Feasibility and Characterization Mortar Blended with High-Amount Basic Oxygen Furnace Slag
Abstract
:1. Introduction
2. Materials and Methods
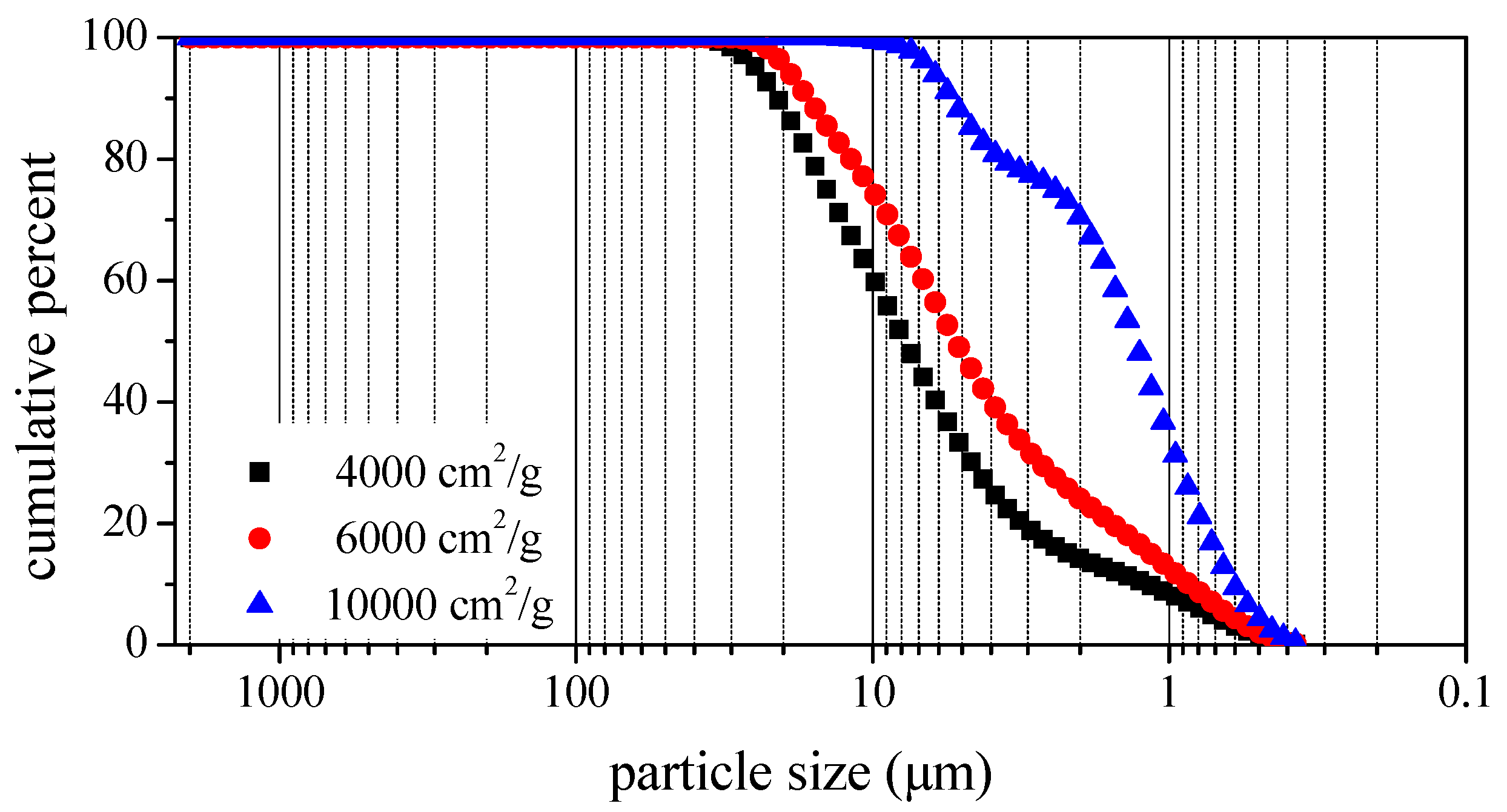
2.1. Materials
2.2. Mix Designs and Specimens
2.3. Test Methods
3. Results and Discussion
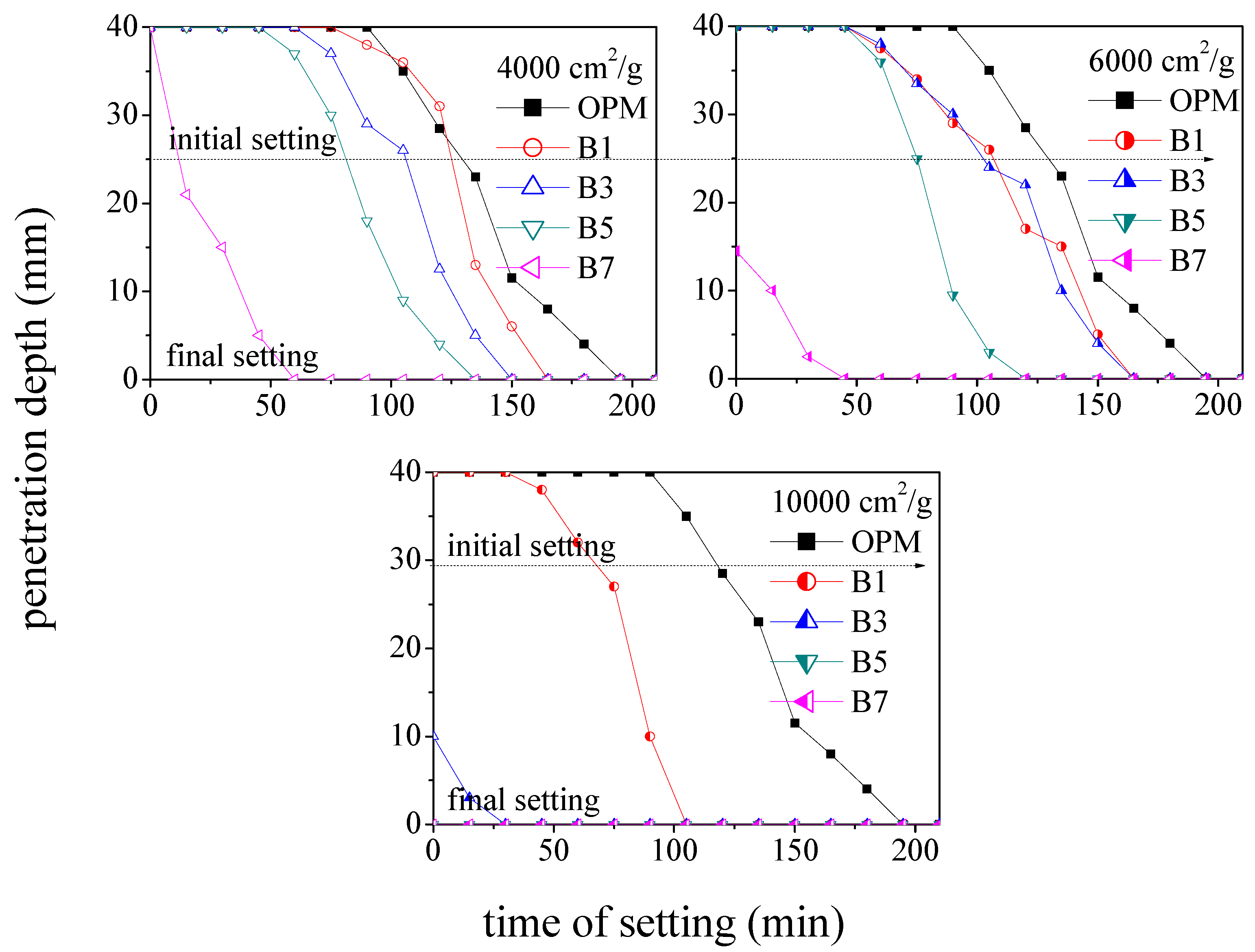
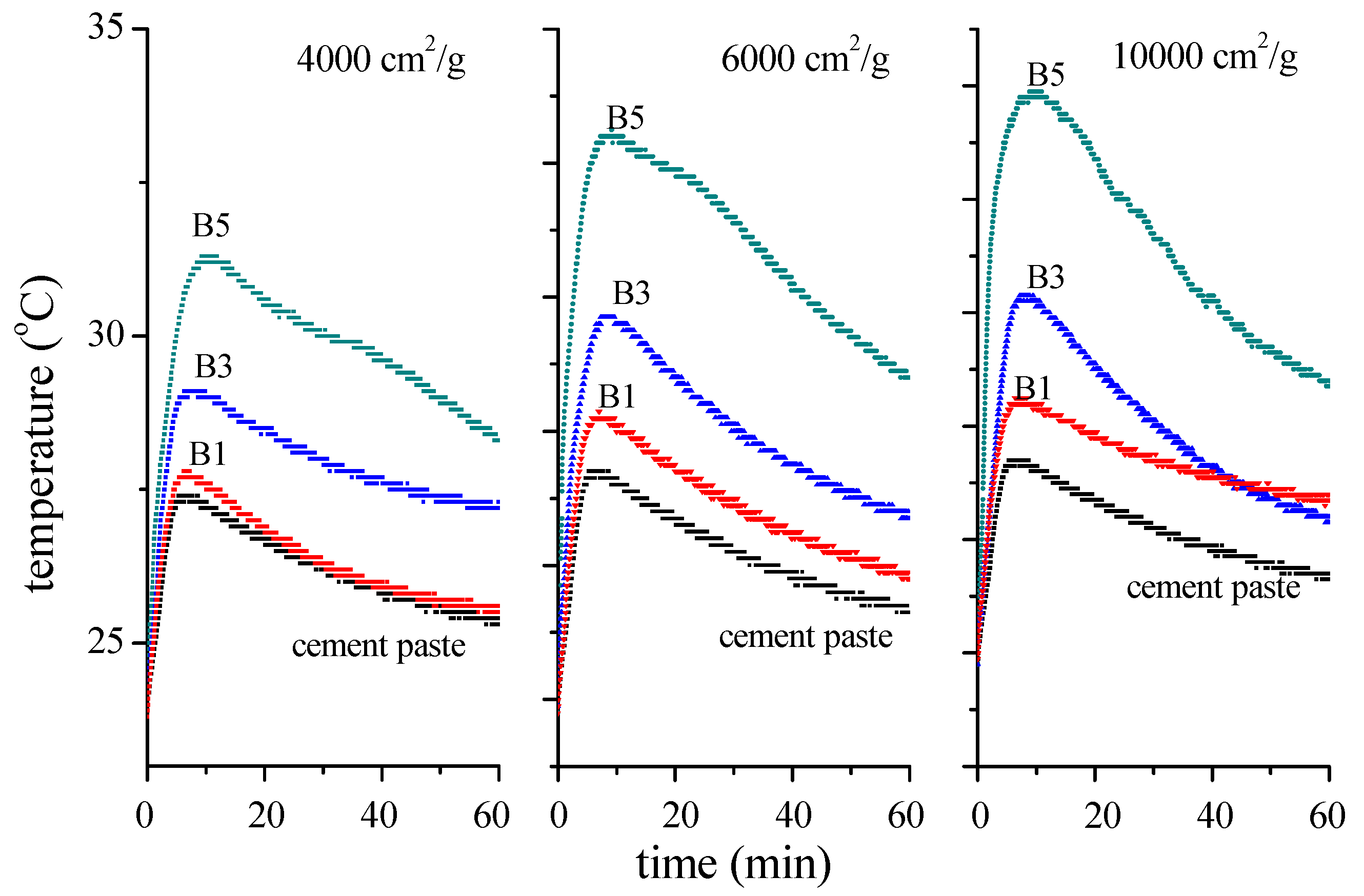
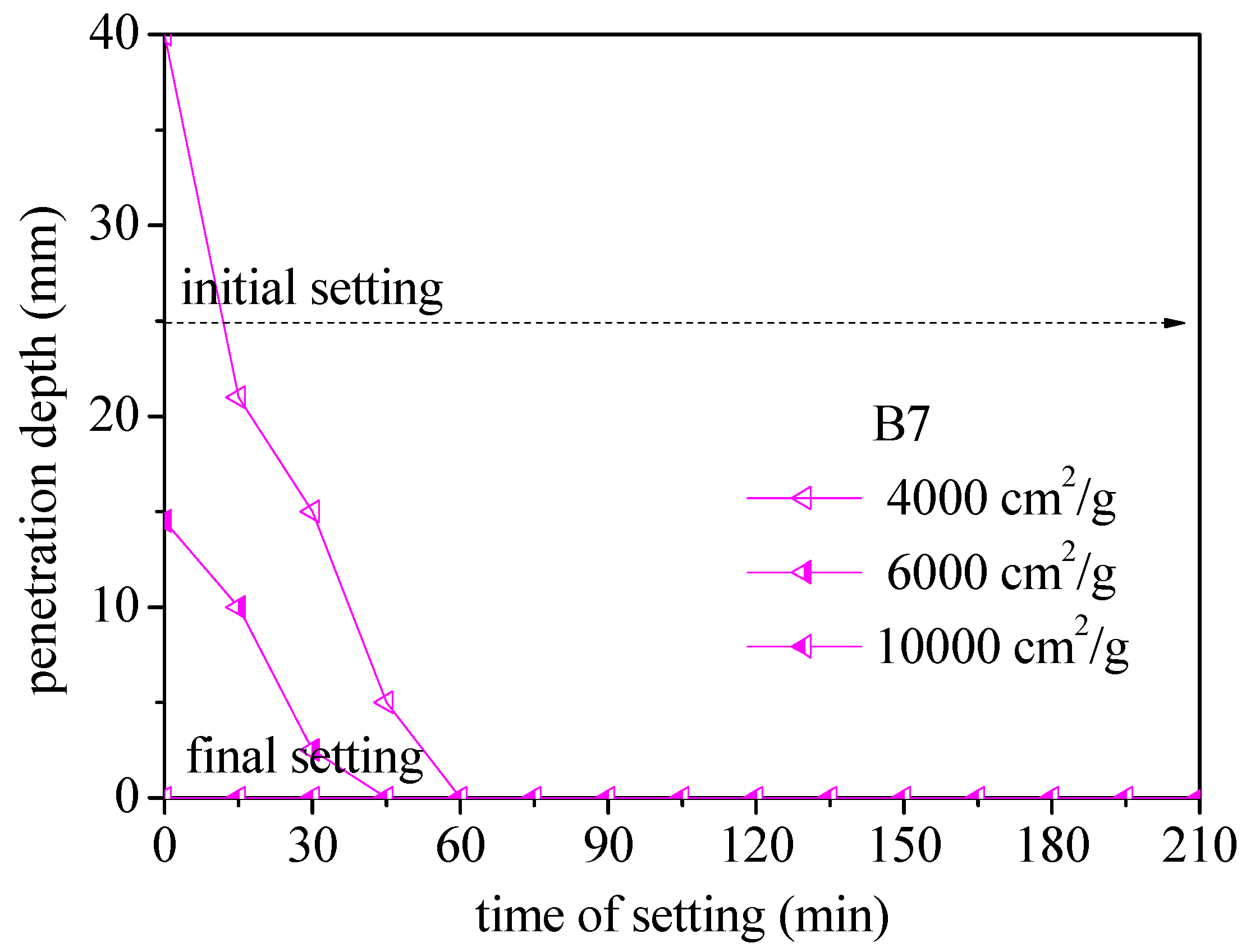
3.1. Effect of BOFS on Setting Time
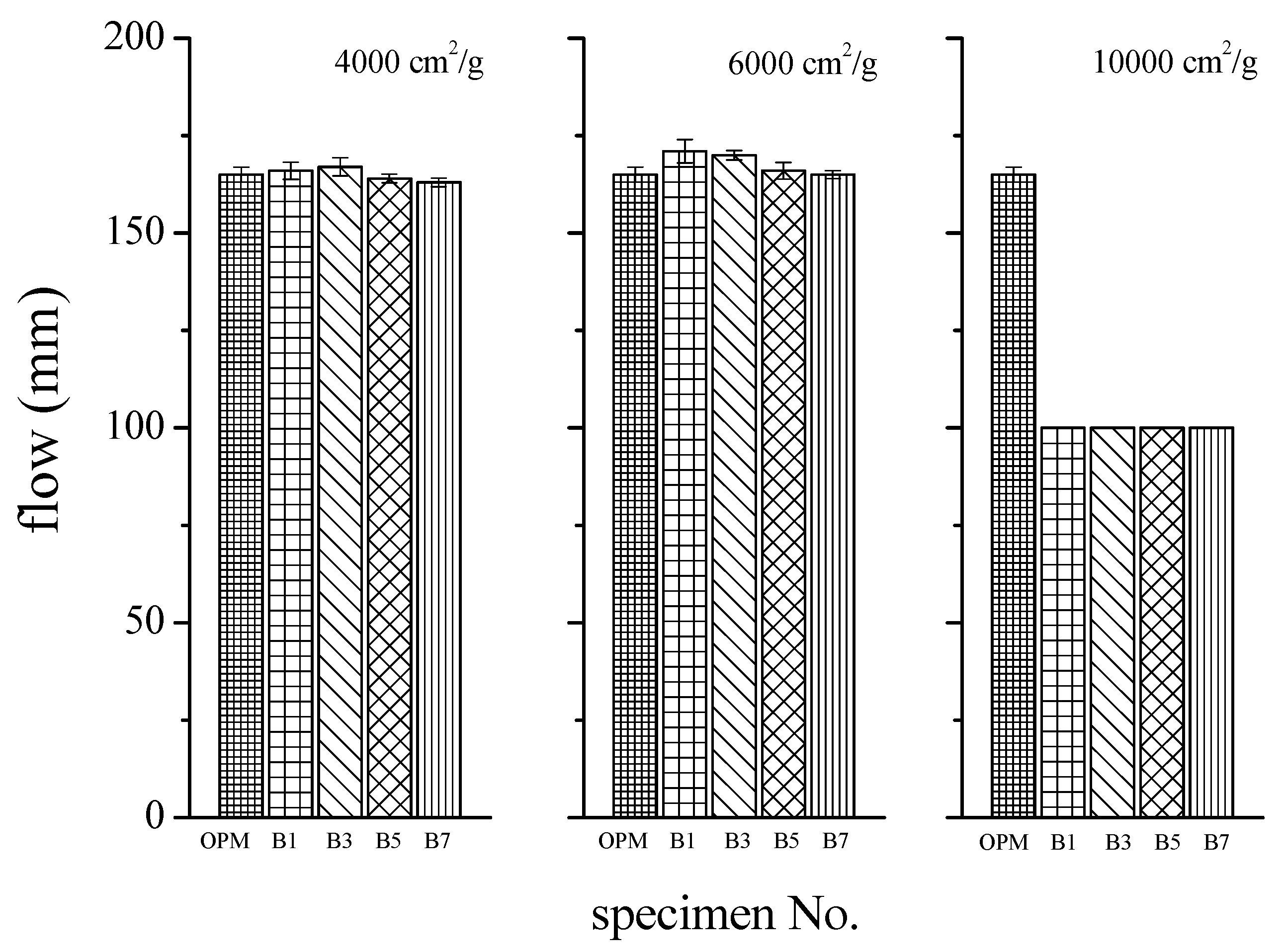
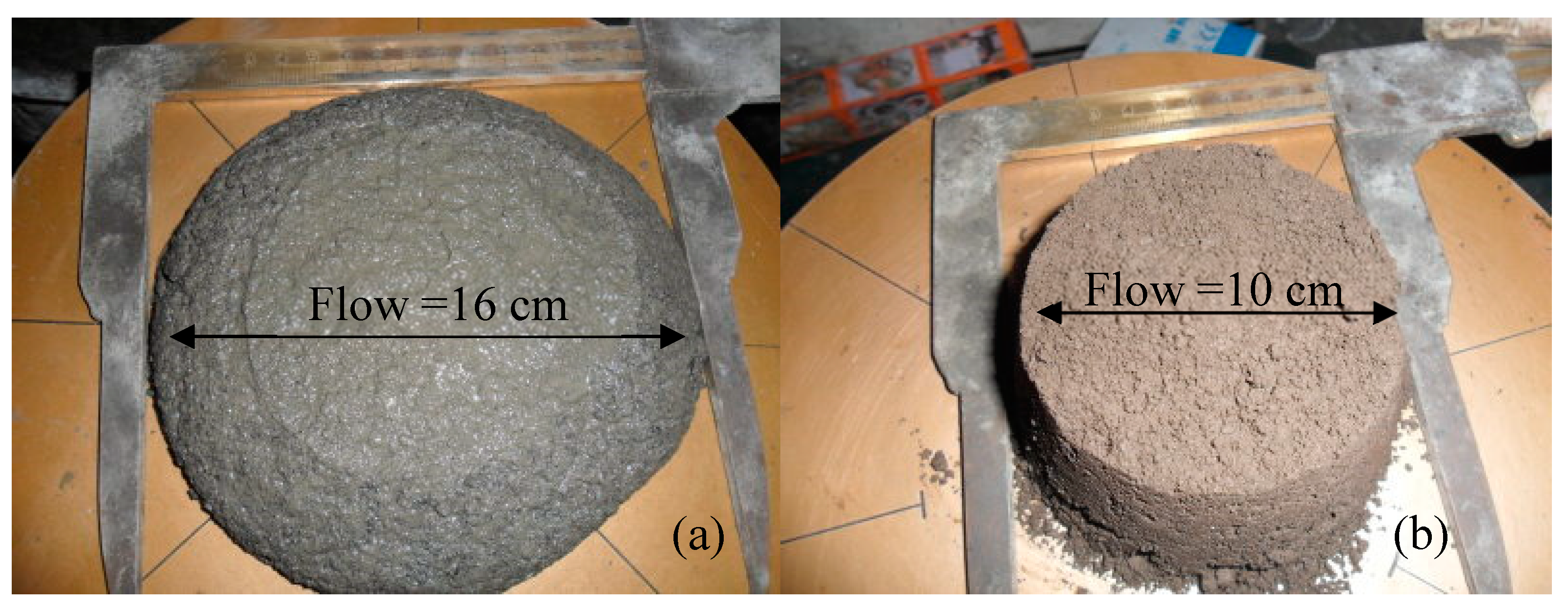
3.2. Effects of BOFS on Flowability
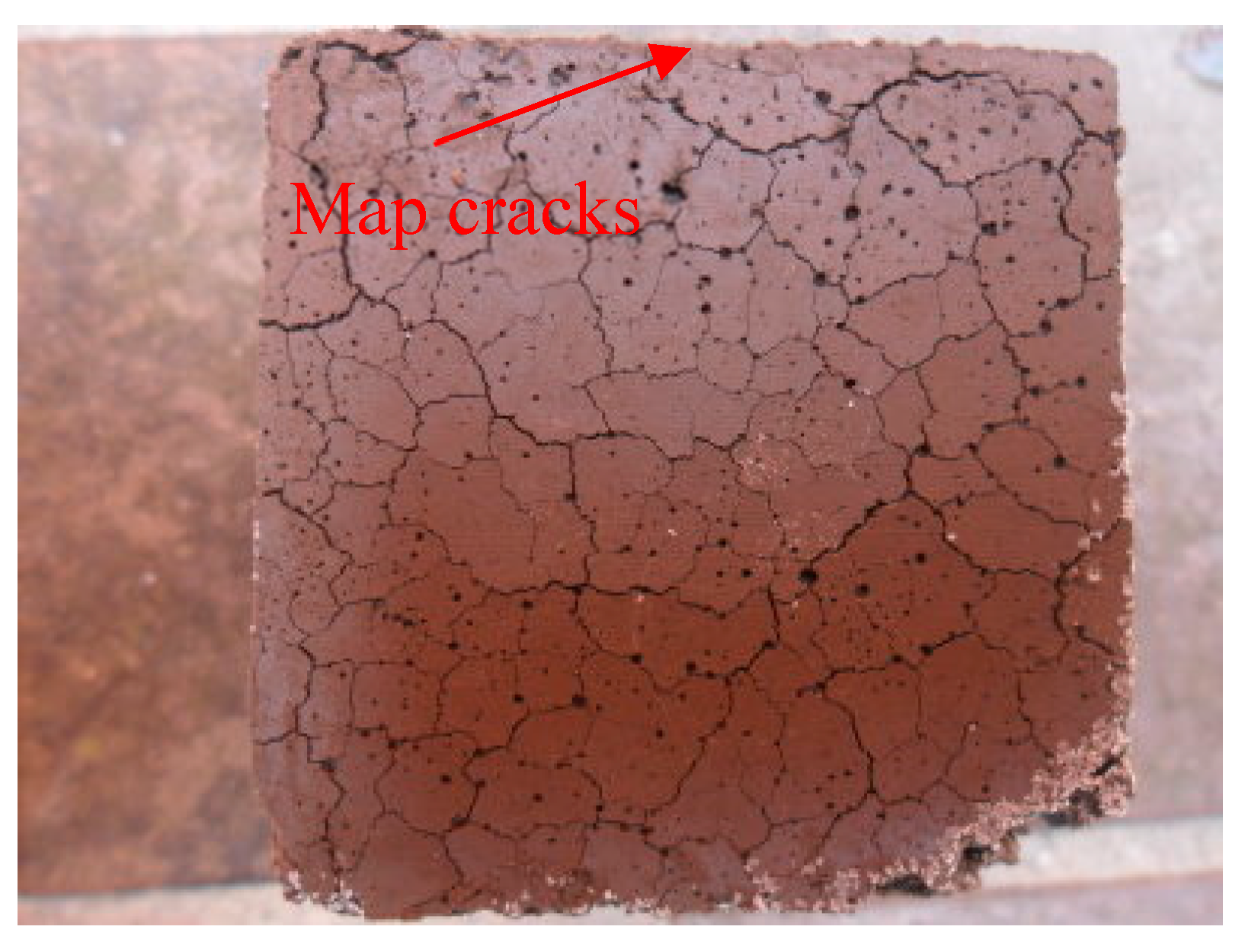
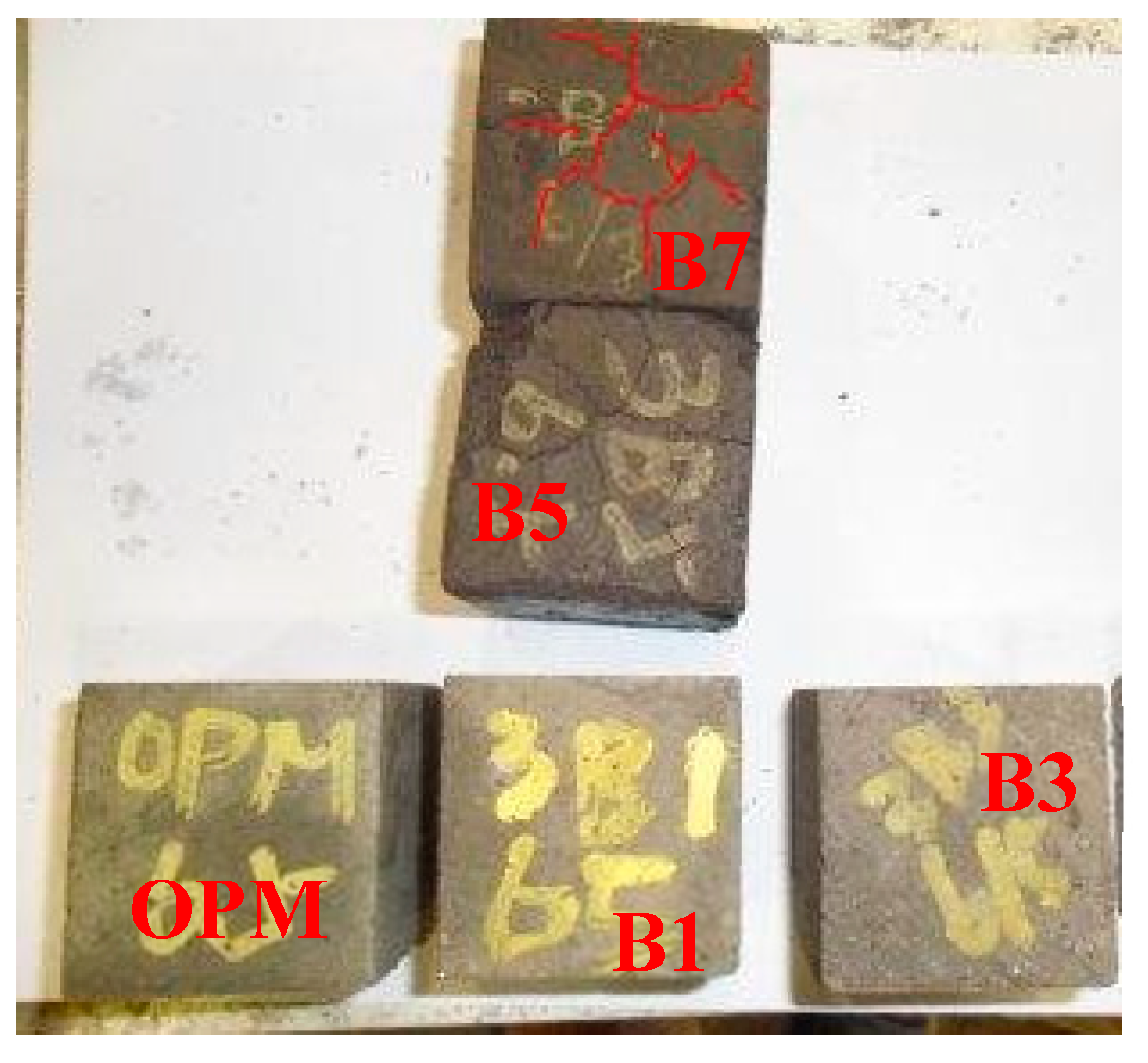
3.3. Effects of BOFS on Length Change
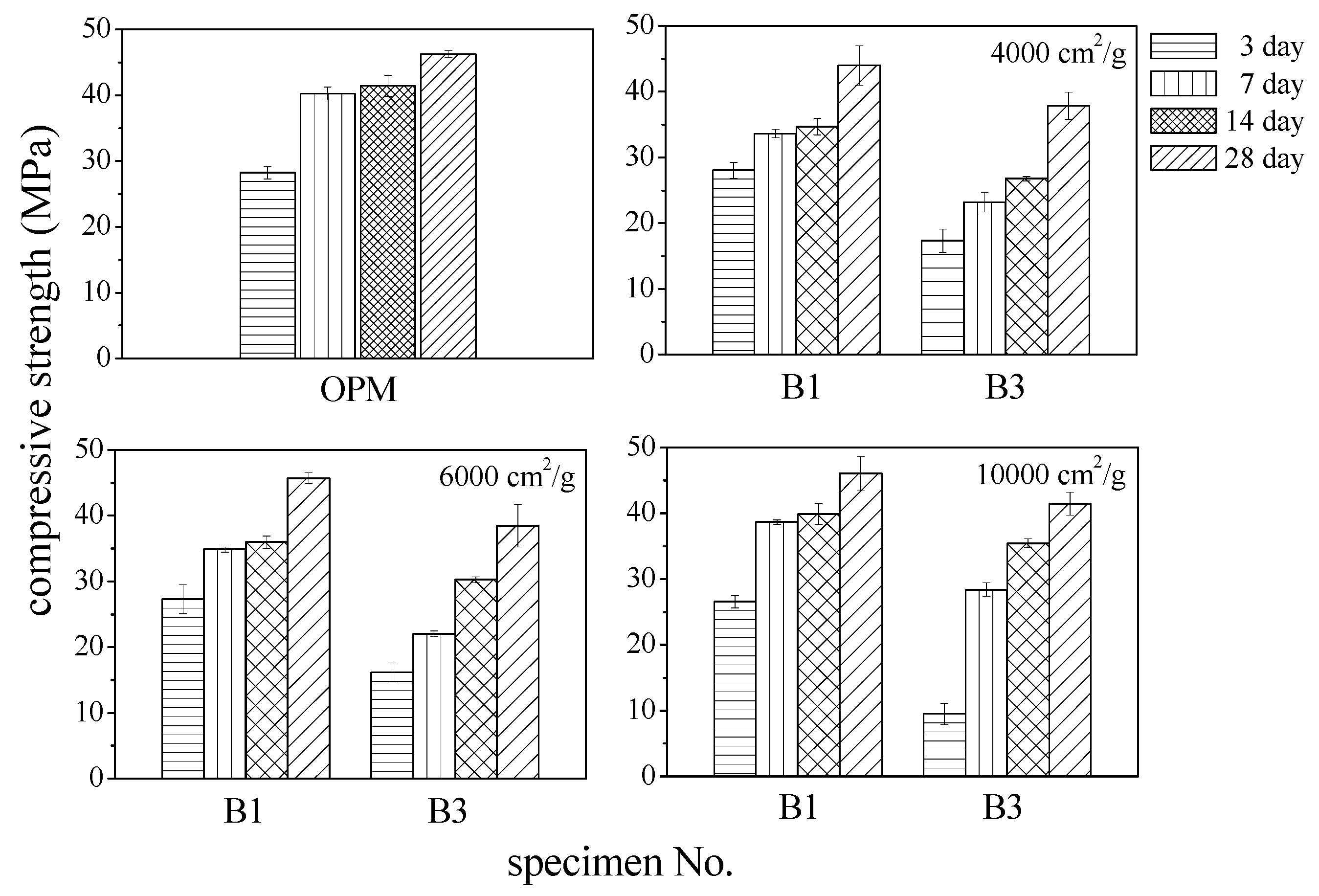
3.4. Compressive Strength
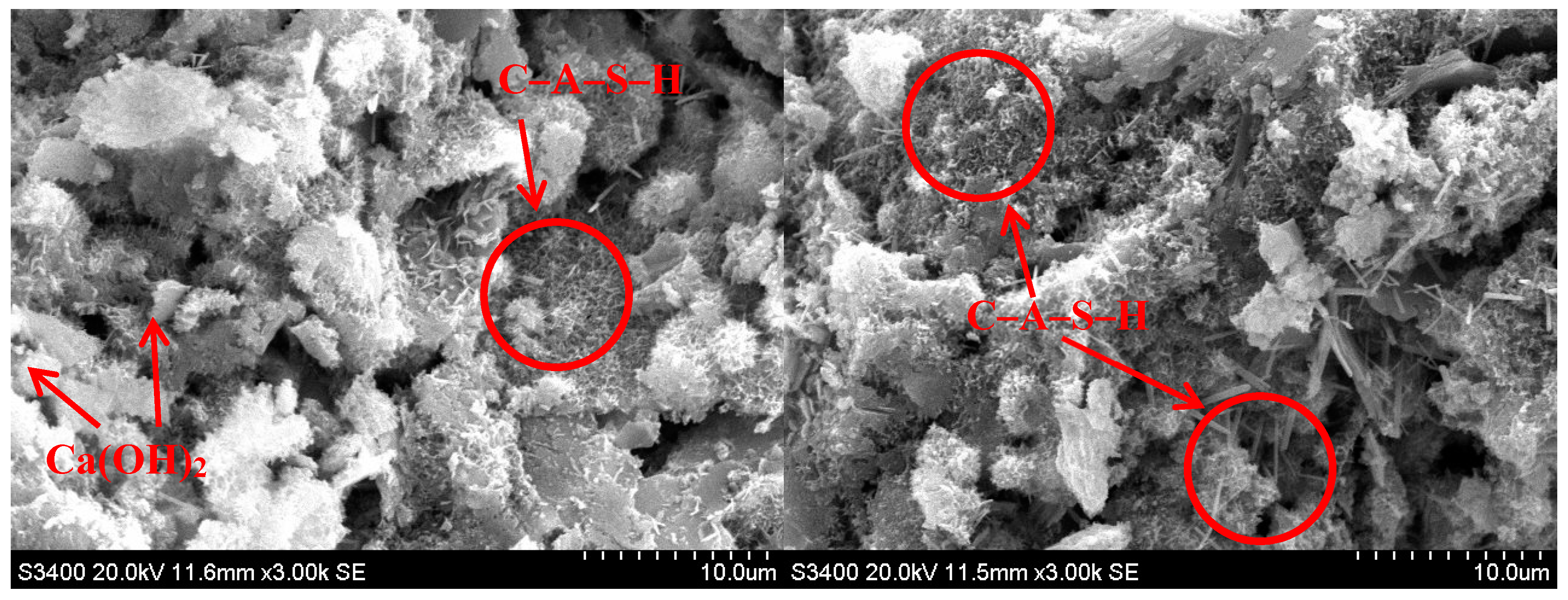
3.5. SEM Observations
4. Conclusions
- Fineness of the BOFS was the primary factor influencing the fluidity of BOFS mortar. Inclusion of 10,000 cm2/g BOFS had a particularly pronounced effect in reducing fluidity. Finer BOFS can reduce the friction between particles and fill the voids between solid particles, thus leading to a decrease in the minimum amount of water needed to initiate flow.
- Increasing the amount of BOFS used as a replacement for cement was shown to speed up the initial and final setting times. The B7 specimens with 6000 cm2/g completed final setting within 45 min. This is 69% faster than OPM specimens (195 min); B5 was 30% faster, B3 was 23% faster, and B1 was 15% faster.
- Replacement of cement with BOFS resulted in increased expansion and cracking, particularly when BOFS was used to replace more than 50%. At 28 days, the expansion ratio of the B3 specimens exceeded that of the B1 specimens by two times.
- BOFS with a specific surface area of 10,000 cm2/g attained compressive strength values close to those of OPM specimens. BOFS with a specific surface area of 6000 cm2/g produced mortar that was 2% weaker than OPM, whereas BOFS with a specific surface area of 4000 cm2/g produced mortar that was 5% weaker.
- Compression strength of the mortar specimens decreased with an increase in cement replacement. Specimens that included 10% BOFS achieved the highest compressive strength at 28 days; however, even this was 5% lower than that of OPM. Increasing BOFS content beyond 50% greatly decreased compressive strength due to increased expansion and cracking. However, the inclusion of fiber in 10% BOFS composites may be used as repair mortar in emergency engineering, especially for BOFS with 6000 cm2/g due to their rapid hardening.
- SEM analysis revealed that the CaO component of cement reacts with H2O, SiO2, and Al2O3 from BOFS to mainly react C–S–H and C–A–S–H, which was a major source of strength development in the BOFS blended materials.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Steel Association. Steel Statistical Yearbook. 2017. Available online: http://www.worldsteel.org/ (accessed on 5 December 2017).
- Gieseler, J. Properties of iron and steel slags regarding their use. In Proceedings of the Sixth International Conference on Molten Slags, Fluxes and Salts: Stockholm, Sweden, Helsinki, Finland, 12–17 June 2000. [Google Scholar]
- Miklos, P. The utilization of electric arc furnace slags in Denmark. Euroslag. Engineering of slags. A scientific and technological challenge. In Proceedings of the 2nd European Slag Conference, Düsseldorf, Germany, 9–10 October 2000. [Google Scholar]
- Tossavainen, M.; Engstrom, F.; Yang, Q.; Menad, N.; Lidstrom Larsson, M.; Bjorkman, B. Characteristics of steel slag under different cooling conditions. Waste Manag. 2007, 27, 1335–1344. [Google Scholar] [CrossRef] [PubMed]
- Tsakiridis, P.E.; Papadimitriou, G.D.; Tsivilis, S.; Koroneos, C. Utilization of steel slag for Portland cement clinker production. J. Hazard. Mater. 2008, 152, 805–811. [Google Scholar] [CrossRef]
- Bernardo, G.; Marroccoli, M.; Nobili, M.; Telesca, A.; Valenti, G.L. The use of oil well-derived drilling waste and electric arc furnace slag as alternative raw materials in clinker production. Resour. Conserv. Recycl. 2007, 52, 95–102. [Google Scholar] [CrossRef]
- Chen, Y.L.; Chang, J.E.; Ko, M.S. Reusing desulfurization slag in cement clinker production and the influence on the formation of clinker phases. Sustainability 2017, 9, 1585. [Google Scholar] [CrossRef]
- Liu, J.; Wang, D. Influence of steel slag-silica fume composite mineral admixture on the properties of concrete. Powder Technol. 2017, 320, 230–238. [Google Scholar] [CrossRef]
- Poulikakos, L.D.; Papadaskalopoulou, C.; Hofko, B.; Gschösser, F.; Falchetto, A.C.; Bueno, M.; Arraigada, M.; Sousa, J.; Ruiz, R.; Petit, C.; et al. Harvesting the unexplored potential of European wastematerials for road construction. Resour. Conserv. Recycl. 2017, 116, 32–44. [Google Scholar] [CrossRef]
- Jia, R.; Liu, J. Simulated experiment study of factors influencing the hydration activity of f-CaO in basic oxygen furnace slag. Adv. Mater. Sci. Eng. 2016, 2016, 7529382. [Google Scholar] [CrossRef]
- Ferreira Neto, J.B.; Fredericci, C.; Faria, J.O.G.; Fabiano, F.; Ribeiro, C.R.; Malynowskyj, A.; Silva, A.L.N.; Quarcioni, V.A.; Lotto, A.A. Modification of Basic Oxygen Furnace Slag for Cement Manufacturing. J. Sustain. Metall. 2017, 3, 720–728. [Google Scholar] [CrossRef]
- Kuo, W.T.; Juang, C.U. Prediction of expansion of electric arc Furnace oxidizing Slag mortar using MNLR and BPN. Comput. Concr. 2017, 20, 111–118. [Google Scholar]
- Shu, C.Y.; Kuo, W.T.; Juang, C.U. Analytical model of expansion for electric arc Furnace oxidizing Slag-containing concrete. Comput. Concr. 2016, 18, 937–950. [Google Scholar] [CrossRef]
- Fernandez, N.P. The Influence of Construction Materials on Life-Cycle Energy Use and Carbon Dioxide Emissions of Medium Size Commercial Buildings. Master’s Thesis, Victoria University, Footscray, Australia, 2008. [Google Scholar]
- Malhotra, V.M. Role of supplementary cementitious materials in reducing greenhouse gas emissions. In Proceedings of the Concrete Technology for a Sustainable Development in the 21st Century, Lofoten, Norway, 1 January 2000; pp. 226–235. [Google Scholar]
- Van Oss, H.G.; Padovani, A.C. Cement Manufacture and the Environment: Part I: Chemistry and Technology. J. Ind. Ecol. 2002, 6, 89–105. [Google Scholar]
- European Cement Association (CEMBUREAU). Activity Report 2016; European Cement Association: Brussels, Belgium, 2016. [Google Scholar]
- Mikulčić, H.; Klemeš, J.J.; Vujanović, M.; Urbaniec, K.; Duić, N. Reducing greenhouse gasses emissions by fostering the deployment of alternative raw materials and energy sources in the cleaner cement manufacturing process. J. Clean. Prod. 2016, 136, 119–132. [Google Scholar] [CrossRef]
- Cho, B.S.; Choi, Y.C. Hydration properties of STS-Refining slag-blended blast furnace slag cement. Adv. Mater. Sci. Eng. 2018, 2018, 5893254. [Google Scholar] [CrossRef]
- Rahman, A.A.; Abd-El-Aziz, M.A.; Aboul-Fetouh, M.; Shehata, K.H. Characteristics of Portland blast-furnace slag cement containing cement kiln dust and active silica. Arab. J. Chem. 2016, 9, S138–S143. [Google Scholar] [CrossRef]
- Bonenfant, D.; Kharoune, L.; Sauvé, S.; Hausler, R. Molecular analysis of carbon dioxide adsorption processes on steel slag oxides. Int. J. Greenh. Gas Control 2009, 3, 20–28. [Google Scholar] [CrossRef]
- Proctor, D.M.; Fehling, K.A.; Shay, E.C.; Wittenborn, J.L.; Green, J.J.; Avent, C.; Bigham, R.D.; Connolly, M.; Lee, B.; Shepker, T.O.; et al. Physical and chemical characteristics of blast furnace, basic oxygen furnace, and electric arc furnace steel industry slags. Environ. Sci. Technol. 2000, 34, 1576–1582. [Google Scholar] [CrossRef]
- de Sena Costa, B.L.; de Oliveira Freitas, J.C.; Santos, P.H.S.; de Araújo Melo, D.M.; da Silva Araujo, R.G.; de Oliveira, Y.H. Carbonation in oil well Portland cement: Influence of hydration time prior to contact with CO2. Constr. Build. Mater. 2018, 159, 252–260. [Google Scholar] [CrossRef]
- Bouma, R.; Vercauteren, F.; van Os, P.; Goetheer, E.; Berstad, D.; Anantharaman, R. Membrane-assisted CO2 Liquefaction: Performance Modelling of CO2 Capture from Flue Gas in Cement Production. Energy Procedia 2017, 114, 72–80. [Google Scholar] [CrossRef]
- Ding, Y.C.; Cheng, T.W.; Liu, P.C.; Lee, W.H. Study on the treatment of BOF slag to replace fine aggregate in concrete. Constr. Build. Mater. 2017, 146, 644–651. [Google Scholar] [CrossRef]
- Wang, G. Determination of the expansion force of coarse steel slag aggregate. Constr. Build. Mater. 2010, 24, 1961–1966. [Google Scholar] [CrossRef]
- Manso, J.; Polanco, J.; Losanez, M.; Gonzalez, J.J. Durability of concrete made with EAF slag as an aggregate. Cem. Concr. Compos. 2006, 28, 528–534. [Google Scholar] [CrossRef]
- Liu, S.H.; Li, L.H. Influence of fineness on the cementitious properties of steel slag. J. Therm. Anal. Calorim. 2014, 117, 629–634. [Google Scholar] [CrossRef]
- Wang, Q.; Yan, P.Y.; Yang, J.W.; Zhang, B. Influence of steel slag on mechanical properties and durability of concrete. Constr. Build. Mater. 2013, 47, 1414–1420. [Google Scholar] [CrossRef]
- Calmon, J.L.; Tristão, F.A.; Giacometti, M.; Meneguelli, M.; Moratti, M.; Teixeira, J.E.S.L. Effects of BOF steel slag and other cementitious materials on the rheological properties of self-compacting cement pastes. Constr. Build. Mater. 2013, 40, 1046–1053. [Google Scholar] [CrossRef]
- Montgomery, D.G.; Wang, G. Preliminary laboratory of steel slag for blended cement manufacture. Forum Mater. 1991, 15, 374–382. [Google Scholar]
- Ananthi, A.; Karthikeyan, J. Properties of industrial slag as fine aggregate in concrete. Int. J. Eng. Technol. Innov. 2015, 5, 132–140. [Google Scholar]
- Higginson, E.C. The effect of cement fineness on concrete. In Fineness of Cement; Verbeck, G.J., Ed.; ASTM STP 473; American Society for Testing and Materials: Philadelphia, PA, USA, 1970; pp. 71–81. [Google Scholar]
- Hu, J.; Ge, Z.; Wang, K. Influence of cement fineness and water-to-cement ratio on mortar early-age heat of hydration and set times. Constr. Build. Mater. 2014, 50, 657–663. [Google Scholar] [CrossRef]
- Li, L.G.; Kwan, A.K.H. Concrete mix design based on water film thickness and paste film thickness. Cem. Concr. Compos. 2013, 39, 33–42. [Google Scholar] [CrossRef]
- Kim, H.; Jeon, J.; Lee, H. Flow, water absorption, and mechanical characteristics of normal-and high-strength mortar incorporating fine bottom ash aggregates. Constr. Build. Mater. 2012, 26, 249–256. [Google Scholar] [CrossRef]
- Belhadj, E.; Diliberto, C.; Lecomte, A. Properties of hydraulic paste of basic oxygen furnace slag. Cem. Concr. Compos. 2014, 45, 15–21. [Google Scholar] [CrossRef]
- Xie, J.; Wu, S.; Lin, J.; Cai, J.; Chen, Z.; Wei, W. Recycling of basic oxygen furnace slag in asphalt mixture: Material characterization & moisture damage investigation. Constr. Build. Mater. 2012, 36, 467–474. [Google Scholar]
- Zhao, J.; Wang, D.; Wang, X.; Liao, S.; Lin, H. Ultra fine grinding of fly ash with grinding aids: Impact on particle characteristics of ultrafine fly ash and properties of blended cement containing ultrafine fly ash. Constr. Build. Mater. 2015, 78, 250–259. [Google Scholar] [CrossRef]
- Tasi, C.J.; Huang, R.; Lin, W.T.; Wang, H.N. Mechanical and cementitious characteristics of ground granulated blast furnace slag and basic oxygen furnace slag blended mortar. Mater. Des. 2014, 60, 267–273. [Google Scholar]
- Lu, T.H.; Chen, Y.L.; Shih, P.H.; Chang, J.E. Use of basic oxygen furnace slag fines in the production of cementitious mortars and the effects on mortar expansion. Constr. Build. Mater. 2018, 167, 768–774. [Google Scholar] [CrossRef]
- Özkan, Ö.; Sarıbıyık, M. Alkali silica reaction of BOF and BFS wastes combination in cement. J. Civ. Eng. Manag. 2013, 19, 113–120. [Google Scholar] [CrossRef]
- Sun, J. Study of effects of ground steel slag on mechanical performance and soundness of concrete. Coal Ash China 2003, 15, 7–9. [Google Scholar]
- Jiang, Y.; Ling, T.C.; Shi, C.; Pan, S.Y. Characteristics of steel slags and their use in cement and concrete—A review. Resour. Conserv. Recy. 2018, 136, 187–197. [Google Scholar] [CrossRef]
- Wang, Q.; Yan, P. Hydration properties of basic oxygen furnace steel slag. Constr. Build. Mater. 2010, 24, 1134–1140. [Google Scholar] [CrossRef]
- Tasi, C.J.; Huang, R.; Lin, W.T.; Chiang, H.W. Using GGBOS as the alkali activators in GGBS and GGBOS blended cements. Constr. Build. Mater. 2014, 70, 501–507. [Google Scholar]
- Olofinnade, O.M.; Ede, A.N.; Ndambuki, J.M. Experimental investigation on the effect of elevated temperature on compressive strength of concrete containing waste glass powder. Int. J. Eng. Technol. Innov. 2017, 7, 280–291. [Google Scholar]
- Lin, W.T. Characteristic and permeability of cement-based materials containing calcium fluoride sludge. Constr. Build. Mater. 2019, 196, 564–573. [Google Scholar] [CrossRef]




| Chemical Composition (wt.%) | Cement | BOFS |
|---|---|---|
| SiO2 | 21.04 | 12.20 |
| Al2O3 | 5.46 | 4.76 |
| Fe2O3 | 2.98 | 32.20 |
| CaO | 63.56 | 38.90 |
| MgO | 2.52 | 6.26 |
| MnO | - | 2.39 |
| Min No. | w/b | Water | Cement | BOFS | Fine Aggregates |
|---|---|---|---|---|---|
| OPM | 0.5 | 258 | 516 | - | 1421 |
| B1 | 259 | 466 | 52 | 1422 | |
| B3 | 260 | 364 | 165 | 1428 | |
| B5 | 261 | 261 | 261 | 1435 | |
| B7 | 261 | 157 | 367 | 1441 |
| Test Target | Specimen Dimensions (mm) | Referenced Standard | |
|---|---|---|---|
| Fresh properties | Flow test | - | ASTM C230 |
| Setting test | - | ASTM C191 | |
| Hydrated temperature test | - | - | |
| Mechanical properties | Compressive strength test | 50 × 50 × 50 | ASTM C109 |
| Durability | Drying shrinkage test | 285 × 25 × 25 | ASTM C596 |
| Micro-structure observations | SEM observation | 10 × 10 × 3 | ASTM C1723 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, W.-T.; Tsai, C.-J.; Chen, J.; Liu, W. Feasibility and Characterization Mortar Blended with High-Amount Basic Oxygen Furnace Slag. Materials 2019, 12, 6. https://doi.org/10.3390/ma12010006
Lin W-T, Tsai C-J, Chen J, Liu W. Feasibility and Characterization Mortar Blended with High-Amount Basic Oxygen Furnace Slag. Materials. 2019; 12(1):6. https://doi.org/10.3390/ma12010006
Chicago/Turabian StyleLin, Wei-Ting, Chia-Jung Tsai, Jie Chen, and Weidong Liu. 2019. "Feasibility and Characterization Mortar Blended with High-Amount Basic Oxygen Furnace Slag" Materials 12, no. 1: 6. https://doi.org/10.3390/ma12010006
APA StyleLin, W.-T., Tsai, C.-J., Chen, J., & Liu, W. (2019). Feasibility and Characterization Mortar Blended with High-Amount Basic Oxygen Furnace Slag. Materials, 12(1), 6. https://doi.org/10.3390/ma12010006






