3.1. Hydrolysates Formed fromTi-Pillaring Solution
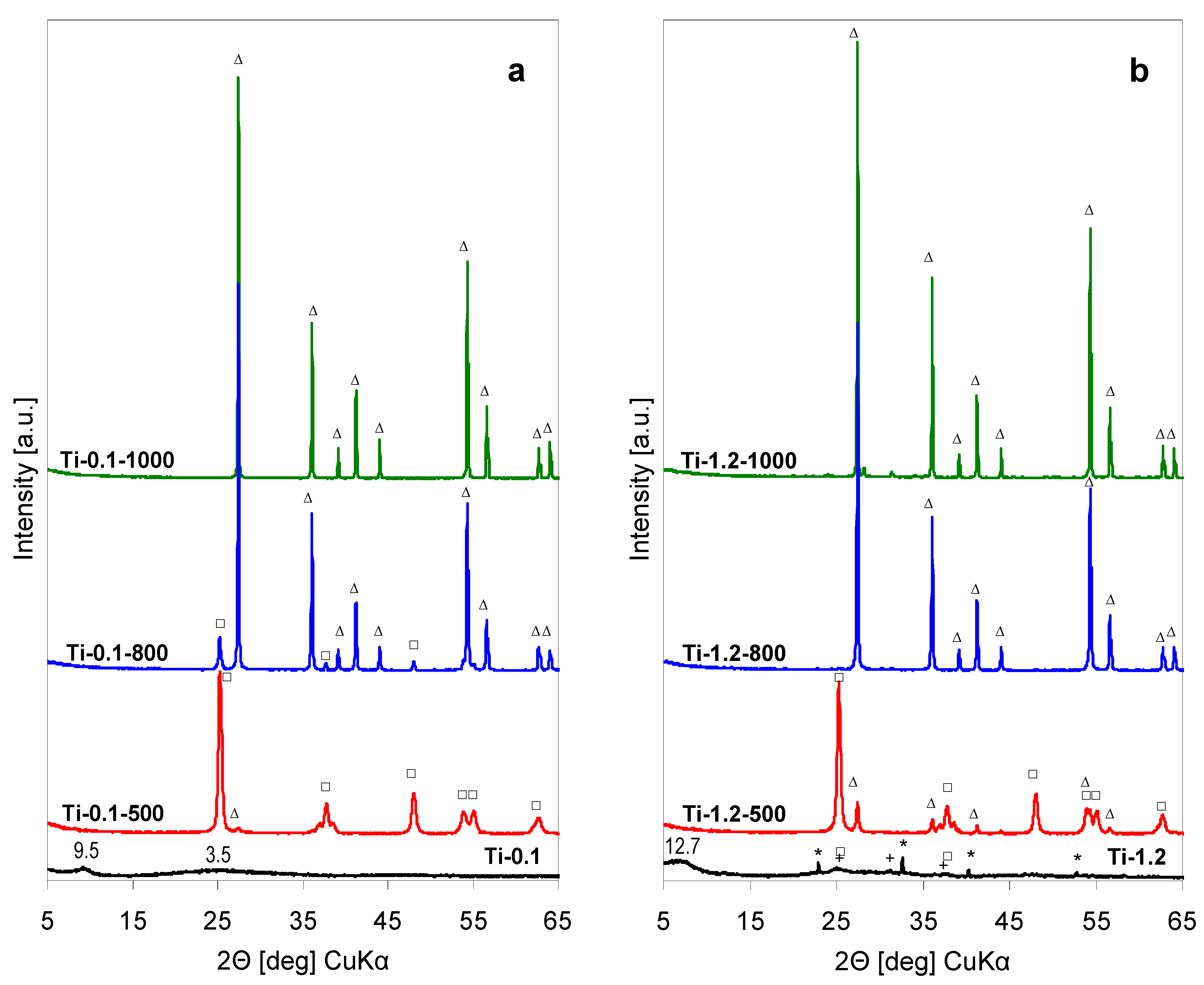
The XRD patterns of precipitates obtained by lyophilization of the as received Ti-pillaring solution (pH = 0.1) are presented in
Figure 1a, while
Figure 1b shows XRD diagrams of lyophilized precipitates obtained from solutions whose pH has been adjusted to 1.2, the value observed upon preparation of Ti-pillared montmorillonite. The XRD pattern of the as received lyophilized Ti-0.1 sample shows only a broad reflection around 2Θ≈ 9.3°, corresponding to ca. 9.5 Å interplanar distance, and an even broader, low intensity bump centered around 2Θ ≈ 25° (d ≈ 3.5 Å), which points to the chiefly amorphous character of the solid components. The low angle reflection may be taken as an indication that part of the amorphous solid tends to form a layered structure, e.g., related to the lepidocrocite-type protonated titanate [
13]. The feature with d ≈ 3.5 Å may be attributed to an amorphous phase from which a titania polymorph is going to nucleate. After calcination of the precipitate at 500 °C (sample Ti-0.1-500) the XRD pattern shows a set of intense reflections characteristic of crystalline anatase (A), accompanied by trace amounts of rutile (R). Material calcined at 800 °C contains a higher relative share of rutile, and after heat treatment at 1000 °C, rutile is the only detected crystalline TiO
2 phase.
The XRD pattern of Ti-1.2 precipitate obtained from pillaring solution whose pH was raised with NH
3aq to the value observed during Ti-PILC formation, shows that some ammonium chloride has been formed in the process, as indicated by the set of sharp reflections characteristic of this salt. The broad reflection at 2Θ ≈ 7° (d ≈ 12.5 Å) indicates that also here an amorphized layered structure is formed, probably of similar nature as in Ti-0.1, but with higher content of water in the interlayer [
13]. The titania-related phase, although still poorly crystalline, is better defined than in the case of Ti-0.1 sample. The most pronounced reflection around 2Θ ≈ 25° (d ≈ 3.5 Å) and the reflection at 2Θ ≈ 37.5° (d ≈ 2.39 Å) suggests nucleation of anatase and/or brookite. A contribution of the latter may be inferred from the faint reflection around 2Θ ≈ 31° (d ≈ 2.88 Å) diagnostic of brookite polymorph. XRD pattern of Ti-1.2-500 sample shows that after calcination at 500 °C the material contains predominantly anatase, but transformation to rutile is more advanced than in the Ti-0.1-500 counterpart. Rutile is the only crystalline phase found in samples calcined at 800 and 1000 °C. Thus, a comparison of the thermal evolution of both precipitates shows that transformation of anatase into rutile is slightly facilitated in the material obtained from alkalized pillaring solution.
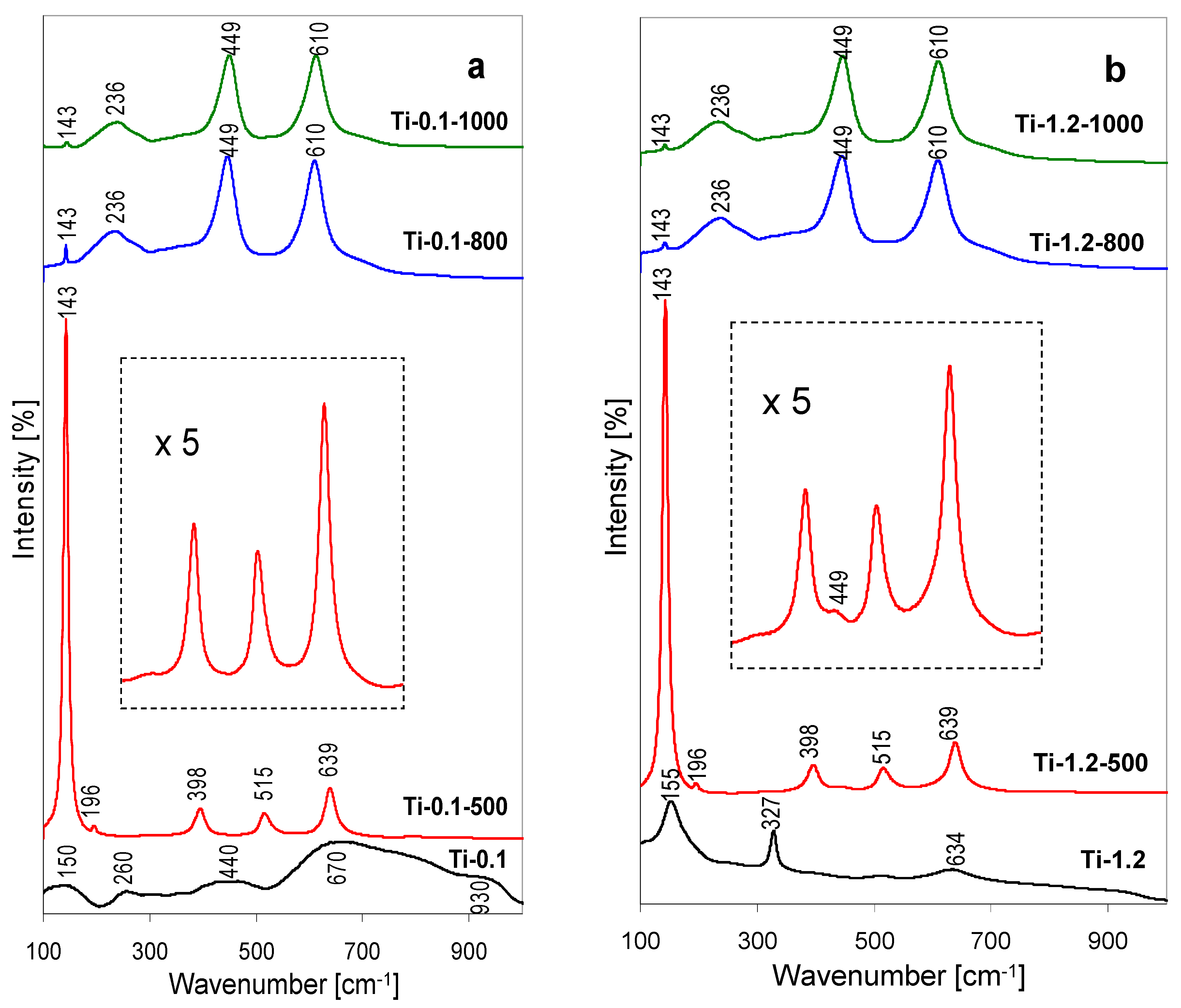
Structure of the precipitates was also studied with Raman spectroscopy, more powerful than XRD for detecting short range order in amorphous nanodomains [
14]. Therefore, Raman analysis was expected to shed more light on the structure of the thermally untreated Ti-0.1 and Ti-1.2 precipitates, essentially amorphous from the XRD point of view. Various titania polymorphs and layered titanates are known to exhibit characteristic Raman spectra, and the present interpretation of the data follows previous assignments [
15,
16,
17,
18]. The Raman spectra monitoring thermal evolution of Ti-0.1 precipitate, are shown in
Figure 2a, those obtained for the Ti-1.2 series, in
Figure 2b.
The Raman spectrum of the lyophylized Ti-0.1 sample, shown in
Figure 2a, displays several ill-defined broad bands, centered around 150, 260, 440, 670, 930 cm
−1. All, except the first one, may be considered as envelopes of broadened bands stemming from amorphized lepidocrocite-type titanate phase [
18], whose presence has been suggested on the base of the XRD pattern (
Figure 1a). The feature around 150 cm
−1 appears in the range where the most intense bands of anatase or brookite appear, and is attributed to the amorphous titania structures responsible for the broad XRD bump centered at d = 3.5 Å in
Figure 1a.
The spectrum of Ti-1.2 precipitate, obtained from the pillaring solution after pH adjustment, shows better resolved features. The set of bands at 155, 327 and 634 cm
−1 points to the formation of poorly crystalline brookite [
16,
17], thus substantiating the tentative assignment derived from the XRD data. Indeed, Pottier, et al. [
19] reported that Ti(OH)
2(Cl)
2(OH
2)
2 species formed during hydrolysis of TiCl
4, in strongly acidic medium enriched with chloride ions, initially tend to form the brookite phase. In this spectral range the dominant band of NH
4Cl appears around 180 cm
−1, but the amount of impurity is too small to make an impact on the recorded Raman spectrum. The spectra obtained for both series of thermally treated samples essentially agree with the conclusions drawn from XRD examination. Thus, the spectra of samples after calcination at 500 °C are dominated by the bands at 143, 196, 398, 515 and 639 cm
−1 characteristics of anatase [
15]. The presence of rutile admixture can be detected in Ti-1.2-500 as a shoulder at 449 cm
−1, clearly visible in the magnified part of the spectrum in
Figure 2b. The trace of rutile indicated by XRD in Ti-0.1-500 is apparently too small to reveal its presence in the Raman spectrum (magnified fragment in
Figure 2a). The spectra of samples calcined at higher temperatures are dominated by the bands typical of rutile, i.e., a weak one at 143 cm
−1 and three strong features at 236, 449 and 610 cm
−1, which agrees with the XRD analysis pointing to the occurrence of anatase to rutile phase transformation [
15]. The presence of some anatase remnants, revealed by XRD in sample Ti-0.1-800, may be inferred from the slightly higher, than in other samples, intensity of the 143 cm
−1 band, which indicates a contribution from the coincident most intense band of anatase (
Figure 2a).
SEM images of samples Ti-0.1-1000 and Ti-1.2-1000 presented in
Figure 3a,d, respectively, show that after calcination at 1000 °C the morphology of both materials is quite similar, with particles size being, in general, ≤0.5 μm.
3.2. Hydrolysates Formed from Zr-Pillaring Solution
Figure 4a shows the XRD patterns of precipitates obtained from the as received Zr-pillaring solution (pH = 1.0),
Figure 4b gathers XRD data obtained for the solids prepared from the Zr-pillaring agent whose pH has been raised to 1.5, the value observed during Zr-PILC synthesis. The XRD pattern of the as received lyophilized Zr-1.0 sample appears essentially amorphous. It is dominated by a broad reflection around 2Θ ≈ 8°, corresponding to ca. 11 Å interplanar distance. The most intense reflections of various ZrOCl
2 hydrates appear in the 7° < 2Θ < 8.6° range (e.g., ZrOCl
2·8H
2O, JCPDS 00-032-1498, ZrOCl
2·6H
2O, JCPDS 00-047-0815, ZrOCl
2·4H
2O, JCPDS 00-018-1497). Matsui and Ohgai [
20], in their extensive study of ZrOCl
2 hydrolysis, referred to such a product as chlorine-containing hydrous zirconia. Calcination of this precipitate at 500 °C leads to crystallization of tetragonal ZrO
2 polymorph (
t-ZrO
2). In addition, in the XRD pattern of Zr-0.1-500 a trace of peak at 2Θ = 28.2° (d = 3.162 Å), corresponding to the most intense reflection of the monoclinic zirconia (
m-ZrO
2), is observed, which shows that a small degree of transformation of the metastable
t-ZrO
2 to the stable
m-ZrO
2 has already occurred. After calcination at 800 °C the transformation is almost complete and the material is composed predominantly of
m-ZrO
2, with only a trace of unreacted tetragonal phase, manifested by the remainder of the most intense reflection of
t-ZrO
2 at 2Θ = 30.2° (d = 2.957 Å). Sample calcined at 1000 °C contains only
m-ZrO
2.
The XRD pattern of Zr-1.5 precipitate obtained from pillaring solution whose pH was adjusted to 1.5 with ammonia, shows that, similarly as in the case of Ti-1.2, some ammonium chloride has been formed. Otherwise, the diffractogram displays broad reflection at 2Θ ≈ 7.5° (d ≈ 11.7 Å), together with a broad bump centered around 2Θ ≈ 25° (d ≈ 3.5 Å), i.e. at a lower angle than that expected for amorphous ZrO
2 (broad envelope around 2Θ = 30 °C [
21]). This shows that, even after an increase of pH, the precipitate bears feature of chlorine-containing hydrous zirconia [
20] rather than of an amorphous precursor of
t-ZrO
2. The latter phase appears as the only crystalline product after calcination at 500 °C. In Zr-1.5-800 the transformation of most of the tetragonal phase to
m-ZrO
2 is visible, although the intensity of the remaining 2Θ = 30.2° reflection of
t-ZrO
2 is stronger than in the Zr-1.0-800 counterpart. Its very weak trace persists even in Zr-1.5-1000, although monoclinic zirconia is the predominant crystalline phase found in this material.
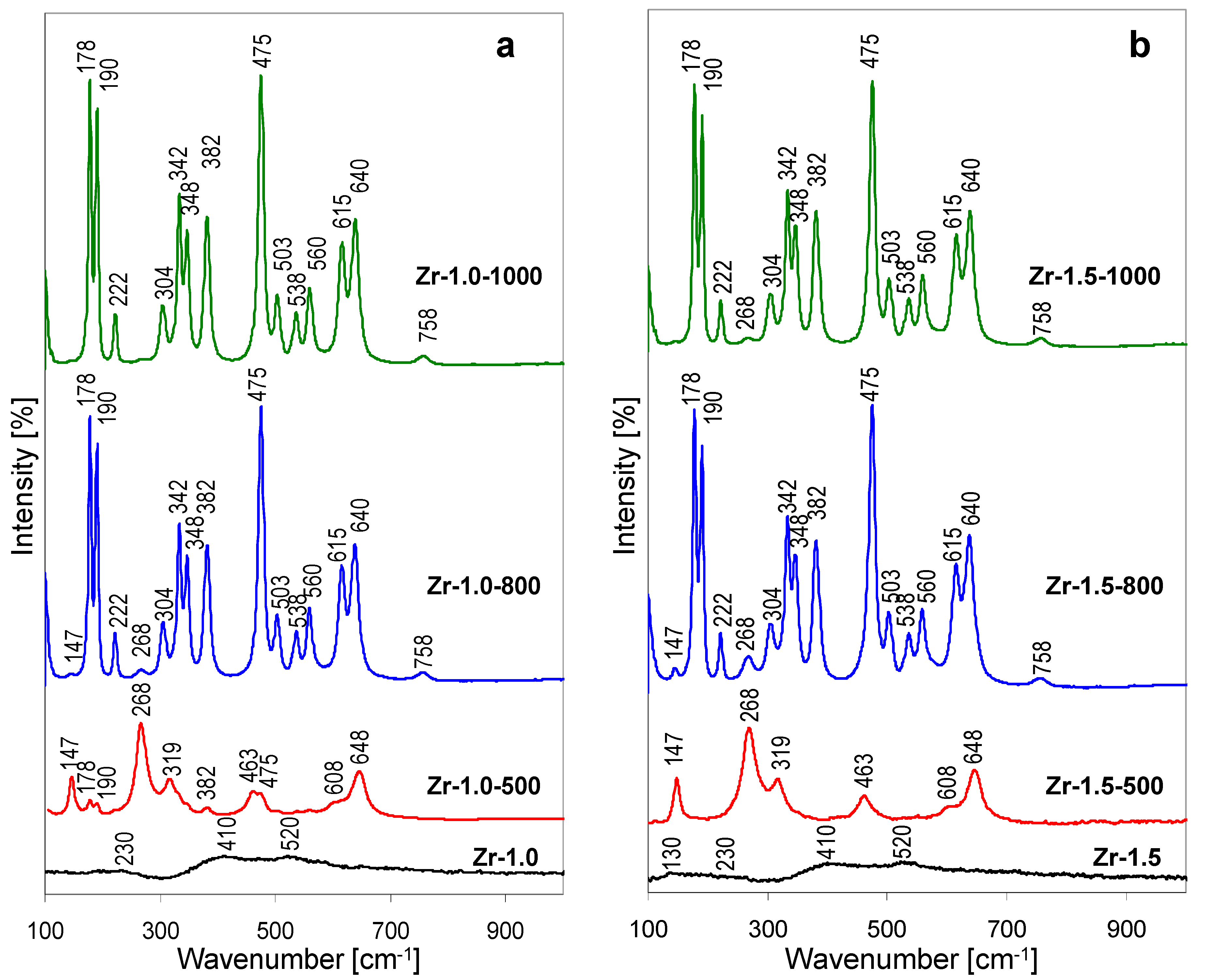
The Raman spectra of the thermal evolution of Zr-1.0 sample are shown in
Figure 5a, those recorded for the Zr-1.5 series, in
Figure 5b. The spectra of Zr-1.0 and Zr-1.5 are quite similar and display several broad bands, centered around 130, 230, 410 and 520 cm
−1. Similarly, as in the case of Ti-1.2, no bands attributable to NH
4Cl impurity are visible. The observed features are similar to those reported previously for non-crystalline solids obtained from freshly hydrolyzed ZrOCl
2 [
22,
23]. The intermediate character of Zr-1.0 and Zr-1.5 precipitates, referred to as chlorine-containing hydrous zirconia, is evidenced by the fact that the observed broad bands are different from those characteristics of ZrOCl
2 hydrate precursor (460 and 590 cm
−1) [
21], but do not yet display features typical of zirconia polymorphs (see thermal evolution of Raman spectra). In contrast, the spectra of thermally treated Zr-1.0-500 and Zr-1.5-500 show well defined bands characteristic of tetragonal zirconia (147, 268, 319, 463, 608, 648 cm
−1) [
21,
24]. In accordance with the XRD findings, the spectrum of Zr-1.0-500 shows some low intensity features characteristic of the most intense bands of
m-ZrO
2 (178, 190, 382, 475 cm
−1). The Raman spectra recorded for samples treated at 800 and 1000 °C are similar, and reflect the transformation of most, or all of the calcined material to monoclinic ZrO
2 polymorph. In agreement with XRD analysis, small amounts of
t-ZrO
2 are still visible in both samples calcined at 800 °C (low intensity bands at 147 and 268 cm
−1), albeit with higher intensity in the Zr-1.5-800 sample. A trace of tetragonal zirconia can be still detected in the spectrum of Zr-1.5-1000 (weak band at 268 cm
−1) bands, thus confirming that alkalization of Zr-pillaring solution yields hydrolysate in which tetragonal to monoclinic zirconia transformation is retarded.
SEM images of samples Zr-0.1-1000 and Zr-1.2-1000, presented in
Figure 3b,e, respectively, show that in both materials’ zirconia grains consist of variously shaped agglomerates.
3.3. Hydrolysates Formed from Ti, Zr-Pillaring Solution
XRD patterns of precipitate recovered by lyophilization from the as received Ti, Zr-pillaring solution (pH = 0.6) and of the products of its thermal evolution are shown in
Figure 6a. Those recorded for the materials obtained from the Ti, Zr-pillaring agent after adjustment of pH to 1.2, i.e., the value observed during Ti, Zr-PILC synthesis, are gathered in
Figure 6b.
The XRD pattern of lyophilized Ti, Zr-0.6 precipitate shows a set of reflexes pointing to the presence of a certain amount of variously hydrated ZrOCl2. Formation of these phases shows that in the as received Ti, Zr-pillaring solution the tendency to form mixed species, if at all present, is limited. Moreover, the zirconyl chloride phases are better defined than in the case of Zr-1.0 precipitate obtained from the Zr-pillaring solution, which is attributed to the slower hydrolysis of the salt in the conditions of lower pH characteristic for mixed pillaring agent. The peaks of zirconyl chloride hydrates overlap with a component showing a broad maximum around 2Θ ≈ 6.0°, corresponding to an interplanar distance of ca. 14.7 Å, and an elevated background centered around 2Θ ≈ 28° (d ≈ 3.2 Å), indicating formation of a quasi-amorphous layered precursor of unknown composition. XRD analysis of sample calcined at 500 °C shows that the material is multiphase, with the evident presence of anatase form of titania, monoclinic polymorph of zirconia and mixed zirconium titanate (ZrTiO4). Moreover, in view of the strong overlap of XRD pattern of tetragonal zirconia with that of ZrTiO4, some contribution of the former cannot be completely excluded. The observed multiphase composition indicates that in the precipitate formed from the as received Ti, Zr-pillaring solution, there is a strong tendency to form separate Zr- and Ti-based phases.
Calcination at 800 and 1000 °C temperatures confirms this observation, as separate phases appear to evolve independently (
Figure 6a). Thus, the phase composition of the sample calcined at 800 °C is the same as after calcination at 500 °C, except for all phases showing better resolved reflections, consistent with their higher crystallinity. Noteworthy, in the case of material obtained from Zr-pillaring solution, the monoclinic form of ZrO
2 is clearly visible only after calcination at ≥ 800 °C (see
Section 3.2), while in the material obtained from Ti, Zr pillaring agent,
m-ZrO
2 appears already at 500 °C. Teng, et al. [
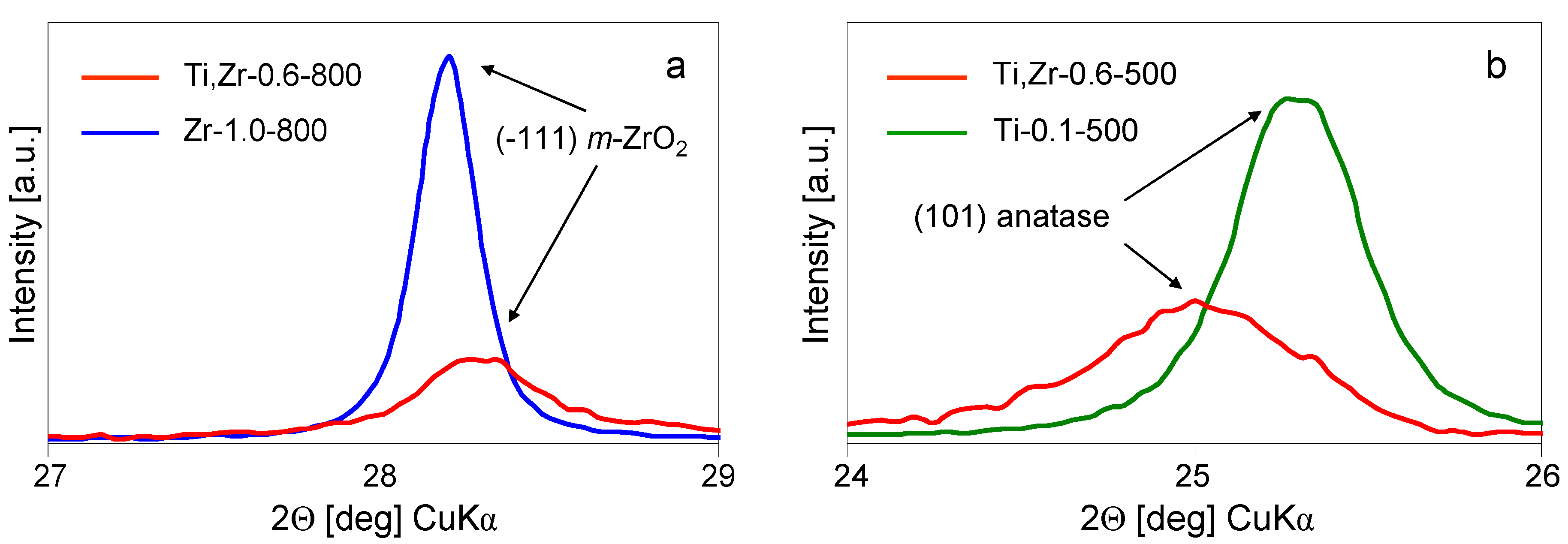
25] reported that partial isomorphous substitution of Zr with Ti facilitates tetragonal to monoclinic polymorph transition. Indeed, in the present study a shift in the position of (-111) reflection of
m-ZrO
2 (indexed according to JCPDS 37-1484) to higher 2Θ values, with respect to monoclinic zirconia obtained from Zr-pillaring solution, is observed (
Figure 7a). This points to a decrease of the corresponding interplanar distance and is consistent with a degree of substitution of large Zr
4+ cations (ionic radius 0.72 Å) with smaller Ti
4+ species (ionic radius 0.605 Å). After calcination at 1000 °C, the transformation of anatase to rutile is observed, albeit not complete, as some anatase can still be detected. This shows that phase transformation in titania obtained from mixed Ti, Zr-pillaring solution occurs at temperature ca. 200 °C higher than in the sample prepared from Ti-pillaring agent (see
Section 3.1). Also, here the likely explanation is that in anatase formed from Ti, Zr-pillaring solution some Ti is substituted with Zr, the effect known to slow down the anatase to rutile transformation [
25,
26,
27,
28]. Indeed, the (101) reflection of anatase (indexed according to JCPDS 21-1272), observed in the sample obtained from mixed Ti, Zr-pillaring solution, is shifted to slightly lower 2Θ value (
Figure 7b), which is consistent with the partial replacement of Ti
4+ with larger Zr
4+ ions.
XRD patterns of solids obtained from pillaring solution whose pH was adjusted to 1.2 with ammonia addition (
Figure 6b), reveal a completely different nature of lyophilized Ti, Zr-1.2 precipitate and of products of its thermal treatment at 500, 800 and 1000 °C. Apart from the sharp reflections, due to formation of some NH
4Cl, the Ti, Zr-1.2 precursor has a quasi-amorphous character. The broad peak at 2Θ ≈ 5.9° (d ≈ 15.0 Å) points to the nucleation of a layered type of structure, whose other feature is an elevated background around 2Θ ≈ 28° (d ≈ 3.2 Å). In Ti, Zr-1.2-500 the low 2Θ reflection disappears, but the sample is still strongly amorphous, except for two peaks emerging from the broadly elevated background, assignable to the most intense reflections of ZrTiO
4. Full set of reflections characteristic of zirconium titanate becomes visible after calcination at 800 and 1000 °C. Thus, in contrast to the multiphase nature of solids evolving from Ti, Zr-0.6 sample, ZrTiO
4 is the only phase crystallizing from the quasi-amorphous Ti, Zr-1.2 precipitate.
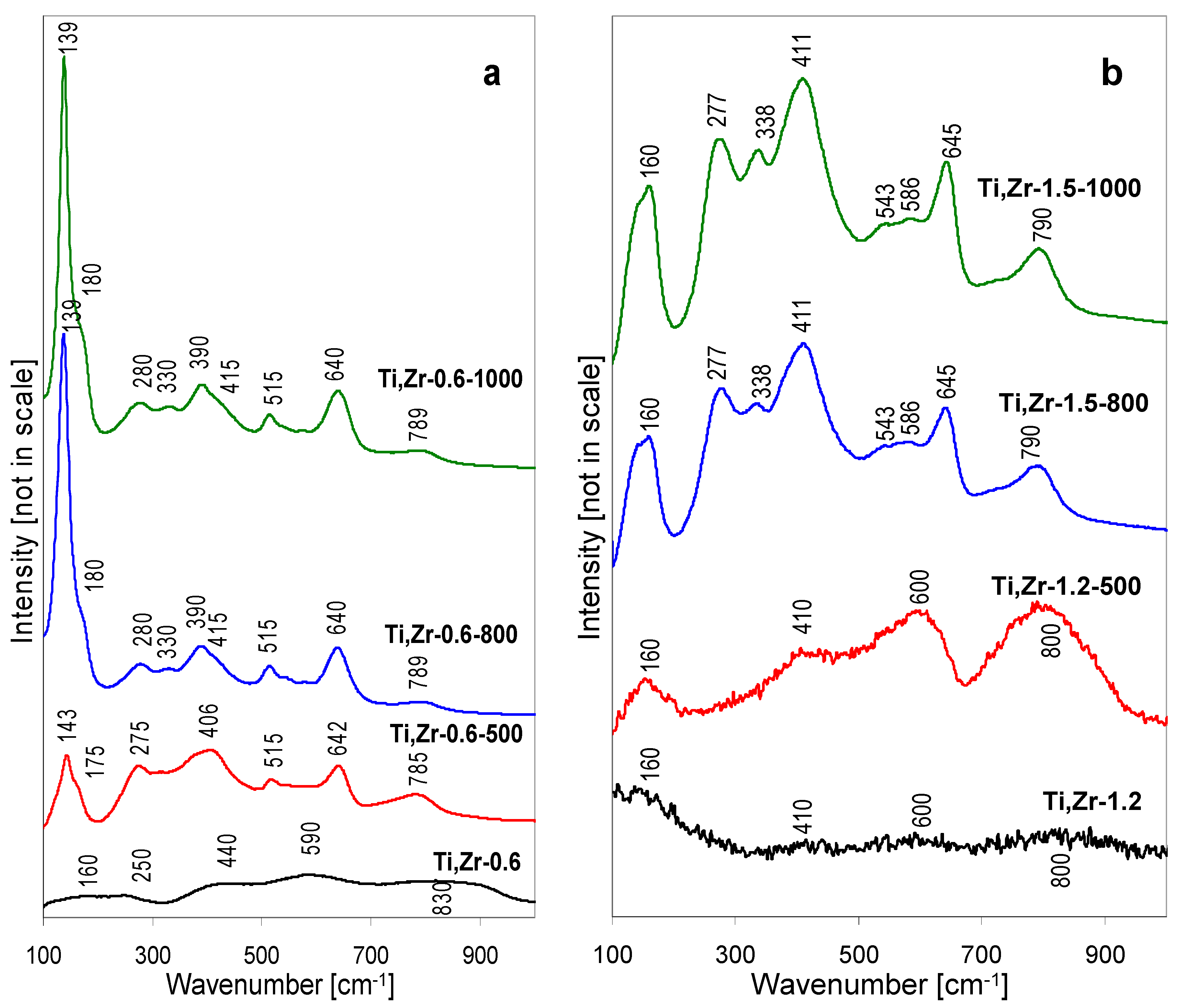
The Raman spectra of Ti, Zr-0.6 and Ti, Zr-1.2 series of samples are gathered in
Figure 8a,b, respectively. The spectrum of lyophilized Ti, Zr-0.6 precipitate shows only very weak, broad bands. The features around 440 and 590 cm
−1 may be attributed to zirconyl chloride hydrates, whose presence has been identified by XRD, the other, around 160, 250, and 830 cm
−1, are assigned to the quasi-amorphous layered structure. It is likely that the amorphous phase contains both Ti and Zr, because the band at ca. 830 cm
−1 has been previously attributed to the formation of Ti-O-Zr in amorphous Zr-rich oxide-like material [
29]. The Raman spectra of Ti, Zr-0.6 sample calcined at 500, 800 and 1000 °C confirm the XRD findings as to the multiphase nature of these solids (
Figure 8a).
Thus, after thermal treatment at 500 °C, the Ti, Zr-0.6-500 sample shows several bands, of which the ones at 143, 406, 515 and 642 may be attributed to anatase. Formation of zirconium titanate component is also documented by Raman spectrum. According to literature, the most intense Raman bands of this phase are found at ~160, ~275, ~340, ~410, ~640, and ~790 cm
−1, weaker ones at ~540, 590 cm
−1 [
12,
29,
30,
31,
32,
33]. The bands tend to be quite broad, which has been attributed to the random distribution of Zr and Ti atoms at equivalent structural positions, combined with anionic defects and/or minor variation of stoichiometry in polycrystalline materials [
29,
34]. The bands around 275 and 785 cm
−1 may be taken as diagnostic for this compound, as they do not overlap with vibrations stemming from other phases. The ZrTiO
4 modes at 410 and 640 cm
−1 contribute to the intensity of 406 and 642 cm
−1 maximum. The shoulder at 170 cm
−1 may also be attributed to ZrTiO
4. However, no Raman bands of monoclinic ZrO
2, whose presence is documented by XRD, can be detected, even in the spectral ranges where no overlap is expected (e.g., lack of feature around 475 cm
−1, very strong in
m-ZrO
2). As demonstrated by Livraghi, et al. [
35], the scattering properties of ZrO
2 are much poorer than those of anatase, hence its bands are easily obscured in the systems containing both components. After calcination at 800 and 1000 °C the overall character of the Raman spectra is preserved, except that the main features become more pronounced, due to the better crystalline order of the sample components. Noteworthy, the ~140 cm
−1 band of anatase is also the strongest on the spectrogram of a sample calcined at 1000 °C, where, as evidenced by X-ray data, most of the anatase has been transformed into rutile, which confirms the exceptionally strong scattering properties of this modification of titania. For the same reason no rutile bands can be clearly distinguished in the spectrum of Ti, Zr-0.6-1000, even the least overlapping mode around 450 cm
−1.
Raman spectra of samples obtained from titanium-zirconium pillared solutions with pH raised to 1.2, i.e., the value observed during pillaring of montmorillonite, are different (
Figure 8b). The spectrum of the lyophilized Ti, Zr-1.2 precipitate is poorly resolved, showing, similarly to other thermally untreated materials, only very broad bands, barely elevated from the background, at ca. 160, 410, 600 and 800 cm
−1. As in other materials whose pH has been adjusted with ammonia addition, the amount of NH
4Cl formed in the precipitate is not sufficient to mark its presence in the Raman spectrum. Changes observed upon thermal treatment show a close relation between the ensuing spectra, as the bands do not disappear but gradually become better resolved to yield the spectrum characteristic of ZrTiO
4. Thus, in the Ti, Zr-1.2-500 sample the faint features visible in the spectrum of Ti, Zr-1.2 become more intense, in accordance with the XRD data which show that in this material the crystallization of ZrTiO
4 from amorphous phase has just begun. The Raman spectra of Ti, Zr-1.2-800 and Ti, Zr-1.2-1000 show the full set of bands expected for ZrTiO
4 (160, 277, 338, 411, 543, 586, 645 and 790 cm
−1). No bands that might indicate the presence of other oxide components are present, which confirms the single-phase nature of the material produced from Ti, Zr-1.2 precipitate. Moreover, the obvious kinship between the spectra is an indication that already in the thermally untreated quasi-amorphous precursor the short order interatomic bonding is closely related to that developed in the zirconium titanate.
SEM analysis of Ti, Zr-0.6-1000 and Ti, Zr-1.2-1000 samples reveals significant differences in morphology of both materials (
Figure 3c,f, respectively). Thus, Ti, Zr-0.6-1000 is composed of grains with various shapes, ranging from small rounded particles to larger rectangular blocks. The inhomogeneous composition of Ti, Zr-0.6 series has been independently confirmed by the results of scanning electron microscope/energy dispersive spectrometer (SEM/EDS) analysis. Backscattered electrons (BSE) imaging, which strongly depends on the atomic number of the scattering elements, has been employed for analysis of the samples. BSE images are particularly suitable for detecting areas of different composition because the sample components containing heavier elements appear brighter. An example of the BSE image of Ti, Zr-0.6-1000 is shown in
Figure 9a. The polycrystalline material is clearly composed of areas of different contrast, indicating differences in chemical composition of various parts of the specimen. The EDS analysis confirms that brighter regions are zirconium-rich, while darker contain mainly titanium. On the other hand, EDS analysis of Ti, Zr-1.2-1000 sample shows that the material is essentially homogeneous as far as the chemical composition is concerned, and no meaningful fluctuations around the expected Ti/Zr ratio equal 1 are observed (
Figure 9b). The results support the conclusion drawn on the basis of the XRD and Raman data, that the mixed oxide material obtained from Ti, Zr-1.2 precursor is structurally and compositionally uniform.
Comparison of the data obtained for materials synthesized from mixed Ti, Zr-0.6 and Ti, Zr-1.2 precursors points to the critical influence of the pH of the solutions on the nature of mixed oxide materials derived from the lyophilized precipitates upon thermal treatment. For the former series an inhomogeneous polycrystalline material is produced, with the tendency to form, next to zirconium titanate, separate Ti- and Zr-based phases. On the contrary, in the solids prepared from Ti, Zr-1.2, the ZrTiO
4 phase, accommodating both elements, is formed exclusively. Since the pH value of 1.2 has been selected to imitate the acidity generated during preparation of Ti, Zr-pillared montmorillonite, the results of the current experiments back the conclusion presented in our earlier work [
2] that pillars possess a TiZrO
4-like nature rather than constitute a mixture of titania and zirconia props.