Diameter of Carbon Nanotube-Directed Self-Assembly of Amphiphilic Block Copolymers
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of MPEG-PDLLA/c-MWCNT Nanocomposites
2.2.2. Characterization of MPEG-PDLLA/c-MWCNT Nanocomposites
2.2.3. In Vitro Drug Release
3. Results and Discussion
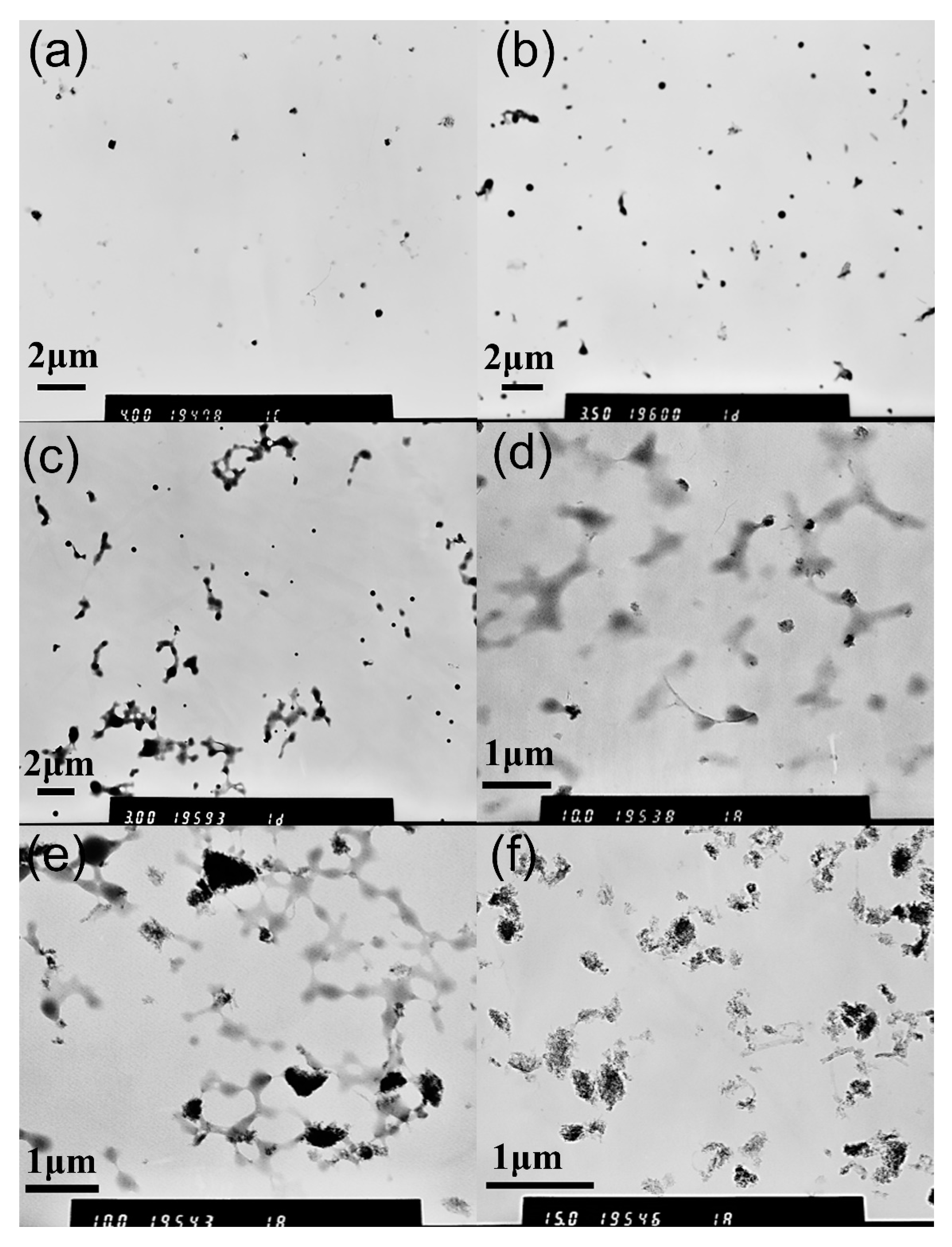
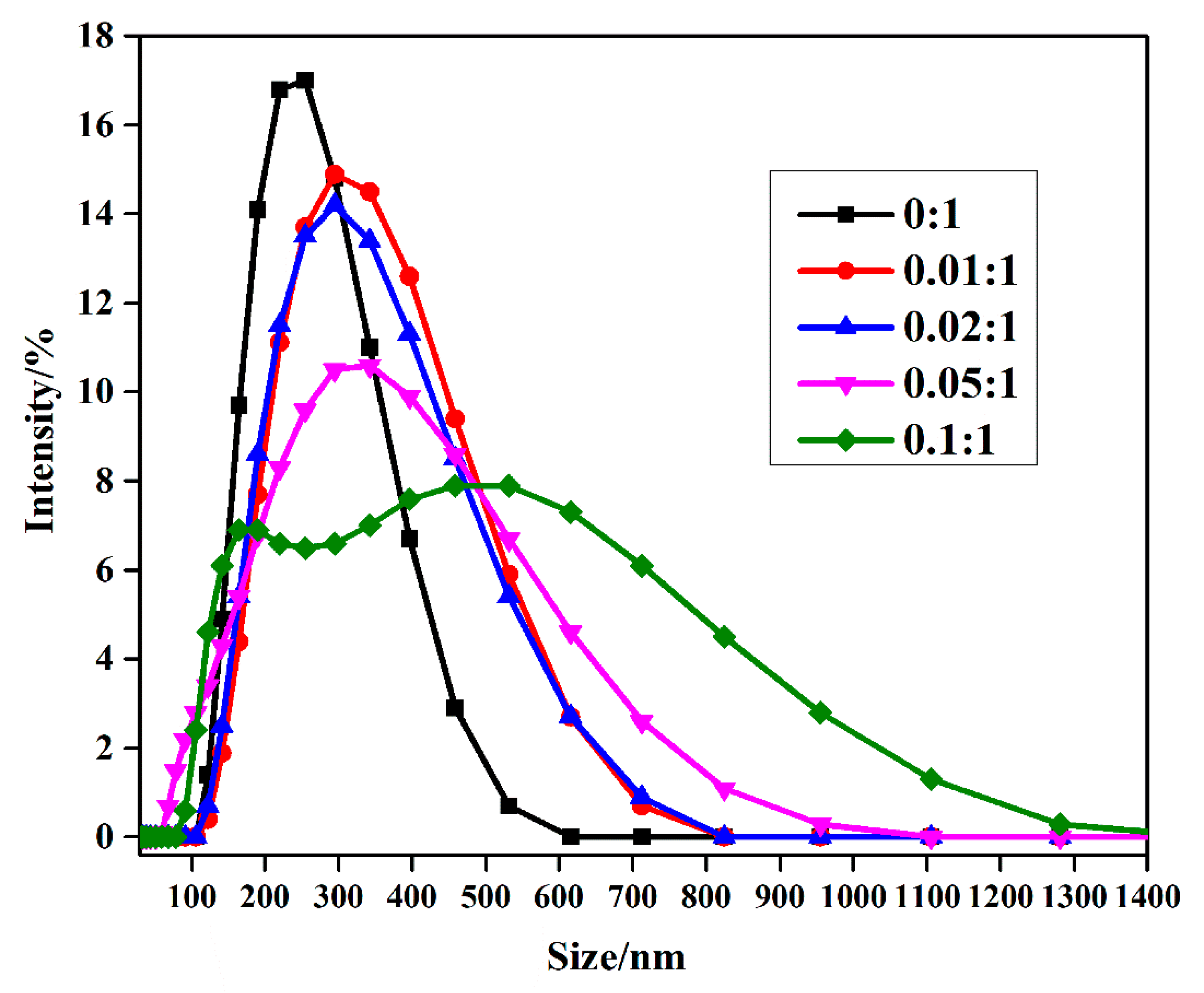
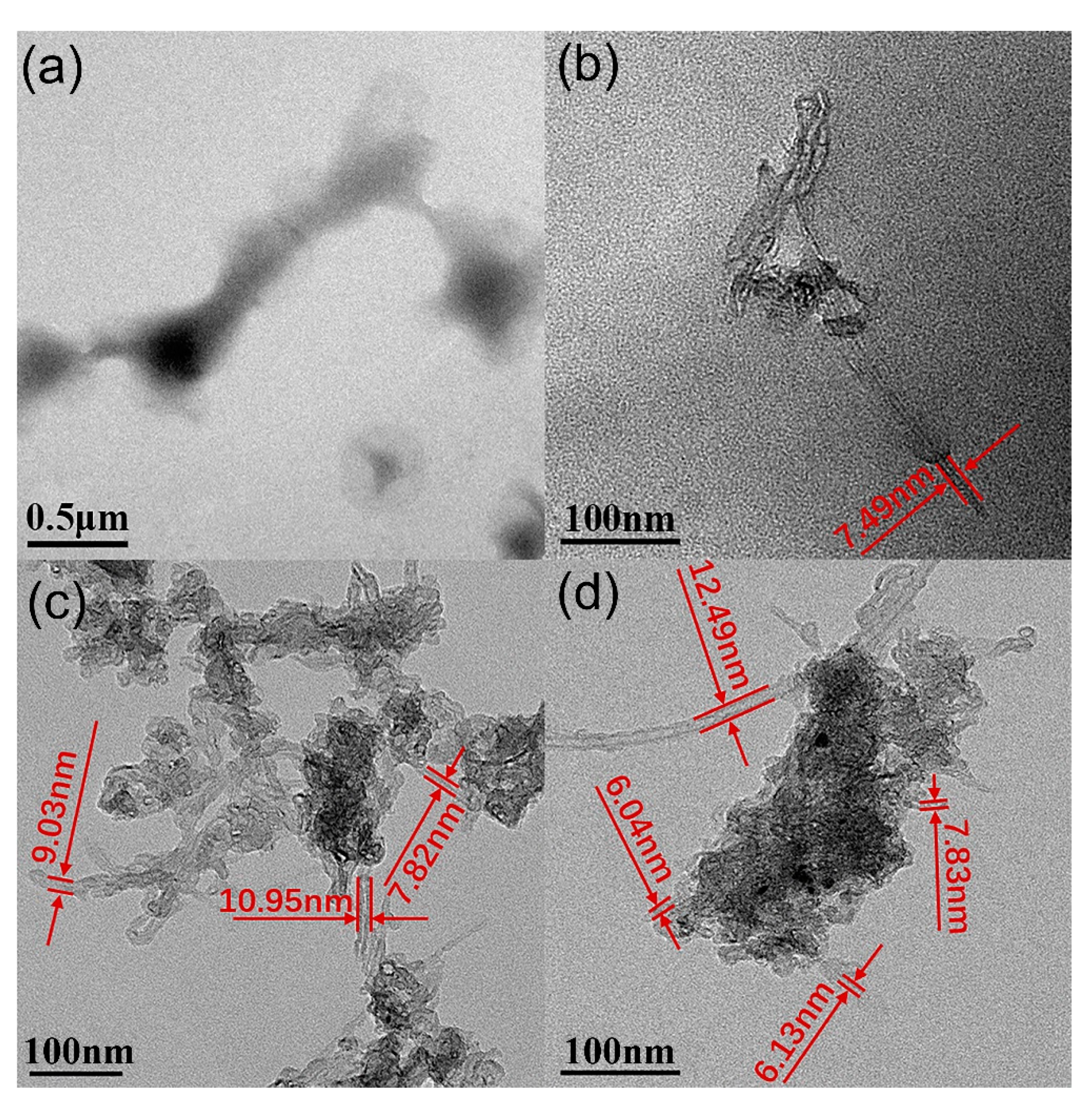
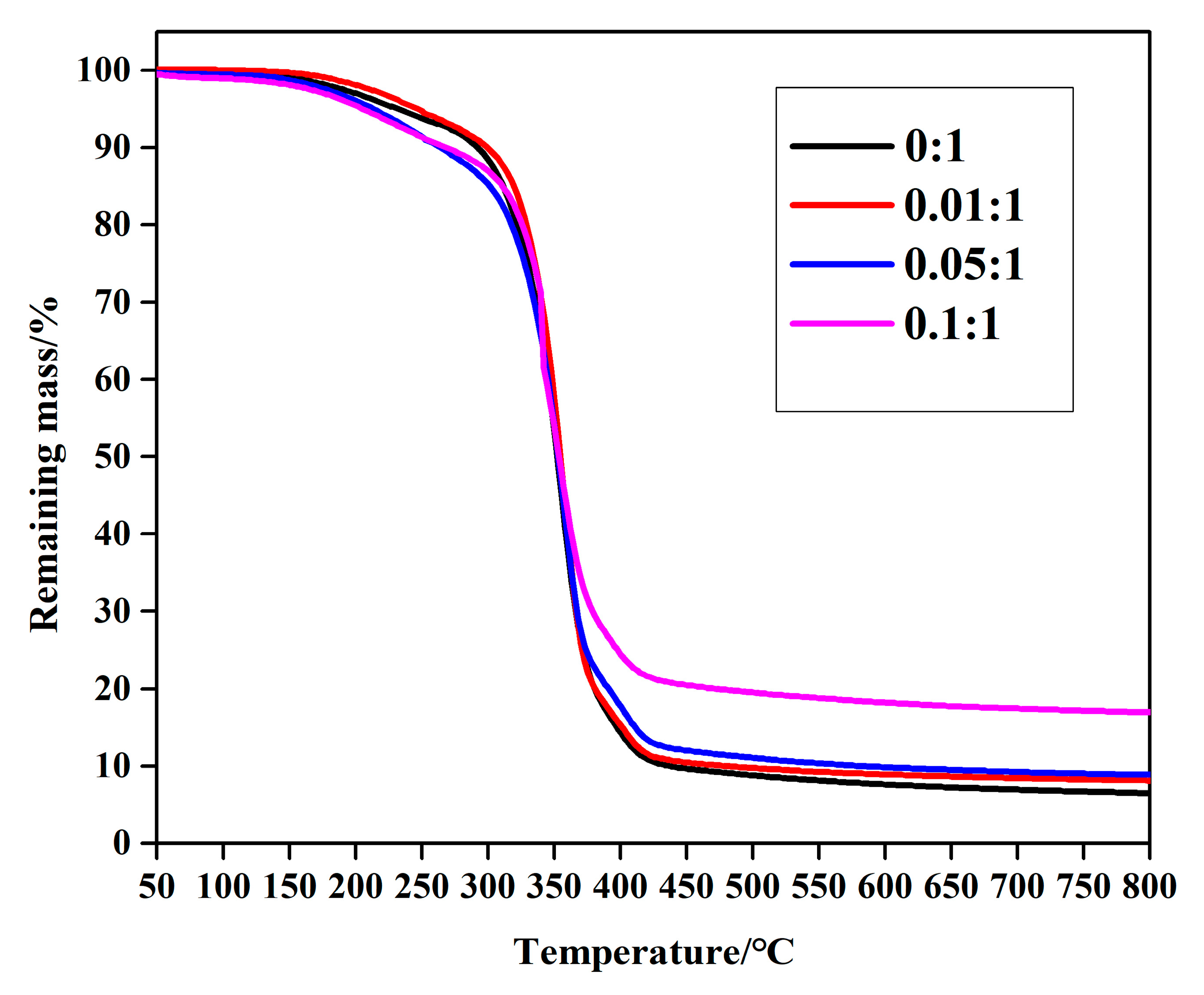
3.1. Characterization of MPEG-PDLLA/c-MWCNT Nanocomposites
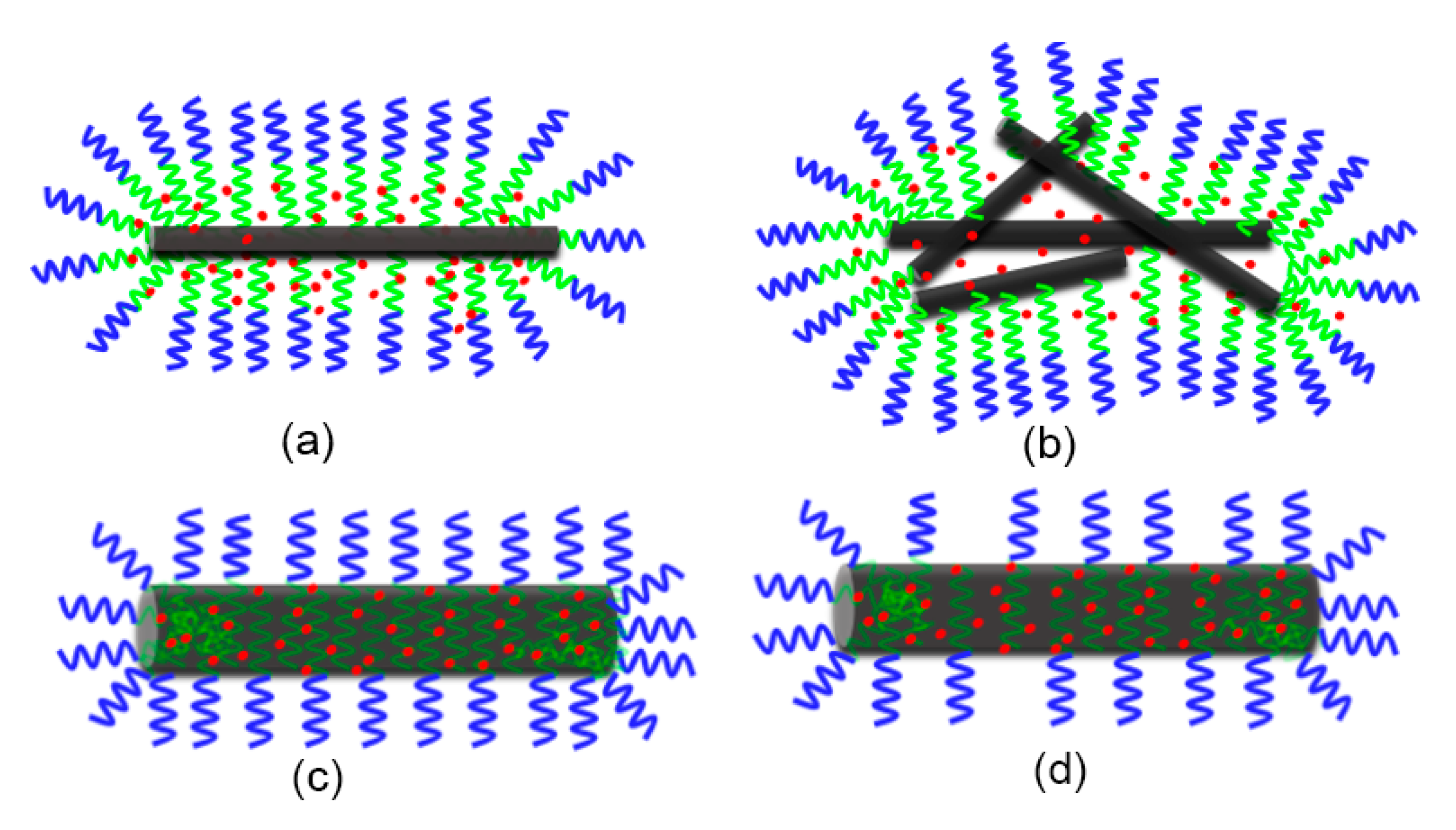
3.2. Proposed Possible Structures for MPEG-PDLLA/c-MWCNT Nanocomposites
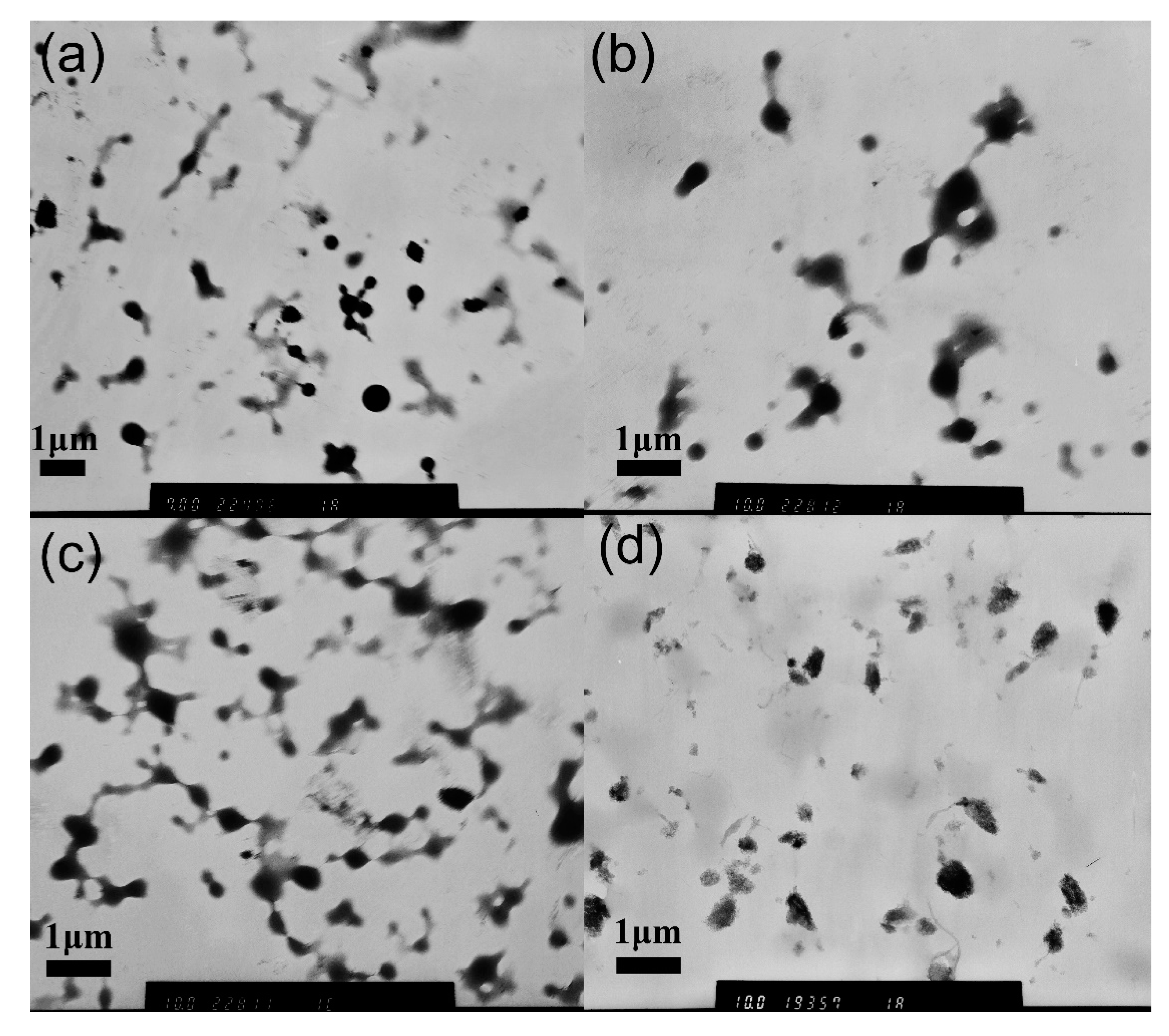
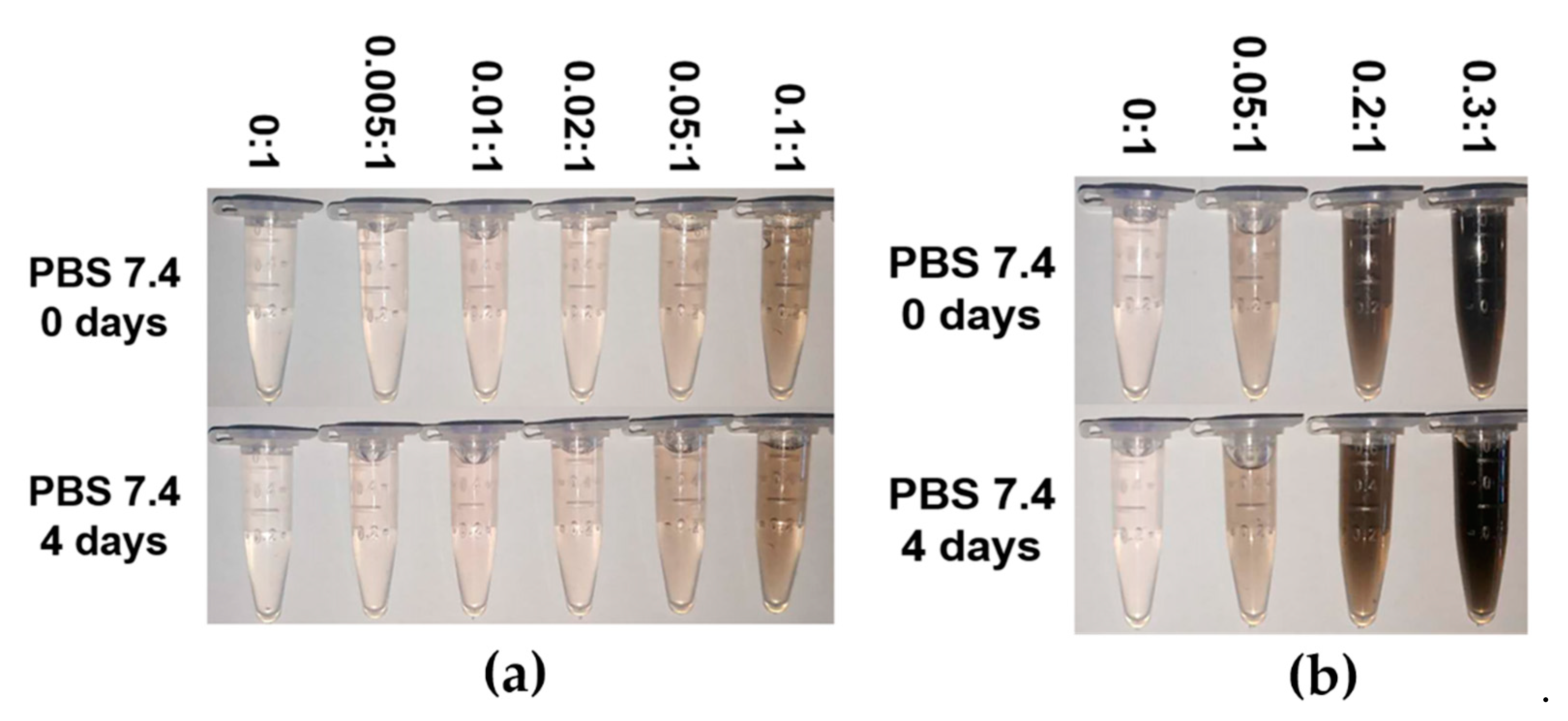
3.3. Stability of MPEG-PDLLA/c-MWCNT Nanocomposites
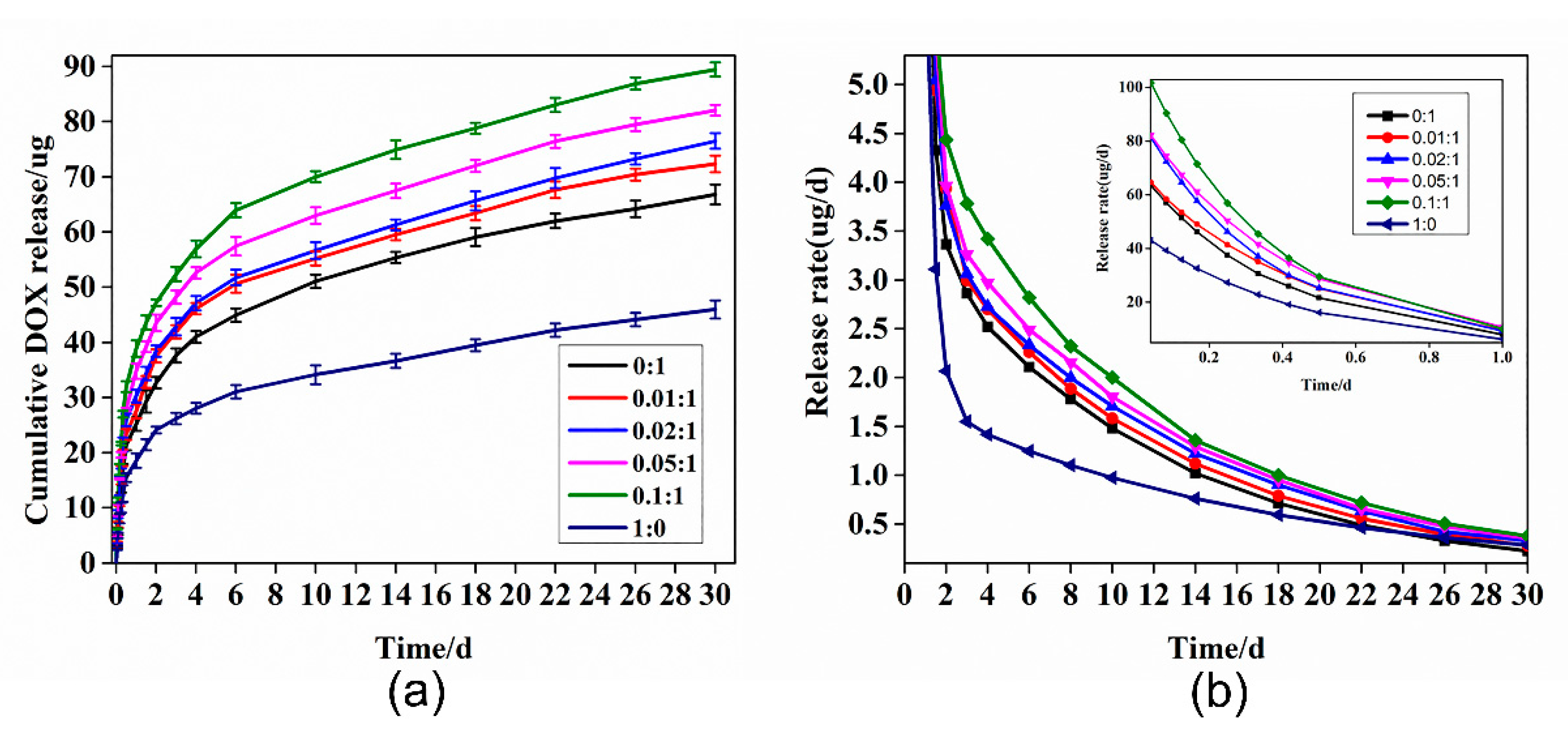
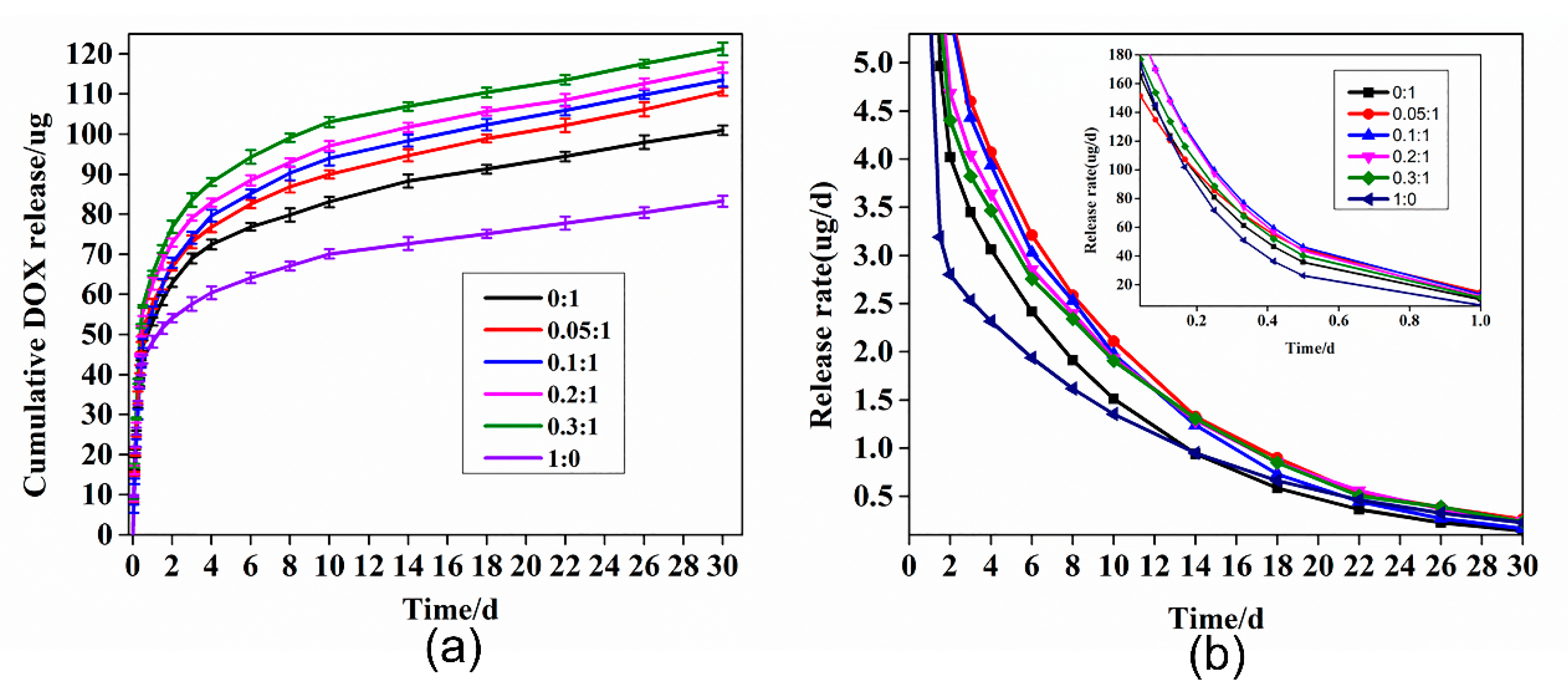
3.4. Drug Loading and In Vitro Release Studies
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Epps, T.H., III; O’Reilly, R.K. Block copolymers: Controlling nanostructure to generate functional materials—Synthesis, characterization, and engineering. Chem. Sci. 2016, 7, 1674–1689. [Google Scholar] [CrossRef]
- Letchford, K.; Burt, H. A review of the formation and classification of amphiphilic block copolymer nanoparticulate structures: Micelles, nanospheres, nanocapsules and polymersomes. Eur. J. Pharm. Biopharm. 2007, 65, 259–269. [Google Scholar] [CrossRef]
- Mai, Y.; Eisenberg, A. Self-assembly of block copolymers. Chem. Soc. Rev. 2012, 41, 5969–5985. [Google Scholar] [CrossRef]
- Moughton, A.O.; Hillmyer, M.A.; Lodge, T.P. Multicompartment Block Polymer Micelles. Macromolecules 2012, 45, 2–19. [Google Scholar] [CrossRef]
- Zhu, J.; Hayward, R.C. Spontaneous generation of amphiphilic block copolymer micelles with multiple morphologies through interfacial instabilities. J. Am. Chem. Soc. 2008, 130, 7496–7502. [Google Scholar] [CrossRef] [PubMed]
- Rosler, A.; Vandermeulen, G.W.M.; Klok, H.A. Advanced drug delivery devices via self-assembly of amphiphilic block copolymers. Adv. Drug Deliv. Rev. 2001, 53, 95–108. [Google Scholar] [CrossRef]
- Nasongkla, N.; Bey, E.; Ren, J.; Ai, H.; Khemtong, C.; Guthi, J.S.; Chin, S.-F.; Sherry, A.D.; Boothman, D.A.; Gao, J. Multifunctional polymeric micelles as cancer-targeted, MRI-ultrasensitive drug delivery systems. Nano Lett. 2006, 6, 2427–2430. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Grailer, J.J.; Rowland, I.J.; Javadi, A.; Hurley, S.A.; Steeber, D.A.; Gong, S. Multifunctional SPIO/DOX-loaded wormlike polymer vesicles for cancer therapy and MR imaging. Biomaterials 2010, 31, 9065–9073. [Google Scholar] [CrossRef]
- Jain, T.K.; Richey, J.; Strand, M.; Leslie-Pelecky, D.L.; Flask, C.A.; Labhasetwar, V. Magnetic nanoparticles with dual functional properties: Drug delivery and magnetic resonance imaging. Biomaterials 2008, 29, 4012–4021. [Google Scholar] [CrossRef]
- Balazs, A.C.; Emrick, T.; Russell, T.P. Nanoparticle polymer composites: Where two small worlds meet. Science 2006, 314, 1107–1110. [Google Scholar] [CrossRef]
- Mai, Y.; Eisenberg, A. Selective Localization of Preformed Nanoparticles in Morphologically Controllable Block Copolymer Aggregates in Solution. Acc. Chem. Res. 2012, 45, 1657–1666. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, W.; Zhu, J. Encapsulation of inorganic nanoparticles into block copolymer micellar aggregates: Strategies and precise localization of nanoparticles. Polymer 2014, 55, 1079–1096. [Google Scholar] [CrossRef]
- Gao, X.H.; Cui, Y.Y.; Levenson, R.M.; Chung, L.W.K.; Nie, S.M. In vivo cancer targeting and imaging with semiconductor quantum dots. Nat. Biotechnol. 2004, 22, 969–976. [Google Scholar] [CrossRef] [PubMed]
- Berret, J.F.; Schonbeck, N.; Gazeau, F.; El Kharrat, D.; Sandre, O.; Vacher, A.; Airiau, M. Controlled clustering of superparamagnetic nanoparticles using block copolymers: Design of new contrast agents for magnetic resonance imaging. J. Am. Chem. Soc. 2006, 128, 1755–1761. [Google Scholar] [CrossRef]
- Hickey, R.J.; Haynes, A.S.; Kikkawa, J.M.; Park, S.-J. Controlling the Self-Assembly Structure of Magnetic Nanoparticles and Amphiphilic Block-Copolymers: From Micelles to Vesicles. J. Am. Chem. Soc. 2011, 133, 1517–1525. [Google Scholar] [CrossRef] [PubMed]
- Kao, J.; Thorkelsson, K.; Bai, P.; Zhang, Z.; Sun, C.; Xu, T. Rapid fabrication of hierarchically structured supramolecular nanocomposite thin films in one minute. Nat. Commun. 2014, 5. [Google Scholar] [CrossRef]
- Mun, J.H.; Cha, S.K.; Kim, H.; Moon, H.-S.; Kim, J.Y.; Jin, H.M.; Choi, Y.J.; Baek, J.E.; Shin, J.; Kim, S.O. Nanodomain Swelling Block Copolymer Lithography for Morphology Tunable Metal Nanopatterning. Small 2014, 10, 3742–3749. [Google Scholar] [CrossRef]
- McDevitt, M.R.; Chattopadhyay, D.; Kappel, B.J.; Jaggi, J.S.; Schiffman, S.R.; Antczak, C.; Njardarson, J.T.; Brentjens, R.; Scheinberg, D.A. Tumor targeting with antibody-functionalized, radiolabeled carbon nanotubes. J. Nucl. Med. 2007, 48, 1180–1189. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Cai, W.; He, L.; Nakayama, N.; Chen, K.; Sun, X.; Chen, X.; Dai, H. In vivo biodistribution and highly efficient tumour targeting of carbon nanotubes in mice. Nat. Nanotechnol. 2007, 2, 47–52. [Google Scholar] [CrossRef]
- Cherukuri, P.; Gannon, C.J.; Leeuw, T.K.; Schmidt, H.K.; Smalley, R.E.; Curley, S.A.; Weisman, R.B. Mammalian pharmacokinetics of carbon nanotubes using intrinsic near-infrared fluorescence. Proc. Natl. Acad. Sci. USA 2006, 103, 18882–18886. [Google Scholar] [CrossRef] [PubMed]
- De La Zerda, A.; Zavaleta, C.; Keren, S.; Vaithilingam, S.; Bodapati, S.; Liu, Z.; Levi, J.; Smith, B.R.; Ma, T.-J.; Oralkan, O.; et al. Carbon nanotubes as photoacoustic molecular imaging agents in living mice. Nat. Nanotechnol. 2008, 3, 557–562. [Google Scholar] [CrossRef]
- de la Zerda, A.; Liu, Z.; Bodapati, S.; Teed, R.; Vaithilingam, S.; Khuri-Yakub, B.T.; Chen, X.; Dai, H.; Gambhir, S.S. Ultrahigh Sensitivity Carbon Nanotube Agents for Photoacoustic Molecular Imaging in Living Mice. Nano Lett. 2010, 10, 2168–2172. [Google Scholar] [CrossRef]
- Goodman, S.M.; Ferguson, N.; Dichiara, A.B. Lignin-assisted double acoustic irradiation for concentrated aqueous dispersions of carbon nanotubes. RSC Adv. 2017, 7, 5488–5496. [Google Scholar] [CrossRef]
- Khandare, J.J.; Jalota-Badhwar, A.; Satavalekar, S.D.; Bhansali, S.G.; Aher, N.D.; Kharas, F.; Banerjee, S.S. PEG-conjugated highly dispersive multifunctional magnetic multi-walled carbon nanotubes for cellular imaging. Nanoscale 2012, 4, 837–844. [Google Scholar] [CrossRef]
- Mahajan, S.; Patharkar, A.; Kuche, K.; Maheshwari, R.; Deb, P.K.; Kalia, K.; Tekade, R.K. Functionalized carbon nanotubes as emerging delivery system for the treatment of cancer. Int. J. Pharm. 2018, 548, 540–558. [Google Scholar] [CrossRef] [PubMed]
- Biju, V. Chemical modifications and bioconjugate reactions of nanomaterials for sensing, imaging, drug delivery and therapy. Chem. Soc. Rev. 2014, 43, 744–764. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.-C.; Chang, X.; Liu, L.; Zhao, F.; Zhao, Y. Chemistry of carbon nanotubes in biomedical applications. J. Mater. Chem. 2010, 20, 1036–1052. [Google Scholar] [CrossRef]
- Mazzaglia, A.; Scala, A.; Sortino, G.; Zagami, R.; Zhu, Y.; Sciortino, M.T.; Pennisi, R.; Pizzo, M.M.; Neri, G.; Grassi, G.; et al. Intracellular trafficking and therapeutic outcome of multiwalled carbon nanotubes modified with cyclodextrins and polyethylenimine. Colloids Surf. B-Biointerfaces 2018, 163, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Sun, Z.; Wang, X.; Leng, X. An innovative MWCNTs/DOX/TC nanosystem for chemo-photothermal combination therapy of cancer. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 2271–2280. [Google Scholar] [CrossRef]
- Kharissova, O.V.; Kharisov, B.I.; de Casas Ortiz, E.G. Dispersion of carbon nanotubes in water and non-aqueous solvents. RSC Adv. 2013, 3, 24812–24852. [Google Scholar] [CrossRef]
- Bilalis, P.; Katsigiannopoulos, D.; Avgeropoulos, A.; Sakellariou, G. Non-covalent functionalization of carbon nanotubes with polymers. RSC Adv. 2014, 4, 2911–2934. [Google Scholar] [CrossRef]
- Yao, X.P.; Li, J.; Kong, L.; Wang, Y. Surface functionalization of carbon nanotubes by direct encapsulation with varying dosages of amphiphilic block copolymers. Nanotechnology 2015, 26, 10. [Google Scholar] [CrossRef]
- Kubotera, A.; Saito, R. Architectural effect of poly(acrylic acid) and poly(amide imide) block copolymers on dispersion of carbon nanotubes in water. J. Appl. Polym. Sci. 2016, 133. [Google Scholar] [CrossRef]
- Li, B.; Li, L.; Wang, B.; Li, C.Y. Alternating patterns on single-walled carbon nanotubes. Nat. Nanotechnol. 2009, 4, 358–362. [Google Scholar] [CrossRef] [PubMed]
- Ha, J.-M.; Jang, H.-S.; Lim, S.-H.; Choi, S.-M. Selective distributions of functionalized single-walled carbon nanotubes in a polymeric reverse hexagonal phase. Soft Matter 2015, 11, 5821–5827. [Google Scholar] [CrossRef]
- Szleifer, I.; Yerushalmi-Rozen, R. Polymers and carbon nanotubes—dimensionality, interactions and nanotechnology. Polymer 2005, 46, 7803–7818. [Google Scholar] [CrossRef]
- Dieckmann, G.R.; Dalton, A.B.; Johnson, P.A.; Razal, J.; Chen, J.; Giordano, G.M.; Muñoz, E.; Musselman, I.H.; Baughman, R.H.; Draper, R.K. Controlled Assembly of Carbon Nanotubes by Designed Amphiphilic Peptide Helices. J. Am. Chem. Soc. 2003, 125, 1770–1777. [Google Scholar] [CrossRef]
- Star, A.; Stoddart, J.F. Dispersion and Solubilization of Single-Walled Carbon Nanotubes with a Hyperbranched Polymer. Macromolecules 2002, 35, 7516–7520. [Google Scholar] [CrossRef]
- O’Connell, M.J.; Bachilo, S.M.; Huffman, C.B.; Moore, V.C.; Strano, M.S.; Haroz, E.H.; Rialon, K.L.; Boul, P.J.; Noon, W.H.; Kittrell, C.; et al. Band Gap Fluorescence from Individual Single-Walled Carbon Nanotubes. Science 2002, 297, 593–596. [Google Scholar] [CrossRef]
- Nativ-Roth, E.; Shvartzman-Cohen, R.; Bounioux, C.; Florent, M.; Zhang, D.; Szleifer, I.; Yerushalmi-Rozen, R. Physical Adsorption of Block Copolymers to SWNT and MWNT: A Nonwrapping Mechanism. Macromolecules 2007, 40, 3676–3685. [Google Scholar] [CrossRef]
- Shvartzman-Cohen, R.; Levi-Kalisman, Y.; Nativ-Roth, E.; Yerushalmi-Rozen, R. Generic Approach for Dispersing Single-Walled Carbon Nanotubes: The Strength of a Weak Interaction. Langmuir 2004, 20, 6085–6088. [Google Scholar] [CrossRef] [PubMed]
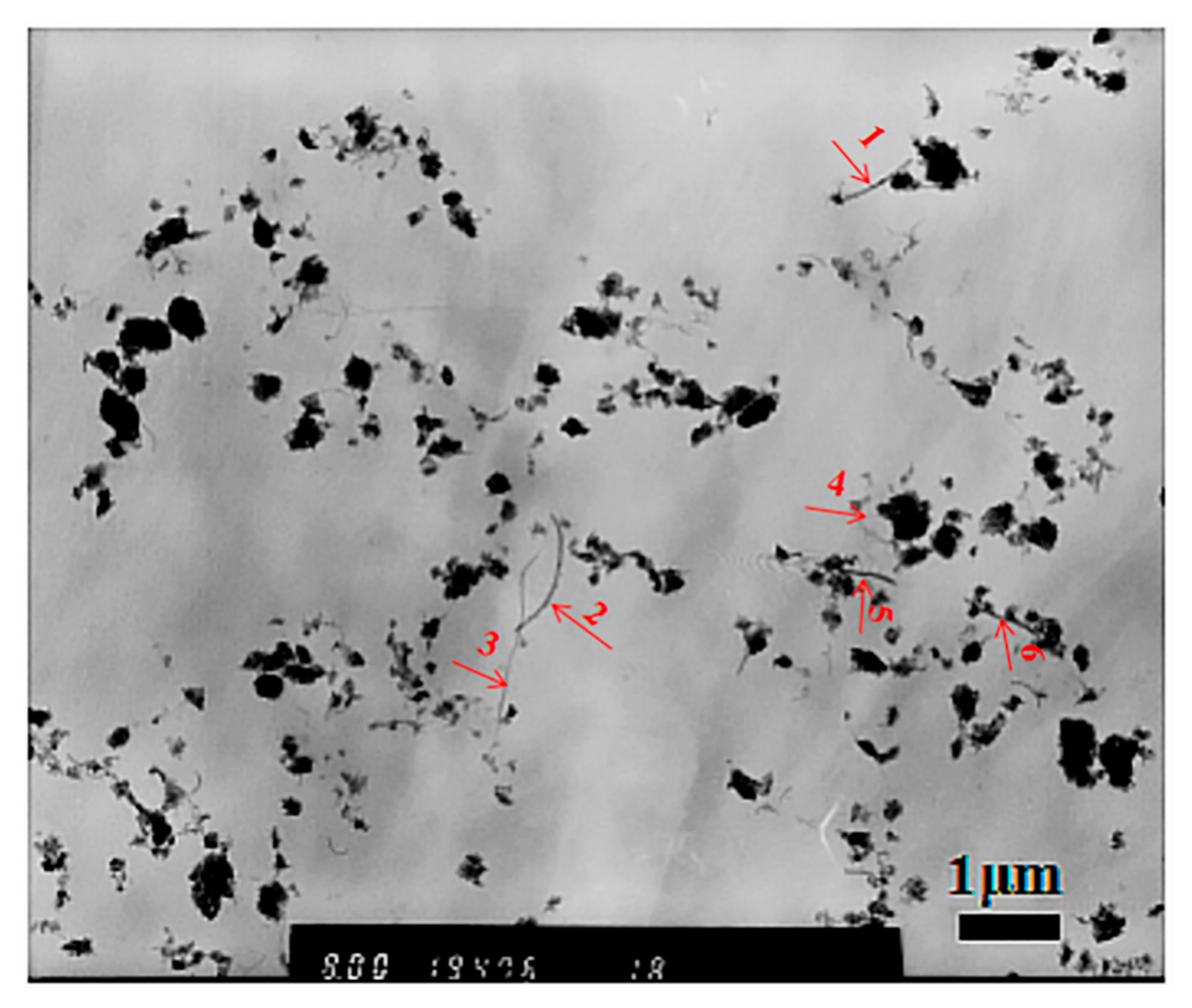


| Number | Size/nm |
|---|---|
| 1 | 30 |
| 2 | 22 |
| 3 | 15 |
| 4 | 14 |
| 5 | 23 |
| 6 | 20 |
| c-MWCNT Addition | Drug Entrapment (DE%) |
|---|---|
| 0:1 | 4.35 ± 0.20 |
| 0.01:1 | 4.72 ± 0.25 |
| 0.02:1 | 4.51 ± 0.15 |
| 0.05:1 | 5.38 ± 0.32 |
| 0.1:1 | 6.22 ± 0.35 |
| 1:0 | 2.53 ± 0.10 |
| c-MWCNT Addition | Drug Entrapment (DE%) |
|---|---|
| 0:1 | 4.35 ± 0.20 |
| 0.05:1 | 4.97 ± 0.27 |
| 0.1:1 | 5.71 ± 0.31 |
| 0.2:1 | 5.13 ± 0.23 |
| 0.3:1 | 6.86 ± 0.36 |
| 1:0 | 3.65 ± 0.13 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Tao, S.; Chu, Y.; Xu, X.; Tan, Q. Diameter of Carbon Nanotube-Directed Self-Assembly of Amphiphilic Block Copolymers. Materials 2019, 12, 1606. https://doi.org/10.3390/ma12101606
Wang Z, Tao S, Chu Y, Xu X, Tan Q. Diameter of Carbon Nanotube-Directed Self-Assembly of Amphiphilic Block Copolymers. Materials. 2019; 12(10):1606. https://doi.org/10.3390/ma12101606
Chicago/Turabian StyleWang, Zihao, Susu Tao, Yanyan Chu, Xiaoyan Xu, and Qinggang Tan. 2019. "Diameter of Carbon Nanotube-Directed Self-Assembly of Amphiphilic Block Copolymers" Materials 12, no. 10: 1606. https://doi.org/10.3390/ma12101606
APA StyleWang, Z., Tao, S., Chu, Y., Xu, X., & Tan, Q. (2019). Diameter of Carbon Nanotube-Directed Self-Assembly of Amphiphilic Block Copolymers. Materials, 12(10), 1606. https://doi.org/10.3390/ma12101606




