Nano-Carriers Based on pH-Sensitive Star-Shaped Copolymers for Drug-Controlled Release
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization
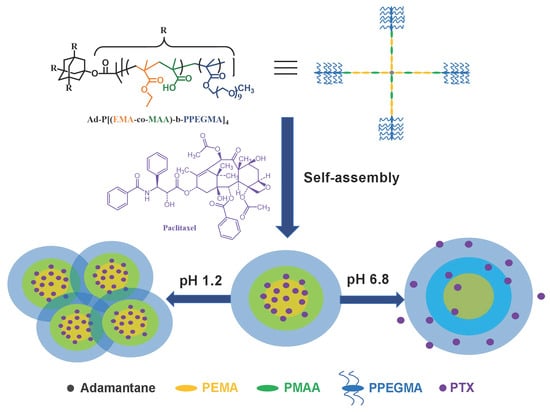
2.3. Synthesis of Ad-P[(EMA-co-MAA)-b-PPEGMA]4
2.3.1. Synthesis of Ad-P[(EMA-co-tBMA)]4
2.3.2. Synthesis of Ad-P[(EMA-co-tBMA)-b-PPEGMA]4
2.3.3. Hydrolysis of Ad-P[(EMA-co-tBMA)-b-PPEGMA]4
2.4. Critical Micelle Concentration (CMC) Measurement
2.5. Study of the pH-Sensitive of the Blank Micelles
2.6. Preparation of PTX-Loaded Micelles
2.7. In Vitro PTX Release Study
2.8. In Vitro Cytotoxicity Test
3. Results and Discussion
3.1. Synthesis and Characterization of Ad-P[(EMA-co-MAA)-b-PPEGMA]4
3.2. pH-Sensitive Behaviors of Blank Micelles
3.3. In Vitro PTX Controlled Release
3.4. In Vitro Cytotoxicity Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Khan, J.; Alexander, A.; Ajazuddin, A.; Swarnlata, S.; Shailendra, S. Exploring the role of polymeric conjugates toward anti-cancer drug delivery: Current trends and future projections. Int. J. Pharm. 2018, 548, 500–514. [Google Scholar] [CrossRef]
- Cabral, H.; Miyata, K.; Osada, K.; Kataoka, K. Block copolymer micelles in nanomedicine applications. Chem. Rev. 2018, 118, 6844–6892. [Google Scholar] [CrossRef] [PubMed]
- Marzbali, M.Y.; Khosroushahi, A.Y. Polymeric micelles as mighty nanocarriers for cancer gene therapy: A review. Cancer Chemoth. Pharm. 2017, 79, 637–649. [Google Scholar] [CrossRef]
- George, A.; Shah, P.A.; Shrivastav, P.S. Natural biodegradable polymers based nano-formulations for drug delivery: A review. Int. J. Pharm. 2019, 561, 244–264. [Google Scholar] [CrossRef]
- Kowalczuk, A.; Trzcinska, R.; Trzebicka, B.; Müller, A.H.E.; Dworak, A.; Tsvetanov, C.B. Loading of polymer nanocarriers: Factors, mechanisms and applications. Prog. Polym. Sci. 2014, 39, 43–86. [Google Scholar] [CrossRef]
- Karimi, M.; Ghasemi, A.; Zangabad, P.S.; Rahighi, R.; Basri, S.M.M.; Mirshekari, H.; Amiri, M.; Pishabad, Z.S.; Aslani, A.; Bozorgomid, M.; et al. Smart micro/nanoparticles in stimulus-responsive drug/gene delivery systems. Chem. Soc. Rev. 2016, 45, 1457–1501. [Google Scholar] [CrossRef]
- Gawde, K.A.; Sau, S.; Tatiparti, K.; Kashaw, S.K.; Mehrmohammadi, M.; Azmi Asiyer, A.K. Paclitaxel and di-fluorinated curcumin loaded in albumin nanoparticles for targeted synergistic combination therapy of ovarian and cervical cancers. Colloids Surf. B Biointerfaces 2018, 167, 8–19. [Google Scholar] [CrossRef] [PubMed]
- Meng, H.; Wang, M.Y.; Liu, H.Y.; Liu, X.S.; Situ, A.; Wu, B.; Ji, Z.X.; Chang, C.H.; Nel, A.E. Use of a lipid-coated mesoporous silica nanoparticle platform for synergistic gemcitabine and paclitaxel delivery to human pancreatic cancer in mice. ACS Nano 2015, 9, 3540–3557. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Yao, W.D.; Rao, Y.F.; Lu, X.Y.; Gao, J.Q. pH-Responsive carriers for oral drug delivery: Challenges and opportunities of current platforms. Drug Deliv. 2017, 24, 569–581. [Google Scholar] [CrossRef]
- Liu, J.; Huang, Y.R.; Kumar, A.; Tan, A.; Jin, S.B.; Mozhi, A.; Liang, X.J. pH-Sensitive nano-systems for drug delivery in cancer therapy. Biotechnol. Adv. 2014, 32, 693–710. [Google Scholar] [CrossRef] [PubMed]
- Felber, A.E.; Dufresne, M.H.; Leroux, J.C. pH-Sensitive vesicles, polymeric micelles, and nanospheres prepared with polycarboxylates. Adv. Drug Deliver. Rev. 2012, 64, 979–992. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.Q.; Lin, W.J.; Zhao, B.; Wen, X.F.; Guo, X.D.; Zhang, L.J. Synthesis and physciochemical characterization of amphiphilic triblock copolymer brush containing pH-sensitive linkage for oral delivery. Langmuir 2012, 28, 8251–8259. [Google Scholar] [CrossRef]
- Yang, Y.Q.; Zheng, L.S.; Guo, X.D.; Qian, Y.; Zhang, L.J. pH-Sensitive micelles self-assembled from amphiphilic copolymer brush for delivery of poorly water-soluble drugs. Biomacromolecules 2011, 12, 116–122. [Google Scholar] [CrossRef]
- Yang, Y.Q.; Guo, X.D.; Lin, W.J.; Zhang, L.J.; Zhang, C.Y. Amphiphilic copolymer brush with random pH-sensitive/hydrophobic structure: Synthesis and self-assembled micelles for sustained drug delivery. Soft Matter 2011, 8, 454–464. [Google Scholar] [CrossRef]
- Ren, J.M.; McKenzie, T.G.; Fu, Q.; Wong, E.H.H.; Xu, J.T.; An, Z.S.; Shanmugam, S.; Davis, T.P.; Boyer, C.; Qiao, G.G. Star polymers. Chem. Rev. 2016, 116, 6743–6836. [Google Scholar] [CrossRef]
- Wu, W.; Wang, W.G.; Li, J.S. Star polymers: Advances in biomedical applications. Prog. Polym. Sci. 2015, 46, 55–85. [Google Scholar] [CrossRef]
- Yang, Y.Q.; Zhao, B.; Li, Z.D.; Lin, W.J.; Zhang, C.Y.; Guo, X.D.; Wang, J.F.; Zhang, L.J. pH-Sensitive micelles self-assembled from multi-arm star triblock co-polymers poly(ε-caprolactone)-b-poly(2-(diethylamino)ethyl methacrylate)-b-poly(poly(ethylene glycol) methyl ether methacrylate) for controlled anticancer drug delivery. Acta Biomater. 2013, 9, 7679–7690. [Google Scholar] [CrossRef]
- Lin, W.J.; Yao, N.; Li, H.R.; Hanson, S.; Han, W.Q.; Wang, C.; Zhang, L.J. Co-delivery of imiquimod and plasmid DNA via an amphiphilic pH-responsive star polymer that forms unimolecular micelles in water. Polymers 2016, 8, 397. [Google Scholar] [CrossRef]
- Lin, W.J.; Hanson, S.; Han, W.Q.; Zhang, X.F.; Yao, N.; Li, H.R.; Zhang, L.J.; Wang, C. Well-defined star polymers for co-delivery of plasmid DNA and imiquimod to dendritic cells. Acta Biomater. 2017, 48, 378–389. [Google Scholar] [CrossRef]
- Le Devedec, F.; Strandman, S.; Baille, W.E.; Zhu, X.X. Functional star block copolymers with a cholane core:Thermo-responsiveness and aggregation behavior. Polymer 2013, 54, 3898–3903. [Google Scholar] [CrossRef]
- Shao, Y.; Jia, Y.G.; Shi, C.Y.; Luo, J.T.; Zhu, X.X. Block and random copolymers bearing cholic acid and oligo(ethylene glycol) pendant groups: Aggregation, thermosensitivity, and drug loading. Biomacromolecules 2014, 15, 1837–1844. [Google Scholar] [CrossRef]
- Cunningham, A.J.; Robinson, M.; Banquy, X.; Leblond, J.; Zhu, X.X. Bile acid-based drug delivery systems for enhanced doxorubicin encapsulation: Comparing hydrophobic and ionic interactions in drug loading and release. Mol. Pharm. 2018, 15, 1266–1276. [Google Scholar] [CrossRef]
- Zheng, Y.C.; Gao, Z.G.; Sheng, Y.; Han, J.; Gao, Y. POSS-based amphiphiles: Synthesis and use in self-assembling nanosystems and nanomaterials. Curr. Org. Chem. 2017, 21, 2849–2876. [Google Scholar] [CrossRef]
- Xu, Y.T.; He, K.W.; Wang, H.C.; Li, M.; Shen, T.; Liu, X.Y.; Yuan, C.H.; Dai, L.Z. Self-assembly behavior and pH-stimuli-responsive property of POSS-based amphiphilic block copolymers in solution. Micromachines 2018, 9, 258. [Google Scholar] [CrossRef]
- Li, L.; Lu, B.B.; Fan, Q.K.; Wu, J.N.; Wei, L.L.; Hou, J.; Guo, X.H.; Liu, Z.Y. Synthesis and self-assembly behavior of pH-responsive star-shaped POSS-(PCL-P(DMAEMA-co-PEGMA))16 inorganic/organic hybrid block copolymer for the controlled intracelluar delivery of doxorubicin. RSC Adv. 2016, 6, 61630–61640. [Google Scholar] [CrossRef]
- Stimac, A.; Sekutor, M.; Mlinaric-Majerski, K.; Frkanec, L.; Frkanec, R. Adamantane in drug delivery systems and surface recognition. Molecules 2017, 22, 297. [Google Scholar] [CrossRef]
- Bagrii, E.I.; Nekhaev, A.I.; Maksimov, A.L. Oxidative functionalization of adamantanes (review). Petrol. Chem. 2017, 57, 183–197. [Google Scholar] [CrossRef]
- Grillaud, M.; Bianco, A. Multifunctional adamantane derivatives as new scaffolds for the multipresentation of bioactive peptides. J. Pept. Sci. 2015, 21, 330–345. [Google Scholar] [CrossRef]
- Yang, H.Y.; Guo, J.W.; Tong, R.; Yang, C.F.; Chen, J.K. pH-Sensitive micelles based on star copolymer Ad-(PCL-b-PDEAEMA-b-PPEGMA)(4) for controlled drug delivery. Polymers 2018, 10, 443. [Google Scholar] [CrossRef]
- Fu, S.Q.; Guo, J.W.; Zhu, D.Y.; Yang, Z.; Yang, C.F.; Xian, J.X.; Li, X. Novel halogen-free flame retardants based on adamantane for polycarbonate. RSC Adv. 2015, 5, 67054–67065. [Google Scholar] [CrossRef]
- Owen, S.C.; Chan, D.P.Y.; Shoichet, M.S. Polymeric micelle stability. Nano Today 2012, 7, 53–65. [Google Scholar] [CrossRef]
- Holappa, S.; Andersson, T.; Kantonen, L.; Plattner, P.; Tenhu, H. Soluble polyelectrolyte complexes composed of poly(ethylene oxide)-block-poly(sodium methacrylate) and poly(methacryloyloxyethyl trimethylammonium chloride). Polymer 2003, 44, 7907–7916. [Google Scholar] [CrossRef]
- Yang, X.L.; Fan, R.R.; Wang, W.L.; Wang, J.X.; Le, Y. Design and synthesis of pH-sensitive polymeric micelles for oral delivery of poorly water-soluble drugs. J. Biomater. Sci. Polym. Ed. 2016, 27, 1341–1353. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.M.; Li, L.D.; Shang, L.; Zhou, Q.H.; Lin, J. Preparation of pH-sensitive micelles from miktoarm star block copolymers by ATRP and their application as drug nanocarriers. React. Funct. Polym. 2016, 107, 28–34. [Google Scholar] [CrossRef]
- Jones, M.C.; Ranger, M.; Leroux, J.C. pH-Sensitive unimolecular polymeric micelles: Synthesis of a novel drug carrier. Bioconjug. Chem. 2003, 14, 774–781. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.L.; Liu, J.J.; Zhang, J.H.; Deng, L.D.; Dong, A.J. pH-Sensitive nanoparticles prepared from amphiphilic and biodegradable methoxy poly(ethylene glycol)-block-(polycaprolactone-graft-poly(methacrylic acid)) for oral drug delivery. Polym. Chem. 2013, 4, 1430–1438. [Google Scholar] [CrossRef]
- Chen, Q.; Lin, W.J.; Wang, H.Y.; Wang, J.F.; Zhang, L.J. PDEAEMA-based pH-sensitive amphiphilic pentablock copolymers for controlled anticancer drug delivery. RSC Adv. 2016, 6, 68018–68027. [Google Scholar] [CrossRef]
- Yang, C.F.; Xue, Z.L.; Liu, Y.L.; Xiao, J.Y.; Chen, J.R.; Zhang, L.J.; Guo, J.W.; Lin, W.J. Delivery of anticancer drug using pH-sensitive micelles from triblock copolymer MPEG-b-PBAE-b-PLA. Mater. Sci. Eng. C 2018, 84, 254–262. [Google Scholar] [CrossRef]
- Lin, W.J.; Nie, S.Y.; Chen, Q.; Qian, Y.; Wen, X.F.; Zhang, L.J. Structure-property relationship of pH-sensitive (PCL)2(PDEA-b-PPEGMA)2 micelles: Experiment and DPD simulation. AIChE J. 2014, 60, 3634–3646. [Google Scholar] [CrossRef]
- Rostamizadeh, K.; Manafi, M.; Nosrati, H.; Manjili, H.K.; Danafar, H. Methotrexate-conjugated mPEG-PCL copolymers: A novel approach for dual triggered drug delivery. New J. Chem. 2018, 42, 5937–5945. [Google Scholar] [CrossRef]
- Huang, Y.; Thanneeru, S.; Zhang, Q.; He, J. A new design of cleavable acetal-containing amphiphilic block copolymers triggered by light. J. Polym. Sci. Part A Polym. Chem. 2018, 56, 1815–1824. [Google Scholar] [CrossRef]
- Li, Y.H.; Zhang, X.; Zhang, J.P.; Mu, X.; Duan, Q.; Wang, T.H.; Tian, H.Y. Synthesis and characterization of a hyperbranched grafting copolymer PEI-g-PLeu for gene and drug co-delivery. J. Mater. Sci. Mater. Med. 2018, 29, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Khatri, H.; Chokshi, N.; Rawal, S.; Patel, M.M. Fabrication, characterization and optimization of artemether loaded PEGylated solid lipid nanoparticles for the treatment of lung cancer. Mater. Res. Express 2019, 6. [Google Scholar] [CrossRef]












| Sample | Mn, GPC1 | Mw/Mn1 | Mn, th2 | CMC 3 (mg/L) | |
|---|---|---|---|---|---|
| AdP-1 | Ad-P[(EMA11-co-MAA9)-b-PPEGMA10]4 | 21.4 × 103 | 1.33 | 25.1 × 103 | 5.0 |
| AdP-2 | Ad-P[(EMA11-co-MAA12)-b-PPEGMA7]4 | 20.9 × 103 | 1.50 | 23.5 × 103 | 3.9 |
| PTX/Copolymer (mg/mg) | Dh (nm) | PDI | Zata Potential (mV) | DLC (%) | EE (%) | |
|---|---|---|---|---|---|---|
| AdP-1 | 0/40 | 150.8 | 0.155 | −28 | – | – |
| 10/40 | 196.1 | 0.227 | −23 | 11.8 | 32.9 | |
| 13/40 | 265.2 | 0.233 | −18.6 | 14.6 | 33.3 | |
| 20/40 | 294.8 | 0.248 | −25 | 15.9 | 26.1 | |
| AdP-2 | 0/40 | 160.2 | 0.183 | −30 | – | – |
| 10/40 | 258 | 0.197 | −26.8 | 14.7 | 38.9 | |
| 13/40 | 300.6 | 0.258 | −21.3 | 18.9 | 36 | |
| 20/40 | 330 | 0.210 | −24.1 | 16.8 | 35.7 |
| Model | Equation | R2 | R |
|---|---|---|---|
| AdP-1 at pH 1.2 | |||
| Zero order | Y = 0.1048x + 10.979 | 0.5189 | 0.7203 |
| First order | Y = 10.38e0.009x | 0.4083 | 0.6390 |
| Hixson-Crowell | Y = 0.0002x3 − 0.0224x2 + 0.8466x + 7.2662 | 0.8838 | 0.9401 |
| Peppas | Y = 7.7009x0.2103 | 0.8609 | 0.9278 |
| AdP-2 at pH 1.2 | |||
| Zero order | Y = 0.1328x + 11.281 | 0.6486 | 0.8053 |
| First order | Y = 10.77e0.01x | 0.5034 | 0.7100 |
| Hixson-Crowell | Y = 0.0002x3 − 0.0205x2 + 0.8156x + 7.8419 | 0.8949 | 0.9460 |
| Peppas | Y = 8.009x0.2215 | 0.9159 | 0.9570 |
| AdP-1 at pH 6.8 | |||
| Zero order | Y = 0.6742x + 24.549 | 0.9045 | 0.9511 |
| First order | Y = 24.52e0.017x | 0.7829 | 0.8848 |
| Hixson-Crowell | Y = 0.0004x3 − 0.0481x2 + 2.3484x + 15.822 | 0.9968 | 0.9984 |
| Peppas | Y = 17.443x0.3092 | 0.9811 | 0.9905 |
| AdP-2 at pH 6.8 | |||
| Zero order | Y = 0.7379x + 28.67 | 0.8665 | 0.9310 |
| First order | Y = 28.054e0.016 | 0.7291 | 0.8539 |
| Hixson-Crowell | Y = 0.0005x3 − 0.0625x2 + 2.9045x + 17.412 | 0.9883 | 0.9941 |
| Peppas | Y = 19.648x0.3128 | 0.9651 | 0.9824 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, W.; Guo, J.; Wen, W.; Jia, Y.-G.; Liu, S. Nano-Carriers Based on pH-Sensitive Star-Shaped Copolymers for Drug-Controlled Release. Materials 2019, 12, 1610. https://doi.org/10.3390/ma12101610
Jiang W, Guo J, Wen W, Jia Y-G, Liu S. Nano-Carriers Based on pH-Sensitive Star-Shaped Copolymers for Drug-Controlled Release. Materials. 2019; 12(10):1610. https://doi.org/10.3390/ma12101610
Chicago/Turabian StyleJiang, Wenzhao, Jianwei Guo, Weiqiu Wen, Yong-Guang Jia, and Sa Liu. 2019. "Nano-Carriers Based on pH-Sensitive Star-Shaped Copolymers for Drug-Controlled Release" Materials 12, no. 10: 1610. https://doi.org/10.3390/ma12101610
APA StyleJiang, W., Guo, J., Wen, W., Jia, Y.-G., & Liu, S. (2019). Nano-Carriers Based on pH-Sensitive Star-Shaped Copolymers for Drug-Controlled Release. Materials, 12(10), 1610. https://doi.org/10.3390/ma12101610