Space Charge Characteristics of Polypropylene Modified by Rare Earth Nucleating Agent for β Crystallization
Abstract
1. Introduction
2. Materials and Methods
2.1. Material Preparation
2.2. Characterization and Testing Scheme
3. Results and Discussion
3.1. Crystalline Features
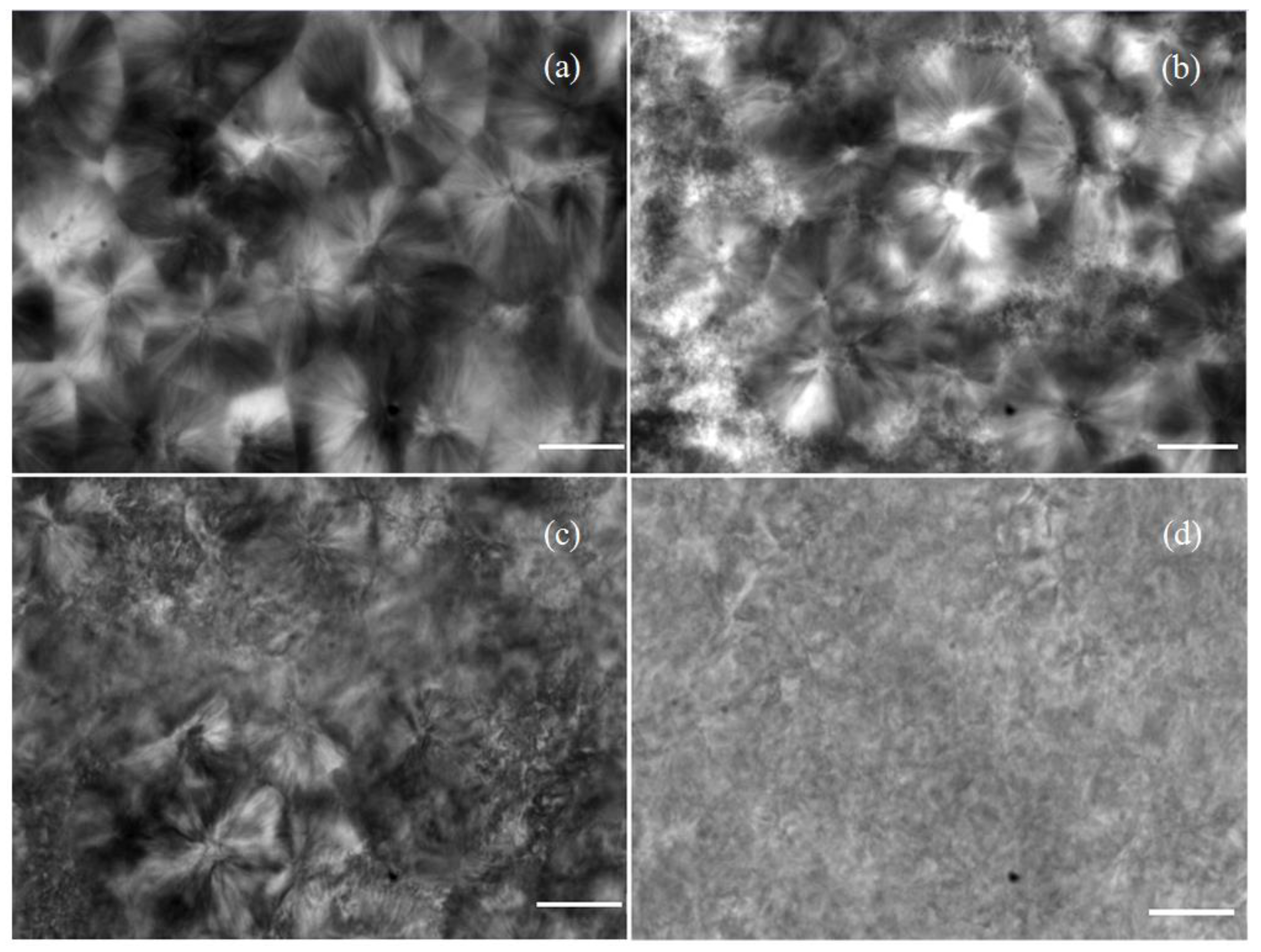
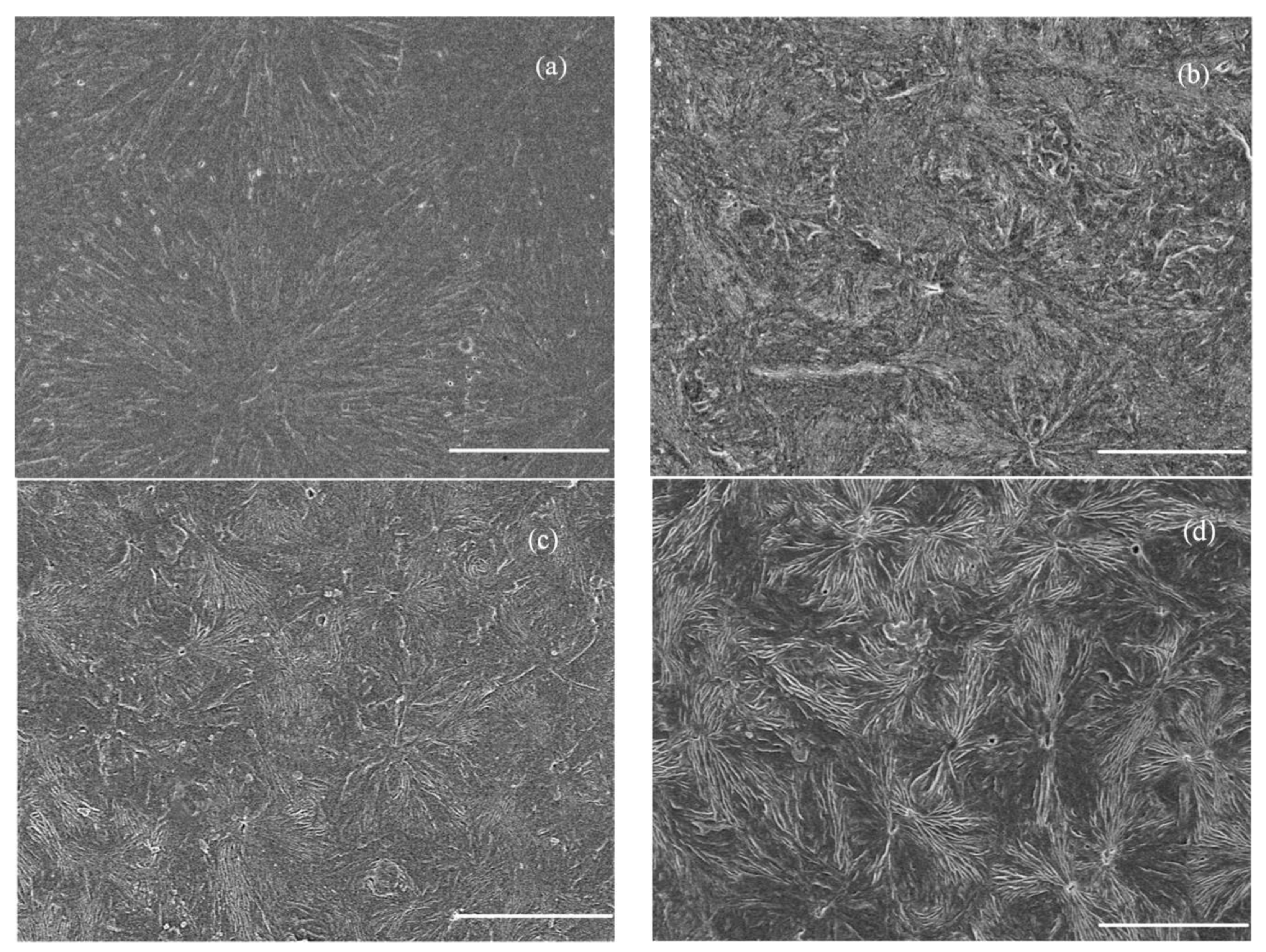
3.2. Crystalline Morphology
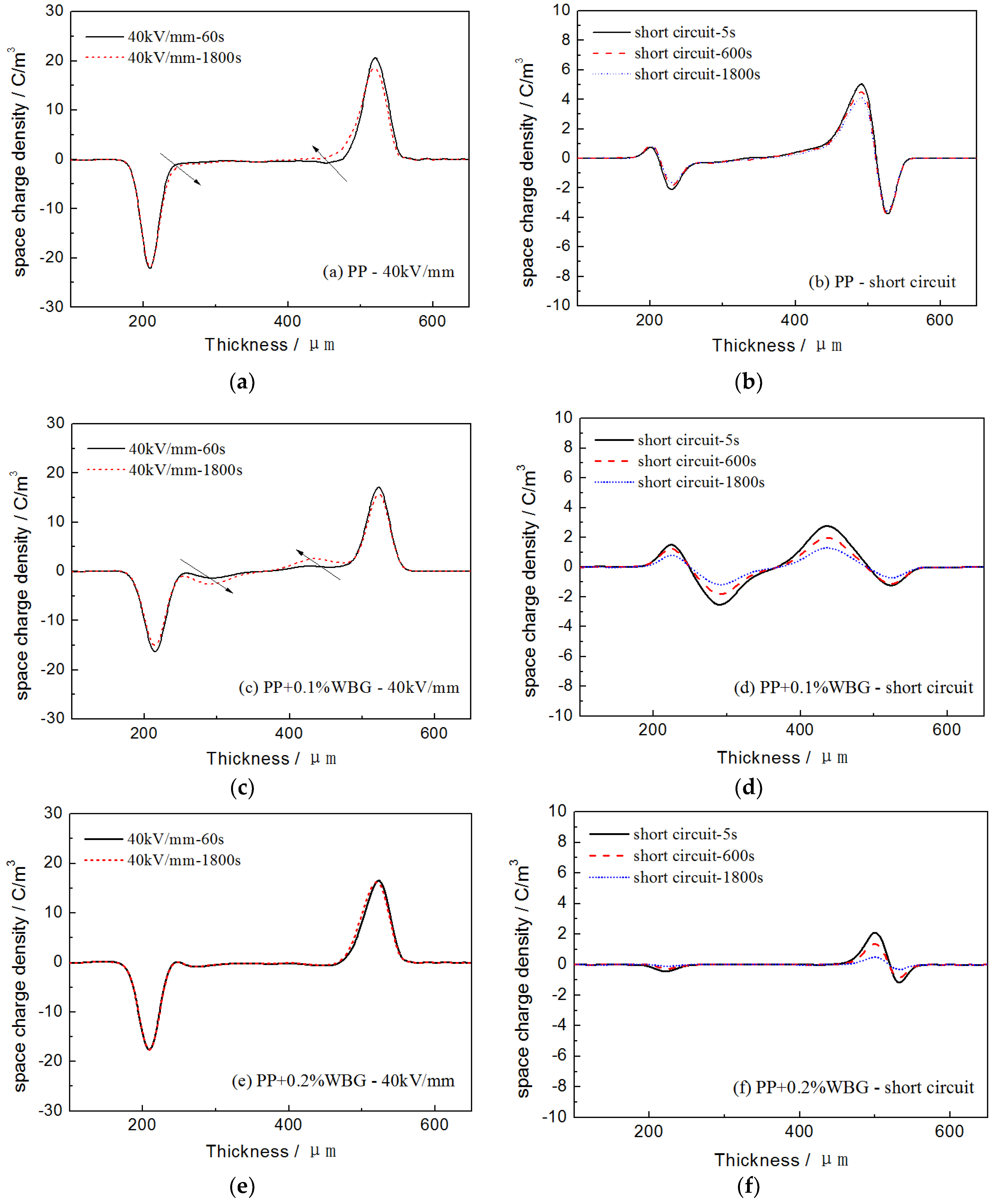
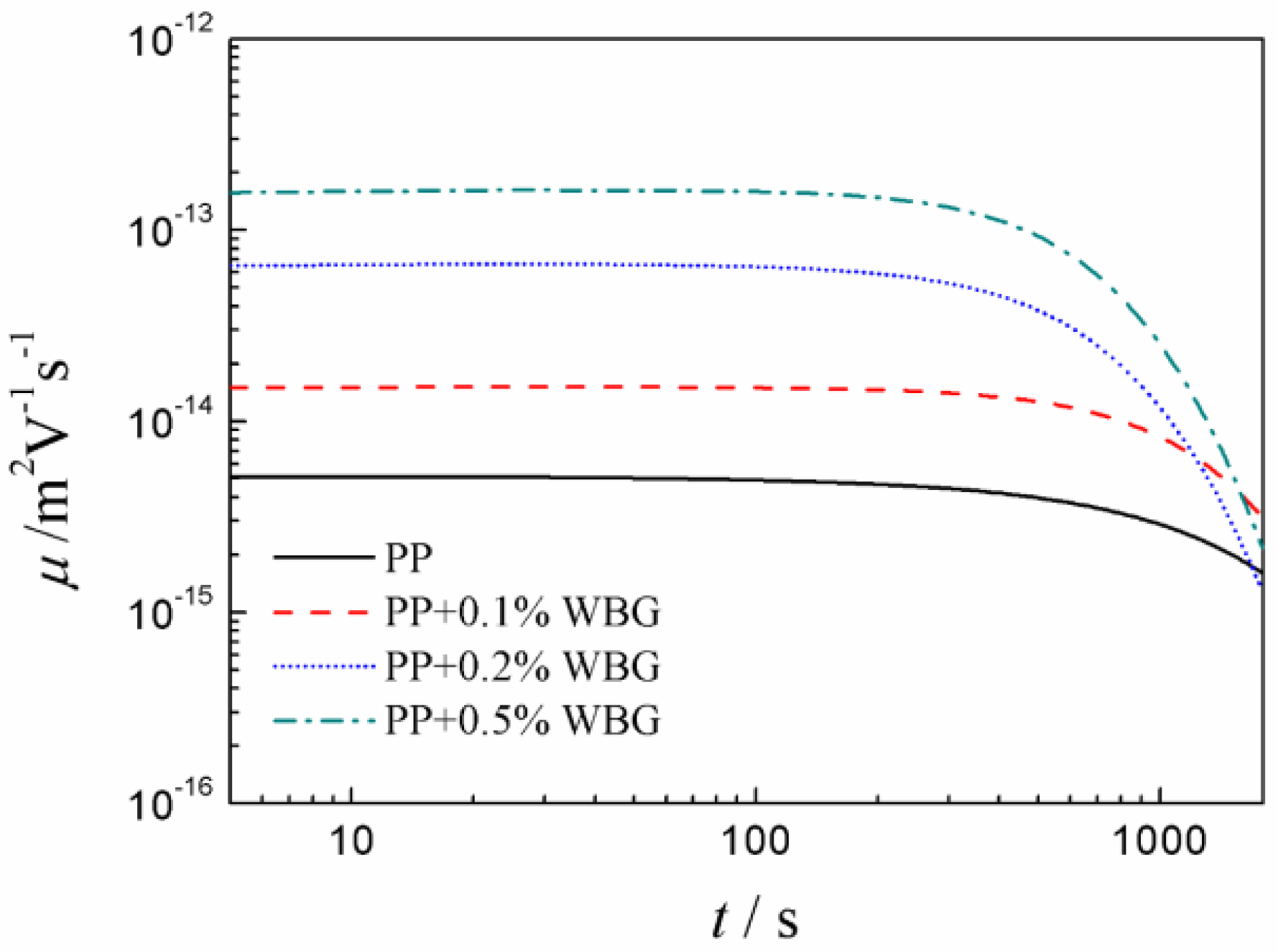
3.3. Space Charge Characteristics
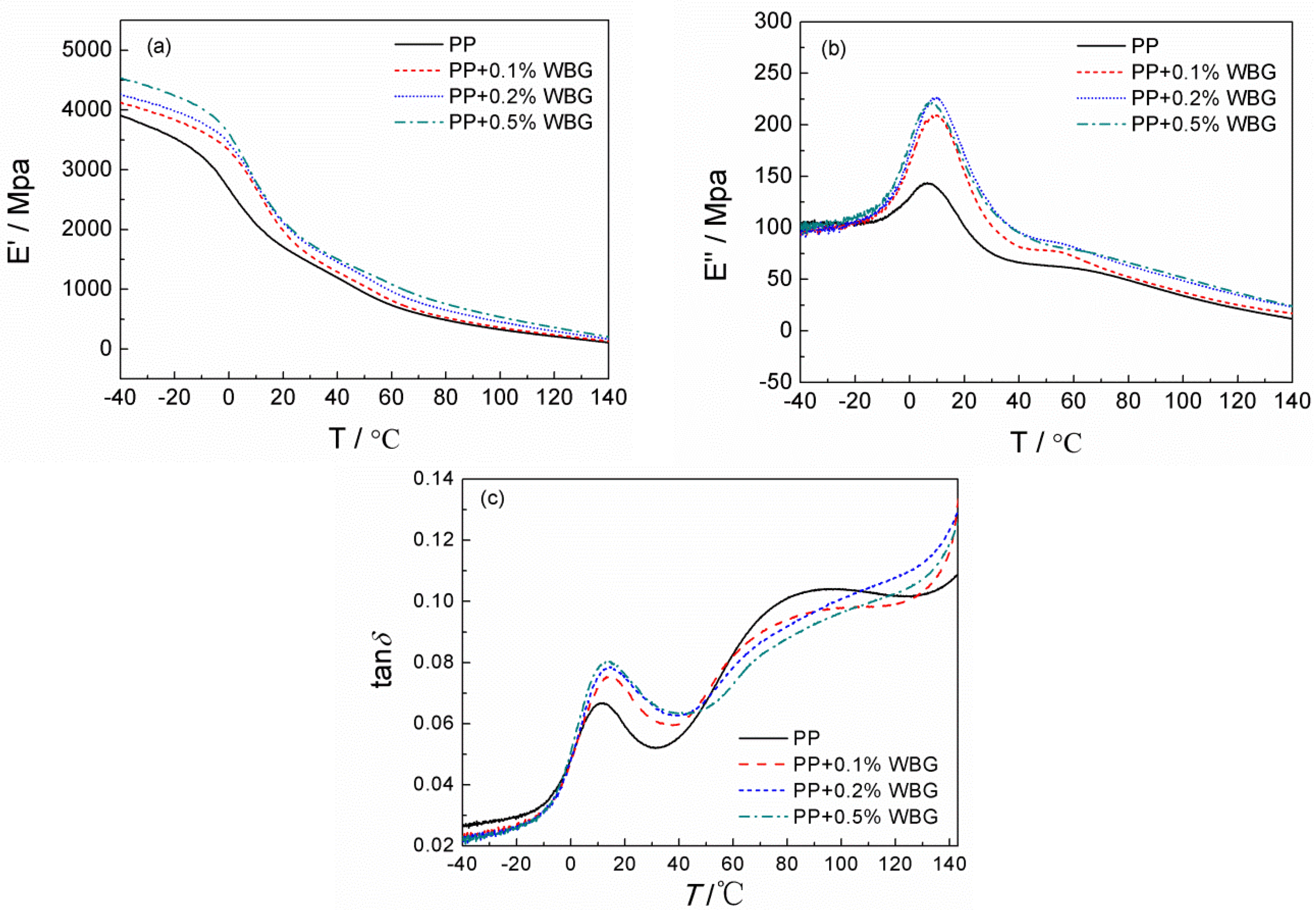
3.4. Mechanical Relaxation Properties
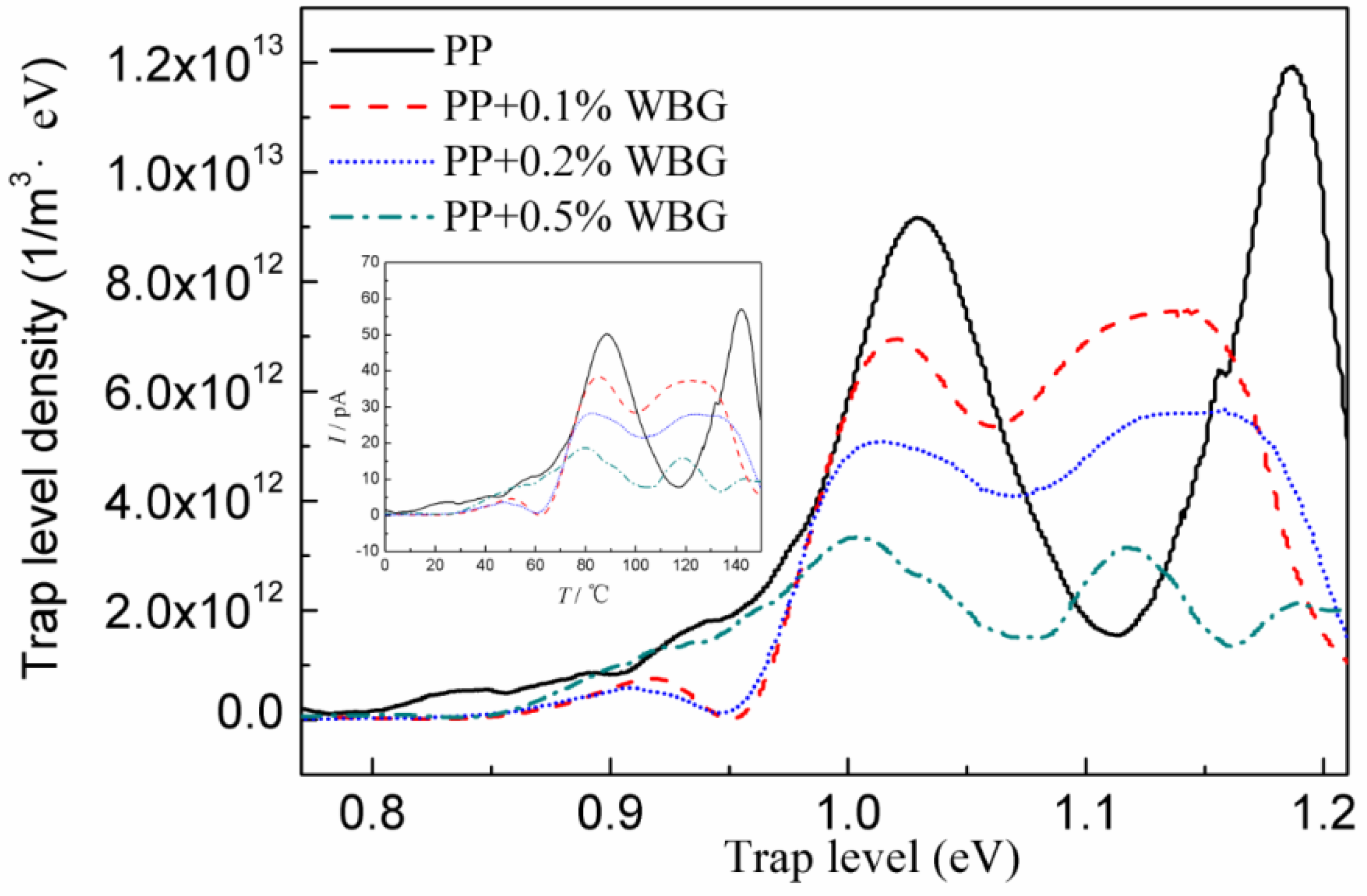
3.5. Charge Trap Characteristics
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Montanari, G.C.; Seri, P.; Lei, X.; Ye, H.; Zhuang, Q.; Morshuis, P.; Stevens, G.; Vaughan, A. Next generation polymeric high voltage direct current cables—A quantum leap needed? IEEE Electr. Insul. Mag. 2018, 34, 24–31. [Google Scholar] [CrossRef]
- He, J.; Chen, G. Insulation materials for HVDC polymeric cables. IEEE Trans. Dielectr. Electr. Insul. 2017, 24, 1307. [Google Scholar] [CrossRef]
- Chi, X.; Cheng, L.; Liu, W.; Zhang, X.; Li, S. Characterization of Polypropylene Modified by Blending Elastomer and Nano-Silica. Materials 2018, 11, 1321. [Google Scholar] [CrossRef] [PubMed]
- Green, C.D.; Vaughan, A.S.; Stevens, G.C.; Pye, A.; Sutton, S.J.; Geussens, T.; Fairhurst, M.J. Thermoplastic cable insulation comprising a blend of isotactic polypropylene and a propylene-ethylene copolymer. IEEE Trans. Dielectr. Electr. Insul. 2015, 22, 639–648. [Google Scholar] [CrossRef]
- Natta, G.; Corradini, P. Structure and properties of isotactic polypropylene. Il Nuovo Cimento (1955–1965) 1960, 15, 40–51. [Google Scholar] [CrossRef]
- Meille, S.V.; Ferro, D.R.; Brueckner, S.; Lovinger, A.J.; Padden, F.J. Structure of beta-Isotactic Polypropylene: A Long-Standing Structural Puzzle. Macromolecules 1994, 27, 2615–2622. [Google Scholar] [CrossRef]
- Valdo Meille, S.; Brückner, S. Non-parallel chains in crystalline γ-isotactic polypropylene. Nature 1989, 340, 455–457. [Google Scholar] [CrossRef]
- Norton, D.R. The spherulitic and lamellar morphology of melt-crystallized isotactic polypropylene. Polymer 1985, 26, 704–716. [Google Scholar] [CrossRef]
- Jin, Y.; Hiltner, A.; Baer, E.; Masirek, R.; Piorkowska, E.; Galeski, A. Formation and transformation of smectic polypropylene nanodroplets. J. Polym. Sci. Part B Polym. Phys. 2010, 44, 1795–1803. [Google Scholar] [CrossRef]
- Luo, F.; Geng, C.; Wang, K.; Deng, H.; Chen, F.; Fu, Q.; Na, B. New Understanding in Tuning Toughness of β-Polypropylene: The Role of β-Nucleated Crystalline Morphology. Macromolecules 2009, 42, 9325–9331. [Google Scholar] [CrossRef]
- Karger-Kocsis, J.; Varga, J.; Ehrenstein, G.W. Comparison of the fracture and failure behavior of injection-molded α- and β-polypropylene in high-speed three-point bending tests. J. Appl. Polym. Sci. 2015, 64, 2057–2066. [Google Scholar] [CrossRef]
- Karger-Kocsis, J. Towards phase transformation toughened semicrystalline polymers. Polym. Bull. 1996, 36, 119–124. [Google Scholar] [CrossRef]
- Grein, C.; Plummer, C.J.G.; Kausch, H.H.; Germain, Y.; Béguelin, P. Influence of β nucleation on the mechanical properties of isotactic polypropylene and rubber modified isotactic polypropylene. Polymer 2002, 43, 3279–3293. [Google Scholar] [CrossRef]
- Krache, R.; Benavente, R.; López-Majada, J.M.; Pereña, J.M.; Cerrada, M.L.; Pérez, E. Competition between α, β, and γ Polymorphs in a β-Nucleated Metallocenic Isotactic Polypropylene. Macromolecules 2007, 40, 897–909. [Google Scholar] [CrossRef]
- Bai, H.; Wang, Y.; Liu, L.; Zhang, J.; Han, L. Nonisothermal crystallization behaviors of polypropylene with α/β nucleating agents. J. Polym. Sci. Part B Polym. Phys. 2010, 46, 1853–1867. [Google Scholar] [CrossRef]
- Hanley, T.L.; Burford, R.P.; Fleming, R.J.; Barber, K.W. A general review of polymeric insulation for use in HVDC cables. IEEE Electr. Insul. Mag. 2003, 19, 13–24. [Google Scholar] [CrossRef]
- Maeno, Y.; Hirai, N.; Ohki, Y.; Tanaka, T.; Okashita, M.; Maeno, T. Effects of crosslinking byproducts on space charge formation in crosslinked polyethylene. IEEE Trans. Dielectr. Electr. Insul. 2005, 12, 90–97. [Google Scholar] [CrossRef]
- Choo, W.; Chen, G.; Swingler, S.G. Electric field in polymeric cable due to space charge accumulation under DC and temperature gradient. IEEE Trans. Dielectr. Electr. Insul. 2011, 18, 596–606. [Google Scholar] [CrossRef]
- Dissado, L.A.; Laurent, C.; Montanari, G.C.; Morshuis, P.H.F. Demonstrating a threshold for trapped space charge accumulation in solid dielectrics under DC field. IEEE Trans. Dielectr. Electr. Insul. 2005, 12, 612–620. [Google Scholar] [CrossRef]
- Gradys, A.; Sajkiewicz, P.; Minakov, A.A.; Adamovsky, S.; Schick, C.; Hashimoto, T.; Saijo, K. Crystallization of polypropylene at various cooling rates. Mater. Sci. Eng. A 2005, 413, 442–446. [Google Scholar] [CrossRef]
- Supaphol, P.; Spruiell, J.E. Crystalline memory effects in isothermal crystallization of syndiotactic polypropylene. J. Appl. Polym. Sci. 2015, 75, 337–346. [Google Scholar] [CrossRef]
- Kolesov, S.N. The Influence of Morphology on the Electric Strength of Polymer Insulation. IEEE Trans. Dielectr. Electr. Insul. 1980, EI-15, 382–388. [Google Scholar] [CrossRef]
- Thyssen, A.; Almdal, K.; Thomsen, E.V. Electret stability related to the crystallinity in polypropylene. IEEE Trans. Dielectr. Electr. Insul. 2017, 24, 3038–3046. [Google Scholar] [CrossRef]
- Way, J.L.; Atkinson, J.R.; Nutting, J. The effect of spherulite size on the fracture morphology of polypropylene. J. Mater. Sci. 1974, 9, 293–299. [Google Scholar] [CrossRef]
- Rowe, S.W.; Tobazeon, R. Characterization of spherulite boundaries and general observations of solvent action on crystallized polypropylene and polybutene. J. Mater. Sci. 1981, 16, 2608–2612. [Google Scholar] [CrossRef]
- Ceres, B.V.; Schultz, J.M. Dependence of electrical breakdown on spherulite size in isotactic polypropylene. J. Mater. Sci. 2010, 29, 4183–4197. [Google Scholar] [CrossRef]
- Zhao, Y.; Vaughan, A.S.; Sutton, S.J.; Swingler, S.G. On the crystallization, morphology and physical properties of a clarified propylene/ethylene copolymer. Polymer 2001, 42, 6587–6597. [Google Scholar] [CrossRef]
- Vaughan, A.S.; Zhaob, Y.; Barréa, L.L.; Sutton, S.J.; Swingler, S.G. On Additives, Morphological Evolution and Dielectric Breakdown in Low Density Polyethylene. Eur. Polym. J. 2003, 39, 355–365. [Google Scholar] [CrossRef]
- Wu, Y.H.; Zha, J.W.; Li, W.K.; Wang, S.J.; Dang, Z.M. A remarkable suppression on space charge in isotatic polypropylene by inducing the β-crystal formation. Appl. Phys. Lett. 2015, 115, 112901. [Google Scholar] [CrossRef]
- Jones, A.T.; Aizlewood, J.M.; Beckett, D.R. Crystalline forms of isotactic polypropylene. Macromol. Chem. Phys. 1964, 75, 134–158. [Google Scholar] [CrossRef]
- Mazzanti, G.; Montanari, G.C.; Alison, J.M. A space-charge based method for the estimation of apparent mobility and trap depth as markers for insulation degradation-theoretical basis and experimental validation. IEEE Trans. Dielectr. Electr. Insul. 2003, 10, 187–197. [Google Scholar] [CrossRef]
- Jourdan, C.; Cavaille, J.Y.; Perez, J. Mechanical relaxations in polypropylene: A new experimental and theoretical approach. J. Polym. Sci. Part B Polym. Phys. 1989, 27, 2361–2384. [Google Scholar] [CrossRef]
- Ieda, M. Electrical Conduction and Carrier Traps in Polymeric Materials. IEEE Trans. Dielectr. Electr. Insul. 1984, 19, 162–178. [Google Scholar] [CrossRef]
- Suh, K.S.; Tanaka, J.; Damon, D. What is TSC? IEEE Electr. Insul. Mag. 2016, 8, 13–20. [Google Scholar] [CrossRef]
- Tian, F.; Bu, W.; Shi, L.; Yang, C.; Wang, Y.; Lei, Q. Theory of modified thermally stimulated current and direct determination of trap level distribution. J. Electrost. 2011, 69, 7–10. [Google Scholar] [CrossRef]
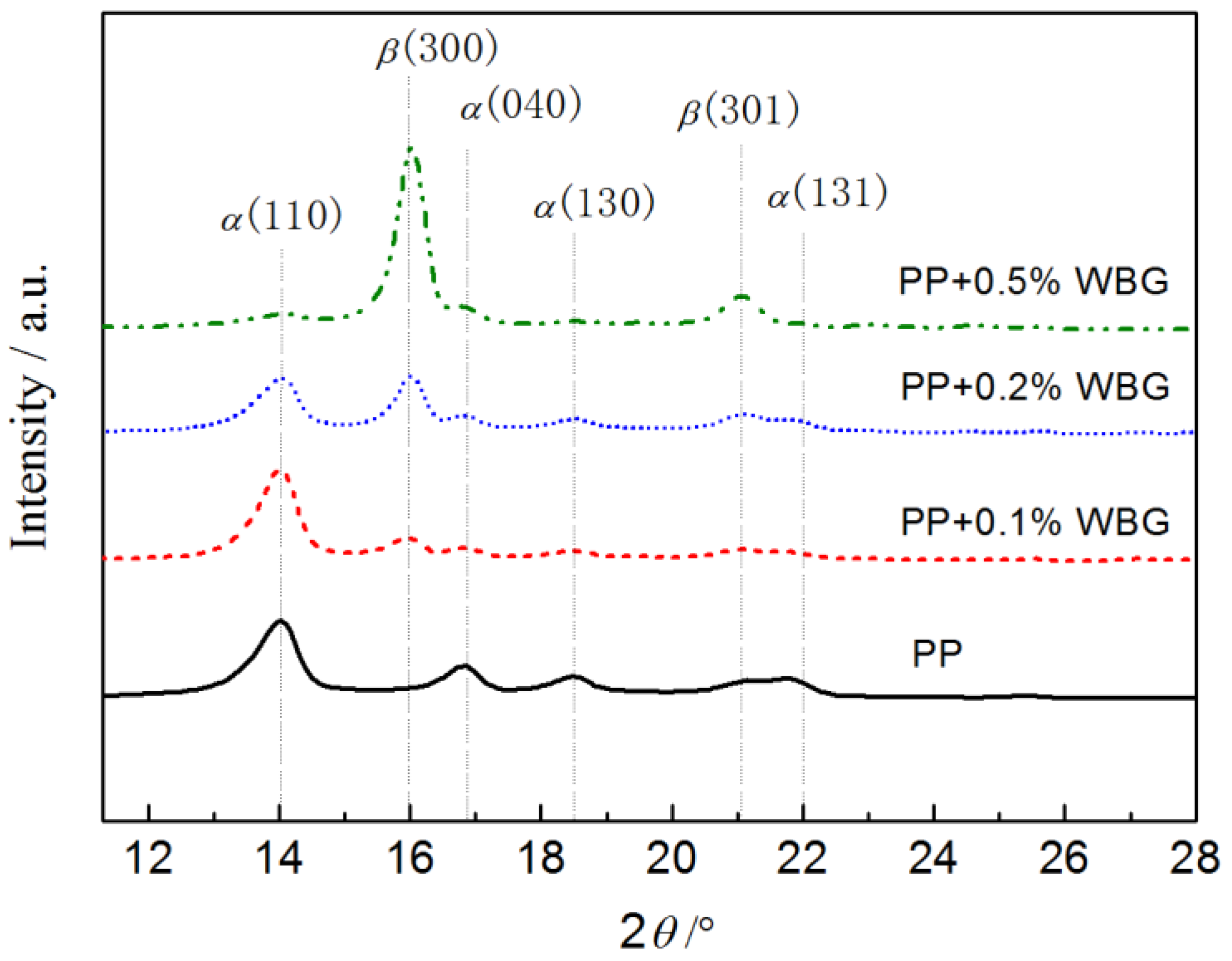



| Samples | Diffraction Peak Intensity | ||||
|---|---|---|---|---|---|
| Hα(110) | Hα(040) | Hα(130) | Hβ(300) | Kβ, % | |
| PP | 35,170 | 11,764 | 7011 | 0 | 0 |
| PP + 0.1% WBG | 36,343 | 2721 | 2683 | 7811 | 15.8 |
| PP + 0.2% WBG | 22,738 | 4538 | 4186 | 24,546 | 43.8 |
| PP + 0.5% WBG | 5963 | 3645 | 445 | 85,655 | 89.5 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, J.; Gao, M.; Zhao, H.; Liu, S.; Hu, M.; Xie, S. Space Charge Characteristics of Polypropylene Modified by Rare Earth Nucleating Agent for β Crystallization. Materials 2019, 12, 42. https://doi.org/10.3390/ma12010042
Yang J, Gao M, Zhao H, Liu S, Hu M, Xie S. Space Charge Characteristics of Polypropylene Modified by Rare Earth Nucleating Agent for β Crystallization. Materials. 2019; 12(1):42. https://doi.org/10.3390/ma12010042
Chicago/Turabian StyleYang, Jiaming, Mingze Gao, Hong Zhao, Shilin Liu, Ming Hu, and Shuhong Xie. 2019. "Space Charge Characteristics of Polypropylene Modified by Rare Earth Nucleating Agent for β Crystallization" Materials 12, no. 1: 42. https://doi.org/10.3390/ma12010042
APA StyleYang, J., Gao, M., Zhao, H., Liu, S., Hu, M., & Xie, S. (2019). Space Charge Characteristics of Polypropylene Modified by Rare Earth Nucleating Agent for β Crystallization. Materials, 12(1), 42. https://doi.org/10.3390/ma12010042





