Nanotechnology Meets Oncology: Nanomaterials in Brain Cancer Research, Diagnosis and Therapy
Abstract
1. Cancer: From Macro to Nano
Glioblastoma
2. Nanomaterials in Biomedical Research
2.1. Metallic Nanoparticles
2.1.1. Silver Nanoparticles
2.1.2. Gold Nanoparticles
2.1.3. Magnetic Nanoparticles
2.1.4. Platinum Nanoparticles
2.2. Inorganic Nanoparticles
Quantum Dots
2.3. Organic Nanoparticles
2.3.1. Liposomes
2.3.2. Block Copolymere Micelles
2.3.3. Dendrimers
2.3.4. Polymers
2.4. Biological Nanoparticles
2.4.1. Nanobodies
2.4.2. Extracellular Vesicles
3. Nanomaterials and Brain Cancer
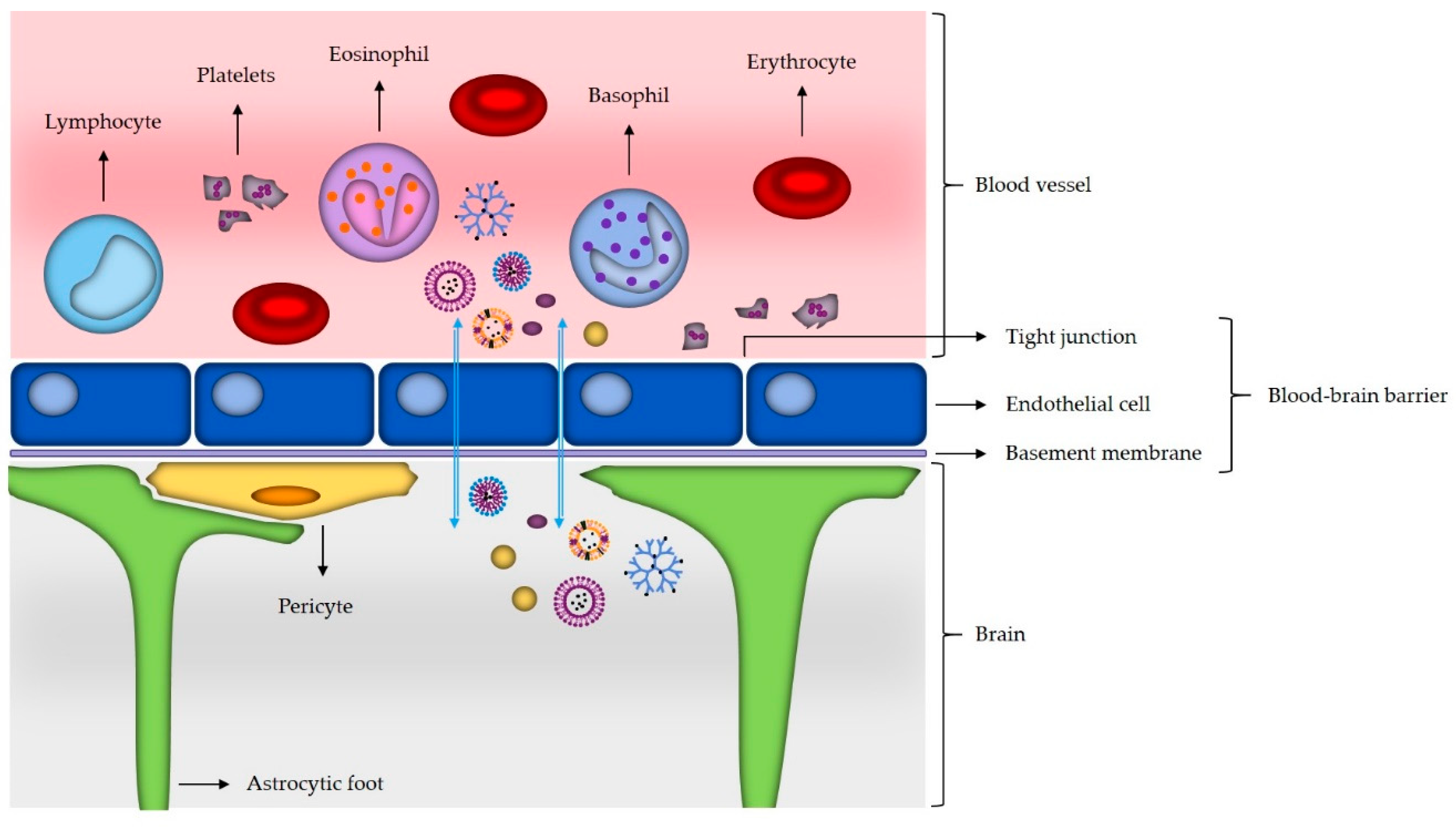
3.1. Mechanisms of Nanomaterials Transport across the Blood–Brain Barrier
3.2. Use of Nanoparticles in Glioblastoma Targeting
- Phase I: dosing, toxicity and excretion in healthy subjects;
- Phase II: safety, efficacy in large patient groups;
- Phase III: multi-centered randomized placebo-controlled trials;
- Phase IV: post-marketing studies, requested by health care professionals or the FDA.
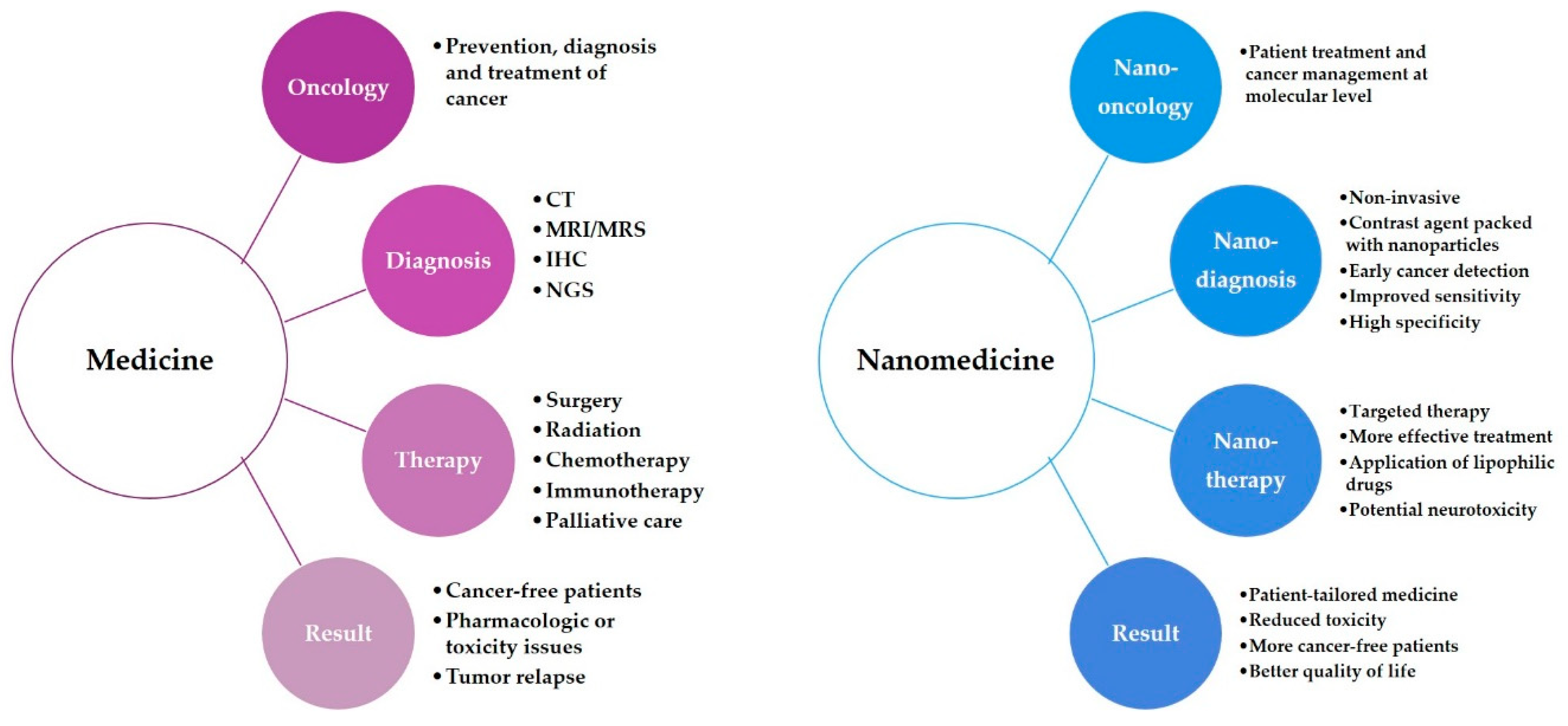
4. Nanotechnology
4.1. Nanodiagnostics
4.2. Nanotherapy
5. Nanomedicine
5.1. Nanooncology
5.2. Challenges, Social Concerns and Safety Issues of Nanomedicine
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kaufmann, J.K.; Chiocca, E.A. Glioma virus therapies between bench and bedside. Neuro-Oncology 2014, 16, 334–351. [Google Scholar] [CrossRef]
- Silva, C.O.; Pinho, J.O.; Lopes, J.M.; Almeida, A.J.; Gaspar, M.M.; Reis, C. Current trends in cancer nanotheranostics: Metallic, polymeric, and lipid-based systems. Pharmaceutics 2019, 11, 22. [Google Scholar] [CrossRef] [PubMed]
- Peer, D.; Karp, J.M.; Hong, S.; Farokhzad, O.C.; Margalit, R.; Langer, R. Nanocarriers as an emerging platform for cancer therapy. Nat. Nanotechnol. 2007, 2, 751–760. [Google Scholar] [CrossRef] [PubMed]
- Quader, S.; Kataoka, K. Nanomaterial-enabled cancer therapy. Mol. Ther. J. Am. Soc. Gene Ther. 2017, 25, 1501–1513. [Google Scholar] [CrossRef] [PubMed]
- Jain, K.K. Nanomedicine: Application of nanobiotechnology in medical practice. Med. Princ. Pract. Int. J. Kuwait Univ. Health Sci. Cent. 2008, 17, 89–101. [Google Scholar] [CrossRef] [PubMed]
- Jain, K.K. Nanobiotechnology and personalized medicine. Prog. Mol. Biol. Transl. Sci. 2011, 104, 325–354. [Google Scholar]
- Lukianova-Hleb, E.Y.; Kim, Y.S.; Belatsarkouski, I.; Gillenwater, A.M.; O’Neill, B.E.; Lapotko, D.O. Intraoperative diagnostics and elimination of residual microtumours with plasmonic nanobubbles. Nat. Nanotechnol. 2016, 11, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Piktel, E.; Niemirowicz, K.; Watek, M.; Wollny, T.; Deptula, P.; Bucki, R. Recent insights in nanotechnology-based drugs and formulations designed for effective anti-cancer therapy. J. Nanobiotechnology 2016, 14, 39. [Google Scholar] [CrossRef]
- Jabir, N.R.; Anwar, K.; Firoz, C.K.; Oves, M.; Kamal, M.A.; Tabrez, S. An overview on the current status of cancer nanomedicines. Curr. Med. Res. Opin. 2018, 34, 911–921. [Google Scholar] [CrossRef]
- Tran, S.; DeGiovanni, P.J.; Piel, B.; Rai, P. Cancer nanomedicine: A review of recent success in drug delivery. Clin. Transl. Med. 2017, 6, 44. [Google Scholar] [CrossRef]
- Wicki, A.; Witzigmann, D.; Balasubramanian, V.; Huwyler, J. Nanomedicine in cancer therapy: Challenges, opportunities, and clinical applications. J. Control. Release Off. J. Control. Release Soc. 2015, 200, 138–157. [Google Scholar] [CrossRef]
- Bobo, D.; Robinson, K.J.; Islam, J.; Thurecht, K.J.; Corrie, S.R. Nanoparticle-based medicines: A review of fda-approved materials and clinical trials to date. Pharm. Res. 2016, 33, 2373–2387. [Google Scholar] [CrossRef]
- Hua, S.; de Matos, M.B.C.; Metselaar, J.M.; Storm, G. Current trends and challenges in the clinical translation of nanoparticulate nanomedicines: Pathways for translational development and commercialization. Front. Pharmacol. 2018, 9, 790. [Google Scholar] [CrossRef]
- Jain, K.K. Nanobiotechnology-based drug delivery to the central nervous system. Neuro-Degener. Dis. 2007, 4, 287–291. [Google Scholar] [CrossRef] [PubMed]
- Jain, K.K. Nanobiotechnology-based strategies for crossing the blood–brain barrier. Nanomedicine 2012, 7, 1225–1233. [Google Scholar] [CrossRef]
- Teleanu, D.M.; Chircov, C.; Grumezescu, A.M.; Volceanov, A.; Teleanu, R.I. Blood–brain delivery methods using nanotechnology. Pharmaceutics 2018, 10, 269. [Google Scholar] [CrossRef]
- Teleanu, D.M.; Chircov, C.; Grumezescu, A.M.; Volceanov, A.; Teleanu, R.I. Impact of nanoparticles on brain health: An up to date overview. J. Clin. Med. 2018, 7, 490. [Google Scholar] [CrossRef]
- Bailey, C.P.; Figueroa, M.; Mohiuddin, S.; Zaky, W.; Chandra, J. Cutting edge therapeutic insights derived from molecular biology of pediatric high-grade glioma and diffuse intrinsic pontine glioma (dipg). Bioengineering 2018, 5, 88. [Google Scholar] [CrossRef]
- Lim, M.; Xia, Y.; Bettegowda, C.; Weller, M. Current state of immunotherapy for glioblastoma. Nat. Reviews. Clin. Oncol. 2018, 15, 422–442. [Google Scholar] [CrossRef]
- Pinel, S.; Thomas, N.; Boura, C.; Barberi-Heyob, M. Approaches to physical stimulation of metallic nanoparticles for glioblastoma treatment. Adv. Drug Deliv. Rev. 2019, 138, 344–357. [Google Scholar] [CrossRef] [PubMed]
- Qiao, C.; Yang, J.; Shen, Q.; Liu, R.; Li, Y.; Shi, Y.; Chen, J.; Shen, Y.; Xiao, Z.; Weng, J.; et al. Traceable nanoparticles with dual targeting and ros response for rnai-based immunochemotherapy of intracranial glioblastoma treatment. Adv. Mater. 2018, 30, e1705054. [Google Scholar] [CrossRef]
- Marumoto, T.; Saya, H. Molecular biology of glioma. Adv. Exp. Med. Biol. 2012, 746, 2–11. [Google Scholar]
- Samec, N.; Jovcevska, I.; Stojan, J.; Zottel, A.; Liovic, M.; Myers, M.P.; Muyldermans, S.; Sribar, J.; Krizaj, I.; Komel, R. Glioblastoma-specific anti-tufm nanobody for in-vitro immunoimaging and cancer stem cell targeting. Oncotarget 2018, 9, 17282–17299. [Google Scholar] [CrossRef]
- Jain, K.K. Role of nanobiotechnology in the personalized management of glioblastoma multiforme. Nanomedicine 2011, 6, 411–414. [Google Scholar] [CrossRef]
- Miernicki, M.; Hofmann, T.; Eisenberger, I.; von der Kammer, F.; Praetorius, A. Legal and practical challenges in classifying nanomaterials according to regulatory definitions. Nat. Nanotechnol. 2019, 14, 208–216. [Google Scholar] [CrossRef]
- D'Mello, S.R.; Cruz, C.N.; Chen, M.L.; Kapoor, M.; Lee, S.L.; Tyner, K.M. The evolving landscape of drug products containing nanomaterials in the united states. Nat. Nanotechnol. 2017, 12, 523–529. [Google Scholar] [CrossRef]
- Uchegbu, I.F.; Siew, A. Nanomedicines and nanodiagnostics come of age. J. Pharm. Sci. 2013, 102, 305–310. [Google Scholar] [CrossRef]
- Zhang, L.; Gu, F.X.; Chan, J.M.; Wang, A.Z.; Langer, R.S.; Farokhzad, O.C. Nanoparticles in medicine: Therapeutic applications and developments. Clin. Pharm. 2008, 83, 761–769. [Google Scholar] [CrossRef]
- Drummen, G.P. Quantum dots-from synthesis to applications in biomedicine and life sciences. Int. J. Mol. Sci. 2010, 11, 154–163. [Google Scholar] [CrossRef]
- Wei, L.; Lu, J.; Xu, H.; Patel, A.; Chen, Z.S.; Chen, G. Silver nanoparticles: Synthesis, properties, and therapeutic applications. Drug Discov. Today 2015, 20, 595–601. [Google Scholar] [CrossRef]
- Jovcevska, I.; Zupanec, N.; Kocevar, N.; Cesselli, D.; Podergajs, N.; Stokin, C.L.; Myers, M.P.; Muyldermans, S.; Ghassabeh, G.H.; Motaln, H.; et al. Trim28 and beta-actin identified via nanobody-based reverse proteomics approach as possible human glioblastoma biomarkers. PLoS ONE 2014, 9, e113688. [Google Scholar] [CrossRef] [PubMed]
- Pedone, D.; Moglianetti, M.; De Luca, E.; Bardi, G.; Pompa, P.P. Platinum nanoparticles in nanobiomedicine. Chem. Soc. Rev. 2017, 46, 4951–4975. [Google Scholar] [CrossRef] [PubMed]
- Marchioni, M.; Jouneau, P.-H.; Chevallet, M.; Michaud-Soret, I.; Deniaud, A. Silver nanoparticle fate in mammals: Bridging in vitro and in vivo studies. Coord. Chem. Rev. 2018, 364, 118–136. [Google Scholar] [CrossRef]
- Kim, J.S.; Kuk, E.; Yu, K.N.; Kim, J.H.; Park, S.J.; Lee, H.J.; Kim, S.H.; Park, Y.K.; Park, Y.H.; Hwang, C.Y.; et al. Antimicrobial effects of silver nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2007, 3, 95–101. [Google Scholar] [CrossRef]
- Duran, N.; Duran, M.; de Jesus, M.B.; Seabra, A.B.; Favaro, W.J.; Nakazato, G. Silver nanoparticles: A new view on mechanistic aspects on antimicrobial activity. Nanomedicine 2016, 12, 789–799. [Google Scholar] [CrossRef]
- Morones, J.R.; Elechiguerra, J.L.; Camacho, A.; Holt, K.; Kouri, J.B.; Ramirez, J.T.; Yacaman, M.J. The bactericidal effect of silver nanoparticles. Nanotechnology 2005, 16, 2346–2353. [Google Scholar] [CrossRef]
- Ghosh, P.; Han, G.; De, M.; Kim, C.K.; Rotello, V.M. Gold nanoparticles in delivery applications. Adv. Drug Deliv. Rev. 2008, 60, 1307–1315. [Google Scholar] [CrossRef]
- Pissuwan, D.; Valenzuela, S.M.; Cortie, M.B. Therapeutic possibilities of plasmonically heated gold nanoparticles. Trends Biotechnol. 2006, 24, 62–67. [Google Scholar] [CrossRef]
- Gharatape, A.; Salehi, R. Recent progress in theranostic applications of hybrid gold nanoparticles. Eur. J. Med. Chem. 2017, 138, 221–233. [Google Scholar] [CrossRef]
- Jiang, X.M.; Wang, L.M.; Wang, J.; Chen, C.Y. Gold nanomaterials: Preparation, chemical modification, biomedical applications and potential risk assessment. Appl. Biochem. Biotechnol. 2012, 166, 1533–1551. [Google Scholar] [CrossRef]
- Aslan, B.; Ozpolat, B.; Sood, A.K.; Lopez-Berestein, G. Nanotechnology in cancer therapy. J. Drug Target. 2013, 21, 904–913. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Huo, M.; Chen, Y.; Shi, J. Tumor microenvironment-enabled nanotherapy. Adv. Healthc. Mater. 2018, 7, e1701156. [Google Scholar] [CrossRef] [PubMed]
- Aminabad, N.S.; Farshbaf, M.; Akbarzadeh, A. Recent advances of gold nanoparticles in biomedical applications: State of the art. Cell Biochem. Biophys. 2018. [Google Scholar] [CrossRef]
- Dulinska-Litewka, J.; Lazarczyk, A.; Halubiec, P.; Szafranski, O.; Karnas, K.; Karewicz, A. Superparamagnetic iron oxide nanoparticles-current and prospective medical applications. Materials 2019, 12, 617. [Google Scholar] [CrossRef]
- Duguet, E.; Vasseur, S.; Mornet, S.; Devoisselle, J.M. Magnetic nanoparticles and their applications in medicine. Nanomedicine 2006, 1, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Indhira, T.K.; Lakshmi, P.K. Magnetic nanoparticles—A review. Int. J. Pharm. Sci. Nanotech. 2010, 3, 1035–1042. [Google Scholar]
- Salunkhe, A.B.; Khot, V.M.; Pawar, S.H. Magnetic hyperthermia with magnetic nanoparticles: A status review. Curr. Top. Med. Chem. 2014, 14, 572–594. [Google Scholar] [CrossRef] [PubMed]
- Pankhurst, Q.A.; Connolly, J.; Jones, S.K.; Dobson, J. Applications of magnetic nanoparticles in biomedicine. J. Phys. D Appl. Phys. 2003, 36, R167–R181. [Google Scholar] [CrossRef]
- Rhyner, M.N.; Smith, A.M.; Gao, X.; Mao, H.; Yang, L.; Nie, S. Quantum dots and multifunctional nanoparticles: New contrast agents for tumor imaging. Nanomedicine 2006, 1, 209–217. [Google Scholar] [CrossRef]
- Bilan, R.; Nabiev, I.; Sukhanova, A. Quantum dot-based nanotools for bioimaging, diagnostics, and drug delivery. Chembiochem 2016, 17, 2103–2114. [Google Scholar] [CrossRef]
- Mazumder, S.; Dey, R.; Mitra, M.K.; Mukherjee, S.; Das, G.C. Review: Biofunctionalized quantum dots in biology and medicine. J. Nanomater. 2009, 2009, 38. [Google Scholar] [CrossRef]
- Zrazhevskiy, P.; Gao, X. Multifunctional quantum dots for personalized medicine. Nano Today 2009, 4, 414–428. [Google Scholar] [CrossRef]
- Matea, C.T.; Mocan, T.; Tabaran, F.; Pop, T.; Mosteanu, O.; Puia, C.; Iancu, C.; Mocan, L. Quantum dots in imaging, drug delivery and sensor applications. Int. J. Nanomed. 2017, 12, 5421–5431. [Google Scholar] [CrossRef] [PubMed]
- Chinnathambi, S.; Chen, S.; Ganesan, S.; Hanagata, N. Silicon quantum dots for biological applications. Adv. Healthc. Mater. 2014, 3, 10–29. [Google Scholar] [CrossRef] [PubMed]
- Malam, Y.; Loizidou, M.; Seifalian, A.M. Liposomes and nanoparticles: Nanosized vehicles for drug delivery in cancer. Trends Pharm. Sci. 2009, 30, 592–599. [Google Scholar] [CrossRef] [PubMed]
- Bozzuto, G.; Molinari, A. Liposomes as nanomedical devices. Int. J. Nanomed. 2015, 10, 975–999. [Google Scholar] [CrossRef] [PubMed]
- Torchilin, V.P. Recent advances with liposomes as pharmaceutical carriers. Nat. Rev. Drug Discov. 2005, 4, 145–160. [Google Scholar] [CrossRef] [PubMed]
- Fenske, D.B.; Cullis, P.R. Liposomal nanomedicines. Expert Opin. Drug Deliv. 2008, 5, 25–44. [Google Scholar] [CrossRef] [PubMed]
- Allen, T.M.; Cullis, P.R. Liposomal drug delivery systems: From concept to clinical applications. Adv. Drug Deliv. Rev. 2013, 65, 36–48. [Google Scholar] [CrossRef] [PubMed]
- Immordino, M.L.; Dosio, F.; Cattel, L. Stealth liposomes: Review of the basic science, rationale, and clinical applications, existing and potential. Int. J. Nanomed. 2006, 1, 297–315. [Google Scholar]
- Xia, Y.; Xu, C.; Zhang, X.; Ning, P.; Wang, Z.; Tian, J.; Chen, X. Liposome-based probes for molecular imaging: From basic research to the bedside. Nanoscale 2019, 11, 5822–5838. [Google Scholar] [CrossRef]
- Gaucher, G.; Dufresne, M.H.; Sant, V.P.; Kang, N.; Maysinger, D.; Leroux, J.C. Block copolymer micelles: Preparation, characterization and application in drug delivery. J. Control. Release 2005, 109, 169–188. [Google Scholar] [CrossRef]
- Kataoka, K.; Harada, A.; Nagasaki, Y. Block copolymer micelles for drug delivery: Design, characterization and biological significance. Adv. Drug Deliv. Rev. 2001, 47, 113–131. [Google Scholar] [CrossRef]
- Gong, J.; Chen, M.; Zheng, Y.; Wang, S.; Wang, Y. Polymeric micelles drug delivery system in oncology. J. Control. Release 2012, 159, 312–323. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Park, K. Polymeric micelles and alternative nanonized delivery vehicles for poorly soluble drugs. Int. J. Pharm. 2013, 453, 198–214. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Shi, X. Dendrimers in cancer therapeutics and diagnosis. Curr. Drug Metab. 2012, 13, 1097–1109. [Google Scholar] [CrossRef] [PubMed]
- Kesharwani, P.; Jain, K.; Jain, N.K. Dendrimer as nanocarrier for drug delivery. Prog. Polym. Sci. 2014, 39, 268–307. [Google Scholar] [CrossRef]
- Bugno, J.; Hsu, H.J.; Hong, S. Tweaking dendrimers and dendritic nanoparticles for controlled nano-bio interactions: Potential nanocarriers for improved cancer targeting. J. Drug Target. 2015, 23, 642–650. [Google Scholar] [CrossRef] [PubMed]
- Kono, K. Dendrimer-based bionanomaterials produced by surface modification, assembly and hybrid formation. Polym. J. 2012, 44, 531. [Google Scholar] [CrossRef]
- Kim, Y.; Park, E.J.; Na, D.H. Recent progress in dendrimer-based nanomedicine development. Arch. Pharm. Res. 2018, 41, 571–582. [Google Scholar] [CrossRef]
- Palmerston Mendes, L.; Pan, J.; Torchilin, V.P. Dendrimers as nanocarriers for nucleic acid and drug delivery in cancer therapy. Molecules 2017, 22, 1401. [Google Scholar] [CrossRef]
- Park, J.H.; Lee, S.; Kim, J.-H.; Park, K.; Kim, K.; Kwon, I.C. Polymeric nanomedicine for cancer therapy. Prog. Polym. Sci. 2008, 33, 113–137. [Google Scholar] [CrossRef]
- Liechty, W.B.; Kryscio, D.R.; Slaughter, B.V.; Peppas, N.A. Polymers for drug delivery systems. Annu. Rev. Chem. Biomol. Eng. 2010, 1, 149–173. [Google Scholar] [CrossRef]
- George, A.; Shah, P.A.; Shrivastav, P.S. Natural biodegradable polymers based nano-formulations for drug delivery: A review. Int. J. Pharm. 2019, 561, 244–264. [Google Scholar] [CrossRef]
- Makadia, H.K.; Siegel, S.J. Poly lactic-co-glycolic acid (plga) as biodegradable controlled drug delivery carrier. Polymers 2011, 3, 1377–1397. [Google Scholar] [CrossRef]
- Gao, S.; Xu, Y.; Asghar, S.; Chen, M.; Zou, L.; Eltayeb, S.; Huo, M.; Ping, Q.; Xiao, Y. Polybutylcyanoacrylate nanocarriers as promising targeted drug delivery systems. J. Drug Target. 2015, 23, 481–496. [Google Scholar] [CrossRef]
- Revets, H.; De Baetselier, P.; Muyldermans, S. Nanobodies as novel agents for cancer therapy. Expert Opin. Biol. Ther. 2005, 5, 111–124. [Google Scholar] [CrossRef]
- Van Audenhove, I.; Gettemans, J. Nanobodies as versatile tools to understand, diagnose, visualize and treat cancer. EBioMedicine 2016, 8, 40–48. [Google Scholar] [CrossRef]
- Steeland, S.; Vandenbroucke, R.E.; Libert, C. Nanobodies as therapeutics: Big opportunities for small antibodies. Drug Discov. Today 2016, 21, 1076–1113. [Google Scholar] [CrossRef]
- Vincke, C.; Gutierrez, C.; Wernery, U.; Devoogdt, N.; Hassanzadeh-Ghassabeh, G.; Muyldermans, S. Generation of single domain antibody fragments derived from camelids and generation of manifold constructs. Methods Mol. Biol. 2012, 907, 145–176. [Google Scholar]
- Muyldermans, S. Nanobodies: Natural single-domain antibodies. Annu. Rev. Biochem. 2013, 82, 775–797. [Google Scholar] [CrossRef]
- Siontorou, C.G. Nanobodies as novel agents for disease diagnosis and therapy. Int. J. Nanomed. 2013, 8, 4215–4227. [Google Scholar] [CrossRef]
- Kijanka, M.; Dorresteijn, B.; Oliveira, S.; van Bergen en Henegouwen, P.M. Nanobody-based cancer therapy of solid tumors. Nanomedicine 2015, 10, 161–174. [Google Scholar] [CrossRef]
- Allegra, A.; Innao, V.; Gerace, D.; Vaddinelli, D.; Allegra, A.G.; Musolino, C. Nanobodies and cancer: Current status and new perspectives. Cancer Investig. 2018, 36, 221–237. [Google Scholar] [CrossRef] [PubMed]
- van Niel, G.; D'Angelo, G.; Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228. [Google Scholar] [CrossRef]
- Zaborowski, M.P.; Balaj, L.; Breakefield, X.O.; Lai, C.P. Extracellular vesicles: Composition, biological relevance, and methods of study. Bioscience 2015, 65, 783–797. [Google Scholar] [CrossRef]
- Quezada, C.; Torres, A.; Niechi, I.; Uribe, D.; Contreras-Duarte, S.; Toledo, F.; San Martin, R.; Gutierrez, J.; Sobrevia, L. Role of extracellular vesicles in glioma progression. Mol. Asp. Med. 2018, 60, 38–51. [Google Scholar] [CrossRef]
- Minciacchi, V.R.; Freeman, M.R.; Di Vizio, D. Extracellular vesicles in cancer: Exosomes, microvesicles and the emerging role of large oncosomes. Semin Cell Dev. Biol. 2015, 40, 41–51. [Google Scholar] [CrossRef]
- Hersh, D.S.; Wadajkar, A.S.; Roberts, N.; Perez, J.G.; Connolly, N.P.; Frenkel, V.; Winkles, J.A.; Woodworth, G.F.; Kim, A.J. Evolving drug delivery strategies to overcome the blood brain barrier. Curr. Pharm. Des. 2016, 22, 1177–1193. [Google Scholar] [CrossRef]
- Shilo, M.; Motiei, M.; Hana, P.; Popovtzer, R. Transport of nanoparticles through the blood–brain barrier for imaging and therapeutic applications. Nanoscale 2014, 6, 2146–2152. [Google Scholar] [CrossRef]
- Zhang, T.T.; Li, W.; Meng, G.; Wang, P.; Liao, W. Strategies for transporting nanoparticles across the blood–brain barrier. Biomater. Sci. 2016, 4, 219–229. [Google Scholar] [CrossRef]
- Eugenin, E.A.; Clements, J.E.; Zink, M.C.; Berman, J.W. Human immunodeficiency virus infection of human astrocytes disrupts blood–brain barrier integrity by a gap junction-dependent mechanism. J. Neurosci. Off. J. Soc. Neurosci. 2011, 31, 9456–9465. [Google Scholar] [CrossRef]
- Cena, V.; Jativa, P. Nanoparticle crossing of blood–brain barrier: A road to new therapeutic approaches to central nervous system diseases. Nanomedicine 2018, 13, 1513–1516. [Google Scholar] [CrossRef]
- Saraiva, C.; Praca, C.; Ferreira, R.; Santos, T.; Ferreira, L.; Bernardino, L. Nanoparticle-mediated brain drug delivery: Overcoming blood–brain barrier to treat neurodegenerative diseases. J. Control. Release Off. J. Control. Release Soc. 2016, 235, 34–47. [Google Scholar] [CrossRef]
- Ulbrich, K.; Hekmatara, T.; Herbert, E.; Kreuter, J. Transferrin- and transferrin-receptor-antibody-modified nanoparticles enable drug delivery across the blood–brain barrier (bbb). Eur. J. Pharm. Biopharm. 2009, 71, 251–256. [Google Scholar] [CrossRef]
- Zhou, Y.; Peng, Z.; Seven, E.S.; Leblanc, R.M. Crossing the blood–brain barrier with nanoparticles. J. Control. Release Off. J. Control. Release Soc. 2018, 270, 290–303. [Google Scholar] [CrossRef]
- Betzer, O.; Shilo, M.; Opochinsky, R.; Barnoy, E.; Motiei, M.; Okun, E.; Yadid, G.; Popovtzer, R. The effect of nanoparticle size on the ability to cross the blood–brain barrier: An in vivo study. Nanomedicine 2017, 12, 1533–1546. [Google Scholar] [CrossRef]
- Rempe, R.; Cramer, S.; Huwel, S.; Galla, H.J. Transport of poly(n-butylcyano-acrylate) nanoparticles across the blood–brain barrier in vitro and their influence on barrier integrity. Biochem. Biophys. Res. Commun. 2011, 406, 64–69. [Google Scholar] [CrossRef]
- Hadjipanayis, C.G.; Machaidze, R.; Kaluzova, M.; Wang, L.; Schuette, A.J.; Chen, H.; Wu, X.; Mao, H. Egfrviii antibody-conjugated iron oxide nanoparticles for magnetic resonance imaging-guided convection-enhanced delivery and targeted therapy of glioblastoma. Cancer Res. 2010, 70, 6303–6312. [Google Scholar] [CrossRef]
- Wang, X.; Tu, M.; Tian, B.; Yi, Y.; Wei, Z.; Wei, F. Synthesis of tumor-targeted folate conjugated fluorescent magnetic albumin nanoparticles for enhanced intracellular dual-modal imaging into human brain tumor cells. Anal. Biochem. 2016, 512, 8–17. [Google Scholar] [CrossRef]
- Jain, K.K. Recent advances in nanooncology. Technol. Cancer Res. Treat. 2008, 7, 1–13. [Google Scholar] [CrossRef]
- Kolosnjaj-Tabi, J.; Wilhelm, C. Magnetic nanoparticles in cancer therapy: How can thermal approaches help? Nanomedicine 2017, 12, 573–575. [Google Scholar] [CrossRef]
- Cheng, Y.; Dai, Q.; Morshed, R.A.; Fan, X.; Wegscheid, M.L.; Wainwright, D.A.; Han, Y.; Zhang, L.; Auffinger, B.; Tobias, A.L.; et al. Blood–brain barrier permeable gold nanoparticles: An efficient delivery platform for enhanced malignant glioma therapy and imaging. Small 2014, 10, 5137–5150. [Google Scholar] [CrossRef]
- Yuan, B.O.; Zhao, Y.; Dong, S.; Sun, Y.; Hao, F.; Xie, J.; Teng, L.; Lee, R.J.; Fu, Y.; Bi, Y.E. Cell-penetrating peptide-coated liposomes for drug delivery across the blood–brain barrier. Anticancer Res. 2019, 39, 237–243. [Google Scholar] [CrossRef]
- Mamot, C.; Drummond, D.C.; Noble, C.O.; Kallab, V.; Guo, Z.; Hong, K.; Kirpotin, D.B.; Park, J.W. Epidermal growth factor receptor-targeted immunoliposomes significantly enhance the efficacy of multiple anticancer drugs in vivo. Cancer Res. 2005, 65, 11631–11638. [Google Scholar] [CrossRef]
- Miura, Y.; Takenaka, T.; Toh, K.; Wu, S.; Nishihara, H.; Kano, M.R.; Ino, Y.; Nomoto, T.; Matsumoto, Y.; Koyama, H.; et al. Cyclic rgd-linked polymeric micelles for targeted delivery of platinum anticancer drugs to glioblastoma through the blood–brain tumor barrier. ACS Nano 2013, 7, 8583–8592. [Google Scholar] [CrossRef]
- Jain, K.K. Use of nanoparticles for drug delivery in glioblastoma multiforme. Expert Rev. Neurother. 2007, 7, 363–372. [Google Scholar] [CrossRef]
- Taghizadehghalehjoughi, A.; Hacimuftuoglu, A.; Cetin, M.; Ugur, A.B.; Galateanu, B.; Mezhuev, Y.; Okkay, U.; Taspinar, N.; Taspinar, M.; Uyanik, A.; et al. Effect of metformin/irinotecan-loaded poly-lactic-co-glycolic acid nanoparticles on glioblastoma: In vitro and in vivo studies. Nanomedicine 2018, 13, 1595–1606. [Google Scholar] [CrossRef]
- Qu, J.; Zhang, L.; Chen, Z.; Mao, G.; Gao, Z.; Lai, X.; Zhu, X.; Zhu, J. Nanostructured lipid carriers, solid lipid nanoparticles, and polymeric nanoparticles: Which kind of drug delivery system is better for glioblastoma chemotherapy? Drug Deliv. 2016, 23, 3408–3416. [Google Scholar] [CrossRef]
- Wu, M.; Fan, Y.; Lv, S.; Xiao, B.; Ye, M.; Zhu, X. Vincristine and temozolomide combined chemotherapy for the treatment of glioma: A comparison of solid lipid nanoparticles and nanostructured lipid carriers for dual drugs delivery. Drug Deliv. 2016, 23, 2720–2725. [Google Scholar] [CrossRef]
- Song, S.; Mao, G.; Du, J.; Zhu, X. Novel rgd containing, temozolomide-loading nanostructured lipid carriers for glioblastoma multiforme chemotherapy. Drug Deliv. 2016, 23, 1404–1408. [Google Scholar] [CrossRef]
- Li, Y.; He, H.; Jia, X.; Lu, W.L.; Lou, J.; Wei, Y. A dual-targeting nanocarrier based on poly(amidoamine) dendrimers conjugated with transferrin and tamoxifen for treating brain gliomas. Biomaterials 2012, 33, 3899–3908. [Google Scholar] [CrossRef]
- Bai, C.Z.; Choi, S.; Nam, K.; An, S.; Park, J.S. Arginine modified pamam dendrimer for interferon beta gene delivery to malignant glioma. Int. J. Pharm. 2013, 445, 79–87. [Google Scholar] [CrossRef]
- Rufino-Ramos, D.; Albuquerque, P.R.; Carmona, V.; Perfeito, R.; Nobre, R.J.; Pereira de Almeida, L. Extracellular vesicles: Novel promising delivery systems for therapy of brain diseases. J. Control. Release 2017, 262, 247–258. [Google Scholar] [CrossRef]
- Puzar Dominkus, P.; Ferdin, J.; Plemenitas, A.; Peterlin, B.M.; Lenassi, M. Nef is secreted in exosomes from nef.Gfp-expressing and hiv-1-infected human astrocytes. J. Neurovirol. 2017, 23, 713–724. [Google Scholar] [CrossRef]
- Bastos, N.; Ruivo, C.F.; da Silva, S.; Melo, S.A. Exosomes in cancer: Use them or target them? Semin Cell Dev. Biol. 2018, 78, 13–21. [Google Scholar] [CrossRef]
- Alvarez-Erviti, L.; Seow, Y.; Yin, H.; Betts, C.; Lakhal, S.; Wood, M.J. Delivery of sirna to the mouse brain by systemic injection of targeted exosomes. Nat. Biotechnol. 2011, 29, 341–345. [Google Scholar] [CrossRef]
- Hadla, M.; Palazzolo, S.; Corona, G.; Caligiuri, I.; Canzonieri, V.; Toffoli, G.; Rizzolio, F. Exosomes increase the therapeutic index of doxorubicin in breast and ovarian cancer mouse models. Nanomedicine 2016, 11, 2431–2441. [Google Scholar] [CrossRef]
- Zhu, Q.; Ling, X.; Yang, Y.; Zhang, J.; Li, Q.; Niu, X.; Hu, G.; Chen, B.; Li, H.; Wang, Y.; et al. Embryonic stem cells-derived exosomes endowed with targeting properties as chemotherapeutics delivery vehicles for glioblastoma therapy. Adv. Sci. (Weinh) 2019, 6, 1801899. [Google Scholar] [CrossRef]
- Gourlay, J.; Morokoff, A.P.; Luwor, R.B.; Zhu, H.J.; Kaye, A.H.; Stylli, S.S. The emergent role of exosomes in glioma. J. Clin. Neurosci. 2017, 35, 13–23. [Google Scholar] [CrossRef]
- Xu, H.L.; Yang, J.J.; ZhuGe, D.L.; Lin, M.T.; Zhu, Q.Y.; Jin, B.H.; Tong, M.Q.; Shen, B.X.; Xiao, J.; Zhao, Y.Z. Glioma-targeted delivery of a theranostic liposome integrated with quantum dots, superparamagnetic iron oxide, and cilengitide for dual-imaging guiding cancer surgery. Adv. Healthc. Mater. 2018, 7, e1701130. [Google Scholar] [CrossRef]
- Wu, B.; Wan, B.; Lu, S.T.; Deng, K.; Li, X.Q.; Wu, B.L.; Li, Y.S.; Liao, R.F.; Huang, S.W.; Xu, H.B. Near-infrared light-triggered theranostics for tumor-specific enhanced multimodal imaging and photothermal therapy. Int. J. Nanomed. 2017, 12, 4467–4478. [Google Scholar] [CrossRef]
- Ventola, C.L. Progress in nanomedicine: Approved and investigational nanodrugs. P T 2017, 42, 742–755. [Google Scholar]
- Grodzinski, P.; Silver, M.; Molnar, L.K. Nanotechnology for cancer diagnostics: Promises and challenges. Expert Rev. Mol. Diagn. 2006, 6, 307–318. [Google Scholar] [CrossRef]
- Jain, K.K. Nanodiagnostics: Application of nanotechnology in molecular diagnostics. Expert Rev. Mol. Diagn. 2003, 3, 153–161. [Google Scholar] [CrossRef]
- Baptista, P.V. Nanodiagnostics: Leaving the research lab to enter the clinics? Diagnosis 2014, 1, 305–309. [Google Scholar] [CrossRef]
- Kim, B.Y.; Rutka, J.T.; Chan, W.C. Nanomedicine. N. Engl. J. Med. 2010, 363, 2434–2443. [Google Scholar] [CrossRef]
- Yoon, J.W.; Jiang, W.; Rutka, J.T.; Huang, Y.; Kim, B.Y.S. Perspectives of nanotechnology in the management of gliomas. Prog. Neurol Surg. 2018, 32, 196–210. [Google Scholar]
- Jain, K.K. Advances in the field of nanooncology. BMC Med. 2010, 8, 83. [Google Scholar] [CrossRef]
- Alharbi, K.K.; Al-Sheikh, Y.A. Role and implications of nanodiagnostics in the changing trends of clinical diagnosis. Saudi J. Biol. Sci. 2014, 21, 109–117. [Google Scholar] [CrossRef]
- Jain, K.K. Role of nanodiagnostics in personalized cancer therapy. Clin. Lab. Med. 2012, 32, 15–31. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.A.; Arlauckas, S.; Weissleder, R. Prediction of anti-cancer nanotherapy efficacy by imaging. Nanotheranostics 2017, 1, 296–312. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, C.; Suares, D.; Yergeri, M.C. Tumor microenvironment targeted nanotherapy. Front. Pharmacol. 2018, 9, 1230. [Google Scholar] [CrossRef]
- Sheng, W.Y.; Huang, L. Cancer immunotherapy and nanomedicine. Pharm. Res. 2011, 28, 200–214. [Google Scholar] [CrossRef]
- Emerich, D.F.; Thanos, C.G. Nanotechnology and medicine. Expert Opin. Biol. Ther. 2003, 3, 655–663. [Google Scholar] [CrossRef] [PubMed]
- Jain, K.K. The role of nanobiotechnology in the development of personalized medicine. Med. Princ Pr. 2011, 20, 1–3. [Google Scholar] [CrossRef]
- Jain, K.K. Role of nanobiotechnology in the development of personalized medicine. Nanomedicine 2009, 4, 249–252. [Google Scholar] [CrossRef]
- Lalatsa, A.; Lee, V.; Malkinson, J.P.; Zloh, M.; Schatzlein, A.G.; Uchegbu, I.F. A prodrug nanoparticle approach for the oral delivery of a hydrophilic peptide, leucine(5)-enkephalin, to the brain. Mol. Pharm. 2012, 9, 1665–1680. [Google Scholar] [CrossRef]
- Siew, A.; Le, H.; Thiovolet, M.; Gellert, P.; Schatzlein, A.; Uchegbu, I. Enhanced oral absorption of hydrophobic and hydrophilic drugs using quaternary ammonium palmitoyl glycol chitosan nanoparticles. Mol. Pharm. 2012, 9, 14–28. [Google Scholar] [CrossRef]
- Lammers, T.; Hennink, W.E.; Storm, G. Tumour-targeted nanomedicines: Principles and practice. Br. J. Cancer 2008, 99, 392–397. [Google Scholar] [CrossRef]
- Wang, M.D.; Shin, D.M.; Simons, J.W.; Nie, S. Nanotechnology for targeted cancer therapy. Expert Rev. Anticancer Ther. 2007, 7, 833–837. [Google Scholar] [CrossRef] [PubMed]
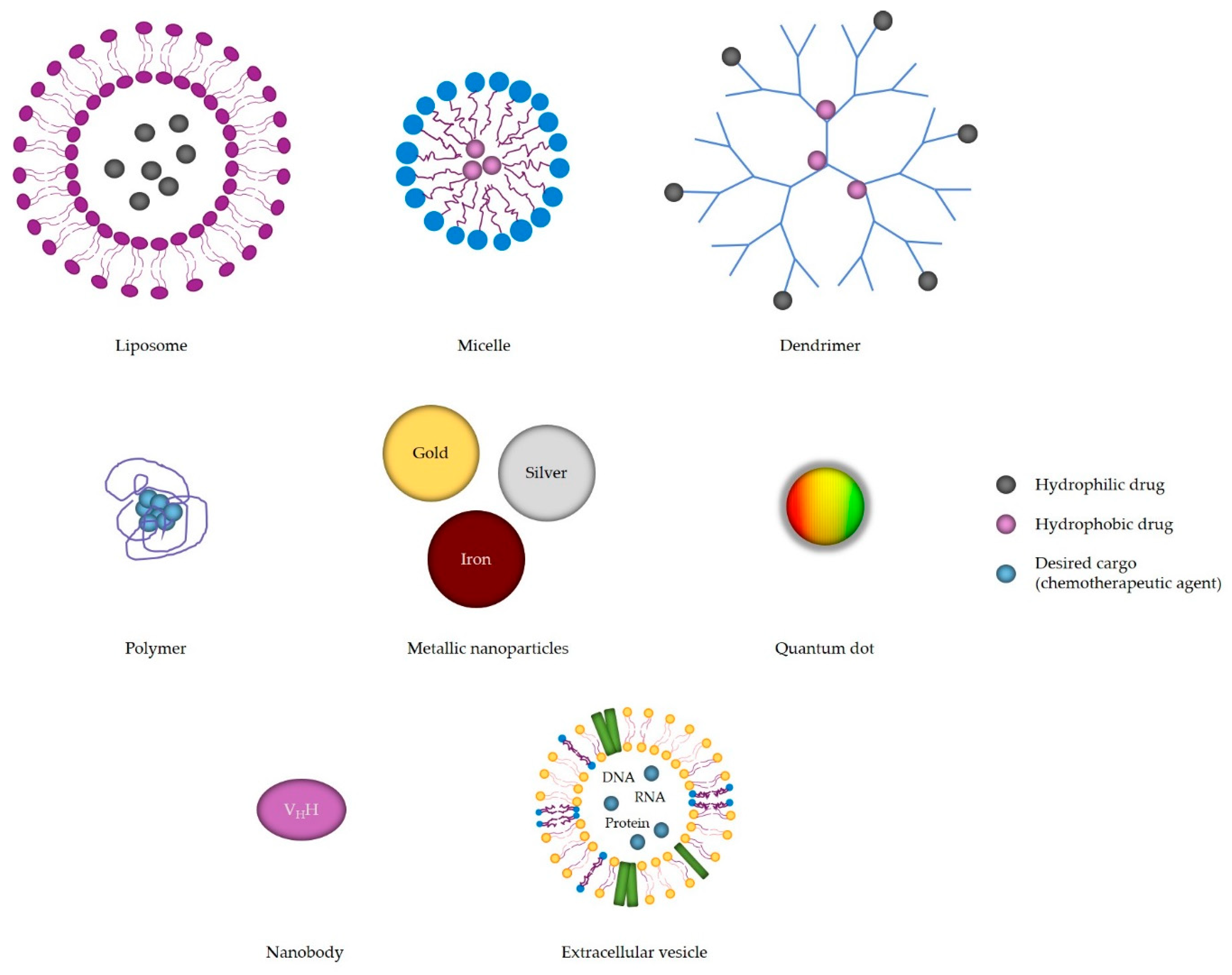
| Nanomaterial | Advantages |
|---|---|
| Silver nanoparticles | Bactericidal properties Antiviral function |
| Gold nanoparticles | Plasmon resonance Absorb light in near infrared region Easy surface-conjugation with antibodies Suitable for passive and active targeting Can be used as drug carriers CT contrast agent |
| Magnetic nanoparticles | Active when external magnetic field is applied Selective destruction of cancer cells in hypoxic areas as a result of heat release |
| Platinum nanoparticles | Protection mechanisms against ROS |
| Quantum dots | Bright Photostable Broad excitation and narrow emission spectra Signal amplification is not needed |
| Liposomes | Suitable for packing neutral, hydrophilic and hydrophobic drugs Engineered to release cargo upon suitable pH, redox potential, ultrasound and electromagnetic field Passive and active targeting PEG-coating increases biocompatibility, water solubility and half-life, and lowers toxicity Suitable as imaging tools (CT) |
| Block copolymere micelles | Carriers of water-insoluble drugs Stable High loading capacity |
| Dendrimers | Monodispersity Very small size PEG-conjugation decreases toxicity, enhances biocompatibility and EPR effect, and increases half-life Slow drug release Controlled release upon pH, glutathione or enzyme stimuli High tumor accumulation Suitable for antibody and nucleic acid delivery Use in diagnostics as contrast agents in MR, CT and fluorescence imaging |
| Polymers | Improved pharmacokinetic and pharmacodynamic characteristics Controlled drug release upon diffusion-control, solvent-activation, chemical control or external triggers (pH, temperature and redox potential) Biodegradable, biocompatible and non-toxic |
| Nanobodies | High antigen affinity and specificity Economic production High stability at elevated temperatures and non-physiological pH Water solubility Low immunogenicity PEG- or albumin-binding increases half-life Better tumor penetration and distribution Suitable for use in PET and SPECT |
| Extracellular vesicles | Carriers of different cell proteins, viral proteins, nucleic acids and lipids Biocompatible Drug carriers Less toxic and immunogenic Present in blood and cerebrospinal fluid |
| Disease | Agent | Clinical Trial Number | Phase |
|---|---|---|---|
| Recurrent high grade glioma Newly diagnosed glioblastoma | ABI-009 (Nab-Rapamycin) | NCT03463265 | II |
| Recurrent high grade glioma | NL CPT-11 (Nanoliposomal CPT-11) | NCT00734682 | I completed |
| Recurrent high grade glioma | Ferumoxytol | NCT00769093 | I |
| Glioblastoma | 9-ING-41 | NCT03678883 | I/II |
| Recurrent high grade glioma | Liposomal irinotecan | NCT02022644 | I |
| Recurrent malignant glioma or solid tumors and brain metastases | 2B3-101 | NCT01386580 | I/II |
| Children and adolescents with refractory or relapsed malignant glioma | Myocet | NCT02861222 | I |
| Glioblastoma and diffuse intristic pontine glioma | Doxorubicin | NCT02758366 | II |
| Recurrent glioblastoma or gliosarcoma | NU-0129 | NCT03020017 | I |
| Recurrent glioblastoma | SGT-53 | NCT02340156 | II |
| Recurrent glioblastoma | RNL (rhenium nanoliposomes) | NCT01906385 | I |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zottel, A.; Videtič Paska, A.; Jovčevska, I. Nanotechnology Meets Oncology: Nanomaterials in Brain Cancer Research, Diagnosis and Therapy. Materials 2019, 12, 1588. https://doi.org/10.3390/ma12101588
Zottel A, Videtič Paska A, Jovčevska I. Nanotechnology Meets Oncology: Nanomaterials in Brain Cancer Research, Diagnosis and Therapy. Materials. 2019; 12(10):1588. https://doi.org/10.3390/ma12101588
Chicago/Turabian StyleZottel, Alja, Alja Videtič Paska, and Ivana Jovčevska. 2019. "Nanotechnology Meets Oncology: Nanomaterials in Brain Cancer Research, Diagnosis and Therapy" Materials 12, no. 10: 1588. https://doi.org/10.3390/ma12101588
APA StyleZottel, A., Videtič Paska, A., & Jovčevska, I. (2019). Nanotechnology Meets Oncology: Nanomaterials in Brain Cancer Research, Diagnosis and Therapy. Materials, 12(10), 1588. https://doi.org/10.3390/ma12101588






