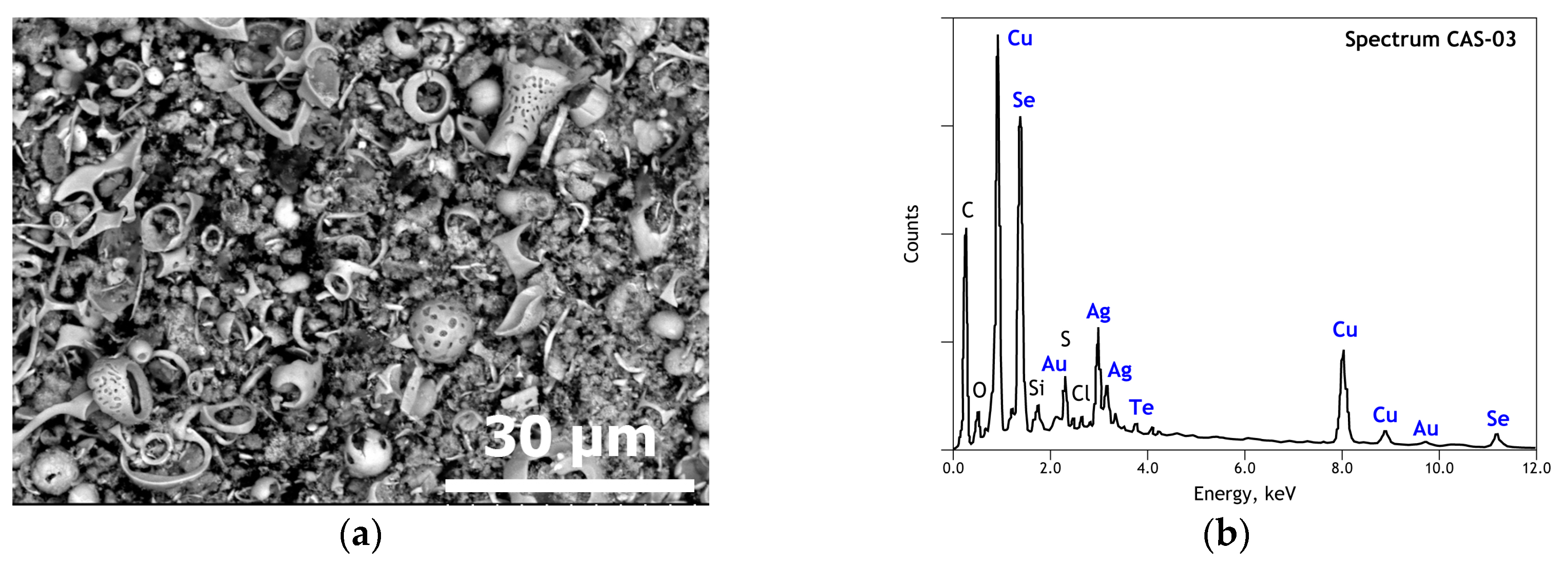
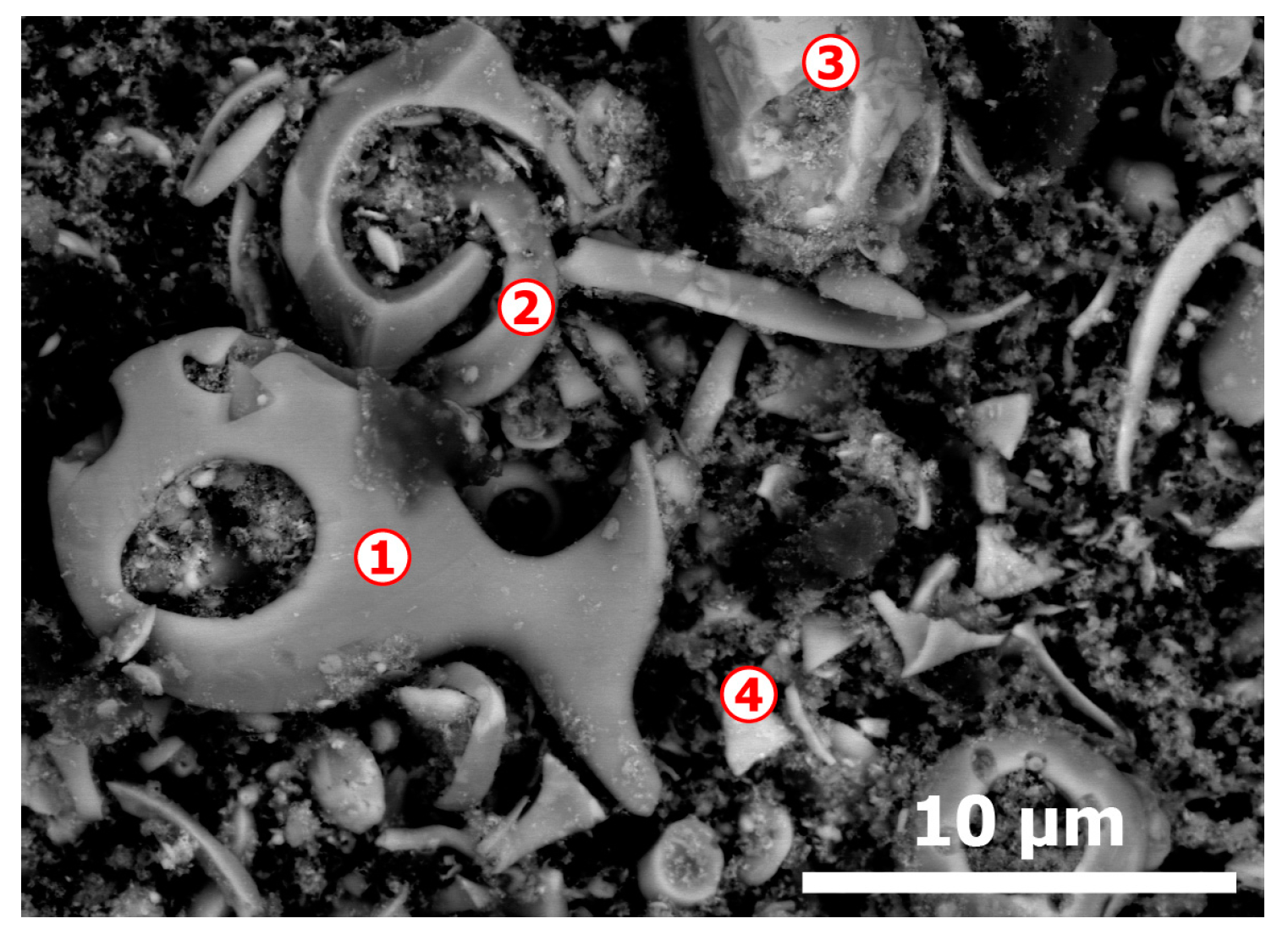
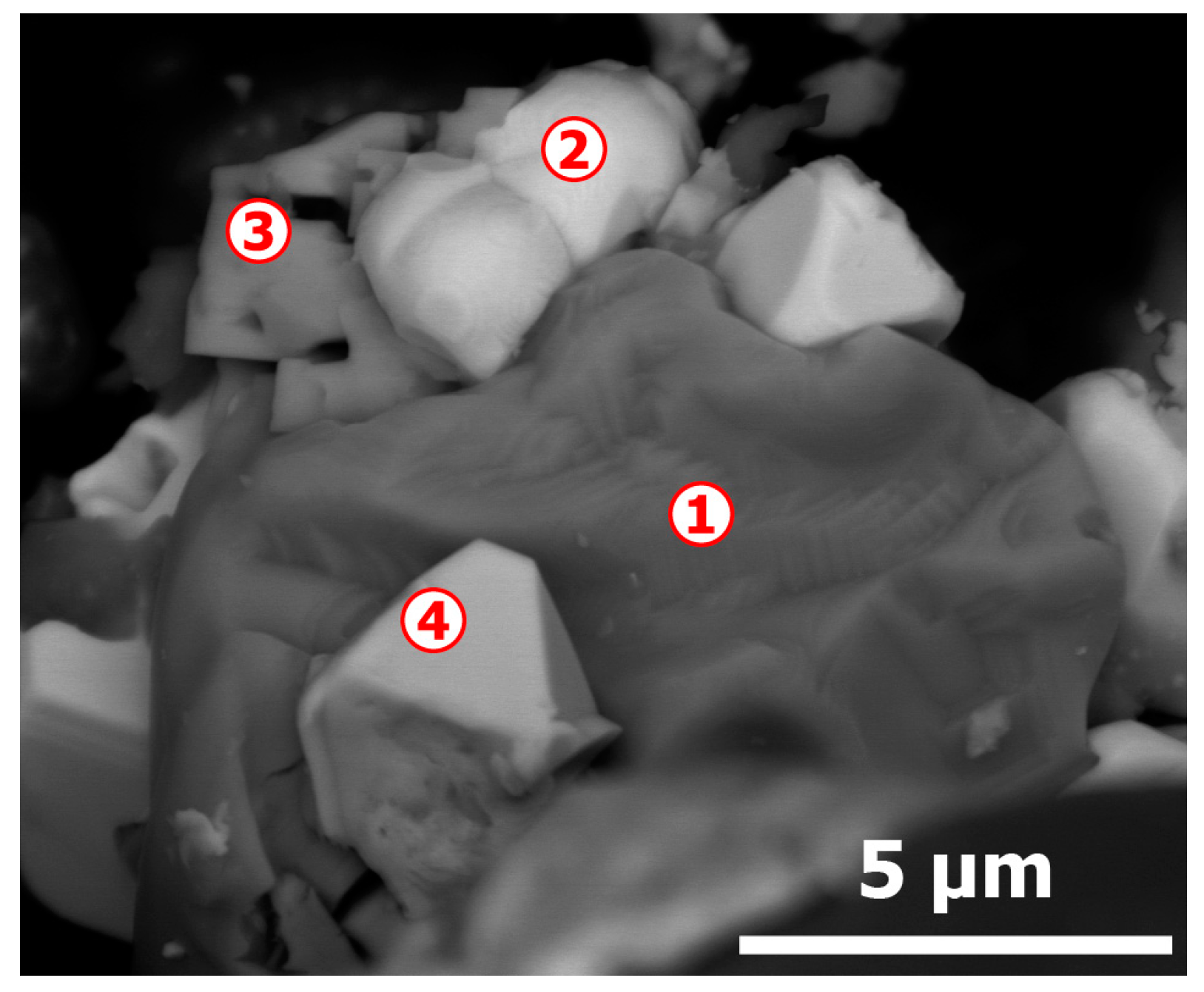
| Elements | Spot n° 1 | Spot n° 2 | Spot n° 3 | Spot n° 4 | ||||
|---|---|---|---|---|---|---|---|---|
| wt % 1 | at % 1 | wt % | at % | wt % | at % | wt % | at % | |
| O | - | - | - | - | - | - | 0.49 | 2.55 |
| Si | - | - | - | - | - | - | 1.01 | 3.00 |
| S | 2.01 | 4.12 | 2.87 | 6.01 | 1.72 | 4.36 | 1.46 | 3.79 |
| Cl | - | - | - | - | 0.63 | 1.45 | - | - |
| Cu | 72.20 | 74.48 | 58.55 | 61.88 | 22.21 | 28.47 | 30.79 | 40.28 |
| Se | 25.78 | 21.40 | 35.48 | 30.17 | 32.57 | 33.59 | 20.34 | 21.42 |
| Ag | - | - | 3.11 | 1.93 | 40.88 | 30.89 | 27.05 | 20.84 |
| Te | - | - | - | - | 1.98 | 1.27 | 0.67 | 0.43 |
| Au | - | - | - | - | - | - | 18.19 | 7.68 |
| Elements | Spot n° 1 | Spot n° 2 | Spot n° 3 | Spot n° 4 | ||||
|---|---|---|---|---|---|---|---|---|
| wt % 1 | at % 1 | wt % | at % | wt % | at % | wt % | at % | |
| O | 18.35 | 46.66 | 1.48 | 9.34 | 15.35 | 47.05 | - | - |
| Al | 0.59 | 0.89 | - | - | 0.67 | 1.22 | - | - |
| Si | 0.67 | 0.97 | 0.72 | 2.57 | 1.13 | 1.97 | - | - |
| Cu | 80.39 | 51.48 | 4.56 | 7.22 | 45.96 | 35.47 | 4.38 | 7.80 |
| Ag | - | - | 78.61 | 73.39 | 1.57 | 0.71 | 78.56 | 82.40 |
| Au | - | - | 14.62 | 7.48 | - | - | 17.06 | 9.80 |
| Te | - | - | - | - | 35.33 | 13.58 | - | - |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kanari, N.; Allain, E.; Shallari, S.; Diot, F.; Diliberto, S.; Patisson, F.; Yvon, J. Thermochemical Route for Extraction and Recycling of Critical, Strategic and High Value Elements from By-Products and End-of-Life Materials, Part I: Treatment of a Copper By-Product in Air Atmosphere. Materials 2019, 12, 1625. https://doi.org/10.3390/ma12101625
Kanari N, Allain E, Shallari S, Diot F, Diliberto S, Patisson F, Yvon J. Thermochemical Route for Extraction and Recycling of Critical, Strategic and High Value Elements from By-Products and End-of-Life Materials, Part I: Treatment of a Copper By-Product in Air Atmosphere. Materials. 2019; 12(10):1625. https://doi.org/10.3390/ma12101625
Chicago/Turabian StyleKanari, Ndue, Eric Allain, Seit Shallari, Frederic Diot, Sebastien Diliberto, Fabrice Patisson, and Jacques Yvon. 2019. "Thermochemical Route for Extraction and Recycling of Critical, Strategic and High Value Elements from By-Products and End-of-Life Materials, Part I: Treatment of a Copper By-Product in Air Atmosphere" Materials 12, no. 10: 1625. https://doi.org/10.3390/ma12101625
APA StyleKanari, N., Allain, E., Shallari, S., Diot, F., Diliberto, S., Patisson, F., & Yvon, J. (2019). Thermochemical Route for Extraction and Recycling of Critical, Strategic and High Value Elements from By-Products and End-of-Life Materials, Part I: Treatment of a Copper By-Product in Air Atmosphere. Materials, 12(10), 1625. https://doi.org/10.3390/ma12101625






